Abstract
Background
Second primary cancer incidence is rising among breast cancer survivors. We examined the risks of non-breast second primaries, in combination and at specific cancer sites, through a systematic review and meta-analysis.
Methods
We conducted a systematic search of PubMed, Embase, and Web of Science, seeking studies published by March 2022. We included studies that reported standardized incidence ratios (SIRs), with associated standard errors, assessing the combined risk of second non-breast primaries following breast cancer. We performed meta-analyses of combined second primary risks, stratifying by age, follow-up duration, and geographic region. We also assessed second primary risks at several specific sites, stratifying by age. The inverse variance method with DerSimonian–Laird estimators was used in all meta-analyses, assuming a random-effects model. Associated biases and study quality were evaluated using the Newcastle–Ottawa scale.
Results
One prospective and twenty-seven retrospective cohort studies were identified. SIRs for second non-breast primaries combined ranged from 0.84 to 1.84. The summary SIR estimate was 1.24 (95% CI 1.14–1.36, I2: 99%). This varied by age: the estimate was 1.59 (95% CI 1.36–1.85) when breast cancer was diagnosed before age 50, which was significantly higher than in women first diagnosed at 50 or over (SIR: 1.13, 95% CI 1.01–1.36, p for difference: < 0.001). SPC risks were also significantly higher when based on Asian, rather than European, registries (Asia—SIR: 1.47, 95% CI 1.29–1.67. Europe—SIR: 1.16, 95% CI 1.04–1.28). There were significantly increased risks of second thyroid (SIR: 1.89, 95% CI 1.49–2.38), corpus uteri (SIR: 1.84, 95% CI 1.53–2.23), ovary (SIR: 1.53, 95% CI 1.35–1.73), kidney (SIR: 1.43, 95% CI 1.17–1.73), oesophagus (SIR: 1.39, 95% CI 1.26–1.55), skin (melanoma) (SIR: 1.34, 95% CI 1.18–1.52), blood (leukaemia) (SIR: 1.30, 95% CI 1.17–1.45), lung (SIR: 1.25, 95% CI 1.03–1.51), stomach (SIR: 1.23, 95% CI 1.12–1.36) and bladder (SIR: 1.15, 95% CI 1.05–1.26) primaries.
Conclusions
Breast cancer survivors are at significantly increased risk of second primaries at many sites. Risks are higher for those diagnosed with breast cancer before age 50 and in Asian breast cancer survivors compared to European breast cancer survivors. This study is limited by a lack of data on potentially confounding variables. The conclusions may inform clinical management decisions following breast cancer, although specific clinical recommendations lie outside the scope of this review.
Similar content being viewed by others
Background
Multiple studies have compared the risk of second primary cancers (SPCs) following a first breast cancer (BC) to the corresponding first cancer risks in the general population [1,2,3,4,5,6,7,8,9,10,11,12,13,14,15,16,17,18,19,20,21,22,23,24,25,26,27,28,29,30,31,32,33]. Although most of these studies report an elevated risk [1, 2, 4,5,6, 8,9,10,11,12,13,14,15,16,17,18,19,20,21,22,23,24,25,26,27,28,29,30,31,32,33], the magnitudes of the reported associations vary widely. Since a 2015 review reported a 17% increase in SPC risks following BC [34], many new studies have been published [1, 5, 6, 9, 12, 16, 17, 19, 20, 23, 24, 27, 32]. In addition, BC is both increasing in incidence and improving in survival outcomes [35,36,37], exacerbating the public health problem posed by SPCs in BC survivors. Updated pooled estimates of SPC risks following BC are hence due.
Most published studies to date drew their data from European or North American population-based cancer registries [1,2,3,4,5,6,7,8,9,10,11,12,13,14,15,16,17, 28,29,30,31, 33], although several also drew their data from Asian registries [18,19,20,21,22,23,24,25,26,27, 32]. Many studies have found BC survivors to be at increased risk of melanoma [1, 7, 13, 14, 29,30,31, 33], thyroid cancer [1, 15, 19, 20, 23,24,25, 27, 29,30,31, 33, 38], and several cancers of the urogenital and gastrointestinal systems [1, 2, 4, 6,7,8,9,10,11,12,13,14,15,16,17,18,19,20,21,22,23,24,25,26,27,28,29,30,31,32,33], although the estimated magnitude of these risks varies.
A systematic review of the latest published evidence on SPC risks is helpful in guiding clinical management following BC. This could lead to improvements in SPC prevention and early detection.
In this review, we examine the latest evidence regarding the combined risks of developing SPCs following a first primary BC. We also evaluate the variability in SPC risks caused by confounding variables such as patient characteristics and demographic information. Finally, we identify which cancer sites may drive the combined risk of SPCs and quantify the magnitude of these site-specific risks.
Methods
Exposure, outcome and measures of association
The exposure was the diagnosis of a primary BC. The outcome was the later diagnosis of a non-breast SPC. The measure of association was the standardized incidence ratio (SIR) comparing the incidence of second non-breast primaries among BC survivors to the incidence of first non-breast primaries in the general population.
To ensure the review accurately assessed second primary risks, a key condition of inclusion was that a study should have made a clear effort to differentiate SPCs from recurrences or metastatic developments of the first primary BC. For example, guidance on the topic is provided by the Surveillance, Epidemiology and End Results (SEER) programme [39]. Separate guidelines are also provided by the International Association of Cancer Registries (IACR)/International Agency for Research on Cancer (IARC) [40, 41]. However, a study by Coyte et al. found counts of second breast primaries following a first BC to differ between the SEER and IARC/IACR guidelines and counts of all other primaries to agree very closely [42]. Since the SEER guidelines entail standard practice in North America and the IARC/IACR guidelines entail standard practice in all other areas, it was anticipated that most studies would use these guidelines, and therefore that we would have been unable to draw meaningful conclusions about second primary BC risk. As a result, only second non-breast cancers were considered as an outcome in this review. To make use of more data, we did not restrict on the types of efforts to differentiate SPCs from recurrences or metastases that studies made.
Data sources and search strategy
Embase, PubMed, and Web of Science were searched on 11th March 2022 using the below queries:
Embase
(Breast Neoplasms/ or “breast cancer”) and (Neoplasms, Second Primary/ or “second cancer” or “second primary”) and risk
PubMed
(“Breast Neoplasms”[MeSH] OR “breast cancer”) AND (“Neoplasms, Second Primary”[MeSH] OR “second cancer” OR “second primary”) AND risk
Web of science
(TS = ((“breast cancer” OR “breast neoplasm”) AND (“second cancer” or “second primary”) AND risk)) OR (AB = ((“breast cancer” OR “breast neoplasm”) AND (“second cancer” or “second primary”) AND risk))
Inclusion and exclusion criteria
To be included in the review, a study had to provide all information needed to extract a SIR and associated standard error evaluating the combined risk of non-breast SPCs in female BC survivors. It also had to take clearly described steps to discern SPCs from recurrences or metastases of the first BC, use data predominantly on those aged 15 and above at BC diagnosis, and be written in English.
A study would be excluded if it evaluated SPC risks only in survivors of a non-invasive BC or only following a specific treatment of the first BC. Studies would also be excluded if data on third or subsequent primaries could not be excluded from their SPC risk estimates or if their data overlapped entirely with another accepted study.
Studies with data that partly but not fully overlapped were included in the review. In this case, the study with a greater sample size was the only one included in any meta-analyses. If this could not be established, the study including the most recent data was the one included.
There is a particularly close data link between the Swedish Family Cancer Database and the Swedish national cancer registry [43]. The same is true of the Taiwanese Registry of Catastrophic Illness and the national cancer registry of Taiwan [44]. We therefore considered data from these centres to overlap. Similarly, data from the Osaka Medical Centre for Cancer and Cardiovascular Diseases (OMCC) are primarily a subset of Osaka Cancer Registry (OCR) data [45]. Accordingly, if a study based on OMCC data overlapped with a study based on OCR data, the latter was considered the larger study if there was missing information on sample size.
Data extraction
Title and abstract screening was performed by two authors as part of an independent double-screening process. Conflicts regarding twelve studies were resolved by another author. We closely read the full text, swept the bibliographies, and whenever applicable searched the PubMed “cited by” sections of each the studies that passed the title and abstract screening in search of additional studies.
Statistical analysis
We assumed there would be some between-study variance in SIRs not attributable to sampling error, and therefore assumed a random-effects model in all meta-analyses [46], using the generic inverse variance method with DerSimonian–Laird estimators [47, 48]. Standard errors were extracted routinely [49] and were used to weight the studies in meta-analyses [46]. We used Byar’s approximation to calculate confidence intervals (CIs), unless CIs could be taken directly from a study [49].
We firstly performed an unstratified meta-analysis. We quantified the heterogeneity (variation in true effect sizes between studies [46, 47]) in these results by inspecting Cochran’s Q [48] and the I2 statistic [50, 51]. Cochran’s Q is the sum of squared differences between the estimate of the pooled effect size and the effect sizes reported by each study, weighted by the inverse variances of the studies [46]. The I2 statistic is the percentage by which the observed value of Cochran’s Q exceeds the value expected under the null hypothesis of no between-study heterogeneity [46].
We also performed leave-one-out analyses to identify which studies were the main drivers of heterogeneity [46], which we defined as the studies causing Cochran’s Q to decrease by over 10% once they were removed from the unstratified meta-analysis. We also defined outlier studies to be studies which reported SIRs with 95% confidence intervals that lay wholly outside the confidence interval around the summary SIR generated by the unstratified meta-analysis [46]. We then performed two further meta-analyses after, respectively, eliminating all the main drivers of heterogeneity and all outlier studies, to assess the remaining heterogeneity and the effect on the summary SIR. We examined publication bias by visually assessing funnel plots and performing Egger’s test [52].
We also performed further meta-analyses stratifying on (1) age at BC diagnosis—under 50 years and 50 years or above. Data on those diagnosed before age 56 and at age 56 or over were, respectively, included in the younger and older strata if no stratification at 50 was provided, (2) follow-up time duration following BC diagnosis—under 5 years or 5 years and over. We also performed a second meta-analysis stratifying at 10 years, (3) geographic region—the continent of the data centre (i.e., hospital, registry) used in a particular study.
We evaluated for differences in risks by age, follow-up duration, and geographic region using the Cochran’s Q statistic, by considering each stratum as a subgroup, and by comparing the resulting statistic to a chi-squared distribution [46].
We also examined the Cochran’s Q and I2 statistics in each stratum for each stratified meta-analysis, to assess if a particular risk factor explained some of the heterogeneity in the unstratified analysis of non-breast SPC risks.
We extracted SIRs that quantified SPC risks at specific sites, together with associated standard errors, from the studies included in the unstratified meta-analysis. We then estimated summary SIRs for SPC risks at these sites by conducting meta-analyses of the relevant site-specific SIRs. This was done to elucidate which cancer sites were driving the combined risks of all non-breast SPCs. We first examined site-specific risks for all ages. We then stratified by age at BC diagnosis, using the same stratification points as in the analyses of combined non-breast primary risks. These analyses were performed for each of the 20 non-breast cancer sites with the highest incidence among women worldwide in 2020, excluding non-melanoma skin cancer and excluding oral cavity and lip cancer due to SPC risks at this site often being combined with other head and neck sites [6, 23, 33]. These sites are the bladder, the blood (leukaemia, myeloma, and non-Hodgkin’s lymphoma), the brain and central nervous system (CNS), the cervix uteri, the corpus uteri, the colorectum, the gallbladder, the kidney, the liver, the lung, the oesophagus, the ovary, the pancreas, the skin (melanoma), the stomach, the thyroid, and the vulva [53].
Forest plots were generated as a visual aid to accompany each meta-analysis. We evaluated the methodological quality of each study using the Newcastle–Ottawa scale (NOS) [54], as recommended by the Cochrane Collaboration [47] (details in Additional file 1). RStudio version 4.1.2 was used for all analyses [55]. We defined statistical significance to be present when a p value of under 0.05 was observed.
Results
Results of literature search
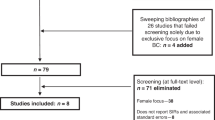
In total, 112 studies were accepted for review at the full-text level after passing the title and abstract screening stage. Sixty-five of these were selected from the 2011 studies returned after the database searches. Thirty-eight of the 112 studies were found following sweeps of the bibliographies of 69 studies: the 65 studies previously mentioned, and 4 additional studies which only failed the title and abstract sweeping due to exclusively examining male BC survivors. We identified the final 9 of the 112 studies after sweeping the “cited by” section of PubMed for 66 of these 69 studies, as the remaining three studies [56,57,58] were unavailable in PubMed. In this way, we hoped to capture additional relevant literature published both before and after the studies identified through the database searches. Following close reading, we included 28 of the 112 studies in this review. Reasons for exclusions of the remaining 84 studies, as well as a full explanation of the search process, are shown in Fig. 1.
All studies included were cohort studies, only one of which was prospective [12]. Three studies were hospital-based [13, 15, 20], and the remainder were wholly or predominantly registry-based. The centre/centres (hospital or registry/registries) were European in fourteen studies [1,2,3,4,5, 7, 9,10,11,12,13,14, 16, 17], Asian in ten studies [18,19,20,21,22,23,24,25,26,27], and North American in three studies [6, 8, 15]. One study [33] drew their cohort from registries based across four continents. Since the bulk of the cohort was taken from European registries, this study was treated as European for the purposes of any stratifications based on geographic region. Three [4, 5, 12] studies used data from multiple countries in Europe, although all the data drawn from non-German centres in Chen et al. [5] fully overlapped with larger studies [17, 33]. Therefore, we only included the German data from Chen et al. in this review.
The longest follow-up period was 57 years [17]. The shortest was 11 years [12, 26].
Six studies set minimum ages at first cancer diagnosis, at age 15 years [5, 11, 16, 23] and age 20 years [18, 20]. Six studies set maximum ages: at age 39 years [16], age 79 years [23, 25, 26], age 84 years [7], and age 89 years [2]. The used cohort in one study [12] was taken from a pre-existing larger observational cohort study. The original larger cohort included participants between ages 35 years and 70 years at recruitment without regard to cancer status. The subset of the participants from this larger cohort who subsequently developed a first primary BC formed the cohort included in this review. All remaining nineteen studies imposed no age-related restrictions when selecting their cohorts.
Fifteen studies excluded data on second primaries occurring within some given follow-up duration following the first BC diagnosis [2,3,4, 8,9,10,11, 13, 18, 20,21,22,23, 25, 26]. All other studies included data on second primaries diagnosed immediately following the first BC, although the study by the AIRTUM Working Group [1] also gave a separate analysis excluding SPCs diagnosed in the first 2 months of follow-up. The data excluding the earlier SPCs were explicitly stated as less prone to bias by the authors, so these were the data used in any statistical analyses.
All but one study [5] gave site-specific risks of second primaries.
The reported SIRs ranged from 0.84 [3] to 1.84 [23]. All but five [3, 7, 18, 20, 23] estimated SIRs ranging between 1.00 and 1.50.
The characteristics of all 28 studies are detailed in Table 1 and Table 2. The NOS scores assigned to each study may be seen in Additional file 1, together with an explanation of the methods used.
Results of meta-analyses
Unstratified results
The unstratified meta-analysis consisted of nineteen studies [1, 3, 5,6,7, 9,10,11, 13,14,15, 18,19,20, 23, 24, 26, 27, 33]. All but two [3, 7] reported an increase in SPC risks following a first primary BC.
The summary SIR was estimated as 1.24 (95% CI 1.14–1.36, Fig. 2). Significant evidence for heterogeneity was found (Q: 1839.32, I2: 99%, p < 0.001).
Following leave-one-out analyses, we found the studies by Diab et al. [6], Odani et al. [23], Mellemkjær et al. [33], Evans et al. [7], and Hung et al. [19] to contribute the most to heterogeneity, with Cochran’s Q falling by 40%, 23%, 20%, 15%, and 13% in the meta-analyses consisting of all studies in the unstratified meta-analysis other than the respective study under investigation. Eliminating all these studies did not appreciably affect the summary SIR estimate (SIR: 1.24, 95% CI 1.13–1.35), and there remained significant evidence for heterogeneity (Q: 154.89, I2: 92%, p < 0.001).
We identified 7 outlier studies [3, 6, 7, 15, 18, 19, 23]. Eliminating all outlier studies also had little effect on the SIR estimate (SIR: 1.25, 95% CI 1.19–1.31), and significant evidence for heterogeneity was still present (Q: 166.23, I2: 93%, p < 0.001).
Examining a funnel plot and performing Egger’s test revealed no significant evidence of publication bias (Additional file 1).
Effects of geographic region
We found significant evidence that summary SIRs varied by geographic region (SIR: 1.47, 95% CI 1.29–1.67 for Asian studies vs. 1.16 (1.04–1.28) for European studies vs. 1.03 (1.02–1.04) for North American studies, p for difference: < 0.001, Fig. 3).
Significant heterogeneity was found for the Asian subgroup analysis (Q: 222.36, I2: 97%, p < 0.001) and for the European subgroup analysis (Q: 561.95, I2: 98%, p < 0.001). No significant evidence for heterogeneity was found in the North American subgroup analysis (Q: 0.09, I2: 0%, p: 0.77).
There was significant evidence that Asian BC survivors had higher SPC risks in comparison with European BC survivors, for whom the largest amount of data was available (p for difference: 0.005). There was also significant evidence that American BC survivors were at lower risks of SPCs compared to European BC survivors (p for difference: 0.027).
Effects of age at BC onset
Eight studies were included in the age-stratified meta-analyses [1, 6, 7, 11, 13, 14, 19, 33]. One small study also stratified by age at breast cancer diagnosis but was not included in this analysis due to a discrepancy between the number of SPCs reported in total and within each age stratum [20]. SPC risks were significantly elevated in both age groups compared to the risks of first primaries, and there was significant evidence for a difference in summary SIRs between these groups (SIR: 1.59, 95% CI 1.36–1.85 for those aged under 50 at first BC diagnosis vs. 1.13 (95% CI 1.01–1.26) for those aged over 50 at first BC diagnosis, p for difference: < 0.001, Fig. 4). Heterogeneity was present in both strata (Aged under 50 at first BC diagnosis: Q: 318.11, I2: 98%, p < 0.001. Aged 50 or over at first BC diagnosis: Q: 717.72, I2: 99%, p < 0.001).
Effects of follow-up time duration
Stratification of BC survivors by follow-up duration revealed no significant evidence for a difference in SPC risks. Full results may be seen in the Additional file 1.
Second primary risks at specific sites
Point estimates of summary SIRs estimating SPC risks unstratified by age at the nineteen examined sites ranged from 0.80 (for the brain and CNS) to 1.89 (for the thyroid). BC survivors were found to be at significantly lower risk of brain and CNS cancers (SIR: 0.80, 95% CI 0.71–0.91), and there was a suggestion of decreased cervix uteri cancer risk (SIR: 0.88, 95% CI 0.77–1.00). In contrast, there was significant evidence for elevated second primary bladder (SIR: 1.15, 95% CI 1.05–1.26), corpus uteri (SIR: 1.84, 95% CI 1.53–2.23), kidney (SIR: 1.43, 95% CI 1.17–1.73), blood (leukaemia) (SIR: 1.30, 95% CI 1.17–1.45), lung (SIR: 1.25, 95% CI 1.03–1.51), skin (melanoma) (SIR: 1.34, 95% CI 1.18–1.52), oesophagus (SIR: 1.39, 95% CI 1.26–1.55), ovary (SIR: 1.53, 95% CI 1.35–1.73), stomach (SIR: 1.23, 95% CI 1.12–1.36), and thyroid (SIR: 1.89, 95% CI 1.49–2.38) cancer risks following BC.
We found BC survivors first diagnosed with BC at under age 50 to be at elevated risk of second primaries at the bladder (SIR: 1.32, 95% CI 1.17–1.48), blood (leukaemia) (SIR: 1.91, 95% CI 1.77–2.05), corpus uteri (SIR: 1.40, 95% CI 1.12–1.76), kidney (SIR: 1.29, 95% CI 1.15–1.43), lung (SIR: 1.65, 95% CI 1.49–1.82), oesophagus (SIR: 2.21, 95% CI 1.89–2.60), ovary (SIR: 2.24, 95% CI 1.59–3.13), pancreas (SIR: 1.35, 95% CI 1.16–1.57), skin (melanoma) (SIR: 1.34, 95% CI 1.23–1.45), stomach (SIR: 1.90, 95% CI 1.75–2.06), and thyroid (SIR: 2.06, 95% CI 1.83–2.31).
We found there to be significantly increased risks of second primaries at three sites in BC survivors diagnosed with BC at age 50 or over: the corpus uteri (SIR: 1.75, 95% CI 1.29–2.37), the oesophagus (SIR: 1.20, 95% CI 1.06–1.37), and the skin (melanoma) (SIR: 1.25, 95% CI 1.17–1.35).
BC survivors diagnosed with breast cancer before age 50 were at significantly increased risk of second primary lung cancer compared to BC survivors diagnosed with breast cancer at age 50 or over (SIR: 1.65, 95% CI 1.49–1.82 for those aged under 50 at first BC diagnosis vs. 0.81 (95% CI 0.55–1.20) for those aged over 50 at first BC diagnosis, p for difference: < 0.001). They were also at significantly increased risks of second primaries at the pancreas (SIR: 1.35, 95% CI 1.16–1.57 vs. 0.92 (95% CI 0.81–1.04), p for difference: < 0.001), blood (leukaemia) (SIR: 1.91, 95% CI 1.77–2.05 vs. 1.34 (95% CI 0.99–1.81), p for difference: 0.026), oesophagus (SIR: 2.21, 95% CI 1.89–2.60 vs. 1.20 (95% CI 1.06–1.37), p for difference: < 0.001), ovary (SIR: 2.24, 95% CI 1.59–3.13 vs. 1.04 (95% CI 0.93–1.16), p for difference < 0.001), stomach (SIR: 1.90, 95% CI 1.75–2.06 vs. 1.10 (95% CI 0.91–1.34), p for difference < 0.001), and thyroid (SIR: 2.06, 95% CI 1.83–2.31 vs. 1.17 (95% CI 0.90–1.52), p for difference < 0.001).
Full results may be seen in Table 3.
Discussion
In this review, we found significant evidence for elevated SPC risks among BC survivors, particularly when first diagnosed with BC at under age 50 or in Asian hospitals/registries. Risks of second primary bladder, kidney, blood, lung, skin (melanoma), oesophagus, ovary, stomach, thyroid, and corpus uteri cancers were significantly increased, whereas risks of brain and CNS and cervix uteri SPCs were significantly decreased.
This review has several strengths. The studies were of high quality (Additional file 1), and we found no significant evidence for publication bias (Additional file 1). It includes an array of studies with large sample sizes [1, 4,5,6,7, 14, 17, 19, 21, 33], long follow-up periods [1, 2, 4, 6,7,8, 10, 13, 16, 17, 20, 21, 25, 27, 33], and recently updated data [1, 5, 6, 16, 17, 19, 20, 23]. Another strength is the inclusion of several studies from outside Europe and North America [18,19,20,21,22,23,24,25,26,27], allowing comparisons between regions with different demographics and BC incidence rates [59].
There are two main weaknesses of this review. The first is the high level of heterogeneity observed, and the second is the underreporting of potentially confounding risk factors.
Regarding the first point, much of the heterogeneity was contributed by Diab et al. [6], a very large study from North America, and the only study that was explicitly stated to use the SEER multiple tumour coding rules. It is therefore possible that the differences between such rules could account for some of the between-study differences in SPC risks, such as the significantly decreased SPC risks among North American studies compared to European studies. This would be at odds with the small study by Coyte et al. [42], which found non-breast SPC counts to be close to identical under both the SEER and the IARC/IACR rules. Larger studies comparing SPC counts observed under these two common sets of guidelines would help clarify this issue. Any differences in the ratio of the screening intensity for non-breast second primaries among BC survivors and the screening intensity for non-breast first cancers, or in the rates of risk-reducing surgeries performed in BC survivors, between North American and European populations could also partly explain these differences in SPC risks. However, this information was not reported in the studies. However, even if such discrepancies do account for the majority of the heterogeneity contributed by Diab et al., this would not explain the rest of the heterogeneity, which remained significant even following the elimination of four further studies identified as major drivers of heterogeneity [7, 19, 23, 33].
To investigate whether the definition of SPC influences the results, we also performed a meta-analyses including only studies using IACR/IARC coding rules to identify second primaries [1, 2, 5, 9,10,11, 14, 18, 23, 24, 26, 27, 33]. The summary SIR estimate was similar to the meta-analysis including all studies (All studies: SIR = 1.24, 95%CI = 1.14–1.36 vs. IARC/IACR studies: SIR = 1.27, 95%CI = 1.14–1.41), and there remained significant evidence of heterogeneity (All studies: Cochran’s Q: 1839.32, I2: 99%, p value: < 0.0001 vs. IARC/IACR studies: Cochran’s Q: 507.29, I2: 98%, p value: < 0.0001).
It is likely that including studies from three different continents contributed to heterogeneity, since SPC risks in these continents were found to vary significantly. Similarly, if ages at BC diagnoses varied widely between studies, then this would account for some of the heterogeneity, as younger age groups were found to be at significantly increased risk in comparison with those older. However, although heterogeneity was attenuated, it remained significant among Asian and European studies as well as in both younger age and older age groups, so these points cannot fully explain the observed heterogeneity.
It is also possible that differences in the treatments administered between studies could affect SPC risks [60,61,62] and thus contribute to heterogeneity. Unfortunately, this could not be assessed in this review since treatment effects were generally unreported. Information on other important variables also tended to be unavailable. For example, there was a paucity of information reported on obesity, tobacco intake, alcohol intake, the pathology of the initial BC, or family history of BC, which are known to influence cancer risks. We cannot therefore rule out confounding in the results due to these unreported confounding variables, nor can we rule out that unreported risk factors contributed to the significant heterogeneity observed.
It is known that cancer survivors may be more prone to being diagnosed with second cancers simply due to increased surveillance for cancer development, rather than a genuine increase in risk compared to the general population. This is known as “detection bias”, and we cannot rule out that it may have affected some results in this review [1]. However, many studies were included that excluded SPCs diagnosed within some time period following the first BC [2,3,4, 8,9,10,11, 13, 18, 20,21,22,23, 25, 26] when detection bias is likely to be most pronounced [1]. Therefore, detection bias is unlikely to be a major weakness of this review.
It is also possible that some of the observed variability in SIRs between studies could be due to differences in analytical methods and differences in the data quality control processes or the definition of second primary cancers used across registries. For example, Diab et al. calculated SIRs using the SEER database, a population-based data set of very high quality [6, 63] and with a very limited amount of missing data [64]. Several large studies also drew their data from large European registries of similar standard [1, 5, 9, 14, 33]. All studies in the meta-analyses which reported the specific data source used to calculate the SIRs used population-based registry data, which in principle would be of similar good quality [1, 10, 11, 14, 15, 19, 20, 24, 27, 33]. However, most did not report on the exact quality control processes applied and the data missingness. Furthermore, a large study included in the meta-analyses included second and subsequent primaries in the calculations of reference incidences used to generate expected cancer counts [1], whereas others included only first cancers [5, 9], although this information was generally not reported. Excluding these estimates did not have a marked effect on SIR estimates [1].
Finally, although every effort was made to capture all relevant studies, it cannot be ruled out that some studies were not found or were excluded erroneously.
This review adds to the previously published review [34] in several ways. Firstly, the previous review included no studies published since June 2013, whereas this updated review included twelve studies published since [1, 5, 6, 9, 12, 16, 17, 19, 20, 23, 24, 27]. This review also includes studies with cohorts consisting of survivors of any given set of initial cancers provided SPC risks could be extracted for the subset of BC survivors, yielding three new studies published before June 2013 [15, 25, 26]. In total, eighteen of the twenty-eight studies in this review were not included in the previous review [1, 4,5,6, 8, 9, 12, 15,16,17,18,19,20, 23,24,25,26,27], including several large multicentre studies and two sizeable monographs [1, 4,5,6, 8, 9, 12, 16]. Several of the new studies are drawn from Asian registries [18,19,20, 23, 24, 27] and North American registries [6, 8, 15], whereas the previous review did not include any North American studies. This enabled us to assess differences in SPC risks between these geographic regions. Finally, the previous review found follow-up duration to significantly affect SPC risks, whereas this updated review found no significant evidence of this (Additional file 1). The overall summary female SIR of 1.24 (95% CI 1.14–1.36) is slightly higher than the summary SIR reported in the previous review (1.17 (95% CI 1.10–1.25)).
The increased SPC risks could be partly due to treatment effects of the initial BC, such as the administration of hormonal therapy such as tamoxifen, or the administration of chemotherapy or radiotherapy [60,61,62, 65]. The latter may explain the increased risks of second oesophagus and lung primaries in BC survivors diagnosed at under age 50, as radiotherapy confers increasing risks of lung and oesophagus primaries with time since administration [63]. Similarly, chemotherapy is associated with increased leukaemia risk [66, 67] and is more commonly administered to younger BC survivors [68], possibly explaining the significantly higher risks of second primary leukaemias we found for this group. Shared risk factors between breast and other cancers such as obesity will also contribute to the elevated SPC risks among BC survivors [69, 70]. For example, thyroid cancer risks may be elevated by obesity or hormonal risk factors shared with BC [38]. The increased risk of SPCs at the lung [71], in the urogenital system [71] in the gastrointestinal system [71], and at other sites [12, 71, 72] may potentially be associated with increased smoking among BC survivors in comparison with the general population [73].
Germline susceptibility to BC may also raise specific SPC risks [74]. For example, pathogenic variants in known BC susceptibility genes are associated with risks for other cancers. Pathogenic variants in BRCA1/2 have been found to be associated with risks of multiple primary cancers, including pancreatic and stomach cancers [75]. Pathogenic variants in BRCA1/2 are also associated with ovarian cancer risk [76, 77], as are pathogenic variants in PALB2 [78], RAD51C [79, 80], and RAD51D [80, 81]. Such observations may explain the elevated ovarian SPC risks found in this review, particularly among younger BC survivors [82, 83]. There also exist common genetic variants with pleiotropic effects, associated with elevated breast and ovarian cancer risks [84]. Elevated polygenic risk scores are often associated with risks for more than one cancer [84]; for example, a BC polygenic risk score has been associated with colorectal cancer risk [85] and a recent large study found the prevalence of pathogenic protein-truncating variants in established BC susceptibility genes among female BC survivors to be 5.6% [86]. Genetic susceptibility could therefore account for a notable proportion of second primaries following BC in women.
If germline susceptibility does increase SPC risk in female BC survivors, this may partly explain our finding of elevated SPC risks in women diagnosed with BC at under age 50 compared to those diagnosed when older, since genetic susceptibility to BC is associated with earlier BC diagnosis [82, 87]. This finding will also partly account for the increased SPC risks among those diagnosed with BC in Asian registries, as BC is generally diagnosed at younger ages in Asia [88, 89].
The decreased risks of blood (myeloma), brain and CNS, and liver SPCs among BC survivors aged 50 or over at first BC diagnosis may be explained by under-ascertainment of SPCs in older age groups [7]. We also found brain and CNS SPC risks to be significantly decreased when unstratified by age, which may be attributable to misclassifications of second primaries as metastases [90].
Conclusions
In conclusion, this review found that the combined risks of second non-breast cancer following a first primary BC were significantly elevated. Female BC survivors aged under 50 at BC onset or who were from Asian registries/hospitals were found to be at higher risks than other groups. Finally, we found second cancers at the bladder, corpus uteri, kidney, blood, lung, skin (melanoma), oesophagus, ovary, stomach, and thyroid to notably contribute to the observed elevated SPC risks.
The results may lead to increased awareness of the magnitudes and distribution by site of SPC risks following BC. They could also better inform cancer risk management, although specific recommendations would be beyond the scope of this review.
Abbreviations
- SPC:
-
Second primary cancer
- BC:
-
Breast cancer
- SIR:
-
Standardized incidence ratio
- SEER:
-
Surveillance, Epidemiology and End Results
- IACR:
-
International Association of Cancer Registries
- IARC:
-
International Agency for Research on Cancer
- OMCC:
-
Osaka Medical Centre for Cancer and Cardiovascular Diseases
- OCR:
-
Osaka Cancer Registry
- CI:
-
Confidence interval
- CNS:
-
Central nervous system
- NOS:
-
Newcastle–Ottawa Scale
- BRCA1/2:
-
BReast CAncer gene 1/2
- PALB2:
-
Partner and localizer of BRCA2
- RAD51C:
-
RAD51 paralog C
- RAD51D:
-
RAD51 paralog D
References
AIRTUM Working Group. Italian cancer figures, report 2013: multiple tumours. Epidemiol Prev. 37(4–5 Suppl 1):1–152.
Andersson M, Jensen MB, Engholm G, Henrik SH. Risk of second primary cancer among patients with early operable breast cancer registered or randomised in Danish Breast Cancer cooperative Group (DBCG) protocols of the 77, 82 and 89 programmes during 1977–2001. Acta Oncol. 2008;47(4):755–64.
Brenner H, Siegle S, Stegmaier C, Ziegler H. Second primary neoplasms following breast cancer in Saarland, Germany, 1968–1987. Eur J Cancer. 1993;29A(10):1410–4.
Brown LM, Chen BE, Pfeiffer RM, Schairer C, Hall P, Storm H, et al. Risk of second non-hematological malignancies among 376,825 breast cancer survivors. Breast Cancer Res Treat. 2007;106(3):439–51.
Chen T, Fallah M, Jansen L, Castro FA, Krilavicuite A, Katalinic A, et al. Distribution and risk of the second discordant primary cancers combined after a specific first primary cancer in German and Swedish cancer registries. Cancer Lett. 2015;369(1):152–66.
Diab N, Clark G, Langer L, Wang Y, Hamlington B, Brzeskiewicz L, et al. Impact of race and tumor subtype on second malignancy risk in women with breast cancer. Springerplus. 2016;5:14.
Evans HS, Lewis CM, Robinson D, Bell CM, Møller H, Hodgson SV. Incidence of multiple primary cancers in a cohort of women diagnosed with breast cancer in southeast England. Br J Cancer. 2001;84(3):435–40.
Harvey EB, Brinton LA. Second cancer following cancer of the breast in Connecticut, 1935–82. Natl Cancer Inst Monogr. 1985;68:99–112.
Jégu J, Colonna M, Daubisse-Marliac L, Trétarre B, Ganry O, Guizard AV, et al. The effect of patient characteristics on second primary cancer risk in France. BMC Cancer. 2014;15(14):94.
Levi F, Te VC, Randimbison L, la Vecchia C. Cancer risk in women with previous breast cancer. Ann Oncol. 2003;14(1):71–3.
Molina-Montes E, Pollán M, Payer T, Molina E, Dávila-Arias C, Sánchez MJ. Risk of second primary cancer among women with breast cancer: a population-based study in Granada (Spain). Gynecol Oncol. 2013;130(2):340–5.
Ricceri F, Fasanelli F, Giraudo MT, Sieri S, Tumino R, Mattiello A, et al. Risk of second primary malignancies in women with breast cancer: Results from the European prospective investigation into cancer and nutrition (EPIC). Int J Cancer. 2015;137(4):940–8.
Rubino C, de Vathaire F, Diallo I, Shamsaldin A, Lê MG. Increased risk of second cancers following breast cancer: role of the initial treatment. Breast Cancer Res Treat. 2000;61(3):183–95.
Schaapveld M, Visser O, Louwman MJ, de Vries EGE, Willemse PHB, Otter R, et al. Risk of new primary nonbreast cancers after breast cancer treatment: a Dutch population-based study. J Clin Oncol. 2008;26(8):1239–46.
Schottenfeld D, Berg J. Incidence of miltiple primary cancers. IV. Cancers of the female breast and genital organs. J Natl Cancer Inst. 1971;46(1):161–70.
Trama A, Tittarelli A, Barigelletti G, Botta L, Gatta G, Tagliabue G, et al. Excess risk of subsequent malignant neoplasms in adolescent and young adult cancer survivors: results from the first Italian population-based cohort. Cancer. 2022;128(2):364–72.
Zheng G, Hemminki A, Försti A, Sundquist J, Sundquist K, Hemminki K. Second primary cancer after female breast cancer: Familial risks and cause of death. Cancer Med. 2019;8(1):400–7.
Gulhan I, Eser S, Yakut C, Bige O, Ilhan E, Yildirim Y, et al. Second primary gynecologic cancers after breast cancer in Turkish women. Int J Gynecol Cancer. 2009;19(4):648–50.
Hung MH, Liu CJ, Teng CJ, Hu YW, Yeh CM, Chen SC, et al. Risk of second non-breast primary cancer in male and female breast cancer patients: a population-based cohort study. PLoS ONE. 2016;11(2): e0148597.
Jung HK, Park S, Kim NW, Lee JE, Kim Z, Han SW, et al. Development of second primary cancer in Korean breast cancer survivors. Ann Surg Treat Res. 2017;93(6):287–92.
Lee KD, Chen SC, Chan CH, Lu CH, Chen CC, Lin JT, et al. Increased risk for second primary malignancies in women with breast cancer diagnosed at young age: a population-based study in Taiwan. Cancer Epidemiol Biomark Prev. 2008;17(10):2647–55.
Murakami R, Hiyama T, Hanai A, Fujimoto I. Second primary cancers following female breast cancer in Osaka, Japan–a population-based cohort study. Jpn J Clin Oncol. 1987;17(4):293–302.
Odani S, Tabuchi T, Nakata K, Morishima T, Kuwabara Y, Koyama S, et al. Incidence and relative risk of metachronous second primary cancers for 16 cancer sites, Osaka, Japan, 2000–2015: Population-based analysis. Cancer Med. 2022;11(2):507–19.
Silverman BG, Lipshitz I, Keinan-Boker L. Second primary cancers after primary breast cancer diagnosis in Israeli Women, 1992 to 2006. J Glob Oncol. 2017;3(2):135–42.
Tabuchi T, Ito Y, Ioka A, Miyashiro I, Tsukuma H. Incidence of metachronous second primary cancers in Osaka, Japan: update of analyses using population-based cancer registry data. Cancer Sci. 2012;103(6):1111–20.
Tsukuma H, Fujimoto I, Hanai A, Hiyama T, Kitagawa T, Kinoshita N. Incidence of second primary cancers in Osaka residents, Japan, with special reference to cumulative and relative risks. Jpn J Cancer Res. 1994;85(4):339–45.
Utada M, Ohno Y, Hori M, Soda M. Incidence of multiple primary cancers and interval between first and second primary cancers. Cancer Sci. 2014;105(7):890–6.
Schwartz AG, Ragheb NE, Swanson GM, Satariano WA. Racial and age differences in multiple primary cancers after breast cancer: a population-based analysis. Breast Cancer Res Treat. 1989;14(2):245–54.
Prochazka M, Hall P, Granath F, Czene K. Family history of breast cancer and young age at diagnosis of breast cancer increase risk of second primary malignancies in women: a population-based cohort study. Br J Cancer. 2006;95(9):1291–5.
Mellemkjær L, Christensen J, Frederiksen K, Pukkala E, Weiderpass E, Bray F, et al. Risk of primary non-breast cancer after female breast cancer by age at diagnosis. Cancer Epidemiol Biomark Prev. 2011;20(8):1784–92.
Volk N, Pompe-Kirn V. Second primary cancers in breast cancer patients in Slovenia. Cancer Causes Control. 1997;8(5):764–70.
Tabuchi T, Ozaki K, Ioka A, Miyashiro I. Joint and independent effect of alcohol and tobacco use on the risk of subsequent cancer incidence among cancer survivors: a cohort study using cancer registries. Int J Cancer. 2015;137(9):2114–23.
Mellemkjaer L, Friis S, Olsen JH, Scélo G, Hemminki K, Tracey E, et al. Risk of second cancer among women with breast cancer. Int J Cancer. 2006;118(9):2285–92.
Molina-Montes E, Requena M, Sánchez-Cantalejo E, Fernández MF, Arroyo-Morales M, Espín J, et al. Risk of second cancers cancer after a first primary breast cancer: a systematic review and meta-analysis. Gynecol Oncol. 2015;136(1):158–71.
Baeyens-Fernández JA, Molina-Portillo E, Pollán M, Rodríguez-Barranco M, del Moral R, Arribas-Mir L, et al. Trends in incidence, mortality and survival in women with breast cancer from 1985 to 2012 in Granada, Spain: a population-based study. BMC Cancer. 2018;18(1):781.
Dafni U, Tsourti Z, Alatsathianos I. Breast cancer statistics in the European Union: incidence and survival across European countries. Breast Care. 2019;14(6):344–53.
Jemal A, Ward EM, Johnson CJ, Cronin KA, Ma J, Ryerson B, et al. Annual report to the nation on the status of cancer 1975–2014 featuring survival. J Natl Cancer Inst. 2017;109(9):djx030.
Nielsen SM, White MG, Hong S, Aschebrook-Kilfoy B, Kaplan EL, Angelos P, et al. The breast-thyroid cancer link: a systematic review and meta-analysis. Cancer Epidemiol Biomark Prev. 2016;25(2):231–8.
Adamo M, Groves C, Dickie L, Ruhl J. SEER program coding and staging manual 2021. National Cancer Institute, Bethesda, MD 20892: U.S. Department of Health and Human Services National Institutes of Health National Cancer Institute; 2021.
International Association of Cancer Registries. International rules for multiple primary cancers. Asian Pac J Cancer Prev. 6(1):104–6.
Working Group Report. International rules for multiple primary cancers (ICD-0 third edition). Eur J Cancer Prev. 2005;14(4):307–8.
Coyte A, Morrison DS, McLoone P. Second primary cancer risk - the impact of applying different definitions of multiple primaries: results from a retrospective population-based cancer registry study. BMC Cancer. 2014;18(14):272.
Hemminki K, Ji J, Brandt A, Mousavi SM, Sundquist J. The Swedish family-cancer database 2009: prospects for histology-specific and immigrant studies. Int J Cancer. 2010;126(10):2259–67.
Kao WH, Hong JH, See LC, Yu HP, Hsu JT, Chou IJ, et al. Validity of cancer diagnosis in the National Health Insurance database compared with the linked National Cancer Registry in Taiwan. Pharmacoepidemiol Drug Saf. 2018;27(10):1060–6.
Tanaka H, Tsukuma H, Koyama H, Kinoshita Y, Kinoshita N, Oshima A. Second primary cancers following breast cancer in the Japanese female population. Jpn J Cancer Res. 2001;92(1):1–8.
Harrer M, Cuijpers P, Furukawa TA, Ebert DD. Doing meta-analysis with R. Boca Raton: Chapman and Hall/CRC; 2021.
Higgins J, Green S. Cochrane handbook for systematic reviews of interventions. New York: Wiley; 2019.
DerSimonian R, Laird N. Meta-analysis in clinical trials. Control Clin Trials. 1986;7(3):177–88.
Breslow NE, Day NE. Statistical methods in cancer research. Volume II–the design and analysis of cohort studies. IARC Sci Publ. 1987;82:1–406.
Higgins JPT, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta-analyses. BMJ. 2003;327(7414):557–60.
Higgins JPT, Thompson SG. Quantifying heterogeneity in a meta-analysis. Stat Med. 2002;21(11):1539–58.
Egger M, Davey Smith G, Schneider M, Minder C. Bias in meta-analysis detected by a simple, graphical test. BMJ. 1997;315(7109):629–34.
Cancer Today. https://gco.iarc.fr/today/online-analysis-table?v=2020&mode=cancer&mode_population=continents&population=900&populations=900&key=asr&sex=2&cancer=39&type=0&statistic=5&prevalence=0&population_group=0&ages_group%5B%5D=0&ages_group%5B%5D=17&group_cancer=1&include_nmsc=0&include_nmsc_other=1. Estimated number of new cases in 2020, World, females, all ages (excl. NMSC).
Wells G, Shea B, O’Connell D, Peterson J, Welch V, Losos M, et al. The Newcastle-Ottawa Scale (NOS) for assessing the quality of nonrandomised studies in meta-analyses. http://www.ohri.ca/programs/clinical_epidemiology/oxford.asp.
R Core Team. R: A language and environment for statistical computing. Vienna, Austria: R Foundation for Statistical Computing. https://www.R-project.org/.; 2021.
Fournier DM, Bazzell AF. Second primary malignancies in cancer survivors. J Nurse Pract. 2018;14(4):238–44.
van Leeuwen F. Second cancer risk following breast cancer. Eur J Cancer. 2002;38(11):S28-30.
Society of Surgical Oncology 70th Annual Cancer Symposium. Ann Surg Oncol. 2017;24(S1):1–202.
Ferlay J, Colombet M, Soerjomataram I, Parkin DM, Piñeros M, Znaor A, et al. Cancer statistics for the year 2020: an overview. 2021. https://doi.org/10.1002/ijc.33588.
Kirova YM, de Rycke Y, Gambotti L, Pierga JY, Asselain B, Fourquet A, et al. Second malignancies after breast cancer: the impact of different treatment modalities. Br J Cancer. 2008;98(5):870–4.
Bazire L, de Rycke Y, Asselain B, Fourquet A, Kirova YM. Risks of second malignancies after breast cancer treatment: long-term results. Cancer Radiother. 2017;21(1):10–5.
Wei JL, Jiang YZ, Shao ZM. Survival and chemotherapy-related risk of second primary malignancy in breast cancer patients: a SEER-based study. Int J Clin Oncol. 2019;24(8):934–40.
Surveillance Research Program. SEER Quality Improvement Software Tools, Services, and Other Resources. 2016. pp. 1–2.
Plichta JK, Rushing CN, Lewis HC, Rooney MM, Blazer DG, Thomas SM, et al. Implications of missing data on reported breast cancer mortality. Breast Cancer Res Treat. 2022. https://doi.org/10.1007/s10549-022-06764-4.
Grantzau T, Overgaard J. Risk of second non-breast cancer after radiotherapy for breast cancer: a systematic review and meta-analysis of 762,468 patients. Radiother Oncol. 2015;114(1):56–65.
Dong C, Chen L. Second malignancies after breast cancer: the impact of adjuvant therapy. Mol Clin Oncol. 2014;2(3):331–6.
Kaplan HG, Calip GS, Malmgren JA. Maximizing breast cancer therapy with awareness of potential treatment-related blood disorders. Oncologist. 2020;25(5):391–7.
Yang Y, Wei W, Jin L, He H, Wei M, Shen S, et al. Comparison of the characteristics and prognosis between very young women and older women with breast cancer: a multi-institutional report from China. Front Oncol. 2022;12: 783487.
Druesne-Pecollo N, Touvier M, Barrandon E, Chan DSM, Norat T, Zelek L, et al. Excess body weight and second primary cancer risk after breast cancer: a systematic review and meta-analysis of prospective studies. Breast Cancer Res Treat. 2012;135(3):647–54.
Feigelson HS, Bodelon C, Powers JD, Curtis RE, Buist DSM, Veiga LHS, et al. Body mass index and risk of second cancer among women with breast cancer. J Natl Cancer Inst. 2021;113(9):1156–60.
Agudo A, Bonet C, Travier N, González CA, Vineis P, Bueno-de-Mesquita HB, et al. Impact of cigarette smoking on cancer risk in the European prospective investigation into cancer and nutrition study. J Clin Oncol. 2012;30(36):4550–7.
GBD 2019 Cancer Risk Factors Collaborators. The global burden of cancer attributable to risk factors, 2010–19: a systematic analysis for the Global Burden of Disease Study 2019. Lancet. 2022;400(10352):563–91.
Macacu A, Autier P, Boniol M, Boyle P. Active and passive smoking and risk of breast cancer: a meta-analysis. Breast Cancer Res Treat. 2015;154(2):213–24.
Marcheselli R, Marcheselli L, Cortesi L, Bari A, Cirilli C, Pozzi S, et al. Risk of second primary malignancy in breast cancer survivors: a nested population-based case-control study. J Breast Cancer. 2015;18(4):378–85.
Li S, Silvestri V, Leslie G, Rebbeck TR, Neuhausen SL, Hopper JL, et al. Cancer risks associated with BRCA1 and BRCA2 pathogenic variants. J Clin Oncol. 2022;40(14):1529–41.
Momozawa Y, Sasai R, Usui Y, Shiraishi K, Iwasaki Y, Taniyama Y, et al. Expansion of cancer risk profile for BRCA1 and BRCA2 pathogenic variants. JAMA Oncol. 2022;8(6):871–8.
Kuchenbaecker KB, Hopper JL, Barnes DR, Phillips KA, Mooij TM, Roos-Blom MJ, et al. Risks of breast, ovarian, and contralateral breast cancer for BRCA1 and BRCA2 mutation carriers. JAMA. 2017;317(23):2402–16.
Yang X, Leslie G, Doroszuk A, Schneider S, Allen J, Decker B, et al. Cancer risks associated with germline PALB2 pathogenic variants: an international study of 524 families. J Clin Oncol. 2020;38(7):674–85.
Meindl A, Hellebrand H, Wiek C, Erven V, Wappenschmidt B, Niederacher D, et al. Germline mutations in breast and ovarian cancer pedigrees establish RAD51C as a human cancer susceptibility gene. Nat Genet. 2010;42(5):410–4.
Yang X, Song H, Leslie G, Engel C, Hahnen E, Auber B, et al. Ovarian and breast cancer risks associated with pathogenic variants in RAD51C and RAD51D. J Natl Cancer Inst. 2020;112(12):1242–50.
Loveday C, Turnbull C, Ramsay E, Hughes D, Ruark E, Frankum JR, et al. Germline mutations in RAD51D confer susceptibility to ovarian cancer. Nat Genet. 2011;43(9):879–82.
de Sanjosé S, Léoné M, Bérez V, Izquierdo A, Font R, Brunet JM, et al. Prevalence of BRCA1 and BRCA2 germline mutations in young breast cancer patients: a population-based study. Int J Cancer. 2003;106(4):588–93.
Cao AY, Huang J, Hu Z, Li WF, Ma ZL, Tang LL, et al. The prevalence of PALB2 germline mutations in BRCA1/BRCA2 negative Chinese women with early onset breast cancer or affected relatives. Breast Cancer Res Treat. 2009;114(3):457–62.
Kar SP, Beesley J, Amin Al Olama A, Michailidou K, Tyrer J, Kote-Jarai Z, et al. Genome-wide meta-analyses of breast, ovarian, and prostate cancer association studies identify multiple new susceptibility loci shared by at least two cancer types. Cancer Discov. 2016;6(9):1052–67.
Graff RE, Cavazos TB, Thai KK, Kachuri L, Rashkin SR, Hoffman JD, et al. Cross-cancer evaluation of polygenic risk scores for 16 cancer types in two large cohorts. Nat Commun. 2021;12(1):970.
Breast Cancer Association Consortium, Dorling L, Carvalho S, Allen J, González-Neira A, Luccarini C, et al. Breast cancer risk genes - association analysis in more than 113,000 women. N Engl J Med. 2021;384(5):428–39.
Haffty BG, Choi DH, Goyal S, Silber A, Ranieri K, Matloff E, et al. Breast cancer in young women (YBC): prevalence of BRCA1/2 mutations and risk of secondary malignancies across diverse racial groups. Ann Oncol. 2009;20(10):1653–9.
Mousavi-Jarrrahi SH, Kasaeian A, Mansori K, Ranjbaran M, Khodadost M, Mosavi-Jarrahi A. Addressing the younger age at onset in breast cancer patients in Asia: an age-period-cohort analysis of fifty years of quality data from the international agency for research on cancer. ISRN Oncol. 2013;2013: 429862.
Yip CH. Breast cancer in Asia. Methods Mol Biol. 2009;471:51–64.
Drewes AM, Møller ME, Hertzum-Larsen R, Engholm G, Storm HH. Risk of primary brain tumour after breast cancer. Endocr Connect. 2020;9(1):28–33.
Acknowledgements
The authors acknowledge all involved in the CanGene-CanVar research programme (CRUK Catalyst Award CanGene-CanVar (C61296/A27223)). MT was supported by the NIHR Cambridge Biomedical Research Centre (BRC-1215-20014).
Funding
This work was funded by the CRUK Catalyst Award CanGene-CanVar (C61296/A27223). Each person who contributed to the collection, analysis, and interpretation of data and in writing, editing, and giving feedback at the draft phase of this manuscript was funded by this grant.
Author information
Authors and Affiliations
Contributions
IA conducted the database searches, screened the studies at the title and abstract and at the full-text level, performed all data extraction and statistical analyses, and wrote the manuscript. HH also screened the studies at the title and abstract stage, with ES being responsible for resolving conflicts. MT, PP, and AA all edited the manuscript and supervised the research. All authors provided input and suggestions for improvement in the draft phase of the manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Additional file 1.
Additional File.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Allen, I., Hassan, H., Sofianopoulou, E. et al. Risks of second non-breast primaries following breast cancer in women: a systematic review and meta-analysis. Breast Cancer Res 25, 18 (2023). https://doi.org/10.1186/s13058-023-01610-x
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s13058-023-01610-x