Abstract
Most patients with triple-negative breast cancer (TNBC) are not candidates for targeted therapy, leaving chemotherapy as the primary treatment option. Recently, immunotherapy has demonstrated promising results in TNBC, due to its immunogenicity. In addition, a novel antibody–drug conjugate, namely, trastuzumab-deruxtecan, has shown effectiveness in TNBC patients with low-HER2 expression (HER2-low). These novel treatment options raise the question about the potential association between the density of stromal tumor-infiltrating lymphocytes (sTILs) and the level of HER2 expression. We aimed to evaluate the association between the level of HER2 expression (HER2-low versus HER2-0) and density of sTILs in TNBC patients, and how they impact the response to neoadjuvant chemotherapy (NAC). This was a retrospective multicenter study including all TNBC patients diagnosed between 2018 and 2022. Central pathology review included sTILs percentages and level of HER2 expression. Tumors were reclassified as either HER2-0 (HER2 IHC 0) or HER2-low (IHC 1 + or 2 + with negative reflex test). Various clinicopathologic characteristics, including sTILs density, and response to NAC were compared between HER2-0 and HER2-low cases. In total, 753 TNBC patients were included in this study, of which 292 patients received NAC. Interobserver agreement between the original pathology report and central review was moderate (77% had the same IHC status after reclassification in either HER2-0 or HER2-low; k = 0.45). HER2-low TNBC represented about one third (36%) of the tumors. No significant difference in sTILs density or complete pathologic response rate was found between HER2-0 and HER2-low cases (p = 0.476 and p = 0.339, respectively). The density of sTILs (≥ 10% sTILs vs. < 10%) was independently associated with achieving a pCR (p = 0.011). In conclusion, no significant association was found between HER2-low status and density of sTILs nor response to NAC. Nonetheless, sTILs could be an independent biomarker for predicting NAC response in TNBC patients.
Similar content being viewed by others
Introduction
Female breast cancer (BC) is the most commonly diagnosed cancer worldwide [1]. Triple-negative breast cancer (TNBC) constitutes 12–17% of all BCs and it is characterized by the lack of estrogen receptor (ER), progesterone receptor (PR) and human epidermal growth factor receptor 2 (HER2) expression [2, 3]. Because of this, patients with TNBC are not eligible for endocrine or HER2-targeted therapies, leaving chemotherapy as the therapeutic option of choice in most cases [4]. TNBC is considered an aggressive BC subtype with relatively high recurrence, metastatic, and mortality rates [5].
At present, treatment decisions for TNBC are based on baseline prognostic clinicopathologic characteristics. Several studies reported that immune response biomarkers, including the density of stromal tumor-infiltrating lymphocytes (sTILs), have an important prognostic and predictive role in TNBC [6, 7]. Patients with a higher density of sTILs at diagnosis have a better disease free and overall survival compared to TNBC patients with a low density of sTILs [8,9,10]. Several studies including TNBC patients who underwent neoadjuvant chemotherapy (NAC) demonstrated that a high density of sTILs was associated with higher rates of complete pathological response (pCR), independent of other clinicopathological prognostic factors or the chemotherapy regimen [6, 11, 12]. Furthermore, a number of clinical trials have reported that the combination of immunotherapy (e.g., pembrolizumab or atezolizumab) and chemotherapy showed higher rates of pCR than chemotherapy alone [13, 14].
A novel therapeutic option that has demonstrated promising results in a subgroup of TNBC patients is the antibody drug conjugate (ADC) called trastuzumab-deruxtecan (T-DXd) [15]. Notably, this drug showed effectiveness in patients with HER2 overexpression (HER2-positive) but also in those with a low level of HER2 expression (HER2-low) [15]. HER2-low BCs has been defined as tumors with a HER2 immunohistochemistry (IHC) score of 1 + or 2 + with a negative molecular reflex test [16, 17]. In a recent phase III clinical trial including 557 patients with HER2-low BC, from which 63 had TNBC, those who had been treated with T-DXd showed longer progression free survival and overall survival compared to the patients treated with regular chemotherapy [15]. This favorable response of HER2-low tumors to T-DXd has opened the question whether these tumors have clinicopathologic differences compared to TNBC tumors with less HER2 expression (HER2 IHC 0), since this could have consequences for novel treatment strategies, i.e., immunotherapy for immunogenic TNBC and T-DXd for HER2-low TNBC. Several studies reported that within TNBC, HER2-low tumors have a poorer pathologic complete response rate after NAC compared to HER2-0 [18,19,20]. This could be associated with a lower immune response of the former group. In line with this hypothesis, van den Ende et al. reported that a lower density of sTILs was significantly associated with HER2-low tumors in TNBC [21]. However, despite these studies reporting a favorable response to NAC in HER2-0 TNBC, this is still controversial, since a recent meta-analysis found no statistically significant difference in pCR rates between patients with HER2-low and those with HER2-0 TNBC [22]. Literature exploring the differences in pCR and its association to in immunological response in HER2-low and HER2-0 within TNBC is limited [23, 24].
The aim of this study was to assess the difference in sTILs density between HER2-0 and HER2-low TNBC. In addition, we aimed to compare the pathologic response between HER2-0 and HER2-low BC from patients who received NAC.
Methodology
Inclusion criteria
A retrospective multi-institutional study was performed including all patients diagnosed with TNBC according to the original pathology report based on the Dutch National guidelines and the American Society of Clinical Oncology/College of American Pathologists Clinical Practice Guideline (ASCO/CAP) [25], between the 1st of January, 2018 until the 31st of December, 2022. Patients above 18 years of age were included. The type of treatment did not influence eligibility for this study, as both patients who received NAC and those who did not were included. Patients with an equivocal HER2 status were excluded.
Data collection
Tissue slides from all TNBC core biopsies were collected. Demographic and clinicopathologic data from the biopsies and the resection specimens were gathered from the pathology reports of the institutional database LIMS Sympathy Tieto of the Erasmus Medical Center, and Core-UDPS of Pathan B.V, and PAL Laboratory of Pathology Dordrecht.
Several clinical characteristics were collected including sex, age at diagnosis (date of core biopsy diagnosis), and type of performed surgery. Histopathologic features from the core biopsy included histologic subtype (according to the World Health Organization) [26], histologic grade (including number of mitosis), angioinvasion and Ki67 proliferation index. The presence of macro- and micro-metastases in sentinel node and/or an axillary nodal dissection were used to determine the nodal status (positive or negative). If a patient had positive nodal status determined by fine needle aspiration (FNA) before receiving NAC and achieved pCR, nodal status from the pre-NAC FNA was considered for analysis.
Furthermore, data regarding the expression of ER, and PR and HER2 was gathered. Hormone receptor (HR) status was defined as positive if more than 10% of the cancer cells showed nuclear ER or PR staining, according to Dutch Guideline for Breast Cancer Treatment, independent of intensity [27]. Since ASCO/CAP guidelines use a 1% cut-off, we also performed the analyzed according to this cut-off [28]. HER2 status was scored according to international guidelines[25].
Additionally, data regarding pCR (defined as the absence of residual invasive breast cancer on histopathologic evaluation of the resected breast specimen and lymph nodes) was included. An incomplete pathologic response was categorized as < 10% residual tumor, 10–50% residual tumor, > 50% residual tumor or no pathologic response[29].
Consensus meeting
A consensus meeting with all participating pathologists (n = 7) was performed before the start of case scoring. This meeting comprised the presentation of the standardized methodology for evaluating sTILs density in BC according to the recommendations by the International TILs working group[6]. Furthermore, the HER2 IHC criteria of the ASCO/CAP guidelines 2018 were reviewed and some exemplary cases of each IHC score were discussed [25].
HER2 and sTILs scoring
Needle core biopsy slides were stained with Hematoxylin and Eosin staining and the 4B5 HER-2/neu antibody using an automatic immunostainer (Ventana BenchMark Ultra, Roche, Indianapolis, USA). The slides were digitally scanned with the Nanozoomer 2.0-HT (Hamamatsu Photonics, Shizuoka, Japan) which enables Z-stacking. The scanned slides were uploaded to Slide Score B.V. (version 1.2-2022-05-24T15:37:11 (Netherlands Cancer Institute, Amsterdam, The Netherlands). This program blinded the case numbers and randomized the slides for the participating pathologists, who received a personal code to perform the scoring of the density of sTILs and HER2 IHC. sTILs levels were scored according to the recommendations of the International Workgroup for scoring TILs[6]. HER2 IHC was scored according to the 2018 ASCO/CAP guidelines as IHC 0, 1 +, 2 +, 3 + [25]. If the scanned tissue was considered to have insufficient tumor cells for proper scoring, the case was considered as missing data.
The use of coded leftover patient material is in accordance with the Code of Conduct of the Federation of Medical Scientific Societies in the Netherlands[30].
Data analysis
The tumors were reclassified as HER2-0 (IHC 0) or HER2-low (IHC 1 + or 2 + with negative reflex test). Descriptive statistics were used to present patient and tumor characteristics. Data was presented as relative frequencies (percentage) for categorical variables or median and interquartile range (IQR) for continuous variables. Interobserver agreement was assessed with Cohen´s kappa coefficient. The cut-off values of sTILs (10%) and Ki67 (40%) were based on recent studies reporting that above these cut-off values, these markers may predict response to NAC [31,32,33,34,35,36,37]. The association between the HER2 score and the percentage of sTILs was assessed using the Mann Whitney U test (or Chi square, depending on the coding of the variables). pCR was analyzed according to HER2 status using Chi square test. Finally, a univariate and multivariate logistic regression analysis was performed to evaluate the relation between the clinicopathologic characteristics and the pathologic response. Odds ratios was described with 95% confidence interval. A p value < 0.05 was considered statistically significant. The analysis was performed in SPSS (IBM Corp. Released 2021. IBM SPSS Statistics for Windows, Version 28.0. Armonk, New York, USA).
Ethics approval
This work was approved by the Medical Ethics Committee of the Erasmus MC (MEC-2023-0545). The Medical Ethics Committee of the Erasmus MC approved that the rules laid down in the Medical Research Involving Human Subjects Act do not apply to this work. Consequently, informed consent was not needed. The study was performed in accordance with the Declaration of Helsinki.
Results
Patient and tumor characteristics
A total of 977 patients with TNBC were retrieved from the institutional databases from participating medical centers. Among these cases, 753 patients had accessible tumor tissue, enabling analysis of clinicopathologic characteristics. Notably, it was observed that 6% of these patients (n = 48) had multiple synchronous tumors, resulting in a total of 824 core biopsies (Fig. 1).
All included patients were women. The median age at diagnosis was 61 (IQR 49–73) years. From the 583 patients that had available surgery data, 56% (n = 328) underwent breast conserving surgery while 44% (n = 255) underwent mastectomy. The majority of patients (82%, n = 677) had a HR expression of < 1%. The remaining cases (11%, n = 91) were HR-low (1–9% of positive tumor cells). In 56 patients (7%), the percentage of positive cells was < 10% but the exact percentage was missing. Based on the HER2 IHC score from the original pathology report, 64% (n = 527) of the cases had an IHC score of 0, 25% (n = 210) of patients had an IHC score of 1 +, and 11% (n = 91) of patients had an IHC score of 2 + with negative reflex test. This corresponded to 64% (n = 527) of cases with HER2-0 status and 36% (n = 294) of cases with HER2-low status. The median density of sTILs was 10% (IQR 5–20%).
HER2 concordance between multiple tumors
Most of the 48 patients with multiple synchronous tumors (37 out of 48; 77%) had the same IHC score in both tumors. There were 11 cases (23%) in which the tumors had a different IHC score, of which 4 cases had IHC 0 in tumor 1 and IHC 1 + in tumor 2 or vice versa. Six patients had IHC 1 + in tumor 1 and IHC 2 + in tumor 2 or vice versa and 1 patient had IHC 0 in tumor 1 and IHC 2 + in tumor 2. After reclassification of tumors according to the HER2 status (HER2-0 versus HER2 low), 43 of the 48 cases had the same HER2 score in both tumors.
HER2 central review compared to original pathology report
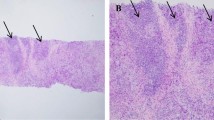
All given HER2 IHC scores from available biopsies were centrally reviewed and compared to the scores from the original pathology report, which resulted in a moderate interobserver agreement (Cohen´s kappa = 0.45). Figure 2A shows the concordance between both IHC scores. In brief, 823 biopsies were analyzed of which 69% (n = 566) had the same IHC score. From the 258 cases that had a different score, 65% (n = 168) had a shift from IHC 0 to 1 + or vice versa; 7% (n = 17) had a shift of 0 to 2 + or vice versa; and 27% (n = 70) had a shift from 1 + to 2 + or vice versa. Notably, there were three cases that were scored as 2 + in the original pathology report that were re-scored as 3 +. These 3 cases had a negative reflex test.
Figure 2B depicts the reclassification of the tumors according to HER2-status (0, low or positive), with a moderate agreement (Cohen’s kappa = 0.52). Around 77% (n = 632) of the cases had the same HER2 score. Among the 23% (n = 191) of cases that had a different HER2 score, 96% (n = 188) changed from HER2-0 to HER2-low or vice versa, and 2% (n = 3) changed from HER2-low to HER2-positive.
Clinicopathologic characteristics according to HER2 status
Table 1 presents the clinicopathologic characteristics from the core biopsy according to HER2 status. Briefly, HER2-low BC was associated with histologic subtype (higher frequency of lobular carcinoma; p < 0.001), lower histologic grade (p < 0.001), lower Ki67 index (p = 0.001), and higher proportion of positive nodal status (p < 0.001). There was no difference in density of sTILs between HER2-0 and HER2-low (p = 0.571). Subgroup analyses restricted to cases with an HR expression of < 1% did not affect these results.
Clinicopathologic characteristics according to sTILs
Table 2 presents the association between clinicopathologic characteristics from the core biopsy and a sTIL density of ≥ 10%. In univariate analysis, age < 50 (p < 0.001), NST histology (p < 0.001), histologic grade 3 (p < 0.001), ≥ 8 mitoses mm2 (p = 0.02), and positive nodal status (p = 0.014) were associated with a higher percentage of sTILs. Subgroup analyses restricted to cases with HR expression of < 1% did not affect these results.
Clinicopathologic features associated with complete pathologic response after NAC
The dataset comprised 292 NAC-treated patients with complete data regarding treatment response in the surgical resection specimen. According to Dutch guidelines for breast cancer treatment, these patients received an antracyclin/taxane-based NAC regimen, supplemented with Carboplatin in patients with stage 3 disease [27].Logistic regression analysis was performed to evaluate the impact of several clinicopathologic characteristics on pCR rate. As presented in Table 3, multivariable analysis showed that having no special type histology (NST) (p = 0.033) and a TIL density of ≥ 10% (p = 0.011) were the only factors independently associated with achieving a pCR. Restricting these analysis to patients with an HR expression of < 1% did not affect these results.
When evaluating the relative differences between sTILs and pCR, it was observed that 50.5% of patients with sTILs ≥ 10% achieved pCR in contrast to 36.5% of those with sTILs < 10% (p = 0.021) (Fig. 3). Moreover, we evaluated the variations in the level of treatment response based on the percentage of sTILs. A trend was seen toward a pCR or < 10% residual tumor and a high percentage of sTILs (≥ 10%), whereas the patients with 10% or more residual tumor more often tend to have lower density of sTILs (< 10%) (p = 0.054). Based on sTILs, we could not select large subgroups of patients with a very high (e.g., > 90%) or very low pCR rate (e.g., < 10%), although all patients with sTILs ≥ 90% who underwent NAC (n = only 3) achieved a pCR.
The response to NAC according to HER2 status was also evaluated. HER2-0 tumors had a higher proportion of patients achieving pCR compared to HER2-low tumors (47.6% vs. 41.9%) but this difference was not statistically significant (p = 0.338).
Discussion
To our knowledge, this is the first comprehensive assessment study aiming to investigate the association between HER2-low status and the immunological response in TNBC. In our analysis, 36% of the TNBC patients had HER2-low status, which is similar to ratios found in other studies [38,39,40]. HER2-low status was associated with a higher rate of lobular histology, a lower histologic grade, lower Ki67, and a higher nodal positivity rate compared to HER2-0 cases. Similar differences had been reported previously [21, 38, 39, 41]. However, the absolute differences are relatively small, so their clinical relevance remains uncertain.
In our study, no correlation between HER2-low status and density of sTILs was found in TNBC. Literature regarding this topic is very limited [38, 42]. Jacot et al. [42] conducted a retrospective study with 296 patients and reported that HER2-low status had no correlation with sTILs density (5% cut-off value) or PD-L1 expression. A national retrospective study conducted in the Netherlands found a slightly lower median for sTILs level in HER2-low TNBC compared to HER2-0 TNBC, although this discrepancy did not achieve statistical significance[38]. It is important to consider that these studies have certain limitation due to their retrospective design and missing data[38]. In contrast, a recent study from van den Ende et al. reported that lower sTILs density (≤ 10%) was independently associated to HER2-low in TNBC patients and these results were supported by gene expression data[21]. These cases were also centrally reviewed, including sTILs density based on whole tissue slides and HER2 status based on tissue microarrays, all from resection specimens. However, this was a historical cohort (1982 to 2003), which could have affected tissue quality and accurate representation of the current HER2 expression levels in BC patients. In our current study, the assessment of sTILs density was performed on material from the core biopsy. It has been reported that there can be an underestimation of the density of sTILs when scoring on biopsies instead of the resection specimen, and that the discordance in the percentage of sTILs tends to be higher in TNBC cases compare to other BCs [43,44,45]. Moreover, in the current study it was reported that HER2-0 tumors were associated with the NST histologic subtype and that these tumors were more often grade 3. In addition, high density of sTILs (≥ 10%) was also significantly associated with the NST histologic subtype and grade 3 tumors. Based on this data, one could hypothesize that there is an indirect correlation between HER2 status and density of sTILs, but no direct significant association was found in this study. Further research of the correlation between HER2 status and the density of sTILs in the resection specimens could provide more insight in the potential association between HER2-low status and sTIL density.
As mentioned above, age, NST type, tumor grade 3, ≥ 8 mitosis per mm2, and higher positive nodal status were features significantly associated with a high density of sTILs in our cohort, which is in line with literature [8, 46,47,48]. However, regardless of the consistent association of these clinicopathologic characteristics with a high density of sTILs, their mutual influence and clinical significance in TNBC remains undefined. Moreover, no significant association was found between Ki67 index and density of sTILs, which is in contrast to several previous studies [10, 46, 48,49,50,51]. The most likely cause for the deviation in outcomes could be the substantial number of cases with missing data for these parameters in our cohort.
As expected, a significant correlation between achieving pCR and a higher density of sTILs (≥ 10%) was found in this study. Several other studies have reported the robust correlation between sTILs and a higher likelihood of achieving pCR [11, 12, 52,53,54,55]. Furthermore, a high density of sTILs has been associated with response to both cytotoxic treatments and immunotherapy, particularly in TNBC [24, 56]. In our study, all patients with sTILs ≥ 90% who underwent NAC achieved pCR, but the absolute number of these patients was very low (n = 3).
On the other hand, HER2-low status had no impact in therapy response to NAC within this study. This is in line with a recent meta-analysis including 15 TNBC studies, that found that HER2-low status had no significant impact on pCR rates compared to HER2-0 [22]. Previous studies showed contradictory results regarding the association between HER2-low status and therapy response. In various uni- and multicenter retrospective cohorts, no significant correlation was found between HER2-low status and pCR in the TNBC subgroup [19, 20, 57]. However, in a much larger nation-wide American cohort, a statistically lower pCR rate was associated with the HER2-low status in TNBC [18]. These conflicting outcomes could potentially be influenced by the cohort size, where statistically significant findings are observed only in exceptionally large cohorts [18, 58]. Additionally, a lack of calibration of the IHC assay has been reported for HER2 detection, especially in the context of HER2-low, which raises doubts about the accurate identification of patients with HER2-low and HER2-0 tumors [59]. Overall, these findings indicate that density of sTILs could be a promising independent biomarker for predicting the response to NAC in TNBC patients, while HER2-low status does not appear to have a relevant effect.
The HER2 score variations between the original pathology report and central review were assessed and the interobserver agreement was moderate, with 23% of the cases having a discordant HER2 status (0 vs. low). Comparable interobserver variability using the current diagnostic guidelines has been described before [40, 60, 61]. At the moment, there is not enough evidence to provide a clear recommendation on the optimal classification method for borderline cases between HER2 IHC 0 and IHC 1 +. To address these complex cases, it is advisable to examine them under high power magnification and have them reviewed by a second or third pathologist [16, 17]. It’s crucial to bear in mind that the ASCO/CAP guidelines were established to define HER2 overexpression and its correlation with the response to trastuzumab, rather than to forecast the response likelihood to T-DXd. Furthermore, HER2 status variability was also observed in this cohort when comparing multiple breast tumors. About 10% of patients with multiple tumors displayed a combination of one HER2-0 tumor and one HER2-low tumor. Alongside interobserver variability, tumor heterogeneity within a single tumor and between different tumors could also be a factor for the variation in HER2 status across multiple tumors [62, 63]. When dealing with multiple tumors, clinical guidelines suggest sampling more than one lesion to accurately categorize the disease [27]. Currently, T-DXd is only approved for non-amplified HER2 metastatic BC [64, 65]. However, if this drug becomes part of treatment regimens for early BC in the future, it is important to consider all these factors.
One of the strengths of this study is that it consists of a large and well-documented cohort of patients, as data is collected from multiple centers, enabling a comprehensive representation of patients with TNBC in the Netherlands. Furthermore, to properly estimate the HER2 protein expression levels, central pathology review was performed. However, this study also has some limitations. Due to the retrospective nature of the cohort, some data was missing regarding certain clinicopathologic features, including pre-NAC tumor stage and chemotherapy regimen. For this reason, the findings of some pathologic characteristics should be interpreted with caution. Moreover, the cohort consisted of recently diagnosed patients, limiting the follow-up time, so we couldn’t look at outcome.
Conclusion
The findings of this study suggest that HER2-low tumors are highly prevalent within TNBC (36%). The HER2-low status is associated with several clinicopathologic characteristics, although the clinical implications are questionable. No significant association was found between HER2-low status and sTILs density nor response to NAC. Nonetheless, the indication that density of sTILs could be an independent biomarker for predicting the response to NAC in TNBC patients was confirmed in this study.
Availability of data and materials
The datasets used and/or analyzed during the current study are available from the corresponding author upon reasonable request.
Abbreviations
- ASCO/CAP:
-
American Society of Clinical Oncology/College of American Pathologists Clinical Practice Guideline
- BC:
-
Breast cancer
- pCR:
-
Complete pathologic response
- FNA:
-
Fine needle aspiration
- HER2:
-
Human epidermal growth factor receptor 2
- HER2-low:
-
Low-HER2 expression
- IHC:
-
Immunohictochemistry
- NAC:
-
Neoadjuvant chemotherapy
- NST:
-
No special type
- sTILs:
-
Stromal tumor-infiltrating lymphocytes
- T-DXd:
-
Trastuzumab deruxtecan
- TNBC:
-
Triple-negative breast cancer
References
Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, et al. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2021;71(3):209–49.
Tsang JYS, Tse GM. Molecular classification of breast cancer. Adv Anat Pathol. 2020;27(1):27–35.
Medina MA, Oza G, Sharma A, Arriaga LG, Hernandez Hernandez JM, Rotello VM, et al. Triple-negative breast cancer: a review of conventional and advanced therapeutic strategies. Int J Environ Res Public Health. 2020;17(6):2078.
Prat A, Pineda E, Adamo B, Galvan P, Fernandez A, Gaba L, et al. Clinical implications of the intrinsic molecular subtypes of breast cancer. Breast. 2015;24(Suppl 2):S26-35.
Griffiths CL, Olin JL. Triple negative breast cancer: a brief review of its characteristics and treatment options. J Pharm Pract. 2012;25(3):319–23.
Salgado R, Denkert C, Demaria S, Sirtaine N, Klauschen F, Pruneri G, et al. The evaluation of tumor-infiltrating lymphocytes (TILs) in breast cancer: recommendations by an International TILs Working Group 2014. Ann Oncol. 2015;26(2):259–71.
van den Ende NS, Nguyen AH, Jager A, Kok M, Debets R, van Deurzen CHM. Triple-negative breast cancer and predictive markers of response to neoadjuvant chemotherapy: a systematic Review. Int J Mol Sci. 2023;24(3):2969.
Gao ZH, Li CX, Liu M, Jiang JY. Predictive and prognostic role of tumour-infiltrating lymphocytes in breast cancer patients with different molecular subtypes: a meta-analysis. BMC Cancer. 2020;20(1):1150.
Liu S, Lachapelle J, Leung S, Gao D, Foulkes WD, Nielsen TO. CD8+ lymphocyte infiltration is an independent favorable prognostic indicator in basal-like breast cancer. Breast Cancer Res. 2012;14(2):R48.
Loi S, Michiels S, Salgado R, Sirtaine N, Jose V, Fumagalli D, et al. Tumor infiltrating lymphocytes are prognostic in triple negative breast cancer and predictive for trastuzumab benefit in early breast cancer: results from the FinHER trial. Ann Oncol. 2014;25(8):1544–50.
Yamaguchi R, Tanaka M, Yano A, Tse GM, Yamaguchi M, Koura K, et al. Tumor-infiltrating lymphocytes are important pathologic predictors for neoadjuvant chemotherapy in patients with breast cancer. Hum Pathol. 2012;43(10):1688–94.
Ono M, Tsuda H, Shimizu C, Yamamoto S, Shibata T, Yamamoto H, et al. Tumor-infiltrating lymphocytes are correlated with response to neoadjuvant chemotherapy in triple-negative breast cancer. Breast Cancer Res Treat. 2012;132(3):793–805.
Mittendorf EA, Zhang H, Barrios CH, Saji S, Jung KH, Hegg R, et al. Neoadjuvant atezolizumab in combination with sequential nab-paclitaxel and anthracycline-based chemotherapy versus placebo and chemotherapy in patients with early-stage triple-negative breast cancer (IMpassion031): a randomised, double-blind, phase 3 trial. Lancet. 2020;396(10257):1090–100.
Schmid P, Cortes J, Pusztai L, McArthur H, Kummel S, Bergh J, et al. Pembrolizumab for early triple-negative breast cancer. N Engl J Med. 2020;382(9):810–21.
Modi S, Jacot W, Yamashita T, Sohn J, Vidal M, Tokunaga E, et al. Trastuzumab deruxtecan in previously treated HER2-low advanced breast cancer. N Engl J Med. 2022;387(1):9–20.
Wolff AC, Somerfield M, Dowsett M, Hammond MEH, Hayes DF, McShane LM, et al. Human epidermal growth factor receptor 2 testing in breast cancer American society of clinical oncology-college of american pathologists guideline update. Arch Pathol Lab Med. 2023;142(11):1364–82.
Tarantino P, Viale G, Press MF, Hu X, Llorca FP, Bardia A, et al. ESMO expert consensus statements (ECS) on the definition, diagnosis, and management of HER2-low breast cancer. Ann Oncol. 2023;34(8):645–59.
Peiffer DS, Zhao F, Chen N, Hahn OM, Nanda R, Olopade OI, et al. Clinicopathologic characteristics and prognosis of ERBB2-low breast cancer among patients in the national cancer database. JAMA Oncol. 2023;9(4):500–10.
Denkert C, Seither F, Schneeweiss A, Link T, Blohmer JU, Just M, et al. Clinical and molecular characteristics of HER2-low-positive breast cancer: pooled analysis of individual patient data from four prospective, neoadjuvant clinical trials. Lancet Oncol. 2021;22(8):1151–61.
de Nonneville A, Houvenaeghel G, Cohen M, Sabiani L, Bannier M, Viret F, et al. Pathological complete response rate and disease-free survival after neoadjuvant chemotherapy in patients with HER2-low and HER2-0 breast cancers. Eur J Cancer. 2022;176:181–8.
van den Ende NS, Smid M, Timmermans A, van Brakel JB, Hansum T, Foekens R, et al. HER2-low breast cancer shows a lower immune response compared to HER2-negative cases. Sci Rep. 2022;12(1):12974.
Molinelli C, Jacobs F, Agostinetto E, Nader-Marta G, Ceppi M, Bruzzone M, et al. Prognostic value of HER2-low status in breast cancer: a systematic review and meta-analysis. ESMO Open. 2023;8(4):101592.
Erber R, Kolberg HC, Schumacher J, Braun M, Fasching PA, Grischke EM, et al. Association between pCR, TILs, and Ki-67 at baseline and after 2 weeks in patients with triple-negative breast cancer (TNBC) treated with atezolizumab and chemotherapy +/− a preceding atezolizumab monotherapy window: a translational analysis of the neoMono trial. J Clin Oncol. 2023;41(16_suppl):595.
Yoder R, Staley JM, Schmitt Z, O’Dea A, Nye LE, Elia M, et al. Differential impact of proliferation signature on efficacy of neoadjuvant chemoimmunotherapy in sTIL-high and sTIL-low triple-negative breast cancer (TNBC): biomarker analysis of the NeoPACT trial. J Clin Oncol. 2023;41(16):507.
Wolff AC, Hammond MEH, Allison KH, Harvey BE, Mangu PB, Bartlett JMS, et al. Human epidermal growth factor receptor 2 testing in breast cancer: American society of clinical oncology/college of American pathologists clinical practice guideline focused update. Arch Pathol Lab Med. 2018;142(11):1364–82.
Tan PH, Ellis I, Allison K, Brogi E, Fox SB, Lakhani S, et al. The 2019 World Health Organization classification of tumours of the breast. Histopathology. 2020;77(2):181–5.
Dutch Clinical Guideline of Breast Cancer [Internet]. Kennisinstituut van de Federatie van Medisch Specialisten. 2020 [cited 04-May-2022]. Available from: https://richtlijnendatabase.nl/richtlijn/borstkanker/pathologie.html.
Allison KH, Hammond MEH, Dowsett M, McKernin SE, Carey LA, Fitzgibbons PL, et al. Estrogen and progesterone receptor testing in breast cancer: ASCO/CAP guideline update. J Clin Oncol. 2020;38(12):1346–66.
Biganzoli L, Marotti L, Hart CD, Cataliotti L, Cutuli B, Kuhn T, et al. Quality indicators in breast cancer care: An update from the EUSOMA working group. Eur J Cancer. 2017;86:59–81.
Human tissue and medical research: code of conduct for responsible use. (2011).
Ochi T, Bianchini G, Ando M, Nozaki F, Kobayashi D, Criscitiello C, et al. Predictive and prognostic value of stromal tumour-infiltrating lymphocytes before and after neoadjuvant therapy in triple negative and HER2-positive breast cancer. Eur J Cancer. 2019;118:41–8.
Kraus JA, Beriwal S, Dabbs DJ, Ahrendt GM, McGuire KP, Johnson RR, et al. Predictors of pathologic complete response after standard neoadjuvant chemotherapy in triple-negative breast carcinoma. Appl Immunohistochem Mol Morphol. 2012;20(4):334–9.
Foldi J, Silber A, Reisenbichler E, Singh K, Fischbach N, Persico J, et al. Neoadjuvant durvalumab plus weekly nab-paclitaxel and dose-dense doxorubicin/cyclophosphamide in triple-negative breast cancer. NPJ Breast Cancer. 2021;7(1):9.
Cerbelli B, Botticelli A, Pisano A, Pernazza A, Campagna D, De Luca A, et al. CD73 expression and pathologic response to neoadjuvant chemotherapy in triple negative breast cancer. Virchows Arch. 2020;476(4):569–76.
Baba M, Takahashi M, Yamashiro K, Yokoo H, Fukai M, Sato M, et al. Strong cytoplasmic expression of NF-kappaB/p65 correlates with a good prognosis in patients with triple-negative breast cancer. Surg Today. 2016;46(7):843–51.
Zuo K, Yuan X, Liang X, Sun X, Liu S, Connell PP, et al. qRT-PCR-based DNA homologous recombination-associated 4-gene score predicts pathologic complete response to platinum-based neoadjuvant chemotherapy in triple-negative breast cancer. Breast Cancer Res Treat. 2022;191(2):335–44.
Wu Q, Ma G, Deng Y, Luo W, Zhao Y, Li W, et al. Prognostic value of Ki-67 in patients with resected triple-negative breast cancer: a meta-analysis. Front Oncol. 2019;9:1068.
Baez-Navarro X, van Bockstal MR, Andrinopoulou ER, van Deurzen CHM. HER2-low breast cancer: incidence, clinicopathologic features, and survival outcomes from real-world data of a large nationwide cohort. Mod Pathol. 2023;36(4):100087.
Tan R, Ong WS, Lee KH, Lim AH, Park S, Park YH, et al. HER2 expression, copy number variation and survival outcomes in HER2-low non-metastatic breast cancer: an international multicentre cohort study and TCGA-METABRIC analysis. BMC Med. 2022;20(1):105.
Schettini F, Chic N, Braso-Maristany F, Pare L, Pascual T, Conte B, et al. Clinical, pathological, and PAM50 gene expression features of HER2-low breast cancer. NPJ Breast Cancer. 2021;7(1):1.
Won HS, Ahn J, Kim Y, Kim JS, Song JY, Kim HK, et al. Clinical significance of HER2-low expression in early breast cancer: a nationwide study from the Korean Breast Cancer Society. Breast Cancer Res. 2022;24(1):22.
Jacot W, Maran-Gonzalez A, Massol O, Sorbs C, Mollevi C, Guiu S, et al. Prognostic value of HER2-low expression in non-metastatic triple-negative breast cancer and correlation with other biomarkers. Cancers (Basel). 2021;13(23):6059.
Cha YJ, Ahn SG, Bae SJ, Yoon CI, Seo J, Jung WH, et al. Comparison of tumor-infiltrating lymphocytes of breast cancer in core needle biopsies and resected specimens: a retrospective analysis. Breast Cancer Res Treat. 2018;171(2):295–302.
Choi H, Ahn SG, Bae SJ, Kim JH, Eun NL, Lee Y, et al. Comparison of programmed cell death ligand 1 status between core needle biopsy and surgical specimens of triple-negative breast cancer. Yonsei Med J. 2023;64(8):518–25.
Kemp Bohan PM, Chick RC, Hickerson AT, Messersmith LM, Williams GM, Cindass JL, et al. Correlation of tumor microenvironment from biopsy and resection specimens in untreated colorectal cancer patients: a surprising lack of agreement. Cancer Immunol Immunother. 2021;70(5):1465–74.
Pujani M, Jain H, Chauhan V, Agarwal C, Singh K, Singh M. Evaluation of Tumor infiltrating lymphocytes in breast carcinoma and their correlation with molecular subtypes, tumor grade and stage. Breast Dis. 2020;39(2):61–9.
Vaid PM, Puntambekar AK, Jumle NS, Banale RA, Ansari D, Reddy RR, et al. Evaluation of tumor-infiltrating lymphocytes (TILs) in molecular subtypes of an Indian cohort of breast cancer patients. Diagn Pathol. 2022;17(1):91.
Loi S, Drubay D, Adams S, Pruneri G, Francis PA, Lacroix-Triki M, et al. Tumor-infiltrating lymphocytes and prognosis: a pooled individual patient analysis of early-stage triple-negative breast cancers. J Clin Oncol. 2019;37(7):559–69.
Kolberg-Liedtke C, Gluz O, Heinisch F, Feuerhake F, Kreipe H, Clemens M, et al. Association of TILs with clinical parameters, Recurrence Score(R) results, and prognosis in patients with early HER2-negative breast cancer (BC)-a translational analysis of the prospective WSG PlanB trial. Breast Cancer Res. 2020;22(1):47.
Macchetti AH, Marana HR, Silva JS, de Andrade JM, Ribeiro-Silva A, Bighetti S. Tumor-infiltrating CD4+ T lymphocytes in early breast cancer reflect lymph node involvement. Clinics (Sao Paulo). 2006;61(3):203–8.
Fujimoto Y, Watanabe T, Hida AI, Higuchi T, Miyagawa Y, Ozawa H, et al. Prognostic significance of tumor-infiltrating lymphocytes may differ depending on Ki67 expression levels in estrogen receptor-positive/HER2-negative operated breast cancers. Breast Cancer. 2019;26(6):738–47.
Zhu M, Yu Y, Shao X, Zhu L, Wang L. Predictors of response and survival outcomes of triple negative breast cancer receiving neoadjuvant chemotherapy. Chemotherapy. 2020;65(3–4):101–9.
Zhang L, Wang XI, Zhang S. Tumor-infiltrating lymphocyte volume is a better predictor of neoadjuvant therapy response and overall survival in triple-negative invasive breast cancer. Hum Pathol. 2018;80:47–54.
Wang K, Xu J, Zhang T, Xue D. Tumor-infiltrating lymphocytes in breast cancer predict the response to chemotherapy and survival outcome: a meta-analysis. Oncotarget. 2016;7(28):44288–98.
Van Bockstal MR, Noel F, Guiot Y, Duhoux FP, Mazzeo F, Van Marcke C, et al. Predictive markers for pathological complete response after neo-adjuvant chemotherapy in triple-negative breast cancer. Ann Diagn Pathol. 2020;49:151634.
El Bairi K, Haynes HR, Blackley E, Fineberg S, Shear J, Turner S, et al. The tale of TILs in breast cancer: a report from the international immuno-oncology biomarker working group. NPJ Breast Cancer. 2021;7(1):150.
de Moura LL, Cesca MG, Tavares MC, Santana DM, Saldanha EF, Guimaraes PT, et al. HER2-low status and response to neoadjuvant chemotherapy in HER2 negative early breast cancer. Breast Cancer Res Treat. 2021;190(1):155–63.
Changchuan J, Stuthi P, Lei D, Qian W, Shipra G, Charles LS. Real-world clinical outcomes in patients with local/regional HER2-low breast cancer: an NCDB analysis. J Clin Oncol. 2022;40(16_suppl):558.
Baez-Navarro X, Salgado R, Denkert C, Lennerz JK, Penault-Llorca F, Viale G, et al. Selecting patients with HER2-low breast cancer: getting out of the tangle. Eur J Cancer. 2022;175:187–92.
Baez-Navarro X, van Bockstal MR, Nawawi D, Broeckx G, Colpaert C, Doebar SC, et al. Interobserver variation in the assessment of immunohistochemistry expression levels in HER2-negative breast cancer: Can we improve the identification of low levels of HER2 expression by adjusting the criteria? An international interobserver study. Mod Pathol. 2023;36(1):100009.
Fernandez AI, Liu M, Bellizzi A, Brock J, Fadare O, Hanley K, et al. Examination of low ERBB2 protein expression in breast cancer tissue. JAMA Oncol. 2022;8(4):1–4.
Hamilton E, Shastry M, Shiller SM, Ren R. Targeting HER2 heterogeneity in breast cancer. Cancer Treat Rev. 2021;100:102286.
Marchio C, Annaratone L, Marques A, Casorzo L, Berrino E, Sapino A. Evolving concepts in HER2 evaluation in breast cancer: Heterogeneity, HER2-low carcinomas and beyond. Semin Cancer Biol. 2021;72:123–35.
Administration UFaD. FDA approves fam-trastuzumab deruxtecan-nxki for HER2-low breast cancer: US Food and Drug Administration; 2022 [updated 05–08–2022. Available from: https://www.fda.gov/drugs/resources-information-approved-drugs/fda-approves-fam-trastuzumab-deruxtecan-nxki-her2-low-breast-cancer
EMA. Enhertu. Trastuzumab deruxtecan. EMA website: European Medicines Agency; 2022.
Aknowledgements
The authors thank AstraZeneca for providing the funds for this research and scientific programmer Jan Hudecek for his assistance during the utilization of Slide Score.
Funding
This work was funded by AstraZeneca. The funder had no role in the design of the study, the interpretation of the results, or the writing of the article.
Author information
Authors and Affiliations
Contributions
C.D. and X.B. contributed to the study concept and design. X.B. and C.D. performed the development of the methodology. X.B. and N.E. performed the writing. A.N., R.S., P.W., J.B., C.S., J.W. contributed to the scoring of the slides. X.B., C.D. and N.E. participated in the acquisition, analysis and/or interpretation of data, and statistical analysis. All authors performed an exhaustive reviews and revisions of the paper. All authors read and approved the final paper.
Corresponding author
Ethics declarations
Competing interests
C. van Deurzen was involved in an advisory board of Astra Zeneca/Daiichi Sankyo and received research funding from Astrazeneca.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Baez-Navarro, X., van den Ende, N.S., Nguyen, A.H. et al. HER2-low and tumor infiltrating lymphocytes in triple-negative breast cancer: Are they connected?. Breast Cancer Res 26, 41 (2024). https://doi.org/10.1186/s13058-024-01783-z
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s13058-024-01783-z