Abstract
Many factors, including reproductive hormones, have been linked to a woman’s risk of developing breast cancer (BC). We reviewed the literature regarding the relationship between ovulatory menstrual cycles (MCs) and BC risk. Physiological variations in the frequency of MCs and interference with MCs through genetic variations, pathological conditions and or pharmaceutical interventions revealed a strong link between BC risk and the lifetime number of MCs. A substantial reduction in BC risk is observed in situations without MCs. In genetic or transgender situations with normal female breasts and estrogens, but no progesterone (P4), the incidence of BC is very low, suggesting an essential role of P4. During the MC, P4 has a strong proliferative effect on normal breast epithelium, whereas estradiol (E2) has only a minimal effect. The origin of BC has been strongly linked to proliferation associated DNA replication errors, and the repeated stimulation of the breast epithelium by P4 with each MC is likely to impact the epithelial mutational burden. Long-lived cells, such as stem cells, present in the breast epithelium, can carry mutations forward for an extended period of time, and studies show that breast tumors tend to take decades to develop before detection. We therefore postulate that P4 is an important factor in a woman’s lifetime risk of developing BC, and that breast tumors arising during hormonal contraception or after menopause, with or without menopausal hormone therapy, are the consequence of the outgrowth of pre-existing neoplastic lesions, eventually stimulated by estrogens and some progestins.
Similar content being viewed by others
Introduction
The absolute lifetime risk of developing breast cancer (BC) in women in the USA is high and has increased from 1 in 11 (9.1%) in 1975 to 1 in 8 (12·9%) in 2021 [1]. Similar, or even higher, lifetime risks have been reported in Europe, as illustrated for the UK with a risk of 1 in 7 (15%) for women born after 1960 [2]. For men, the lifetime risk of acquiring BC is 1 in 833 (0.1%) in the USA, 100 times lower than for women [3]. A recent article describes that the majority of BC in women (58% to 83%) is related to reproductive factors; another 5–10% of BCs are thought to be caused by hereditary germ-line mutations, with the remaining BCs related to environmental factors [4,5,6,7,8]. Of vital importance in this review is the distinguishment between cause and stimulation, when judging the effect of reproductive, genetic and environmental factors in inducing BC.
An important reproductive determinant of developing BC is the cumulative life-time number of menstrual cycles (MCs) and the accompanying repeated intermittent exposure to progesterone (P4) [9, 10]. Women in developed countries have their first menstrual period (menarche) on average at 11 years of age, and their last menses (menopause) occurs on average at 51 years, 40 years later. With an average MC duration of 28 days, a woman has 13 cycles per year, equating to a total of 520 MCs in her lifetime. Assuming that each year of regular menstrual cycling contributes equally to the BC risk, and that hormone fluxes in MCs are a primary determinant of BC risk, a simple calculation finds that MCs carry a relative risk of BC of about 2.5% per year of regular cycling. A highly significant linear relationship between the BC risk and the cumulative number of MCs before a first full term pregnancy as well as for the life time number of MCs has been reported earlier already [11, 12].
Search strategy
We reviewed the literature to investigate the relationship in women between the risk of developing BC and reproductive variables, especially the total lifetime number of MCs a women experiences during her life, and the role of P4 as mutagenic factor causing BC under the following circumstances:
-
1.
physiological variations in the number of MCs related to age at menarche, age at menopause, pregnancies and total lifetime duration of lactation;
-
2.
genetic interference with the occurrence of MCs in hypogonadotropic syndromes including the Turner syndrome (TS), primary congenital hypogonadotropic hypogonadism (CHH), the Kallmann syndrome (KaS) and pure gonadal dysgenesis (PGD), also known in males as the Swyer syndrome (SwS);
-
3.
genetic interference with the hormonal levels or MCs in normogonadotropic syndromes including complete androgen insensitivity syndrome (CAIS) and Mayer–Rokitansky–Küster–Hauser (MRKH) syndrome, also known as Müllerian agenesis;
-
4.
pathological conditions affecting the number of MCs with long-term primary or secondary hypo- or hypergonadotropic amenorrhea and absence of MCs due to anorexia nervosa (AN) and weight loss-related amenorrhea (WLRA), primary ovarian insufficiency (POI) or failure (POF), and early premenopausal oophorectomy for ovarian cancer or extensive endometriosis, and
-
5.
pharmaceutical interventions suppressing the MC and steroid hormone treatments affecting the risk of BC.
In addition, we summarize the molecular actions of P4 in healthy breast epithelium that may stimulate BC development and confirm that the role of estradiol (E2) and other estrogens in the origin of BC is negligible, but that estrogens are more likely to stimulate the growth of existing BCs.
Physiological variations
Menstrual cycles
The average length of a normal MC is 28 days, with a range of 26 to 30 days. A normal MC is characterized endocrinologically by preovulatory and midluteal E2 increases and by P4 secretion during the luteal phase, with P4 levels peaking at the midluteal timepoint. Testosterone (T) levels are stable with a small increase after the preovulatory E2 peak (Fig. 1). When ovulation does not occur in anovulatory cycles, the corpus luteum is not formed and no P4 is synthesized. The primary function of P4, together with E2, is to prepare the endometrium for embryo implantation and support a pregnancy thereafter until term. In addition, during the MC, P4 controls the proliferative effect of E2 on the endometrium, with endometrial shedding and menstrual bleeding occurring when P4 decreases if fertilization does not occur.
Estradiol is essential for ovulation and for the establishment of pregnancy by preparing the endometrium for embryo implantation, while P4 is essential for the establishment and continuation of pregnancy. Testosterone is a precursor of E2, with T levels up to eight times higher than E2 levels (Fig. 1), with large interindividual variations [13, 14]. The function of T in women seems to be primarily related to sexual function, especially desire and arousal, along with a favourable effect on mood and musculoskeletal health [15, 16]. Intermittent exposure to hormones may be more relevant than continuous exposure, since every new hormone peak may initiate a new mutation in breast progenitor and stem cells, and therefore, in this paper we have focused on P4 and E2 and not on T.
Menarche and menopause
Early menarche and late menopause, which correspond with more MCs, are associated with a small increase in the risk of BC [12]. In the Collaborative Group on Hormonal Factors in Breast Cancer (Collaborative Group) cohort of 118,964 women with an average age at menarche of 13.1 years, there was a 5% increase in lifetime risk of breast cancer for every year younger at menarche [12], so these early cycles carry double the average yearly risk of 2.5%. This may be related to a higher propensity for mutations to arise in the developing breast. A later age of menopause is associated with a 2.8–3.5% increased risk of BC for each additional year of MCs, as demonstrated in another Collaborative Group analysis of data from 51 epidemiological studies [12, 17]. The effects of age at menarche and at menopause on the risk of BC are generally recognized as significant although the increases in absolute risk are small.
Pregnancy and lactation
The relationship between BC and pregnancy is complicated. Early pregnancy protects against BC, whereas a first pregnancy after the age of 35 increases the risk [18]. Clinically it is well known that pre-existing estrogen receptor positive (ER+) tumors may grow rapidly during pregnancy. Data from the Collaborative Group Report in 2002 show a 7% decrease in BC risk with each birth [19]. More recent papers confirm the protective effect of a full term pregnancy [20] that is not found with pregnancies ending in first trimester abortion [21]. A full-term pregnancy, including the post-partum MC recovery, will prevent about one year of MCs, and the associated 7% reduction in BC risk is higher than the hypothetical 2.5% per year without MCs. Progesterone is an absolute requirement for the continuation of pregnancy and P4 levels increase from 5 to 20 ng/mL during the luteal phase of the MC (Fig. 1) to levels of 50–300 ng/mL at term pregnancy [22]. The levels of the four natural estrogens estrone (E1), E2, estriol (E3) and estetrol (E4) also increase progressively during pregnancy (Fig. 2), resulting in very high levels at term pregnancy. The seeming contradiction posed by the association of increased P4 and estrogen levels during pregnancy and reduced BC may be explained by the fact that P4 and estrogen levels increase gradually, contrary to the rapidly changing levels during the MC, associated with mutations.
The absence of MCs during lactation is related to a decreased risk of BC correlating with the lifetime total duration of breastfeeding. Figure 3 shows this effect with a 30% decrease in the relative risk (RR) of BC for a total duration of lactation of 6 years, reflecting a 5% decrease in BC risk per year of lactation [19]. This is double the hypothetical decrease based on the 2.5% BC risk reduction per year without MCs. Also age-related lobular involution of the breast is related to a lower risk of BC [23], whereas benign breast disease is a risk factor for BC [24].
Relative risk of breast cancer in parous women in relation to lifetime duration of breastfeeding [19]. RightsLink Elsevier permission
Demographic data
When multiple pregnancies are combined with long periods of lactation as occurs in societies without contraception, the risk of BC decreases considerably. For example, in the Amish population in the USA this way of life is associated with a standardized BC incidence ratio (SIR) of 0·58 (95% confidence interval [CI] 0·39–0·83) [25], reflecting a reduction in the lifetime BC risk of about 40%.
The absolute lifetime risk of developing BC for women in Europe, the USA and Asia experiencing over 500 MCs during their life is 13–15%. Women from sub-Saharan Africa have a 4 to fivefold lower incidence of BC compared to economically advantaged western countries with rates of 15–25 per 100,000, versus 70 and 90 per 100,000 for Western Europe and the USA, respectively (Fig. 4) [26]. This is explained by what Tomasetti and co-workers call “the replicative factors related to BC,” including a protective reproductive history, with late menarche, early first pregnancy, high parity with prolonged breastfeeding, irregular infrequent cycles, fewer ovulatory cycles, more stress-related amenorrhea and early menopause [6]. Recent reviews have found that these replicative causes of BC are responsible for 58–83% of BC, with the remainder attributable to genetic and environmental causes [4,5,6,7,8].
Breast cancer incidence and mortality among women of African nations compared with the USA and other international populations [26]. Rates shown are per 100,000 persons. Open Access reference
Genetic interference
Hypogonadotropic syndromes without menstrual cycles
There are a number of genetic conditions interfering with the occurrence of MCs, as reported in detail previously [27]. These genetic abnormalities are hypogonadotropic syndromes including the Turner syndrome (TS), primary congenital hypogonadotropic hypogonadism (CHH), the Kallmann syndrome (KaS) and the pure gonadal dysgenesis (PGD) or Swyer syndrome (SwS). Normogonadotropic genetic syndromes interfering with MCs are the complete androgen insensitivity syndrome (CAIS), and the Mayer–Rokitansky–Küster–Hauser (MRKH) syndrome, also called Müllerian agenesis. The risk of BC is very low or absent in women with TS, KaS, CHH and PGD, who also have reduced breast development, which may contribute to the very low BC risk. Although treatment with estrogens in these genetic disorders stimulates breast development, it seldomly causes BC and is observed only rarely in TS. The CAIS and MRKH syndrome are described in more detail below since both conditions provide insight in the role of P4 in BC.
Normogonadotropic syndromes without menstrual cycles
The complete androgen insensitivity syndrome (CAIS), previously known as testicular feminization, is an X-linked recessive condition in male genotypes, mostly caused by a defect in the androgen receptor gene, resulting in inactivity of the androgen receptor or by congenital interference with T synthesis. The prevalence of CAIS has been estimated as 1 in 90,000 to 100,000 genomic XY new-borns [28]. In CAIS without functional T, the genotypic male XY embryo develops into a phenotypic female. The external male sexual organs do not develop in spite of hormonally active testicles, located in the abdomen or inguinal canal. There is a short vagina, but regularly no uterus, no oviducts and no ovaries. The testicles synthesize male amounts of T with accompanying T levels, and since T is partially metabolized into active E2, this results in adequate female size and structure breast development [29]. Patients with the CAIS syndrome present with the unique combination of female E2 levels and female size and structure breasts without P4. In individuals with the CAIS syndrome, only a single case of a juvenile fibroadenoma of the breast [30], and no BC has been reported [31].
The Mayer–Rokitansky–Küster–Hauser (MRKH) syndrome or Müllerian agenesis is a congenital malformation characterized by failure of the Müllerian ducts to develop in an XX phenotype female, resulting in the absence of the upper two-thirds of the vagina, the uterus and eventually also the oviducts, all derived from the paramesonephric Müllerian ducts [32]. MRKH occurs in about 1:4000 to 5000 female births [33]. In MRKH women, the karyotype is normal (46, XX), and so is ovarian function and breast development[34]. An important cause of MRKH is the absence of activity of the growth factor WNT family member 4 (WNT4) during a critical period of formation of the internal genitalia, 6 to 8 weeks after conception [34]. As a consequence, the Müllerian ducts fail to develop in the absence of WNT4 [35, 36]. In addition, WNT4 has an essential function in mammary gland development downstream of P4 signalling [37]. In normal male embryos, WNT4 activity is counteracted by the sex-determining region Y (SRY) gene on the Y chromosome causing regression of the Müllerian ducts and development of the mesonephric Wolffian ducts, resulting in the formation of epididymis, vas deferens and seminal vesicles in the male [38]. In women with MRKH, only a single case of BC has been reported in the literature [39]. Remarkably, (1) BC was not mentioned at all in a recent MRKH review [40], (2) MRKH patient organizations in the USA (55,000 members), China (130,000 members) and the Netherlands were not aware of any cases of BC among their members when contacted [27] and (3) BC is not mentioned in an extensive document of the American College of Obstetricians and Gynecologists (ACOG) on Müllerian agenesis, which summarizes the relationship between MRKH and many diseases, but does not mention BC [41].
Pathological effects
Diseases and conditions accompanied by long-term hypo- and hypergonadotropic secondary amenorrhea and absence of MCs include anorexia nervosa (AN) and weight loss-related amenorrhea (WLRA), primary ovarian insufficiency (POI) or failure (POF), and premenopausal oophorectomy in women with an increased genetic risk of BC or ovarian cancer or with extensive endometriosis. Here, we review the evidence regarding the risk of BC in women with these pathological conditions.
Anorexia nervosa and weight loss-related amenorrhea
Long-term functional hypothalamic amenorrhea may be caused by AN and WLRA. Several large studies have reported a decreased risk of BC in AN. Compared to the Swedish general population, 7303 women hospitalized for AN before the age of 40 years had a 53% (95% CI 3‒81%) lower incidence of BC, and in the parous women the risk was 76% lower (95% CI 13‒97%) [42]. In a retrospective cohort study on the incidence of BC among 22,654 women with AN from Sweden, Denmark and Finland, the incidence rate ratio for BC compared to randomly selected persons from population registers, and adjusted for age, parity and age at first child, was 0.61 (95% CI 0.49‒0.77) [43]. In the Sister Study in the USA, including 47,813 women who had a sister with BC, 3% (1569 women) reported a history of an eating disorder [WLRA, AN or bulimia nervosa (BN)]. Compared to sisters without an eating disorder, the sisters with AN or BN had a reduced BC risk, with a hazard ratio (HR) of 0.62 (95% CI 0.42‒0.92) [44]. Although these studies do not provide details about MCs, primary or long-term secondary amenorrhea is an obligatory symptom for the diagnosis of AN according to the DSM-5 [45]. Therefore, one may assume that the women with AN in those studies have substantially reduced numbers of MCs, and this may be a causal factor associated with the observed significant decrease in BC risk in the order of 50‒75% [42, 43]. The plausibility of this hypothesis is supported by the observation that for all cancers except BC, women with AN have a higher incidence ratio or SIR and a higher standardized mortality rate [43, 46].
Polycystic ovary syndrome
The BC risk in women with PCOS is not increased, although these women generally have relatively high E2 and T levels. According to the Rotterdam criteria, anovulation is not required for the diagnosis of PCOS, since hyperandrogenaemia and polycystic ovaries are sufficient for the diagnosis [47]. There is a huge amount of data available on BC and PCOS, but quite remarkably, we have not found specific data in the literature on the BC risk in the subgroup of PCOS women with anovulation, who have reduced exposure to P4 [48].
Primary ovarian insufficiency
Primary ovarian insufficiency (POI) or failure (POF) is generally defined as secondary amenorrhea of at least 12 months duration with elevated follicle-stimulating hormone levels in women younger than 40 years [49,50,51]. Women with POI experience fewer MCs, and therefore, a lower incidence of BC is expected. Menopause before the age of 49 was associated with a decreased BC risk [52], and this effect was observed in Caucasian as well as in Asian women. In a study conducted among 12,134 Dutch women, whose menopause occurred before the age of 40 years, a significantly increased total all-cause mortality was observed after adjustment for confounding factors, as compared with the reference group whose menopause occurred at age 50–54 years [HR 1.40 (95% CI 1.15–1.17)] [53]. However, of note, the corresponding incidence of mortality due to BC was much lower than in the reference group [HR 0.55 (95% CI 0.17–1.82)] [53]. In a Dutch population-based BC-screening cohort of 10,591 women, early menopause (occurring at ages below 45, or at 45–49 years) also showed a protective effect on the risk of BC [HR 0.66 (95% CI 0.43–0.91)] and [HR 0.67 (95% CI 0.48–0.94)], respectively, as compared with menopause occurring at age 55 or older [54]. For example, women who became menopausal below 44 years of age had a 34% lower risk of BC than women aged 55 years. The annual hazard rate in relation to the BC incidence decreased by 2.6% in women who had an earlier menopause [54], which aligns with our calculation of a 2.5% BC risk reduction per year without MCs. In Chinese women, POI was associated with an increased risk of all-cause and cancer mortality [HR 1.29 (95% CI 1.08–1.54) and HR 1.38 (95% CI 1.05–1.81), with or without menopausal hormone therapy (MHT), respectively] [52]. However, POI was inversely associated with the incidence of BC [odds ratio [OR] 0.59 (95% CI 0.38–0.91)]. BC was not addressed at all in a recent review on POI [55], as well as in the recent International Menopause Society white paper on POI [56].
Premenopausal oophorectomy
Prophylactic premenopausal mastectomy and oophorectomy are used widely to prevent BC in women who carry genetic mutations that predispose to an increased risk of BC, such as mutations in the BRCA1 and BRCA2 genes [57]. Risk-reducing salpingo-oophorectomy was associated with a statistically significant reduction in the risk of BC in the combined group of BRCA1/2 mutation carriers [HR 0.49 (95% CI 0.37–0 65)]. Similar risk reductions were observed in BRCA1 mutation carriers [HR 0.47 (95% CI (0.35–0.64)] and in BRCA2 mutation carriers [HR 0.47 (95% CI 0.26–0.84)] [57]. Bilateral oophorectomy for the treatment of ovarian cancer is also associated with a large reduction in BC risk [58]. It would be interesting to know the BC risk after bilateral oophorectomy for extensive endometriosis, but we have not found this information in the literature.
Pharmaceutical effects
The present review is focusing on the effect of the steroid hormones of the natural menstrual cycle (MC) on the risk of breast cancer (BC). The effect of the BC risk of pharmaceutical treatments with exogenous reproductive steroid hormones for hormonal contraception (HC), endometriosis treatment, hormone treatment of transgender individuals and menopausal hormone therapy (MHT) is beyond the scope of this review and deserves a detailed separate analysis. Here we will only briefly summarize the effect of HC, including P-only, on E-only MHT and on transgender treatments.
Menstrual cycle suppression and breast cancer risk
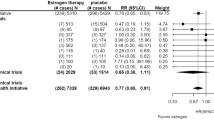
Hormonal contraception (HC) and endocrine treatment of endometriosis are major pharmaceutical applications in Women’s Health, where the natural MC is suppressed and where the relationship between the administration of estrogens and progestogens (including P4 and progestins) and the occurrence of BC has been extensively studied. To judge this relationship, one has to realize that it takes about a decade from a stem cell mutation in the breast until the tumor has grown to a size enabling the diagnosis of a BC [59]. This means that tumors diagnosed during the first decade of HC and endometriosis treatment are most likely due to mutations that occurred during earlier spontaneous MCs as are BCs diagnosed during the first decade after menopause with or without MHT. In case ovulatory MCs would be responsible for the high BC incidence in women indeed, MC suppression by combined E/P oral contraceptives, ovulation inhibiting progestin-only (P-only) contraception or hormonal endometriosis treatment, would protect against BC and one would expect to observe a lower incidence of BC. However, the data show the contrary [60,61,62,63]. As an example, Table 1 shows the results in a recent large Danish HC study including some P-only contraceptives, confirming no decrease in BC and even an increase with duration of use [64].
Estrogen-only MHT in the WHI study and breast cancer risk
A special case is the Women’s Health Initiative (WHI) study, the largest randomized controlled MHT trial ever, which nearly abolished MHT use forever. Table 2 summarizes the BC results of the WHI study. This study is different from many others, since conjugated equine estrogens (CEE) plus medroxyprogesterone acetate (MPA) (E/P) and E-only treatment started on average 12 years after menopause in women with a mean age of 63 years (range 50–79) at the time of enrollment. This late start of MHT is highly relevant, since it means that in most cases already existing BCs had been devoid of estrogens for more than 5 years, so those BCs may have become sensitive to the therapeutic effect of (high dose) estrogen treatment which in this setting, and in the absence of potentially BC stimulating progestins, causes tumor regression (4,5,6,7,8). Initially, the E/P arm of the study was thought to have an increased BC risk which was of concern despite the lack of statistical significance [65]. Retrospectively, this small non-significant increase was likely due to unmasking of existing BCs by the proliferative influence of MPA. Furthermore, later analysis demonstrated that the risk difference in the E/P arm was based on a lower BC risk in women with any prior hormone use [66]. In contrast, from the very beginning, the E-only arm of the WHI showed protection against BC [67], exactly what would be expected, based on the long previous period of estrogen deficiency [4,5,6,7,8]. The most important WHI follow-up paper was the one on long-term all-cause and cause-specific mortality published in JAMA in 2017, summarizing a cumulative follow-up of 18 years [68]. It was found that the median treatment of 5.6 years with the E/P combination and of 7.2 years with E-only in this older age group of postmenopausal women was not associated with an increased risk of all-cause, CV or cancer mortality 18 years later. In the pooled analysis combining E/P and E-only for women 50 to 59 years old when they entered the study, there was a 31% reduction in all-cause mortality [HR 0.69 (95% CI 0.51‒0.94)]. The HR for death due to BC for the E/P combination used in the WHI study was elevated but not statistically significant [1.44 (95% CI 0.97‒2.15)], suggesting a stimulatory, and possibly mutagenic effect of MPA. However, the most impressive BC outcome in this analysis was the highly significant finding that death due to BC (and not just risk) in the E-only CEE group was 45% lower than in the placebo group with a HR of 0.55 (95% CI 0.33‒0.92) (Table 2) [68]. The conclusion is that E-only MHT extends life when MHT is started at least 5 years after menopause (in the WHI study on average at 63 years).
Hormonal treatment of transgender individuals
Both male-to-female (MtF) and female-to-male (FtM) transgender individuals are treated long-term with high doses of, respectively, estrogens and androgens, and the consequences of these steroid hormone treatments have been reported by us in detail previously [27]. Here we summarize the data relevant for this review.
Estrogen treatment of MtF transgenders and BC risk
The MtF gender identity disorder occurs in about 1 in 12,000 born males [69]. These individuals are treated with estrogens for breast development and with antiandrogens or orchidectomy to suppress androgens. Adequate breast development with normal histology is obtained by treatment with high doses of estrogens (HDE) and is continued lifelong [70, 71]. In a study in 2307 MtF transgender individuals, two cases of BC have been reported after exposure to HDE for 5 to 30 years, resulting in an incidence of 4.1/100,000 person-years [70]. A study in MtF transgender individuals in the USA found similar results [72]. A recent nationwide cohort study in the Netherlands reported 15 cases of BC in 2,206 MtF transgenders on HDE treatment with a mean duration of 18 years (range, 7–37 years). Compared to Dutch males, the overall risk of BC was increased [SIR 46.7 (95% CI 27.2–75.4)], but the risk was still much lower than in the female population [SIR 0.3 (95% CI 0.2–0.4)] [73]. These studies show that the incidence of BC in MtF transgenders with fully developed normal breasts, treated long term with HDE without progestins (since they have no uterus), is much lower than the BC risk of cisgender females and somewhat higher than the BC risk of cisgender males [70,71,72,73].
Testosterone treatment of FtM transgenders and BC risk
The FtM gender identity disorder occurs in about 1 in 30,000 born females [69] and concerns genetic and phenotypic females, who feel male and wish to become phenotypic males. In these patients the ovaries, the uterus and the breasts are surgically removed and masculinization is achieved by treatment with high doses of testosterone (HDT). The occurrence of BC in FtM transgenders depends on the completeness of removing all mammary tissue by mastectomy [70, 72, 74] and is comparable to the prevention of BC by mastectomy in high-risk women carrying germline mutations such as BRCA1 and BRCA2 [75]. In the Dutch cohort study, four cases of BC were reported in 1,229 FtM individuals treated with HDT with a SIR vs Dutch cisgender women of 0.2 (95% CI 0.1‒0.5) [73]. In a systematic review of BC in HDT-treated FtM transgender individuals, 17 cases of BC were found originating from eight studies [76], resulting in a BC risk somewhat higher than in cisgender males. These studies show that long-term HDT treatment does not increase the risk of BC significantly.
Carcinogenicity of progesterone
The mammary gland is a highly heterogenous tissue with many different cell lineages of which the differentiation hierarchy has not yet been definitively defined [77]. The adult breast epithelium of the mammary gland contains differentiated ductal, alveolar and myoepithelial cells, and their progenitors. In addition, cells that provide long-term maintenance and regenerative potential, such as multipotent mammary stem cells (MaSCs) and certain short- and long-term progenitors, have been identified [78,79,80,81,82].
While most of these cells, including the stem cells, do not express the ER and progesterone receptor (PR) [77], it has now been well established that E2 and P4 exert their effects on the breast epithelium by inducing the secretion of paracrine factors by a relatively small pool of ER+/PR+ cells [80].
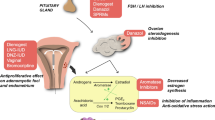
During puberty, the mammary gland undergoes phenotypic and morphological changes, particularly regulated by E2 and to a lesser extent by P4 [83]. However, when female sexual maturity is reached around age 15–17 years, the profound phenotypic changes of the breast epithelium during each MC are mainly induced by P4 [84]. In the follicular phase of the MC, when E2 peaks, ER+ cells upregulate the expression of the PR to increase the pool of PR+ cells, which hardly induces any morphological or molecular changes in the epithelium [85, 86]. When the MC enters the luteal phase, the increasing P4 levels initially induce PR-mediated proliferation of PR+ cells (intrinsic effect), and subsequently of neighboring hormone receptor negative (HR−) cells (extrinsic), including epithelial progenitor cells and MaSCs (Fig. 5) [84, 85]. The cell intrinsic effect is mediated by cyclin D1 and has an overall mild proliferative effect on the breast epithelium, while the extrinsic stimulation of adjacent HR− cells, including MaSCs, by the activated PR+ cells has a much more profound effect. The latter is largely mediated by the paracrine factors RANKL and WNT4, which are direct targets of the PR and play important physiological roles in the breast [87, 88]. Both factors have been shown to be strongly upregulated during the luteal phase in the breast epithelium of healthy premenopausal women, concomitant with genes involved in the cell cycle and DNA replication [86]. The P4-mediated induction of RANKL and WNT4 and the consequent activation and proliferation of MaSCs has been linked to BC initiation [89,90,91]. As stem cells are considered an important cancer cell-of-origin in BC [92], and proliferation (or DNA replication) is a prominent source of mutations in cancer [6], P4 is likely to impact breast carcinogenesis [93].
The progesterone signaling hub in the adult mammary epithelium progesterone, upon binding to its receptor in the ER+/PR+ sensor cells (blue) activates different signaling pathways. It can stimulate cell-intrinsic proliferation by a cyclin D1-dependent mechanism (blue) and induce secreted factors like Amphiregulin, CXCL12, or Calcitonin (blue). Distinct PR+ cells induce WNT4, which acts on the myoepithelium where it activated canonical WNT signaling, which results in the expression of the secreted protease Adamts18 that cleaves fibronectin. As a result the ECM, part of the stem cells niche is biochemically altered with resulting activation of the hippo signaling pathway and increased transcription of FGFR signaling components (red). In other PR+ cells, RANKL is induced that induces the proliferation of neighboring ER−/PR− responder cells (green) [84]. Open Access reference and author permission
Breast cancer has been shown to be a type of cancer largely driven by DNA replication errors, with up to 83% of cases possibly attributable to this factor [6]. Such errors are caused by molecular processes that generate particular mutational signatures/patterns in DNA [94], which have also been identified in the DNA of breast tumors [95,96,97,98]. This revealed that in BC, one of the most important sources of somatic mutations arising during DNA replication stems from the activity of APOBEC (Apolipoprotein B mRNA editing enzyme catalytic) enzymes, which deaminate, and thereby convert, cytosine nucleotides [99].
One of the members of this family of enzymes, APOBEC3B, has been shown to be expressed and to actively generate mutations in breast tumors [100, 101]. APOBEC3B expression strongly correlates with the cell cycle and with the DNA damage response pathway in BC [102]. It introduces mutations during DNA replication, and therefore confers or increases replication stress [99]. Interestingly, this enzyme was shown to be statistically significantly upregulated during the luteal phase in breast epithelium of healthy premenopausal women [86]. This suggests that under the influence of P4, during each MC, increased activity of this enzyme can introduce DNA mutations in breast epithelial cells to increase BC risk. Activity of APOBEC3B has been linked to the generation of mutations in TP53 [100], an important early driver in BC [103]. In addition, APOBEC-mediated mutational signatures have been observed in normal bronchial, colon, oesophageal and bladder epithelium [104,105,106,107].
The proliferative potential of the breast epithelium during the MC decreases with age [108, 109], and after menopause, when exposure to intermittent levels of P4 stops and the levels of E2 decline, it enters a quiescent state. If P4 is an essential component in breast carcinogenesis, how does this hormone impact the increased BC incidence observed in postmenopausal women aged > 60 years? It is plausible that the period between the first MC and menopause is an important source of somatic mutations accumulating in breast epithelial stem cells, a process P4 appears intricately to be involved in.
Studies into the genomic evolution of BC reveal that tumors evolve through several developmental stages before they are diagnosed [96]. Two pre-diagnosis stages are described, of which the first one is by far the longest and is suggested to take decades and slowly accumulates many DNA alterations [103]. The second stage unfolds over a relatively short timeframe (months to a few years) and is postulated to contribute the larger part to tumor size [95]. Therefore, epithelial stem cells that have acquired mutations during the reproductive years as a consequence of P4 action may remain dormant for an extended period of time after menopause. Under the influence of a gradually changing breast tissue microenvironment [110] and perhaps affected by changes in immune competency, pre-malignant lesions may only become evident at an advanced age after menopause.
Considerations
Lifetime number of menstrual cycles and breast cancer risk
We have reviewed the available information on the effect of physiological, genetic, pathological and pharmaceutical variations on the number of MCs, the presence of P4 and E2 peaks, and the risk of BC. We confirm the high correlation reported earlier between the number of MCs and the risk of BC [11, 12]. Early menarche and late menopause are related to a small increase in BC risk, proportional to the higher number of MCs with an even somewhat higher risk of early menarche, which may be due to the higher sensitivity of the developing breasts. Pregnancy and lactation reduce the risk of BC, and the yearly risk reduction of, respectively, 7% and 4.3% is somewhat higher than the expected 2.5% per year based on the absence of MCs alone, suggesting additional protective mechanisms. The substantially lower number of MCs in women with pathological reductions in MCs by AN/WLRA, POI and after premenopausal oophorectomy is accompanied by a large and proportional reduction in the lifetime BC risk of approximately 50% [42, 43, 54, 57], confirming the MC hypothesis.
Genetic interference with menstrual cycles and breast cancer
The risk of BC appears to be very low or absent in individuals with genetic abnormalities interfering with the occurrence of MCs such as TS, KaS, CHH and PGD. Generally, these individuals have little breast development and breast size may have some effect on the BC risk [111], although in normal women no appreciable association between breast size and BC has been found [112]. E/P treatment in individuals with these genetic disorders induces normal breasts, but rarely causes BC. Mastectomy performed in women with BC-related mutations such as BRCA1 and BRCA2, completely prevents the occurrence of BC [75].
Most remarkable and important are the observations in individuals with the CAIS syndrome and the MRKH syndrome. The genotypic male but phenotypic female individuals with the CAIS syndrome have no BC in the presence of substantial estrogen levels and normal female breasts, which could be explained by the absence of P4. An exception to the relationship between MCs, P4 and BC is the apparent absence of BC in women with the MRKH syndrome, who have ovulatory cycles, sometimes as part of PCOS like endocrinology [113], with accompanying normal P4 levels and normal breasts, but no BC. We speculate that this is related to the absence of the WNT4 gene, also responsible for the MRKH syndrome itself, negating P4 induced WNT4 activity. Further research focusing on the role of WNT4 as a causal factor of BC seems relevant.
Progesterone as a causal factor of breast cancer
The major reproductive steroid hormones from the normal menstrual cycle E2, P4 and T have all been implicated as causal as well as stimulatory agents of existing BC. As data have shown that P4 has a much more pronounced effect on the healthy breast epithelium during the MC as compared to E2 and T, we have focused on P4 as causal agent and we have carefully tried to distinguish between cause and stimulation. Based on the BC risk in different populations reviewed in this paper, we found that the lifetime number of MCs and the frequent intermittent exposure to P4 during these MCs are major explanations for the high BC risk in women. The repeated intermittent stimulation of proliferation of the breast epithelium by P4 likely contributes to the accumulation of mutations in certain long-lived cells. Breast cancer may develop because of these mutations, and based on a doubling time of the tumor of about 10–12 years it may take a decade until a BC is large enough to be diagnosed [59]. Breast (mammary) tissue has an increased risk of DNA damage compared to other tissues in the human body due to the extensive remodeling in the breast through a high rate of proliferation, apoptosis and differentiation, occurring throughout a woman’s life. Reproductive events responsible for this enormous remodeling are related to a woman’s reproductive history and include puberty, MCs, pregnancy, lactation and postmenopausal involution. We have investigated the relationship between the risk of developing BC and the number of MCs and the accompanying intermittent luteal phase P4 increases that a woman experiences during her lifetime. The repeated intermittent effect of hormone increases may be more relevant than continuous exposure, since every new hormone peak may cause new mutations and contribute to the accumulation of mutations in long-lived mammary progenitor and stem cells.
The relationship between endogenous P4 and BC has been reviewed recently by Trabert et al. [93]. They found no direct association of circulating P4 levels with BC risk, which may not be surprising in the light of our hypothesis, that intermittent P4 peaks are associated with breast carcinogenesis. They also concluded that preclinical and clinical evidence supports differentiation and proliferative roles of P4 in the adult breast primarily through paracrine actions and points to the importance of understanding the role of P4 in controlling the fate of epithelial cells and early events in breast carcinogenesis.
Environmental factors and breast cancer
The three major causes of BC are generally distinguished as (1) reproductive factors related to a woman’s reproductive history, (2) germline mutations and (3) environmental effects. Contemporary literature suggests that at least 58% and up to 83% of BCs are caused by reproductive factors [4,5,6,7,8]. Our analysis of clinical data strongly suggests that the essential reproductive factor associated with BC is the frequent intermittent exposure to P4 from ovulatory MCs. Crucial is that we have found that BCs hardly ever occur without MCs and without P4, no matter whether environmental factors are present or not. Also, high exposure to E-only in the WHI E-only study and in MtF transgender individuals and high exposure to T-only in FtM transgender individuals is not related to a significant risk of BC, again unrelated to the presence or absence of environmental factors. This suggests that the significance of environmental factors such as exposure to chemicals, alcohol, toxic food contaminants, estrogen-related effects such as high breast density, high E2 levels and exposure to estrogen metabolites, and lifestyle factors such as overweight and (lack of) exercise [5, 7] may have been overemphasized as cause of BC although these factors may certainly stimulate existing BC, which may explain the confusion. The increased incidence of BC in women with high breast density may be related to higher estrogen levels. The increased BC risk related to higher levels of E2, to other non-ovarian estrogens and to estrogen metabolites can all be explained by stronger stimulation of existing BCs. The lower BC risk observed in vigorously exercising female athletes may be related to anovulation and amenorrhea [114, 115], so less P4 with a lower risk of causing BC and less E with less stimulation of existing BC. Also exercise may decrease the body mass index (BMI), and thereby, less estrogens and less estrogen metabolites will be generated from fatty tissue with less BC stimulation as a consequence [116].
Most data on the relationship between environmental factors and the risk of BC are derived from observational studies, and prospective controlled studies on these factors are rare and difficult to perform. Furthermore, except for toxic mutagenic agents such as alcohol, the mutagenicity and mode of action of environmental factors in causing BC are unknown and stimulation of existing BC seems more likely than induction of new BCs. A more detailed analysis of the environmental factors is beyond the scope of this review and deserves a separate analysis.
Key points
The key points resulting from review are presented in Table 3. The relationship between MCs and BC, and of P4 and BC, is summarized in Table 4, including the P4/WNT4 hypothesis based on the MRKH data.
Concluding remarks
Our analysis of clinical and molecular data suggests that P4 and not E2 or T is an important cause of BC by stimulating proliferation of normal breast epithelium during the luteal phase of the MC through the paracrine factors WNT4 and RANKL. Progesterone also appears to upregulate the expression of the DNA mutator enzyme APOBEC3B during this phase of the MC. These effects of P4 likely increase the accumulation of mutations in long-lived mammary stem and progenitor cells. Estrogens, testosterone (as precursor of E2) and most environmental factors that are related to the risk of BC may stimulate the growth of already existing ER+/PR+ BC, but have only mild proliferative effects on normal breast epithelium and, in light of our hypothesis, are therefore unlikely to cause BC. We propose to develop medical strategies in women that avoid exposure to natural P4 and progestins as much as possible.
Availability of data and materials
All data generated or analyzed during this study are included in this published article.
Abbreviations
- ACOG:
-
American College of Obstetricians and Gynaecologists
- AN:
-
Anorexia nervosa
- APOBEC:
-
Apolipoprotein B mRNA editing enzyme catalytic
- BC:
-
Breast cancer
- BN:
-
Bulimia nervosa
- BRCA1/2:
-
Breast cancer genes 1 and 2
- CAIS:
-
Complete androgen insensitivity syndrome
- CEE:
-
Conjugated equine estrogens
- CHH:
-
Congenital hypogonadotropic hypogonadism
- CI:
-
Confidence interval
- DSG:
-
Desogestrel
- E:
-
Estrogen
- E1:
-
Estrone
- E2:
-
Estradiol
- E3:
-
Estriol
- E4:
-
Estetrol
- E/P:
-
Estrogen plus progestin
- ER:
-
Estrogen receptor
- FtM:
-
Female to male
- FU:
-
Follow-up
- HC:
-
Hormonal contraception
- HR:
-
Hazard ratio
- HT:
-
Hormone treatment
- KaS:
-
Kallmann syndrome
- LNG:
-
Levonorgestrel
- MaSCs:
-
Mammary stem cells
- MC:
-
Menstrual cycle
- MHT:
-
Menopausal hormone therapy
- MRKH:
-
Mayer–Rokitansky–Küster–Hauser syndrome
- MPA:
-
Medroxyprogesterone acetate
- MtF:
-
Male to female
- NETA:
-
Norethisterone acetate
- OR:
-
Odds ratio
- P-only:
-
Progestogen-only
- P4:
-
Progesterone
- PCOS:
-
Polycystic ovary syndrome
- PGD:
-
Pure gonadal dysgenesis
- POF:
-
Primary ovarian failure
- POI:
-
Primary ovarian insufficiency
- RR:
-
Relative risk
- SIR:
-
Standardized incidence ratio
- SRY:
-
Sex-determining region Y gene
- SwS:
-
Swyer syndrome
- T:
-
Testosterone
- TS:
-
Turner syndrome
- USA:
-
United States of America
- WHI:
-
Women’s Health Initiative
- WLRA:
-
Weight loss-related amenorrhea
- WNT4:
-
WNT family member 4
- yr:
-
Year
References
NBCC Breast Cancer Facts & Figures. https://www.stopbreastcancer.org/information-center/facts-figures/ (accessed on April 11, 2022).
Cancer Research UK. Breast cancer statistics. https://www.cancerresearchuk.org/health-professional/cancer-statistics/statistics-by-cancer-type/breast-cancer (accessed April 11, 2022).
ACS. Key statistics for breast cancer in men. https://www.cancer.org/cancer/breast-cancer-in-men/about/key-statistics.html (accessed on April 11, 2022).
Prat A, Perou CM. Deconstructing the molecular portraits of breast cancer. Mol Oncol. 2011;5(1):5–23.
Davis JD, Lin SY. DNA damage and breast cancer. World J Clin Oncol. 2011;2(9):329–38.
Tomasetti C, Li L, Vogelstein B. Stem cell divisions, somatic mutations, cancer etiology, and cancer prevention. Science. 2017;355(6331):1330–4.
World Cancer Research Fund/American Institute for Cancer Research. Diet, Nutrition, Physical Activity and Cancer: a Global Perspective. Continuous Update Project Expert Report 2018. https://www.wcrf.org/wp-content/uploads/2021/02/Summary-of-Third-Expert-Report-2018.pdf (accessed on April 25, 2022).
Dowsett M, Folkerd E. Reduced progesterone levels explain the reduced risk of breast cancer in obese premenopausal women: a new hypothesis. Breast Cancer Res Treat. 2015;149(1):1–4.
Henderson BE, Ross RK, Judd HL, Krailo MD, Pike MC. Do regular ovulatory cycles increase breast cancer risk? Cancer. 1985;56(5):1206–8.
Michels-Blanck H, Byers T, Mokdad AH, Will JC, Calle EE. Menstrual patterns and breast cancer mortality in a large U.S. cohort. Epidemiology. 1996;7(5):543–6.
Clavel-Chapelon F. Cumulative number of menstrual cycles and breast cancer risk: results from the E3N cohort study of French women. Cancer Causes Control. 2002;13(9):831–8.
Collaborative Group on Hormonal Factors in Breast Cancer. Menarche, menopause, and breast cancer risk: individual participant meta-analysis, including 118,964 women with breast cancer from 117 epidemiological studies. Lancet Oncol. 2012;13(11):1141–51.
Coelingh Bennink HJT, Zimmerman Y, Laan E, Termeer HMM, Appels N, Albert A, et al. Maintaining physiological testosterone levels by adding dehydroepiandrosterone to combined oral contraceptives: I. Endocrine effects. Contraception. 2017;96(5):322–9.
Bui HN, Sluss PM, Blincko S, Knol DL, Blankenstein MA, Heijboer AC. Dynamics of serum testosterone during the menstrual cycle evaluated by daily measurements with an ID-LC-MS/MS method and a 2nd generation automated immunoassay. Steroids. 2013;78(1):96–101.
Roumen FJME, Zimmerman Y, Van Wijck ATK, Onghena PCBHJT. Mood disturbances during combined oral contraceptive use and the effect of androgen supplementation. Results of a double-blind, placebo-controlled, single-case alternation design pilot study. Eur J Contracept Reprod Health Care. 2017;22(2):147–51.
van Lunsen RHW, Zimmerman Y, Coelingh Bennink HJT, Termeer HMM, Appels N, Fauser B, et al. Maintaining physiologic testosterone levels during combined oral contraceptives by adding dehydroepiandrosterone: II. Effects on sexual function. A phase II randomized, double-blind, placebo-controlled study. Contraception. 2018;98(1):56–62.
Collaborative Group on Hormonal Factors in Breast Cancer. Breast cancer and hormone replacement therapy: collaborative reanalysis of data from 51 epidemiological studies of 52,705 women with breast cancer and 108,411 women without breast cancer. Lancet. 1997;350(9084):1047–59.
Nichols HB, Schoemaker MJ, Cai J, Xu J, Wright LB, Brook MN, et al. Breast cancer risk after recent childbirth: a pooled analysis of 15 prospective studies. Ann Intern Med. 2019;170(1):22–30.
Collaborative Group on Hormonal Factors in Breast Cancer. Breast cancer and breastfeeding: collaborative reanalysis of individual data from 47 epidemiological studies in 30 countries, including 50302 women with breast cancer and 96973 women without the disease. Lancet. 2002;360(9328):187–95.
Meier-Abt F, Bentires-Alj M, Rochlitz C. Breast cancer prevention: lessons to be learned from mechanisms of early pregnancy-mediated breast cancer protection. Cancer Res. 2015;75(5):803–7.
Beral V, Bull D, Doll R, Peto R, Reeves G, Collaborative Group on Hormonal Factors in Breast C. Breast cancer and abortion: collaborative reanalysis of data from 53 epidemiological studies, including 83000 women with breast cancer from 16 countries. Lancet. 2004;363(9414):1007–16.
Speroff L, Fritz M. The clinical gynaecologic endocrinology and infertility. 7th ed. Philadelphia: Lippincott Williams & Wilkins; 2005.
Radisky DC, Visscher DW, Frank RD, Vierkant RA, Winham S, Stallings-Mann M, et al. Natural history of age-related lobular involution and impact on breast cancer risk. Breast Cancer Res Treat. 2016;155(3):423–30.
Hartmann LC, Sellers TA, Frost MH, Lingle WL, Degnim AC, Ghosh K, et al. Benign breast disease and the risk of breast cancer. N Engl J Med. 2005;353(3):229–37.
Westman JA, Ferketich AK, Kauffman RM, MacEachern SN, Wilkins JR 3rd, Wilcox PP, et al. Low cancer incidence rates in Ohio Amish. Cancer Causes Control. 2010;21(1):69–75.
Fregene A, Newman LA. Breast cancer in sub-Saharan Africa: how does it relate to breast cancer in African-American women? Cancer. 2005;103(8):1540–50.
Coelingh Bennink HJT, Egberts JFM, Mol JA, Roes KCB, van Diest PJ. Breast cancer and major deviations of genetic and gender-related structures and function. J Clin Endocrinol Metab. 2020;105(9):e3065–74.
Mendoza N, Motos MA. Androgen insensitivity syndrome. Gynecol Endocrinol. 2013;29(1):1–5.
Galani A, Kitsiou-Tzeli S, Sofokleous C, Kanavakis E, Kalpini-Mavrou A. Androgen insensitivity syndrome: clinical features and molecular defects. Hormones (Athens). 2008;7(3):217–29.
Hoefgen HR, Merritt DF. Invasive ductal carcinoma in a 46, XY partial androgen insensitivity syndrome patient on hormone therapy. J Pediatr Adolesc Gynecol. 2015;28(4):e95–7.
Boehmer AL, Brinkmann O, Bruggenwirth H, van Assendelft C, Otten BJ, Verleun-Mooijman MC, et al. Genotype versus phenotype in families with androgen insensitivity syndrome. J Clin Endocrinol Metab. 2001;86(9):4151–60.
Sultan C, Biason-Lauber A, Philibert P. Mayer–Rokitansky–Kuster–Hauser syndrome: recent clinical and genetic findings. Gynecol Endocrinol. 2009;25(1):8–11.
Choussein S, Nasioudis D, Schizas D, Economopoulos KP. Mullerian dysgenesis: a critical review of the literature. Arch Gynecol Obstet. 2017;295(6):1369–81.
Ledig S, Wieacker P. Clinical and genetic aspects of Mayer–Rokitansky–Kuster–Hauser syndrome. Med Genet. 2018;30(1):3–11.
Prunskaite-Hyyrylainen R, Skovorodkin I, Xu Q, Miinalainen I, Shan J, Vainio SJ. Wnt4 coordinates directional cell migration and extension of the Mullerian duct essential for ontogenesis of the female reproductive tract. Hum Mol Genet. 2016;25(6):1059–73.
Vainio S, Heikkila M, Kispert A, Chin N, McMahon AP. Female development in mammals is regulated by Wnt-4 signalling. Nature. 1999;397(6718):405–9.
Brisken C, Heineman A, Chavarria T, Elenbaas B, Tan J, Dey SK, et al. Essential function of Wnt-4 in mammary gland development downstream of progesterone signaling. Genes Dev. 2000;14(6):650–4.
Kashimada K, Koopman P. Sry: the master switch in mammalian sex determination. Development. 2010;137(23):3921–30.
Kasap E, Gene M, Sahin N, Sivrikoz ON. Mayer–Rokitansky–Kuster–Hauser syndrome accompanied by invasive ductal carcinoma: a case report. Eur J Gynaecol Oncol. 2016;37(5):744–6.
Herlin MK, Petersen MB, Brannstrom M. Mayer–Rokitansky–Kuster–Hauser (MRKH) syndrome: a comprehensive update. Orphanet J Rare Dis. 2020;15(1):214.
ACOG Committee Opinion No. 728, January 2018. Müllerian Agenesis: Diagnosis, Management, and Treatment https://www.acog.org/clinical/clinical-guidance/committee-opinion/articles/2018/01/mullerian-agenesis-diagnosis-management-and-treatment (accessed on April 11, 2022).
Michels KB, Ekbom A. Caloric restriction and incidence of breast cancer. JAMA. 2004;291(10):1226–30.
Mellemkjaer L, Papadopoulos FC, Pukkala E, Ekbom A, Gissler M, Christensen J, et al. Cancer incidence among patients with Anorexia Nervosa from Sweden, Denmark and Finland. PLoS ONE. 2015;10(5): e0128018.
O’Brien KM, Whelan DR, Sandler DP, Weinberg CR. Eating disorders and breast cancer. Cancer Epidemiol Biomarkers Prev. 2017;26(2):206–11.
DSM-5. Diagnostic and Statistical Manual of Mental Disorders. 2013.
Karamanis G, Skalkidou A, Tsakonas G, Brandt L, Ekbom A, Ekselius L, et al. Cancer incidence and mortality patterns in women with anorexia nervosa. Int J Cancer. 2014;134(7):1751–7.
Rotterdam, ESHRE-ASRM-Sponsored PCOS consensus workshop group. Revised 2003 consensus on diagnostic criteria and long-term health risks related to polycystic ovary syndrome (PCOS). Hum Reprod. 2004;19(1):41–7.
Harris HR, Terry KL. Polycystic ovary syndrome and risk of endometrial, ovarian, and breast cancer: a systematic review. Fertil Res Pract. 2016;2:14.
Nelson LM. Clinical practice. Primary ovarian insufficiency. N Engl J Med. 2009;360(6):606–14.
De Vos M, Devroey P, Fauser BC. Primary ovarian insufficiency. Lancet. 2010;376(9744):911–21.
ACOG Committee Opinion No. 698 (reaffirmed 2021). Hormone therapy in primary ovarian insufficiency. https://www.acog.org/clinical/clinical-guidance/committee-opinion/articles/2017/05/hormone-therapy-in-primary-ovarian-insufficiency (accessed on April 11, 2022).
Wu X, Cai H, Kallianpur A, Li H, Yang G, Gao J, et al. Impact of premature ovarian failure on mortality and morbidity among Chinese women. PLoS ONE. 2014;9(3): e89597.
Ossewaarde ME, Bots ML, Verbeek AL, Peeters PH, van der Graaf Y, Grobbee DE, et al. Age at menopause, cause-specific mortality and total life expectancy. Epidemiology. 2005;16(4):556–62.
Monninkhof EM, van der Schouw YT, Peeters PH. Early age at menopause and breast cancer: are leaner women more protected? A prospective analysis of the Dutch DOM cohort. Breast Cancer Res Treat. 1999;55(3):285–91.
Fenton AJ. Premature ovarian insufficiency: pathogenesis and management. J Midlife Health. 2015;6(4):147–53.
Panay N, Anderson RA, Nappi RE, Vincent AJ, Vujovic S, Webber L, et al. Premature ovarian insufficiency: an International Menopause Society White Paper. Climacteric. 2020;23(5):426–46.
Rebbeck TR, Levin AM, Eisen A, Snyder C, Watson P, Cannon-Albright L, et al. Breast cancer risk after bilateral prophylactic oophorectomy in BRCA1 mutation carriers. J Natl Cancer Inst. 1999;91(17):1475–9.
Shuster LT, Rhodes DJ, Gostout BS, Grossardt BR, Rocca WA. Premature menopause or early menopause: long-term health consequences. Maturitas. 2010;65(2):161–6.
Santen RJ, Yue W, Heitjan DF. Modeling of the growth kinetics of occult breast tumors: role in interpretation of studies of prevention and menopausal hormone therapy. Cancer Epidemiol Biomarkers Prev. 2012;21(7):1038–48.
Collaborative Group on Hormonal Factors in Breast Cancer. Breast cancer and hormonal contraceptives: collaborative reanalysis of individual data on 53,297 women with breast cancer and 100,239 women without breast cancer from 54 epidemiological studies. Lancet. 1996;347(9017):1713–27.
Kahlenborn C, Modugno F, Potter DM, Severs WB. Oral contraceptive use as a risk factor for premenopausal breast cancer: a meta-analysis. Mayo Clin Proc. 2006;81(10):1290–302.
Zhu H, Lei X, Feng J, Wang Y. Oral contraceptive use and risk of breast cancer: a meta-analysis of prospective cohort studies. Eur J Contracept Reprod Health Care. 2012;17(6):402–14.
Burchardt NA, Eliassen AH, Shafrir AL, Rosner B, Tamimi R, Kaaks R, et al. Oral contraceptive use by formulation and breast cancer risk by subtype in the Nurses’ Health Study II: a prospective cohort study. Am J Obstet Gynecol. 2022;226(6):821.
Morch LS, Skovlund CW, Hannaford PC, Iversen L, Fielding S, Lidegaard O. Contemporary hormonal contraception and the risk of breast cancer. N Engl J Med. 2017;377(23):2228–39.
Rossouw JE, Anderson GL, Prentice RL, LaCroix AZ, Kooperberg C, Stefanick ML, et al. Risks and benefits of estrogen plus progestin in healthy postmenopausal women: principal results from the Women’s Health Initiative randomized controlled trial. JAMA. 2002;288(3):321–33.
Hodis HN, Sarrel PM. Menopausal hormone therapy and breast cancer: what is the evidence from randomized trials? Climacteric. 2018;21(6):521–8.
Anderson GL, Limacher M, Assaf AR, Bassford T, Beresford SA, Black H, et al. Effects of conjugated equine estrogen in postmenopausal women with hysterectomy: the Women’s Health Initiative randomized controlled trial. JAMA. 2004;291(14):1701–12.
Manson JE, Aragaki AK, Rossouw JE, Anderson GL, Prentice RL, LaCroix AZ, et al. Menopausal hormone therapy and long-term all-cause and cause-specific mortality: the women’s health initiative randomized trials. JAMA. 2017;318(10):927–38.
Gooren LJ. Clinical practice. Care of transsexual persons. N Engl J Med. 2011;364(13):1251–7.
Gooren LJ, van Trotsenburg MA, Giltay EJ, van Diest PJ. Breast cancer development in transsexual subjects receiving cross-sex hormone treatment. J Sex Med. 2013;10(12):3129–34.
Wierckx K, Gooren L, T’Sjoen G. Clinical review: breast development in trans women receiving cross-sex hormones. J Sex Med. 2014;11(5):1240–7.
Brown GR, Jones KT. Incidence of breast cancer in a cohort of 5135 transgender veterans. Breast Cancer Res Treat. 2015;149(1):191–8.
de Blok CJM, Wiepjes CM, Nota NM, van Engelen K, Adank MA, Dreijerink KMA, et al. Breast cancer risk in transgender people receiving hormone treatment: nationwide cohort study in the Netherlands. BMJ. 2019;365: l1652.
Patel JM, Dolitsky S, Bachman GA, Buckley de Meritens A. Gynecologic cancer screening in the transgender male population and its current challenges. Maturitas. 2019;129:40–4.
Balmana J, Diez O, Castiglione M, Group EGW. BRCA in breast cancer: ESMO clinical recommendations. Ann Oncol. 2009;20(Suppl 4):19–20.
Stone JP, Hartley RL, Temple-Oberle C. Breast cancer in transgender patients: a systematic review. Part 2: Female to Male. Eur J Surg Oncol. 2018;44(10):1463–8.
Fu NY, Nolan E, Lindeman GJ, Visvader JE. Stem cells and the differentiation hierarchy in mammary gland development. Physiol Rev. 2020;100(2):489–523.
Shackleton M, Vaillant F, Simpson KJ, Stingl J, Smyth GK, Asselin-Labat ML, et al. Generation of a functional mammary gland from a single stem cell. Nature. 2006;439(7072):84–8.
Stingl J, Eirew P, Ricketson I, Shackleton M, Vaillant F, Choi D, et al. Purification and unique properties of mammary epithelial stem cells. Nature. 2006;439(7079):993–7.
Brisken C, O’Malley B. Hormone action in the mammary gland. Cold Spring Harb Perspect Biol. 2010;2(12): a003178.
Van Keymeulen A, Rocha AS, Ousset M, Beck B, Bouvencourt G, Rock J, et al. Distinct stem cells contribute to mammary gland development and maintenance. Nature. 2011;479(7372):189–93.
Hilton HN, Graham JD, Kantimm S, Santucci N, Cloosterman D, Huschtscha LI, et al. Progesterone and estrogen receptors segregate into different cell subpopulations in the normal human breast. Mol Cell Endocrinol. 2012;361(1–2):191–201.
Macias H, Hinck L. Mammary gland development. Wiley Interdiscip Rev Dev Biol. 2012;1(4):533–57.
Brisken C, Scabia V. 90 Years of progesterone: progesterone receptor signaling in the normal breast and its implications for cancer. J Mol Endocrinol. 2020;65(1):T81–94.
Beleut M, Rajaram RD, Caikovski M, Ayyanan A, Germano D, Choi Y, et al. Two distinct mechanisms underlie progesterone-induced proliferation in the mammary gland. Proc Natl Acad Sci USA. 2010;107(7):2989–94.
Pardo I, Lillemoe HA, Blosser RJ, Choi M, Sauder CA, Doxey DK, et al. Next-generation transcriptome sequencing of the premenopausal breast epithelium using specimens from a normal human breast tissue bank. Breast Cancer Res. 2014;16(2):R26.
Tanos T, Sflomos G, Echeverria PC, Ayyanan A, Gutierrez M, Delaloye JF, et al. Progesterone/RANKL is a major regulatory axis in the human breast. Sci Transl Med. 2013;5(182):182ra55.
Rajaram RD, Buric D, Caikovski M, Ayyanan A, Rougemont J, Shan J, et al. Progesterone and Wnt4 control mammary stem cells via myoepithelial crosstalk. EMBO J. 2015;34(5):641–52.
Schramek D, Leibbrandt A, Sigl V, Kenner L, Pospisilik JA, Lee HJ, et al. Osteoclast differentiation factor RANKL controls development of progestin-driven mammary cancer. Nature. 2010;468(7320):98–102.
Asselin-Labat ML, Vaillant F, Sheridan JM, Pal B, Wu D, Simpson ER, et al. Control of mammary stem cell function by steroid hormone signalling. Nature. 2010;465(7299):798–802.
Gonzalez-Suarez E, Jacob AP, Jones J, Miller R, Roudier-Meyer MP, Erwert R, et al. RANK ligand mediates progestin-induced mammary epithelial proliferation and carcinogenesis. Nature. 2010;468(7320):103–7.
Tharmapalan P, Mahendralingam M, Berman HK, Khokha R. Mammary stem cells and progenitors: targeting the roots of breast cancer for prevention. EMBO J. 2019;38(14): e100852.
Trabert B, Sherman ME, Kannan N, Stanczyk FZ. Progesterone and breast cancer. Endocr Rev. 2020;41(2):320–44.
Alexandrov LB, Kim J, Haradhvala NJ, Huang MN, Tian Ng AW, Wu Y, et al. The repertoire of mutational signatures in human cancer. Nature. 2020;578(7793):94–101.
Nik-Zainal S, Alexandrov LB, Wedge DC, Van Loo P, Greenman CD, Raine K, et al. Mutational processes molding the genomes of 21 breast cancers. Cell. 2012;149(5):979–93.
Nik-Zainal S, Van Loo P, Wedge DC, Alexandrov LB, Greenman CD, Lau KW, et al. The life history of 21 breast cancers. Cell. 2012;149(5):994–1007.
Nik-Zainal S, Davies H, Staaf J, Ramakrishna M, Glodzik D, Zou X, et al. Landscape of somatic mutations in 560 breast cancer whole-genome sequences. Nature. 2016;534(7605):47–54.
Morganella S, Alexandrov LB, Glodzik D, Zou X, Davies H, Staaf J, et al. The topography of mutational processes in breast cancer genomes. Nat Commun. 2016;7:11383.
Cervantes-Gracia K, Gramalla-Schmitz A, Weischedel J, Chahwan R. APOBECs orchestrate genomic and epigenomic editing across health and disease. Trends Genet. 2021;37(11):1028–43.
Burns MB, Lackey L, Carpenter MA, Rathore A, Land AM, Leonard B, et al. APOBEC3B is an enzymatic source of mutation in breast cancer. Nature. 2013;494(7437):366–70.
Venkatesan S, Angelova M, Puttick C, Zhai H, Caswell DR, Lu WT, et al. Induction of APOBEC3 exacerbates DNA replication stress and chromosomal instability in early breast and lung cancer evolution. Cancer Discov. 2021;11(10):2456–73.
Ng JCF, Quist J, Grigoriadis A, Malim MH, Fraternali F. Pan-cancer transcriptomic analysis dissects immune and proliferative functions of APOBEC3 cytidine deaminases. Nucleic Acids Res. 2019;47(3):1178–94.
Gerstung M, Jolly C, Leshchiner I, Dentro SC, Gonzalez S, Rosebrock D, et al. The evolutionary history of 2658 cancers. Nature. 2020;578(7793):122–8.
Yoshida K, Gowers KHC, Lee-Six H, Chandrasekharan DP, Coorens T, Maughan EF, et al. Tobacco smoking and somatic mutations in human bronchial epithelium. Nature. 2020;578(7794):266–72.
Lee-Six H, Olafsson S, Ellis P, Osborne RJ, Sanders MA, Moore L, et al. The landscape of somatic mutation in normal colorectal epithelial cells. Nature. 2019;574(7779):532–7.
Yokoyama A, Kakiuchi N, Yoshizato T, Nannya Y, Suzuki H, Takeuchi Y, et al. Age-related remodelling of oesophageal epithelia by mutated cancer drivers. Nature. 2019;565(7739):312–7.
Lawson ARJ, Abascal F, Coorens THH, Hooks Y, O’Neill L, Latimer C, et al. Extensive heterogeneity in somatic mutation and selection in the human bladder. Science. 2020;370(6512):75–82.
Meyer JS. Cell proliferation in normal human breast ducts, fibroadenomas, and other ductal hyperplasias measured by nuclear labeling with tritiated thymidine. Effects of menstrual phase, age, and oral contraceptive hormones. Hum Pathol. 1977;8(1):67–81.
Potten CS, Watson RJ, Williams GT, Tickle S, Roberts SA, Harris M, et al. The effect of age and menstrual cycle upon proliferative activity of the normal human breast. Br J Cancer. 1988;58(2):163–70.
Fane M, Weeraratna AT. How the ageing microenvironment influences tumour progression. Nat Rev Cancer. 2020;20(2):89–106.
Jansen LA, Backstein RM, Brown MH. Breast size and breast cancer: a systematic review. J Plast Reconstr Aesthet Surg. 2014;67(12):1615–23.
Tavani A, Pregnolato A, La Vecchia C, Negri E, Favero A, Franceschi S. Breast size and breast cancer risk. Eur J Cancer Prev. 1996;5(5):337–42.
Oppelt PG, Muller A, Stephan L, Dittrich R, Lermann J, Buttner C, et al. Hyperandrogenemia and high prolactin in congenital utero-vaginal aplasia patients. Reproduction. 2017;153(5):555–63.
Wyshak G, Frisch RE. Breast cancer among former college athletes compared to non-athletes: a 15-year follow-up. Br J Cancer. 2000;82(3):726–30.
Ackerman KE, Misra M. Amenorrhoea in adolescent female athletes. Lancet Child Adolesc Health. 2018;2(9):677–88.
Matthews CE, Sampson JN, Brenner DR, Moore SC, Courneya KS, Ziegler RG, et al. Effects of exercise and cardiorespiratory fitness on estrogen metabolism in postmenopausal women. Cancer Epidemiol Biomarkers Prev. 2018;27(12):1480–2.
Anedda FM, Velati A, Lello S, Orru M, Paoletti AM, Melis GB, et al. Observational study on the efficacy of tibolone in counteracting early carotid atherosclerotic lesions in postmenopausal women. Horm Res. 2004;61(1):47–52.
Chlebowski RT, Anderson GL, Aragaki AK, Manson JE, Stefanick ML, Pan K, et al. Association of menopausal hormone therapy with breast cancer incidence and mortality during long-term follow-up of the women’s health initiative randomized clinical trials. JAMA. 2020;324(4):369–80.
Levitz M, Young BK. Estrogens in pregnancy. Vitam Horm. 1977;35:109–47.
Tulchinsky D, Hobel CJ, Yeager E, Marshall JR. Plasma estrone, estradiol, estriol, progesterone, and 17-hydroxyprogesterone in human pregnancy. I. Normal pregnancy. Am J Obstet Gynecol. 1972;112(8):1095–100.
Tulchinsky D, Frigoletto FD, Ryan KJ, Fishman J. Plasma estetrol as an index of fetal well-being. J Clin Endocrinol Metab. 1975;40:560–7.
Acknowledgements
The authors thank Cathrin Brisken (SREC—Swiss Institute for Experimental Cancer Research, Lausanne, Switzerland) for her critical review of a draft version of the present manuscript. Amanda Prowse (at Terminal 4 Communications, Hilversum, The Netherlands) and Astrid van den Heuvel (Pantarhei Bioscience) are acknowledged for their contributions to the preparation of the manuscript.
Funding
Not applicable.
Author information
Authors and Affiliations
Contributions
HJTCB, FZS and RDL developed the concept and design of the Review. HJTCB, JFME, IJS, FZS and RDL collected the data. MS, VCJ, PB, KGD, KK, JK, LK and TS interpreted the data. HJTCB, JFME and IJS prepared the manuscript. HJTCB, MS, VCJ, PB, KGD, KK, JK, LK, TS, FZS and RDL were responsible for critical revision of the manuscript, interpretation of the literature and the concluding remarks. All authors gave final approval of the version to be submitted.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
HJTCB is President, IJS is Director R&D and JK is Chief Medical Officer of Pantarhei Oncology (subsidiary of Pantarhei Bioscience), the company developing the fetal estrogen estetrol for the treatment of advanced breast and prostate cancer, but this is outside the scope of the present review. The other authors have nothing to disclose.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Coelingh Bennink, H.J.T., Schultz, I.J., Schmidt, M. et al. Progesterone from ovulatory menstrual cycles is an important cause of breast cancer. Breast Cancer Res 25, 60 (2023). https://doi.org/10.1186/s13058-023-01661-0
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s13058-023-01661-0