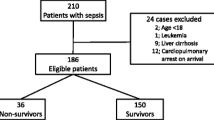
Abstract
Background
Sepsis guidelines suggest immediate start of resuscitation for patients with quick Sequential Organ Failure Assessment (qSOFA) 2 or 3. However, the interpretation of qSOFA 1 remains controversial. We investigated whether measurements of soluble urokinase plasminogen activator receptor (suPAR) may improve risk detection when qSOFA is 1.
Methods
The study had two parts. At the first part, the combination of suPAR with qSOFA was analyzed in a prospective cohort for early risk detection. At the second part, the double-blind, randomized controlled trial (RCT) SUPERIOR evaluated the efficacy of the suPAR-guided medical intervention. SUPERIOR took place between November 2018 and December 2020. Multivariate stepwise Cox regression was used for the prospective cohort, while univariate and multivariate logistic regression was used for the RCT. Consecutive admissions at the emergency department (ED) with suspected infection, qSOFA 1 and suPAR ≥ 12 ng/mL were allocated to single infusion of placebo or meropenem. The primary endpoint was early deterioration, defined as at least one-point increase of admission Sequential Organ Failure Assessment (SOFA) score the first 24 h.
Results
Most of the mortality risk was for patients with qSOFA 2 and 3. Taking the hazard ratio (HR) for death of patients with qSOFA = 1 and suPAR < 12 ng/mL as reference, the HR of qSOFA = 1 and suPAR ≥ 12 ng/mL for 28-day mortality was 2.98 (95% CI 2.11–3.96). The prospective RCT was prematurely ended due to pandemia-related ED re-allocations, with 91 patients enrolled: 47 in the placebo and 44 in the meropenem arm. The primary endpoint was met in 40.4% (n = 19) and 15.9% (n = 7), respectively (difference 24.5% [5.9–40.8]; odds ratio 0.14 [0.04–0.50]). One post hoc analysis showed significant median changes of SOFA score after 72 and 96 h equal to 0 and − 1, respectively.
Conclusions
Combining qSOFA 1 with the biomarker suPAR improves its prognostic performance for unfavorable outcome and can help decision for earlier treatment.
Trial registration EU Clinical Trials Register (EudraCT, 2018-001008-13) and Clinical-Trials.gov (NCT03717350). Registered 24 October 2018.
Similar content being viewed by others
Introduction
Early recognition and start of antibiotics are the cornerstone of sepsis management [1]. Since early recognition often fails, warning scores have been introduced to facilitate early recognition [2,3,4,5]. qSOFA (quick Sequential Organ Failure Assessment Score) is one simplistic approach which integrates mental confusion with increase in the respiratory rate and hypotension; patients with suspicion of infection and at least two of these clinical signs are at nearly threefold greater risk for death after 28 days [6]. Early sepsis resuscitation is warranted for patients with 2 or 3 qSOFA points. However, there is ambivalence how to manage patients with one qSOFA point [7, 8].
Previous studies of our group showed that blood concentrations of the biomarker suPAR (soluble urokinase plasminogen activator receptor) 12 ng/mL or more are an independent predictor of death the first 28 days for patients with infection [9]. suPAR is the soluble form of the membrane-bound receptor uPAR on myeloid cells cleaved after an inflammatory stimulus which promotes chemotaxis and cell migration [10,11,12]. We hypothesized that suPAR may improve the early detection of sepsis in patients with one sign of qSOFA.
Current guidelines of the Surviving Sepsis Campaign do not suggest in favor of qSOFA for the early detection of sepsis [13]. The analysis of data coming from the Hellenic Sepsis Study Group (HSSG) indicates significant risk for death among infections with one qSOFA sign upon admission [7]. This stimulated us to follow an investigational approach including two parts. In the first part, the risk of death was defined among patients with one qSOFA sign and increased suPAR in a retrospective analysis of one large-scale prospective study. In the second part, we designed and conducted the randomized controlled trial with the acronym SUPERIOR (SUPar-guided doublE-blind randomized controlled trial of Initiation Of antibiotics foR presumed infection at the emergency department). The aim of the SUPERIOR trial was to investigate whether early antibiotic treatment for patients with one qSOFA sign and increased suPAR may impact on patients’ outcomes.
Methods
Part 1: prospective HSSG registry
Study design and data source
The HSSG is running one prospective registry of clinical data and biomaterials collected from patients admitted to 39 departments in Greece which are departments of internal medicine, surgery and intensive care units (www.sepsis.gr) (see Additional file 1: Table S1). The samples used for this study were collected between May 2006 and December 2016. Since at that study period, the Sepsis-3 definitions were not yet introduced, patients were re-classified retrospectively for qSOFA signs. The study protocol was approved by the Ethics Committees of the participating hospitals, and patients were enrolled after written informed consent provided by themselves or their first-degree relatives (see Additional file 1: Table S1).
Study population
Participants were aged 18 years or more, with acute pyelonephritis or lung infection or intra-abdominal infection, and had at least two signs of the systemic inflammatory response syndrome (SIRS). Main exclusion criteria were known infection by the human immunodeficiency virus and neutropenia (see Additional file 2).
Variables, exposures and endpoints
Blood was sampled the first 24 h from onset of SIRS. The following information were captured: severity scores, comorbidities, microbiology, administered antibiotics and 28-day outcome. suPAR was measured in duplicate in serum using a CE/IVD enzyme immunosorbent assay (suPARnostic ELISA, ViroGates, Denmark). The lower limit of detection was 0.4 ng/mL.
The aim of the analysis was to investigate whether for patients with one qSOFA sign, suPAR 12 ng/mL or more may be an independent predictor of 28-day outcome.
Statistical analysis
Demographics were expressed as frequencies and 95% confidence intervals (CI) for qualitative data, and as means (SD) or median (quartiles) for quantitative variables with normal or non-normal distribution. Analysis was done by multivariate stepwise Cox regression analysis; hazard ratios (HR) and 95% CI for the independent factors associated with 28-day mortality were defined. The serum suPAR cutoff level with the best trade-off between sensitivity and specificity to predict 28-day mortality among patients with qSOFA equal to 1 was calculated after design of the receiver operator characteristics (ROC) curve using the Youden index. Any value of (two-sided) p less than 0.05 was considered significant. Analysis was performed using IBM SPSS Statistics v23.
Part 2: the SUPERIOR study
Study design and data source
SUPERIOR is a prospective, double-blind RCT conducted in two tertiary University hospitals in Greece. The study protocol was approved by the Ethics Committees of the participating hospitals (approval 276/03–05-2018 by ATTIKON University General Hospital; approval 1060/05–12-2018 by Rion University General Hospital), by the National Ethics Committee of Greece (106/24–07-2018) and by the National Organization for Medicines of Greece (approval 141/ 21–05-2020). The study is prospectively registered at the EU registry database (2018–001008-13) and at Clinicaltrials.gov (NCT03717350). Written informed consent was provided by the patients or legal representatives.
Study population
Included patients were male or female adults admitted to the emergency department (ED) with clinical suspicion of infection, one qSOFA sign and suPAR in the blood 12 ng/mL or more. Main exclusion criteria were: patients scoring 0, 2 or 3 points of qSOFA; pregnancy or lactation, organ transplantation, full-blown sepsis requiring immediate resuscitation as defined by the treating physicians or a decision not to resuscitate (DNR).
Patients meeting all inclusion criteria and none of the exclusion criteria were 1:1 randomized to either one intravenous dose of placebo or 2 g meropenem. Stratified randomization was performed using a computer-generated sequence. Study drug was prepared by an unblinded pharmacist who had access to the electronic study system using separate username and password. The prepared drugs were similar in appearance. The study drug was prepared in a final volume of 100 mL in 0.9% sodium saline and it was infused intravenously within 15 min. The drug was administered by a blinded study nurse.
Variables, exposures and endpoints
suPAR was measured in human EDTA plasma using the rapid suPARnostic® Quick Triage (ViroGates, Denmark), a lateral flow immunoassay which provides results in 20 min. The assay provides a quantitative suPAR measurement in ng/mL, ranging from 2 to 15 ng/mL. If samples were above 15 ng/mL, the sample was diluted 1:1 with dilution buffer and rerun.
The daily clinical assessment including clinical symptoms, laboratory tests, vital-sign assessment tools, type of infection, resolution of infection and survival was performed by blind investigators until hospital discharge or death. The type of infection was classified using pre-defined criteria (see Additional file 1: Table S2). All discharged patients were followed up by telephone calls for clinical condition and health state until day 90. New infections or hospitalizations were recorded. Treatment-emergent adverse events (TEAEs) were recorded and classified into serious and non-serious. Patients who failed study enrollment because of suPAR less than 12 ng/mL were followed up until day 28 for survival.
The primary study endpoint was the early worsening of the patient. This was defined as any increase of total admission SOFA score by at least one point the first 24 h. The key secondary endpoint was the validation of the predictive performance of increased suPAR for 28-day mortality. For this purpose, 28-day mortality was compared between patients failing screening because of suPAR less than 12 ng/mL and patients enrolled in the study and allocated to treatment with placebo.
Other secondary endpoints were the increase of admission total SOFA score by at least two points the first 24 h; mortality by days 7, 28, 60 and 90; time to infection resolution; change of initial antibiotics; duration of hospital stay; and incidence of new infections the first 60 days.
Statistical analysis
The study was powered for the primary endpoint. With the assumptions of 80% power at the 10% level of significance and that the primary endpoint would be achieved in 30% of patients allocated to the placebo arm and 15% of patients allocated to the meropenem arm, 110 patients were needed in each group. The study was analyzed for the intention-to-treat (ITT) population. Categorical variables were expressed as frequencies and 95% CIs. Continuous variables were expressed as mean and standard deviation (SD) or median and interquartile range (IQR), as appropriate. Comparisons for categorical variables were made using the Chi-square test or Fischer’s exact test. For continuous variables, comparisons were made with the nonparametric Mann–Whitney U test or Student’s t test. Univariate and multivariate logistic regression models were performed to identify variables and factors associated with the primary outcome. Results were expressed as odds ratio (OR) and 95% confidence intervals (CI). Hosmer and Lemeshow’s test was used as goodness of fit for multivariable model. Covariates included in the multivariate model were baseline characteristics of study participants which met at least one of two conditions: (a) the p value of comparison between patients achieving or not the primary endpoint was less than 0.100; or (b) the p value of comparison between the two groups was less than 0.100. As the change of SOFA score after 72 h and 96 h is suggested important surrogate for the disease course by others [14, 15], a post hoc analysis was done. In that analysis, the delta SOFA at 72 and 96 h was calculated as the difference between the SOFA score of days 4 and 5 from the SOFA score on admission. Comparisons between groups were done by the Mann–Whitney U test. Any value of P less than 0.05 was considered statistically significant. Analysis was conducted using IBM SPSS Statistics v26.
Results
Part 1: prospective HSSG registry
A total of 2,377 patients from the HSSG registry were analyzed (see Additional file 3: Fig. S1). Patients were divided into four groups: group A including 590 patients with 0 qSOFA signs; group B including 615 patients with one qSOFA sign and suPAR less than 12 ng/mL; group C including 290 patients with one qSOFA sign and suPAR ≥ 12 ng/mL; and group D including 882 patients with 2 or 3 qSOFA signs (Additional file 1: Table S2). ROC analysis among patients with qSOFA equal to 1, defined suPAR 12 ng/mL or more as the best trade-off between sensitivity and specificity. This cutoff had 88.5% negative predictive value to exclude the risk for death after 28 days (see Additional file 4: Fig. S2).
The 28-day mortalities were 7.5% (95% CI 5–10%) for group A, 11.5% (95% CI 9–14%) for group B, 30% (95% CI 25–35%) for group C and 38.7% (95% CI 35–42%) for group D. In the forward stepwise Cox regression analysis patients with one qSOFA point and suPAR less than 12 ng/mL were entered as the reference point. The presence of both one qSOFA point and increased suPAR increased significantly the risk of death (HR: 2.98; 95% CI 2.11–3.96) and even provided similar mortality risk as qSOFA 2 or more (HR: 3.99; 95% CI 3.08–5.16) (Fig. 1A,B). An additive risk for death was found after combining suPAR with any qSOFA point (Additional file 1: Table S3).
Improvement of risk prediction for unfavorable outcome by the qSOFA and suPAR combination. Survival curves provide the analysis of 1787 patients enrolled in the HSSG prospective cohort stratified into three strata of severity by qSOFA score and serum suPAR. The table provides the stepwise Cox regression analysis of survival for each stratum of severity. Cutoffs of APACHE II score, CCI and SOFA score were defined after ROC analyses. CI Confidence interval, AKI acute kidney injury, ARDS acute respiratory distress syndrome, APACHE acute physiology and chronic health evaluation, DIC disseminated intravascular coagulation, HSSG Hellenic Sepsis Study Group, HR hazard ratio, ROC receiver operator characteristics curve, SOFA Sequential Organ Failure Assessment Score, suPAR soluble urokinase plasminogen activator receptor
Part 2: the SUPERIOR study
The first patient was enrolled on November 12, 2018. The study was stopped prematurely by the Sponsor in December 2020. The reason of premature stop was the COVID-19 pandemic during which the function of the ED taking care only of patients infected by SARS-CoV-2 did not logistically allow the study to run. The last visit of the last patient was completed on January 13, 2021. During the study period, 91 patients were enrolled: 47 allocated to the placebo group and 44 to the meropenem group (Fig. 2). Eighty-four patients were enrolled before the start of the pandemic. If the study could run in a similar enrollment rate, the pre-calculated number of patients would have been enrolled in due course. However, the enrollment of only seven patients after the start of the pandemic made evident that the study could not continue at the desired rate of enrollment and led to the decision of premature stop. Baseline characteristics were similar in both groups (Table 1 and Additional file 1: Table S4). The median time from blood drawing until suPAR result was 40 min in both groups; and from blood drawing until start of the study drug was 50 min in both groups.
Primary endpoint
Early worsening of the patient, defined as at least one-point increase of admission SOFA score, was found in 40.4% (95% CI 26–55%) of patients in the placebo group compared with 15.9% (95% CI 5–27%) in the meropenem group (p = 0.011) (Fig. 3A). The relative change of admission SOFA score the first 24 h was higher in the placebo group (p = 0.005) (Additional file 5: Fig. S3).
Primary endpoint of the SUPERIOR trial. The graph shows the percent achievement of the primary endpoint in the two groups of treatment. The primary endpoint is early worsening of the patients defined as at least one-point increase of admission SOFA score after 24 h. The table shows the logistic regression analysis of variables associated with primary outcome. Five covariates were included in the multivariate analysis. Three variables (MH of COPD, chronic intake of corticosteroids and admission APACHE II score) had p value less than 0.100 in the univariate analysis between achievers and non-achievers of the primary endpoint. Two variables (male gender and MH of coronary heart disease) had p value less than 0.100 in the comparisons of the baseline demographics between the two groups of treatment. APACHE Acute physiology and chronic health evaluation, CI confidence interval, COPD chronic obstructive pulmonary disease, MH medical history, n number of patients, OR odds ratio
Multivariate logistic regression analysis was performed for the primary endpoint (Fig. 3B). Univariate analysis showed that medical history of chronic obstructive pulmonary disease (COPD), chronic intake of corticosteroids and APACHE II score were the only variables with p values of comparisons between achievers and non-achievers of the primary endpoint below 0.100 (Additional file 1: Table S5). Comparisons of the baseline demographics between the two groups showed p values less than 0.100 for gender and medical history of coronary heart disease. These five variables were included with the allocation group in the logistic regression model. The model showed that treatment with meropenem was the only variable protecting against early worsening the first 24 h (OR: 0.14, 95% CI 0.04–0.50, p = 0.002) and that medical history of COPD was the only variable favoring early worsening.
Secondary endpoints
Significant differences were found between the two groups of treatment in three secondary endpoints, namely ≥ 2-point increase of admission SOFA score the first 24 h, the rate of infection resolution and the time to infection resolution (Table 2). More precisely, an increase of ≥ 2 points of the admission SOFA score was found in 21.3% (95% CI 9–33%) in the placebo group compared to 4.5% (95% CI 3.2–11%) in the meropenem group (odds ratio 0.17; 95% CI 0.05–0.86, p = 0.028). The rate of resolution of infection was 61.7% (95% CI 47–76%) and 84.1% (95% CI 73–95%), respectively, whereas the time to resolution of infection was significantly shorter in the meropenem group.
Among patients who were not enrolled in the trial, 274 patients failed screening because they had one qSOFA sign but suPAR less than 12 ng/mL; 28-day mortality was 6.6% (95% CI 4.2–7.15). This was significantly lower than the 28-day mortality of patients with one qSOFA sign and suPAR ≥ 12 ng/mL which were allocated to the placebo arm (odds ratio 2.92; 95% CI 1.19–7.16; p = 0.036).
Post hoc analyses
A post hoc analysis revealed that SOFA score after 72 and 96 h decreased significantly more in the meropenem group than the placebo group (Table 3). The empiric treatment administered by physicians as standard of care was modified in 51.1% (95% CI 37.2–64.7%) of patients in the placebo group and 40.9% (95% CI 27.6–55.6%) in the meropenem group [OR = 0.66, 95% CI (0.29–1.52), p = 0.402]. De-escalation of the antibiotics initiated after the study drug was done in 34% (95% CI 22.2–48.3%) of placebo patients compared to 22.7% (95% CI 12.8–37%) of patients allocated to meropenem (Table 3). Source control was performed in 8.5% (95% CI 3.4–19.9%) and 13.6% (95% CI 6.4–26.7%) of patients, respectively; 2.1% (95% CI 0.4–11.1) and 2.3% (95% CI 0.4–11.8) required ICU admission (Table 3).
Safety
No differences in the incidence of serious and non-serious TEAEs were found between the two groups of treatment (Table 4 and Additional file 6: Table S6). Nil TEAE was related to the study drug.
Discussion
This study following a two-stage process showed that combining qSOFA score with suPAR at the ED may guide early administration of meropenem and prevent early deterioration of the patient. Even if this trial ended prematurely due to the changes in ED functions during the pandemic, SUPERIOR managed to be successful in achieving the primary endpoint. In addition, four other main endpoints were met; ≥ 2-point increase of admission SOFA the first 24 h was prevented, the resolution of infection was increased and the time to infection resolution was shortened; and finally, the prognostic performance of the qSOFA/suPAR combination was validated. Following a post hoc analysis, the benefit from meropenem treatment on decrease of the SOFA score after 72 and 96 h was also shown.
Although the new Sepsis-3 definitions have reduced the rate of misclassification of critically ill patients, sepsis is still misdiagnosed or diagnosed late in the ED. The role of commonly used rapid assessment tools outside the ICU for the detection of sepsis or septic shock is controversial [8], even in the most recent Surviving Sepsis Campaign 2021 guidelines, in which the single use of qSOFA score is not recommended [13]. Based on two previous validation analyses of the new Sepsis-3 definitions, the sensitivity of qSOFA score ≥ 2 to predict 28-day hospital mortality is close to 60% [5, 7]. This means that a substantial risk for death exists among patients with one point of qSOFA. The SUPERIOR trial showed how risk prediction in these patients may be improved with the use of suPAR guiding early intervention.
Before the publication of the Sepsis-3 criteria, two large studies on the diagnostic and prognostic value of suPAR in sepsis were conducted in Greece. The first included 180 patients hospitalized in two ICUs with sepsis after ventilator-associated pneumonia and showed that suPAR more than 11.9 ng/mL was an independent predictor of unfavorable outcome [9]. The second included 1,914 patients and showed how the combination of suPAR and APACHE (acute physiology and chronic health evaluation) II score improves risk prediction [16].
This is not the first study proving that the addition of suPAR to other existing clinical scores may improve risk detection at the ED. In the TRIAGE III intervention study from Denmark, suPAR prioritized patients in the ED better than the conventional triage algorithm and improved the prediction of short-term mortality risk [17]. The combination to suPAR to the National Early Warning Score (NEWS) [18] is another example. Therefore, the ability to combine suPAR and other biomarkers with vital-sign-based assessment tools is of great importance as there are patients with no or few clinical signs requiring higher attention [9, 17,18,19,20,21,22]. However, none of these studies proved exactly how the information of early risk detection can guide successful treatment intervention as happened in the double-blind randomized approach of the SUPERIOR RCT.
The present study follows the paradigm generated by the SAVE and SAVE-MORE trials in COVID-19 how the use of suPAR at the ED predicts risk for deterioration and guides early treatment [23, 24]. More precisely, suPAR 6 ng/mL or more increased the likelihood for progression into severe respiratory failure in patients with COVID-19 pneumonia [25]. This risk is substantially attenuated when anakinra, one inhibitor of the interleukin-1 activation, is administered for 10 days guided by the increase of suPAR [23, 24]. During the COVID-19 pandemic, suPAR was proved to predict early deterioration associated with respiratory failure [25] and acute kidney injury [26]. The results of the SUPERIOR trial suggest that for bacterial infections the alert cutoff level of suPAR, prompting intervention, should be 12 ng/mL which is higher than the respective cutoff suggested for viral infections.
SUPERIOR is providing a prospective validation of the qSOFA/suPAR prediction tool generated by the prospective cohort of HSSG. Four main limitations of the SUPERIOR trial need to be mentioned: (a) the small number of study participants due to the premature termination of the study; (b) the limited number of hypotensive patients. Indeed, almost 85% of enrolled patients were tachypneic and few were hypotensive. As such, results cannot be generalized to patients with hypotension where suspicion of infection should guide prompt start of antibiotics [13]; (c) the high prevalence of respiratory tract infections. Indeed, almost half of study participants were sufferers of respiratory tract infections while abdominal tract infections and urinary tract infections were less frequent (25 and 15%, respectively); and (d) the broad-spectrum activity of meropenem. During study design, meropenem was selected due to the high prevalence of infections in the Greek community caused by Gram-negative pathogens producing extended spectrum β-lactamases [27]. The results of the SUPERIOR trial should actually be conceived as the beneficial response after early antibiotic treatment guided by qSOFA/suPAR. With this point of view, the exact type of administered antibiotic may be selected by the results of local surveillance of resistance rates. This may further give rise to the development of larger, international multicenter studies that could corroborate our results.
Conclusions
Sepsis is a deadly disease and several times early diagnosis escapes. The measurement of the biomarker suPAR in patients with one point of qSOFA score admitted in the ED elaborates risk for unfavorable outcome and early deterioration. These patients receive significant benefit from early meropenem treatment.
Availability of data and materials
After publication, data will be made available to other investigators on reasonable requests to the corresponding author.
Abbreviations
- APACHE:
-
Acute physiology and chronic health evaluation
- CI:
-
Confidence intervals
- COVID-19:
-
Coronavirus disease 2019
- COPD:
-
Chronic obstructive pulmonary disease
- DNR:
-
Do not resuscitate
- ED:
-
Emergency department
- HR:
-
Hazard ratio
- HSSG:
-
Hellenic Sepsis Study Group
- ICU:
-
Intensive care unit
- ITT:
-
Intention-to-treat
- IQR:
-
Interquartile range
- NEWS:
-
National Early Warning Score
- OR:
-
Odds ratio
- RCT:
-
Randomized controlled trial
- SD:
-
Standard deviation
- SIRS:
-
Systemic inflammatory response syndrome
- SOFA:
-
Sequential Organ Failure Assessment
- qSOFA:
-
Quick Sequential Organ Failure Assessment
- suPAR:
-
Soluble urokinase plasminogen activator receptor
- TEAEs:
-
Treatment-emergent adverse events
References
Kumar A, Roberts D, Wood KE, et al. Duration of hypotension before initiation of effective antimicrobial therapy is the critical determinant of survival in human septic shock. Crit Care Med. 2006;34(6):1589–96.
Knaus WA, Draper EA, Wagner DP, Zimmerman JE. APACHE II: a severity of disease classification system. CritCareMed. 1985;13(10):818–29.
Vincent JL, Moreno R, Takala J, et al. The SOFA (Sepsis-related Organ Failure Assessment) score to describe organ dysfunction/failure. On behalf of the Working Group on Sepsis-Related Problems of the European Society of Intensive Care Medicine. IntensiveCareMed. 1996;22(7):707–10.
Le Gall JR, Lemeshow S, Saulnier F. A new Simplified Acute Physiology Score (SAPS II) based on a European/North American multicenter study [published correction appears in JAMA 1994 May 4;271(17):1321]. JAMA. 1993;270(24):2957–63.
Fernando SM, Reardon PM, Rochwerg B, et al. Sepsis-3 septic shock criteria and associated mortality among infected hospitalized patients assessed by a rapid response team. Chest. 2018;154(2):309–16.
Singer M, Deutschman CS, Seymour CW, et al. The third international consensus definitions for sepsis and septic shock (Sepsis-3). JAMA. 2016;315(8):801–10.
Giamarellos-Bourboulis EJ, Tsaganos T, Tsangaris I, et al. Validation of the new Sepsis-3 definitions: proposal for improvement in early risk identification. Clin Microbiol Infect. 2017;23(2):104–9.
Tusgul S, Carron PN, Yersin B, et al. Low sensitivity of qSOFA, SIRS criteria and sepsis definition to identify infected patients at risk of complication in the prehospital setting and at the emergency department triage. Scand J Trauma Resusc Emerg Med. 2017;25(1):108.
Giamarellos-Bourboulis EJ, Norrby-Teglund A, Mylona V, et al. Risk assessment in sepsis: a new prognostication rule by APACHE II score and serum soluble urokinase plasminogen activator receptor. Crit Care. 2012;16(4):R149.
Ni W, Han Y, Zhao J, et al. Serum soluble urokinase-type plasminogen activator receptor as a biological marker of bacterial infection in adults: a systematic review and meta-analysis. Sci Rep. 2016;6:39481.
Backes Y, van der Sluijs KF, et al. Usefulness of suPAR as a biological marker in patients with systemic inflammation or infection: a systematic review. Intensive Care Med. 2012;38(9):1418–28.
Rasmussen LJH, Petersen JEV, Eugen-Olsen J. Soluble urokinase plasminogen activator receptor (suPAR) as a biomarker of systemic chronic inflammation. Front Immunol. 2021;12:780641.
Evans L, Rhodes A, Alhazzanni W, et al. Surviving sepsis campaign: international guidelines for management of sepsis and septic shock. Intensive Care Med. 2021;47(11):1181–247.
Moreno R, Vincent JL, Matos R, et al. The use of maximum SOFA score to quantify organ dysfunction/failure in intensive care. Results of a prospective, multicentre study. Working Group on Sepsis related Problems of the ESICM. Intensive Care Med. 1999;25(7):686–96.
Ferreira FL, Bota DP, Bross A, Mélot C, Vincent J. Serial evaluation of the SOFA score to predict outcome in critically ill patients. JAMA. 2001;286(14):1754–8.
Savva A, Raftogiannis M, Baziaka F, et al. Soluble urokinase plasminogen activator receptor (suPAR) for assessment of disease severity in ventilator-associated pneumonia and sepsis. J Infect. 2011;63(5):344–50.
Schultz M, Raamussen LJH, Kallemose T, et al. Availability of suPAR in emergency departments may improve risk stratification: a secondary analysis of the TRIAGE III trial. Scand J Traum Res Emerg Med. 2019;27(1):43.
Rasmussen LJH, Ladelund S, Haupt TH, Ellekilde GE, Eugen-Olsen J, Andersen O. Combining national early warning score with soluble urokinase plasminogen activator receptor (suPAR) improves risk prediction in acute medical patients: a registry-based cohort study. Crit Care Med. 2018;46(12):1961–8.
Kofoed K, Andersen O, Kronborg G, et al. Use of plasma C-reactive protein, procalcitonin, neutrophils, macrophage migration inhibitory factor, soluble urokinase-type plasminogen activator receptor, and soluble triggering receptor expressed on myeloid cells-1 in combination to diagnose infections: a prospective study. Crit Care. 2007;11:R38.
Rubio Díaz R, de Rafael GE, Martín Torres E, et al. Prognostic power of soluble urokinase plasminogen activator receptor (suPAR) for short-term mortality in patients seen in emergency departments due to infections. Rev Esp Quimioter. 2022;35(1):50–62.
Casagranda I, Vendramin C, Callegari T, et al. Usefulness of suPAR in the risk stratification of patients with sepsis admitted to the emergency department. Intern Emerg Med. 2015;10(6):725–30.
Tong-Minh K, Endeman H, Ramakers C, Gommers D, van Gorp E, van der Does Y. Soluble urokinase plasminogen activator receptor and procalcitonin for risk stratification in patients with a suspected infection in the emergency department: a prospective cohort study. Eur J Emerg Med Off J Eur Soc Emerg Med. 2023;10:1097.
Kyriazopoulou E, Poulakou G, Milionis H, et al. Early treatment of COVID-19 with anakinra guided by soluble urokinase plasminogen receptor plasma levels: a double-blind, randomized controlled phase 3 trial. NatMed. 2021;27(10):1752–60.
Kyriazopoulou E, Panagopoulos P, Metallidis S, et al. An open label trial of anakinra to prevent respiratory failure in COVID-19. Elife. 2021;10:e66125.
Rovina N, Akinosoglou K, Eugen-Olsen J, Hayek S, Reiser J, Giamarellos-Bourboulis EJ. Soluble urokinase plasminogen activator receptor (suPAR) as an early predictor of severe respiratory failure in patients with COVID-19 pneumonia. Crit Care. 2020;24(1):187.
Azam TU, Shadid HR, Blakely P, et al. Soluble Urokinase Receptor (SuPAR) in COVID-19-related AKI. J Am SocNephrol. 2020;31(11):2725–35.
Booklet of Sepsis 2017: Definitions, Diagnostic Approach and Treatment recommendations by the Hellenic Sepsis Study Group. http://sepsis.gr/dmsepsis/wp-content/uploads/2019/04/%CE%95%CE%9D%CE%97%CE%9C%CE%95%CE%A1%CE%A9%CE%A4%CE%99%CE%9A%CE%9F-%CE%94%CE%95%CE%9B%CE%A4%CE%99%CE%9F.pdf.
Funding
The work was sponsored by the Hellenic Institute for the Study of Sepsis (HISS) and funded by HISS and ViroGates A/S. ViroGates A/S did not have any role in study design, analysis and interpretation of the data, and decision to publish. HISS designed the study, analyzed the data and decided to publish.
Author information
Authors and Affiliations
Contributions
ΜΕA contributed to data acquisition and drafting the manuscript, revised the manuscript for intellectual content and approved the final version for submission. MEA has accessed and verified the data and is responsible for the decision to submit the manuscript. EG conceptualized the study and approved its final version. EJG-B conceptualized the study, analyzed the data, drafted the manuscript, revised the manuscript for intellectual content and approved the final version for submission. EJG-B has accessed and verified the data, and he is responsible for the decision to submit the manuscript. JE-O conceptualized the study, revised the manuscript for important intellectual content and approved the final version for submission. AK supervised the study and approved the final version for submission. NA, SCh, MS, ChA, KA, KD, GD, KK, PK, VL, AP and AS contributed to data acquisition and gave approval for the version to be published.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
The prospective HSSG study protocol was approved by the Ethics Committees of the participating hospitals, and patients were enrolled after written informed consent provided by themselves or their first-degree relatives (see Additional file 2: Methods for a list of approvals). The SUPERIOR study protocol was approved by the Ethics Committees of the participating hospitals (approval 276/03-05-2018 by ATTIKON University General Hospital; approval 1060/05-12-2018 by Rion University General Hospital), by the National Ethics Committee of Greece (106/24-07-2018) and by the National Organization for Medicines of Greece (approval 141/ 21-05-2020).
Consent for publication
Not applicable.
Competing interests
EJG-Β has received honoraria from Abbott Products Operations, bioMérieux, Brahms GmbH, GSK, InflaRx GmbH and Sobi; independent educational grants from Abbott Products Operations, AbbVie, bioMérieux Inc, InCyte, Johnson & Johnson, MSD, UCD and Sobi; and funding from the Horizon 2020 Marie Skłodowska-Curie International Training Network “the European Sepsis Academy” (granted to the National and Kapodistrian University of Athens), the Horizon 2020 European Grants ImmunoSep and RISC in COVID and the Horizon Health grant EPIC-CROWN-2 (granted to the Hellenic Institute for the Study of Sepsis). JE-O is a co-founder, shareholder and CSO of ViroGates, Denmark, and named inventor on patents on suPAR owned by Copenhagen University Hospital Hvidovre, Denmark. The other authors do not disclose any conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Additional file 1: Table S1.
List of participating sites in the prospective registry. Table S2. Baseline characteristics of 2,377 patients of the HSSG cohort study. Table S3: Survival analysis of patients enrolled in the prospective cohort study stratified into strata of severity by qSOFA score and serum suPAR. Table S4. Antibiotics administered after the study drug. Table S5. Comparison of baseline demographics before randomization according to the achievement of the SUPERIOR primary endpoint or not.
Additional file 2.
Inclusion and exclusion criteria, Methods, and Study design for the prospective registry.
Additional file 3: Figure S1.
Flowchart of the prospective cohort study. HSSG Hellenic Sepsis Study Group, ICU intensive care unit, qSOFA Quick Sequential Organ Failure Assessment Score, n number of patients, suPAR soluble urokinase plasminogen activator receptor.
Additional file 4: Figure S2.
Development of the cutoff of 12ng/mL of suPAR for risk prediction among patients with qSOFA= 1. A Receiver operator characteristics curve of suPAR to predict 28-day mortality among patients outside the ICU with qSOFA equal to one. B Prognostic performance of suPAR 12ng/mL or more to predict 28-day mortality. AUC Area under the curve, ICU intensive care unit, NPV negative predictive value, PPV positive predictive value, qSOFA Quick Sequential Organ Failure Assessment Score, suPAR soluble urokinase plasminogen activator receptor.
Additional file 5: Figure S3.
SOFA changes the first 24 h. n Number of patients, SE standard error.
Additional file 6: Table S6.
Full list of serious and non-serious treatment-emergent adverse events (TEAEs) classified by system organ class and preferred term.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Adami, M.E., Kotsaki, A., Antonakos, N. et al. qSOFA combined with suPAR for early risk detection and guidance of antibiotic treatment in the emergency department: a randomized controlled trial. Crit Care 28, 42 (2024). https://doi.org/10.1186/s13054-024-04825-2
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s13054-024-04825-2