Abstract
Background
Scalp acupuncture (SA) and repetitive transcranial magnetic stimulation (rTMS) are effective for treating cerebral infarction. This study aims to examine the efficacy and safety of SA and electromagnetic convergence stimulation (SAEM-CS), which was developed through collaboration between conventional medical physicians and doctors who practice traditional Korean medicine. SAEM-CS was designed to improve function in patients with cerebral infarction, compared to the improvement after conventional stroke rehabilitation, SA, and rTMS therapeutic approaches.
Methods/design
This study is a prospective, outcome assessor-blinded, randomized controlled clinical trial with a 1:1:1:1 allocation ratio. Participants with motion or sensory disabilities caused by a first-time cerebral infarction (n = 60) that had occurred within 1 month of the study onset will be randomly assigned to control, SA, rTMS, or SAEM-CS groups. All groups will receive two sessions of conventional rehabilitation treatment per day. The SA group will receive SA on the upper limb area of MS6 and MS7 (at the lesional hemisphere) for 20 min, the rTMS group will receive low-frequency rTMS (LF-rTMS) treatment on the hot spot of the M1 region (motor cortex at the contralesional hemisphere) for 20 min, and the SAEM-CS group will receive LF-rTMS over the contralesional M1 region hot spot while receiving simultaneous SA stimulation on the lesional upper limb area of MS6 and MS7 for 20 min. SA, rTMS, and SAEM-CS treatments will be conducted once/day, 5 days/week (excluding Saturdays and Sundays) for 3 weeks, for a total of 15 sessions. The primary outcome will be evaluated using the Fugl‐Meyer Assessment, while other scales assessing cognitive function, activities of daily living, walking, quality of life, and stroke severity are considered secondary outcome measures. Outcome measurements will be conducted at baseline (before intervention), 3 weeks after the first intervention (end of intervention), and 4 weeks after intervention completion.
Discussion
This study aims to explore the efficacy and safety of SAEM-CS on cerebral infarction. Collaborative research combined traditional Korean and conventional medicines, which can be useful in developing new treatment technologies.
Trial registration
KCT0001768. Registered on 14 January 2016.
Similar content being viewed by others
Background
Cerebral infarction (CI) is one of the most commonly reported cerebral vascular diseases, accounting for about 70 % of strokes [1]. The incidence, mortality, and recurrence rates of CI are high, and CI usually leads to serious damage of the central nervous system [2]. Despite a considerable amount of research on effective treatments for stroke, there is still no single intervention that clearly and definitively contributes to stroke recovery. Therefore, stroke treatment strategies should combine multiple disciplines, such as neurology, rehabilitation medicine, and traditional medicine [3]. Scalp acupuncture (SA) is one of several specialized acupuncture techniques, and it involves a filiform needle being used to penetrate specific stimulation areas on the scalp [4]. SA therapy for ischemic and hemorrhagic stroke has been empirically established and is used worldwide [5–8]. The local application of repetitive transcranial magnetic stimulation (rTMS) influences the neural excitability of selected brain areas [9], and it has been reported that low-frequency stimulation (1 Hz) suppresses local neural activities [10, 11], whereas high-frequency stimulation (≥5 Hz) activates local neural activities [12]. Both high-frequency rTMS (HF-rTMS) applied to the lesional hemisphere and low-frequency rTMS (LF-rTMS) applied to the contralesional hemisphere are beneficial for upper limb hemiparesis in patients with chronic stroke [11, 13–17] and in the early phase of stroke [9, 18–20].
Conventional medical physicians and doctors who practice traditional Korean medicine (TKM) tend to focus on each field’s treatment methods in separate medical systems. Unfortunately, efforts to improve the treatment rates of incurable diseases through collaborative research and via the fusion of treatment techniques are lacking. The purpose of this study is to explore the efficacy and safety of SA and electromagnetic convergence stimulation (SAEM-CS), which was developed through a collaborative study between the Department of Physical and Rehabilitation Medicine at Chonnam National University Hospital and the Departments of Acupuncture and Moxibustion Medicine and Traditional Korean Medicine Rehabilitation at Dong-Shin University. Thereafter, we will compare SAEM-CS to conventional stroke rehabilitation, SA, and rTMS therapeutic approaches.
Methods/design
Objective
The objective of this study is to compare the efficacy of SAEM-CS on motor function recovery to physical therapy, rTMS, and SA. Further, we aim to explore the synergistic effect of converging SA and rTMS, which are known to be effective in promoting the recovery of motor function, in patients with CI.
Study design
This study is a prospective, outcome assessor-blinded, single-center, randomized controlled clinical trial with a 1:1:1:1 allocation ratio. This study is a pilot study to investigate the efficacy and safety of SAEM-CS, which is a newly developed treatment. This study is designed as a single-center study to overcome several procedural and organizational difficulties in terms of developing a research team, since SAEM-CS involves the simultaneous conduction of SA and rTMS. Participants (n = 60) who fit the inclusion and exclusion criteria will be randomly allocated into a control group (n = 15), SA group (n = 15), rTMS group (n = 15), and SAEM-CS group (n = 15). All groups will receive conventional stroke rehabilitation therapy twice/day, five times/week (excluding Saturdays and Sundays), for a total of 15 times over the course of a 3-week hospitalization period at Chonnam National University Hospital. In addition, the SA group will receive SA therapy, the rTMS group will receive rTMS therapy, and the SAEM-CS group will receive SAEM-CS therapy once/day. Outcome measures will be determined at baseline (before intervention), 3 weeks after the first intervention (end of the intervention), and 4 weeks after completion of the intervention.
This study was approved by the Ministry of Food and Drug Safety (MFDS), Medical Device Clinical Trial Plan Approval number 516. (See Additional file 1.)
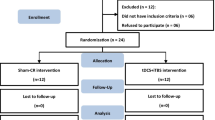
The study design is summarized in Table 1 and Fig. 1.
Participant recruitment
For achieving adequate participant enrollment to reach target sample size, all stroke patients admitted to the Department of Neurology of Chonnam National University Hospital will be screened by Physical and Rehabilitation Medicine doctors. From among the first-ever patients with CI who are hospitalized and have finished treatment of early acute stage CI at the Department of Neurology, those who are assessed as stable by a neurologist and meet the inclusion and exclusion criteria will be recruited for participation in this study.
Patients who are given an explanation about this study by the Clinical Research Coordinator (CRC) and who voluntarily sign a consent form will be transferred to the Department of Physical and Rehabilitation Medicine to participate in this study (see Additional file 2). The CRC will continuously monitor the medical conditions of enrolled participants to improve adherence to intervention protocols.
Inclusion criteria
Patients who meet all of the following conditions will be considered for enrollment: patients who are aged >19 years; who have incipient CI confirmed by a computed tomography or magnetic resonance imaging examination; who are diagnosed by a neurologist or neurosurgeon; who experience a CI that resulted in motor and sensory disorders within 1 month of the study onset; who can undergo rehabilitation therapy after hospitalization in the Department of Physical and Rehabilitation Medicine, Chonnam National University Hospital; who have a modified Rankin Scale (mRS) score of 2–4; and who voluntarily sign an informed consent form.
Exclusion criteria
Subjects whose general condition is not good or fit for SA and rTMS therapies will be excluded. Additional exclusion criteria are as follows: history of brain lesion (e.g., stroke, serious mental illness, loss of consciousness accompanied by head trauma, brain surgery, or seizure disorder); presence of other serious illnesses (e.g., cancer, Alzheimer’s disease, epilepsy, head trauma, or cerebral palsy); transient ischemic attacks; contraindications to electro-magnetic stimulation (e.g., metal implants in the brain, implanted electronic devices in the body, such as non-detachable ferromagnetic metals, metal-sensitive implants less than 30 cm away from the brain like cochlear implants, pacemakers, aneurysm clips or coils, stents, bullet fragments, deep brain stimulation, vagus nerve stimulators, jewelry, or hairpins); continuous convulsion symptoms; previous craniectomy or shunt surgery; elevated intracranial pressure symptoms such as headache, vomiting, nausea, etc.; seizure disorder or epilepsy after CI; history of stroke accompanied by a clear clinical sign; contraindications to SA (e.g., scalp scarring, inflammation from scalp injury, or infection in the treatment region, inability to stop blood flow due to clotting disturbances, such as hemophilia, etc., serious unusual response after acupuncture treatment); women who are pregnant or breastfeeding; patients who disagree with the informed consent; and individuals scheduled for surgery within 2 weeks.
Ethical considerations
The Institutional Review Board of Chonnam National University Hospital approved this study (CNUH-2015-114; see Additional file 3). The purpose and potential risks of this clinical trial will be fully explained to the patients and their families. All patients will be asked to provide written informed consent before participating in this study.
Randomization
After signed informed consent and baseline measurements are obtained, random allocation software (developed by M. Saghaei, M.D., at the Department of Anesthesia, Isfahan University of Medical Sciences, Isfahan, Iran) is used to assign a serial number to the 60 research volunteers and to randomly allocate 15 of them into each group. The serial number codes will be inserted into sealed, opaque envelopes, kept in a double-locked cabinet, and opened in the presence of the patient and a guardian.
Implementation
A CRC will generate the allocation sequence, enroll participants, and assign participants to interventions.
Blinding
We have no choice but to adopt a single blinding (outcome assessor blinding) approach, as sham treatment — where participants and practitioners are not aware of the treatment condition — is impossible due to the characteristics of scalp acupuncture which include scalp penetration. Efforts to maintain objectivity are made by separating the CRC who manages the treatment schedules, the practitioner who conducts the treatments, and the assessor. During the course of this clinical trial, the assessor will not come into contact with any of the participants except at the time of assessment. Further, there is no circumstance where unblinding will be permitted. To prevent risks of bias, such as selection, performance, and attrition caused by non-blinding of participants and practitioners, only individuals without conflicts of interest or preconceived positions are involved in this study. All practitioners will receive training in clinical trials prior to participation in this study.
Intervention
All participants will receive occupational therapy, which focuses on practicing fine and gross motor movements, activities of daily living, task-oriented therapeutic exercises, and muscular electrical stimulation therapy, as needed. Training for swallowing and to improve language will be also performed for dysarthria. These sessions will be conducted for 30 min, twice daily (excluding Saturdays and Sundays) for 3 weeks, for a total of 15 times. SA, rTMS, and SAEM-CS therapies will be conducted once daily for 20 min (excluding Saturdays and Sundays) for 3 weeks, for a total of 15 times (see Table 2).
The SA therapy method will be conducted as follows: one or two needles are horizontally inserted about 3 cm deep at the lesion site and upper limb regions of MS6 (line connecting GV21 and GB6) and MS7 (line connecting GV20 and GB7) [8] under the Standard International Nomenclature in the direction from GV21 to GB6 and GV20 to GB7. Manual stimulation and electroacupuncture are not applied, and the needles (KOS-92 non-magnetic steel acupuncture needles; size 0.25 mm × 30 mm; Dong Bang Acupuncture, Inc., Boryeong, Republic of Korea; Product no: A84010.02) (see Table 3) are left in position for 20 min (see Table 4).
The rTMS method will be conducted as follows: a 70-mm figure 8 coil and a Magstim Rapid stimulator (Magstim Co., Dyfed, UK) is used to deliver 1 Hz of rTMS to the skull of the contralesional hemisphere at the site that elicits the largest motor evoked potentials (MEPs) in the first dorsal interosseous (FDI) muscle of the unaffected upper limb. One LF-rTMS session consists of 1200 pulses and lasts for 20 min. The stimulation intensity is set to 80 % of the motor threshold of the FDI muscle, which is defined as the lowest intensity of stimulation that provokes MEPs [15, 16]. All patients sit in a reclining wheelchair and are asked to relax as much as possible, with their heads strapped to a headrest [9].
The SAEM-CS treatment method will be conducted as follows: the above-described SA and LF-rTMS therapies are performed simultaneously. After SA treatment of MS6 and MS7 on the lesion side, LF-rTMS stimulation is conducted on the contralateral hemisphere of the opposite side of the lesion for 20 min (Fig. 2).
The SA therapy will be conducted by a doctor who is experienced in Korean medicine, is affiliated with Dong-Shin Traditional Korean Medicine University Hospital, and has more than 2 years of clinical experience. rTMS will be conducted by a doctor affiliated with the Department of Physical and Rehabilitation Medicine at Chonnam National University Hospital.
During the clinical trial period, all participants are allowed routine management, existing medications (medications for hypertension, diabetes, hyperlipidemia, and improvement of brain function), and medications to maintain and improve health status. However, patients are not allowed to engage in treatments to improve CI other than the therapies used in this study.
All medical devices will be inspected by coordinators who will manage scalp acupuncture needles (Min-yeong Song, Resident of Department of Korean Medicine Rehabilitation, College of Traditional Korean Medicine, Dong-Shin University) and rTMS (Eom-ji Kim, occupational therapist, Department of Physical and Rehabilitation Medicine at Chonnam National University Hospital). Coordinators will record the results of the check-ups in the management register.
Outcome measurements
Primary and secondary outcome assessments will be conducted at baseline (before intervention), 3 weeks after the first intervention, and 4 weeks after completion of intervention.
Primary outcome
Since the objective of this study is to investigate the efficacy of SAEM-CS on motor function recovery in patients with cerebral infarction, the primary outcome will be assessed via changes on the Fugl‐Meyer Assessment (FMA) scale for motor function. The FMA is widely used as a qualitative measure of motor function and is used in the current study to assess upper and lower limb function. The FMA is categorized into 50 items based on a six-stage recovery process of Brunnstrom’s hemiplegia classification and progress record. This assessment is an ordinal scale, in which 0 is given for unable to perform, 1 for partial performance, and 2 for complete performance. The FMA has a possible total score of 100, 66 points of which apply to the upper limbs and 34 points to the lower limbs [16].
Secondary outcomes
Secondary outcome measures will be assessed changes in the National Institutes of Health Stroke Scale (NIHSS) score, Modified Barthel Index (MBI), Functional Independence Measurement (FIM) score, Korean Mini-Mental State Examination (K-MMSE) score, American Speech-Language-Hearing Association National Outcomes Measurement System (ASHA-NOMS) Swallowing Scale score, Functional Ambulation Categories (FAC), European Quality of Life-5 Dimensions (EQ-5D), Modified Ashworth Scale (MAS) score, Hand Grip Strength Test, MEPs, mRS score, and 9-Hole Peg Test (9HPT).
The NIHSS is a scale that was developed by the United States National Institutes of Health, and provides useful information on the severity, prognosis, and early treatment of stroke [9].
The MBI is an assessment tool used to objectively evaluate performance of everyday movements on 10 items: personal hygiene, bathing, eating, toilet use, ability to climb stairs, ability to dress, bowel/bladder function, walking function, capability of getting into and out of a chair/bed, and mobility [21].
The FIM is an assessment of everyday movement performance that evaluates 13 detailed items of motor FIM and 5 detailed items of cognitive FIM. The FIM has a total possible score of 18–126, and each item is divided into seven stages, allowing for a comparably detailed assessment. Higher FIM scores indicate higher functional independence [22].
The MMSE is a brief global instrument used to assess cognitive abilities and has been translated into Korean (K-MMSE) [23]. The K-MMSE is a quick screening assessment tool that was developed to evaluate cognitive function and dementia in elderly patients with suspected dementia or patients with brain damage. The instrument consists of measures used to assess orientation, recall, attention and calculation, language, judgment, and understanding. A score ≥24 points indicates definitive normal, 20–23 indicates suspected dementia, and ≤19 indicates definitive dementia [24].
The ASHA-NOMS is a seven-stage dysphagia scale developed by the American Speech-Language-Hearing Association to evaluate severity of dysphagia for items such as parenteral hyperalimentation, whether independent eating is possible, use of compensative skills for eating, and diet limitations [25].
The FAC is designed to evaluate walking ability where a patient’s independent walking ability is categorized into six ranks: 0 = unable to walk, 1 = direct assistance required by the therapist, 2 = intermittent and direct assistance required by the therapist, 3 = able to walk independently but requires observation, 4 = able to walk independently but requires assistance on uneven surfaces, and 5 = able to independently walk on even surfaces [26].
The EQ-5D evaluates quality of life in five fields including exercise ability, self-management, daily activities, pain/discomfort, and anxiety/depression. Each field is divided into three levels with 1 indicating a good state of health. The EQ-5D also includes a visual analog scale in which a subject indicates subjective and overall individual health status on a vertical scale where the best health state is 100 points and the poorest health state is 0 points [27].
The MAS assesses muscles by measuring spasticity in the wrist and elbow joints while the joints are maximally bent. Scores range from 0, where there is no increase in muscle tone, to 4, where there is contracture in bent and straight muscles and/or joints [15].
The Hand Grip Strength Test evaluates muscle strength in the hands and is conducted twice in six stages; the higher value (kg) of the two tests is recorded [9].
In the current study, MEPs are evoked by stimulating the primary motor cortex on representation of hand grip muscles without pain, and responses of the FDI muscle are then observed. MEPs are useful to predict functional recovery in CI. Here, the cerebral hemisphere is magnetically stimulated in a figure 8 shape while the patient is lying down. Stimulations increase in intensity from 0 to 100 % until the MEP with the shortest latency occurs. The latency and the amplitude of the MEP responses are recorded [28].
The mRS is a six-point, ordinal hierarchical scale that describes “global disability” with a focus on mobility. The mRS is often used in contemporary stroke studies as a measure of premorbid ability and is used to assist in the selection of patients and as a final outcome measure. The mRS uses a 5-min non-standardized interview, and has six potential scores (0–5), which describe a full range of stroke outcomes; a score of 6 denotes death [29, 30].
The 9HPT is useful in measuring dexterity in relatively well-recovered patients. In the current study, participants are instructed to place nine pegs into nine holes on a board as fast as possible. Scores are computed as pegs/s, averaged over three trials, and normalized to the average score of the unaffected hand (range 0–1; 0 = cannot do) [31].
Incidence of adverse events
Adverse events refer to undesirable and unintentional signs, symptoms, or diseases that appear after treatment in a clinical trial. Importantly, these events may not have a causal relationship with the therapy employed; nevertheless, they should be documented. Adverse events expected from this study include nausea, vomiting, headaches, dizziness, skin irritation, convulsions, and objective worsening of existing symptoms. The CRC will record adverse events, their progress, and their causal relationship with treatments in detail and will report them to the principal investigator (PI) and Institutional Review Board (IRB). If serious adverse events occur, defined as those causing severe disability or malfunction, then appropriate measures will be taken and incidents will be immediately reported to the PI and IRB.
Sample size
The sample size was calculated based on Cohen’s formula. As there are very few precedent studies that have conducted SAEM-CS similar to the present study, and because characteristics of the variables being tested are diverse, it was difficult to select and apply a single effect size. Thus, we set the effect size as 0.25 based on a medium f, which is one of Cohen’s criteria [32]. Accordingly, we have established the number of groups as 4, number of repetitions as 3, effect size as 0.25, significance level as 0.05, and statistical power as 0.8. Therefore, the total sample size required for a repeated measures ANOVA (taking into account interaction time with the treatment method), calculated using G*power, is 40 (10/group) [33].
Also, because the present study is an exploratory clinical trial, we determined that a total of 60 participants, 15 in each group, would be more appropriate. We based this on a previous study that recommended 12 participants per group for pilot studies that lack prior information [34] and other conditions used to determine the sample size for a pilot study [35] that gave a sample size of 12 per group, to which a drop-out rate of 25 % was applied. In order to minimize the dropout, the CRC will manage the treatment schedule of subjects.
Moreover, we considered the feasibility of the study, since participants will be patients with CI (which, as of 2014 average 46 per month) being treated at the Department of Neurology at the Chonnam National University Hospital. Considering the fact that among them, only 10–20 % will receive inpatient treatment at the Department of Rehabilitative Medicine and meet the selection criteria, it reaffirmed our estimation that 60 participants would be appropriate and feasible to achieve the study objectives. This also takes into account recruitment, study period, and drop-out possibilities.
Data monitoring
The Data Monitoring Committee (DMC) is composed of the PI and coordinators who are in charge of scalp acupuncture needles (Min-yeong Song, resident of the Department of Korean Medicine Rehabilitation, College of Traditional Korean Medicine, Dong-Shin University) and rTMS (Eom-ji Kim, an occupational therapist in the Department of Physical and Rehabilitation Medicine at Chonnam National University Hospital). If needed, they will report the monitoring results to the PI. Finally, this study is independent from any sponsors.
Data analysis
Continuous data will be presented as means and standard deviations, while categorical data will be presented as frequencies and percentages. A repeated measures ANOVA will be conducted for the FMA and all secondary outcomes (NIHSS, MBI, FIM, K-MMSE, 9HPT, ASHA-NOMS, FAC, EQ-5D, MAS, Hand Grip Strength, and MEPs). Dependent variables include measured values before and after intervention and the measured values 4 weeks after completion of intervention. An F test will be conducted to detect differences between therapies, and Tukey’s post hoc test will be conducted to identify the groups. Repeated contrast tests will be conducted to account for time differences in each group, and the interaction group and time test will be conducted. A p value of <0.05 will be considered significant, and participants who drop out of the study will be excluded from the analysis. In brief, only complete case analyses will be used; thus, only subjects who complete the three evaluations will be analyzed. All statistical analyses will be performed using SPSS version 22.0 software (SPSS Inc., Chicago, IL, USA).
Data from participants who engage in less than 70 % of protocol adherence (i.e., receive <10 treatments from 15 trials) will be eliminated. Missing values will be implemented by multiple imputations. In addition, statistical differences will be verified by comparing attribution between eliminated and completed participants to check particular factors causing drop-out.
Confidentiality and data management
Participant identification records will be kept confidential until the results of this study are published. All documents related to a clinical trial, such as case report forms (CRFs), will be recorded and labeled with participant identification codes and will not show the name of the participant. The serial number codes will be inserted in sealed, opaque envelopes, kept in a double-locked cabinet, and opened in the presence of the patient and a guardian.
All data of the participants will be recorded in Excel files by the CRC. Additionally, raw data (CRFs) will be kept in a cabinet until the end of this study.
Written informed consent will be obtained from the participants for publication of their individual details and accompanying images in this manuscript. The consent forms will be available for review by the Editor-in-Chief.
Discussion
This protocol, following the Standard Protocol Items: Recommendations for Interventional Trials (SPIRIT) advice and Consolidated Standards Of Reporting Trials (CONSORT) 2010 guidelines, is designed to explore the synergistic effect of SAEM-CS on the recovery of motor function in patients in the acute stage of CI [36] (Additional file 4). We expect that rTMS will enhance the effect of SA and compensate for a commonly encountered problem in SA treatment: specifically, difficulty in twirling the acupuncture needle (as these needles should be twirled more than 200 times/min).
SAEM-CS is a treatment technique that combines LF-rTMS over the M1 region hot spot (motor cortex at the contralesional hemisphere) and SA stimulation of MS6 and MS7 at the upper limb regions of the lesional hemisphere to promote optimal functioning in patients following stoke. Despite other research showing that HF-rTMS applied to the lesional hemisphere more effectively improves motor function than LF-rTMS applied to the contralesional hemisphere in the early phases of stroke [9], LF-rTMS is selected for the combined SAEM-CS approach because there is some concern about complications that can arise due to the simultaneous use of SA and rTMS in the same hemisphere, and since we cannot completely exclude the interfering effects of HF-rTMS, even with non-magnetic needles. rTMS creates a powerful magnetic field near the stimulated area; therefore, all metal materials must be removed [37]. For rTMS and SA therapies that are conducted simultaneously (during SAEM-CS), we use a KOS-92 non-magnetic steel acupuncture needle that contains a much higher percentage of nitrogen (N) than STS304N1; thus, even in high processing volumes, the needle has no magnetic properties. The same method used in the SAEM-CS approach is employed for the SA and rTMS therapies. Thus, manual stimulation and electroacupuncture are not conducted in the SA group, and LF-rTMS is applied to the contralesional hemisphere in the rTMS group.
In the current study, we select patients hospitalized within 1 month after acute stroke in order to increase the homogeneity of the experimental population. This period was also chosen to increase reliability, because the most important period of recovery falls within the acute and subacute stages of ischemic stroke [38].
We expect that SAEM-CS will improve motor function; therefore, the FMA was selected as the primary outcome measure. In order to investigate unexpected effects of SAEM-CS, other scales assessing cognitive function, activities of daily living, walking, quality of life, and stroke severity are employed as secondary outcome measures.
Among TKM and CM professionals, there is a general lack of cooperation for developing techniques aimed to treat obstinate diseases. If the efficacy and safety of the above-described SAEM-CS approach is proven, it will serve as a model for collaborative research and will promote CM and TKM professionals to fuse therapeutic techniques. One hopes that such approaches can improve the treatment rates of incurable diseases.
Dissemination policy
We will report the final data to the Ministry of Health and Welfare through the Korea Health Industry Development Institute, and we will publish the results at the end of this study.
Trial status
This trial is ongoing. Enrollment and trial completion are expected to be finished by the end of May 2017.
Abbreviations
- CI:
-
Cerebral infarction
- SA:
-
Scalp acupuncture
- rTMS:
-
Repetitive transcranial magnetic stimulation
- SAEM-CS:
-
Scalp acupuncture and electromagnetic convergence stimulation
- CM:
-
Conventional medicine
- TKM:
-
Traditional Korean medicine
- FMA:
-
Fugl-Meyer Assessment
- NIHSS:
-
National Institutes of Health Stroke Scale
- MBI:
-
Modified Barthel Index
- FIM:
-
Functional Independence Measurement
- MMSE:
-
Mini-Mental State Examination
- ASHA-NOMS:
-
American Speech-Language-Hearing Association National Outcomes Measurement System
- FAC:
-
Functional Ambulation Categories
- EQ-5D:
-
European Quality of Life-5 Dimensions
- MAS:
-
Modified Ashworth Scale
- MEP:
-
Motor evoked potential
- mRS:
-
Modified Rankin Scale
- 9HPT:
-
9-Hole Peg Test
References
Lou JN. New progress on treating acute cerebral infarction. Pract J Cardiac Cereb Pneumal Vasc Dis. 2010;18:1546–7.
Deng L, Liu XD, Zhang YB, Li JM. Advances in the treatment of acute cerebral infarction. Chin Gen Pract. 2011;14:825–9.
Chen L, Fang J, Ma R, Froym R, Gu X, Li J, et al. Acupuncture for acute stroke: study protocol for a multicenter, randomized, controlled trial. Trials. 2014;15:214–9.
Liu Z, GuYn L, Wang Y, Xie CL, Lin XM. History and mechanism for treatment of intracerebral hemorrhage with scalp acupuncture. Evid Based Complement Altern Med. 2012;2012:895032.
Zheng GQ, Zhao ZM, Wang Y, Gu Y, Li Y, Chen XM, et al. Meta-analysis of scalp acupuncture for acute hypertensive intracerebral hemorrhage. J Altern Complement Med. 2011;17:293–9.
Hsing WT, Imamura M, Weaver K, Fregni F, Azevedo Neto RS. Clinical effects of scalp electrical acupuncture in stroke: a sham-controlled randomized clinical trial. J Altern Complement Med. 2012;18:341–6.
Wang Y, Shen J, Wang XM, Fu DL, Chen CY, Lu LY, et al. Scalp acupuncture for acute ischemic stroke: a meta-analysis of randomized controlled trials. Evid Based Complement Altern Med. 2012;2012:480950.
Lee SJ, Shin BC, Lee MS, Han CH, Kim JI. Scalp acupuncture for stroke recovery: a systematic review and meta-analysis of randomized controlled trials. European J Integr Med. 2013;5:87–99.
Sasaki N, Mizutani S, Kakuda W, Abo M. Comparison of the effects of high- and low-frequency repetitive transcranial magnetic stimulation on upper limb hemiparesis in the early phase of stroke. J Stroke Cerebrovasc Dis. 2013;22:413–8.
Mansur CG, Fregni F, Boggio PS, Riberto M, Gallucci Neto J, Santos CM, et al. A sham stimulation-controlled trial of rTMS of the unaffected hemisphere in stroke patients. Neurology. 2005;64:1802–4.
Fregni F, Boggio PS, Valle AC, Rocha RR, Duarte J, Ferreira MJ, et al. A sham-controlled trial of a 5-day course of repetitive transcranial magnetic stimulation of the unaffected hemisphere in stroke patients. Stroke. 2006;37:2115–22.
Peinemann A, Reimer B, Loer C, Quartarone A, Munchau A, Conrad B, et al. Long-lasting increase in corticospinal excitability after 1800 pulses of subthreshold 5 Hz repetitive TMS to the primary motor cortex. Clin Neurophysiol. 2004;115:1519–26.
Yozbatiran N, Alonso-Alonso M, See J, et al. Safety and behavioral effects of high-frequency repetitive transcranial magnetic stimulation in stroke. Stroke. 2009;40:309–12.
Kim YH, You SH, Ko MH, et al. Repetitive transcranial magnetic stimulation-induced corticomotor excitability and associated motor skill acquisition in chronic stroke. Stroke. 2006;37:1471–6.
Kakuda W, Abo M, Kobayashi K, Momosaki R, Yokoi A, Fukuda A, et al. Anti-spastic effect of low-frequency rTMS applied with occupational therapy in post-stroke patients with upper limb hemiparesis. Brain Inj. 2011;25:496–502.
Kakuda W, Abo M, Shimizu M, Sasanuma J, Okamoto T, Yokoi A, et al. A multi-center study on low-frequency rTMS combined with intensive occuptional therapy for upper limb hemiparesis in post-stroke patients. J Neuroeng Rehab. 2012;9:4–14.
Kirton A, Chen R, Friefeld S, Gunrai C, Pontigon AM, Deveber G. Contralesional repetitive transcranial magnetic stimulation for chronic hemiparesis in subcortical pediatric stroke: a randomised trial. Lancet Neurol. 2008;7:507–13.
Khedr EM, Abdel-Fadeil MR, Farghali A, Qaid M. Role of 1 and 3 Hz repetitive transcranial magnetic stimulation on motor function recovery after acute ischemic stroke. Eur J Neurol. 2009;16:1323–30.
Khedr EM, Etraby AE, Hemeda M, Nasef AM, Razek AA. Long-term effect of repetitive transcranial magnetic stimulation on motor function recovery after acute ischemic stroke. Acta Neurol Scand. 2010;121:30–7.
Chang WH, Kim YH, Bang OY, Kim SI, Park YH, Lee PK. Long-term effects of rTMS on motor recovery in patients after subacute stroke. J Rehabil Med. 2010;42:758–64.
Kim C, Choi HE, Jung HJ, Lee BJ, Lee KH, Lim YJ. Comparison of the effects of 1 Hz and 20 Hz rTMS on motor recovery in subacute stroke patients. Ann Rehabil Med. 2014;38:585–91.
Barros Galvao SC, Borba Costa dos Santos R, Borba dos Santos P, Cabral ME, Monte-Silva K. Efficacy of coupling repetitive transcranial magnetic stimulation and physical therapy to reduce upper-limb spasticity in patients with stroke: a randomized controlled trial. Arch Phys Med Rehabil. 2014;95:222–9.
Han C, Jo SA, Jo I, Kim E, Park MH, Kang Y. An adaptation of the Korean mini-mental state examination (K-MMSE) in elderly Koreans: demographic influence and population-based norms (the AGE study). Arch Gerontol Geriatr. 2008;47:302–10.
Vidovich MR, Lautenschlager NT, Flicker L, Clare L, Almeida OP. The PACE study: a randomised clinical trial of cognitive activity (CA) for older adults with mild cognitive impairment (MCI). Trials. 2009;10:114–21.
Lim KB, Lee HJ, Yoo J, Kwon YG. Effect of low-frequency rTMS and NMES on subacute unilateral hemispheric stroke with dysphagia. Ann Rehabil Med. 2014;38:592–602.
Doruk P. The impact of knee osteoarthritis on rehabilitation outcomes in hemiparetic stroke patients. J Back Musculoskelet Rehabil. 2013;26:207–11.
Golicki D, Niewada M, Buczek J, Karliska A, Kobayashi A, Janssen MF, Pickard AS. Validity of EQ-5D-5 L in stroke. Qual Life Res. 2015;24:845–50.
Lim KB, Kim JA. Activity of daily living and motor evoked potentials in the subacute stroke patients. Ann Rehabil Med. 2013;37:82–7.
Harrison JK, McArthur KS, Quinn TJ. Assessment scales in stroke: clinimetric and clinical considerations. Clin Interv Aging. 2013;8:201–11.
Quinn TJ, McArthur K, Dawson J, Walters MR, Lees KR. Reliability of structured modified Rankin Scale assessment. Stroke. 2010;41:602–3.
Talelli P, Wallace A, Dileone M, Hoad D, Cheeran B, Oliver R, et al. Theta burst stimulation in the rehabilitation of the upper limb: a semirandomized, placebo-controlled trial in chronic stroke patients. Neurorehabil Neural Repair. 2012;26:976–87.
Cohen J. Statistical power analysis for the behavioural sciences. New York: Academic; 1969.
Faul F, Erdfelder E, Lang A-G, Buchner A. G*Power 3: A flexible statistical power analysis program for the social, behavioral, and biomedical sciences. Behav Res Meth. 2007;39:175–91.
Julious SA. Sample size of 12 per group rule of thumb for a pilot study. Pharm Stat. 2015;4:287–91.
Melody A, Hertzog MA. Considerations in determining sample size for pilot studies. Res Nurs Health. 2008;31:180–91.
Chan AW, Tetzlaff JM, Altman DG, Laupacis A, Gøtzsche PC, Krleža-Jerić K, Hróbjartsson A, Mann H, Dickersin K, Berlin JA, Doré CJ, Parulekar WR, Summerskill WS, Groves T, Schulz KF, Sox HC, Rockhold FW, Rennie D, Moher D. SPIRIT 2013 statement: defining standard protocol items for clinical trials. Ann Intern Med. 2013;158(3):200–7.
Rossini S, Hallet M, Rossini PM, Leone AP. Safety ethical considerations and application guidelines for the use of transcranial magnetic stimulation in clinical practice and research. Clin Neurophysio. 2009;120:2008–39.
Jorgensen HS, Nakayama H, Raaschou HO, Vive Larsen J, Stoier M, Olsen TS. Outcome and time course of recovery in stroke. Part II: time course of recovery. The Copenhagen Stroke Study. Arch Phys Med Rehabil. 1995;76:406–12.
Acknowledgements
This study is supported by the Convergence of Conventional Medicine and Traditional Korean Medicine R&D program funded by the Ministry of Health and Welfare through the Korea Health Industry Development Institute (KHIDI) (HI14C0862). The funder had no further role in the study design, data collection, analysis and interpretation of data, writing of the report, or the decision to submit the paper for publication.
Authors’ contributions
JHK and JYH are responsible for the conception and design of the trial, planning analyses of the data, drafting the manuscript, making the final decision to terminate the trial, and approving the final manuscript. JHP, MYS, MKS, DJK, YNY, GCP, JBC, MRC, and JCS participate in data collection and are in charge of the recruitment and treatment of patients. JHC is responsible for planning analyses of the data and analyzing the data of the clinical trial. All authors have access to these interim results, as well as to discuss, revise, and approve the final manuscript.
Competing interests
The authors declare that they have no competing interests.
Consent for publication
Written informed consent was obtained from the participants for publication of their individual details and accompanying images in this manuscript. The consent form is held by the authors and is available for review by the Editor-in-Chief.
Protocol number and version
The protocol number is DSGOH32, version 1.5.
Author information
Authors and Affiliations
Corresponding author
Additional files
Additional file 1:
Medical Device Clinical Trial Plan Approval number 516. (DOCX 527 kb)
Additional file 2:
Informed consent materials given to participants and authorized surrogates. (DOCX 370 kb)
Additional file 3:
The Institutional Review Board of Chonnam National University Hospital approval of this study (CNUH-2015-114). (DOCX 1609 kb)
Additional file 4:
SPIRIT checklist. (DOCX 363 kb)
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated.
About this article
Cite this article
Han, JY., Kim, JH., Park, JH. et al. Scalp acupuncture and electromagnetic convergence stimulation for patients with cerebral infarction: study protocol for a randomized controlled trial. Trials 17, 490 (2016). https://doi.org/10.1186/s13063-016-1611-y
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s13063-016-1611-y