Abstract
Background
Appropriate antibiotic (AB) therapy remains a challenge in the intensive care unit (ICU). Procalcitonin (PCT)-guided AB stewardship could help optimize AB treatment and decrease AB-related adverse effects, but firm evidence is still lacking. Our aim was to compare the effects of PCT-guided AB therapy with standard of care (SOC) in critically ill patients.
Methods
We searched databases CENTRAL, Embase and Medline. We included randomized controlled trials (RCTs) comparing PCT-guided AB therapy (PCT group) with SOC reporting on length of AB therapy, mortality, recurrent and secondary infection, ICU length of stay (LOS), hospital LOS or healthcare costs. Due to recent changes in sepsis definitions, subgroup analyses were performed in studies applying the Sepsis-3 definition. In the statistical analysis, a random-effects model was used to pool effect sizes.
Results
We included 26 RCTs (n = 9048 patients) in the quantitative analysis. In comparison with SOC, length of AB therapy was significantly shorter in the PCT group (MD − 1.79 days, 95% CI: -2.65, − 0.92) and was associated with a significantly lower 28-day mortality (OR 0.84, 95% CI: 0.74, 0.95). In Sepsis-3 patients, mortality benefit was more pronounced (OR 0.46 95% CI: 0.27, 0.79). Odds of recurrent infection were significantly higher in the PCT group (OR 1.36, 95% CI: 1.10, 1.68), but there was no significant difference in the odds of secondary infection (OR 0.81, 95% CI: 0.54, 1.21), ICU and hospital length of stay (MD − 0.67 days 95% CI: − 1.76, 0.41 and MD − 1.23 days, 95% CI: − 3.13, 0.67, respectively).
Conclusions
PCT-guided AB therapy may be associated with reduced AB use, lower 28-day mortality but higher infection recurrence, with similar ICU and hospital length of stay. Our results render the need for better designed studies investigating the role of PCT-guided AB stewardship in critically ill patients.
Graphical abstract

Similar content being viewed by others
Introduction
Inappropriate use of antibiotics (ABs) has serious adverse effects. As a result, antibiotic resistance is emerging, causing approximately 700,000 deaths worldwide in 2014 and is predicted to be the leading cause of death worldwide by 2050—accounting for 10 million deaths per year [1]. Critically ill patients in ICU are at high risk of becoming infected with multidrug-resistant organisms (MDRO) due to their acquired immune deficiency, resulting in unacceptably high morbidity and mortality [2].
In general, more than 50% of critically ill patients are considered as infected. Infection and related sepsis can more than double ICU mortality [3]. However, less than 60% of critically ill patients with an initial diagnosis of sepsis are confirmed to be infected [4]. Despite the known challenges in the differential diagnosis of infection and sepsis, there is an urgent constraint to administer ABs shortly after the onset of sepsis and septic shock [5]. This strategy may inevitably result in unnecessary AB therapy, thus increasing the chance of harm and costs associated with AB treatment.
Procalcitonin (PCT) is one of the most studied inflammatory biomarkers [6] and can distinguish bacterial infections from viral infections in critically ill patients [7, 8]. There is growing evidence that PCT-guided AB therapy can safely reduce antimicrobial consumption—by reducing the number of unnecessary or excessively long therapies. The results of a large, individual patient data meta-analysis in 2017 support the use of PCT in the management of AB stewardship in acute respiratory infections in a variety of clinical settings [9]. However, the evidence is less convincing in other types of infection and sepsis.
The PRORATA study was the first big, multicenter RCT to demonstrate the efficacy and non-inferiority of AB management guided by a predefined PCT protocol in septic critically ill patients [10]. Subsequent trials conducted in ICUs used an approach identical with or similar to PRORATA, but PCT levels for starting and stopping thresholds varied, patient populations were also heterogeneous with medical, surgical, or mixed populations treated for different types of infections, and therefore the overall interpretation and implementation of PCT-guided AB therapy in ICU setting remains challenging. Moreover, with the implementation of the new Sepsis-3 definition [11], study inclusion criteria for sepsis and septic shock have also changed in the most recent clinical trials as compared to the definitions previously used for decades [12, 13]. An updated comprehensive analysis of PCT stewardship in ICU setting, including Sepsis-3 patients, was lacking.
Therefore, we aimed to perform a systematic review and meta-analysis of randomized controlled trials (RCTs) that investigated the effects of PCT-guided AB therapy compared to standard of care (SOC) in critically ill patients.
Methods
We report our systematic review and meta-analysis based on the recommendations of the PRISMA 2020 guideline [14] (see Additional file 1: Table S1), while we followed the Cochrane Handbook [15]. The protocol of the study was registered on PROSPERO (registration number CRD42022374605), and we adhered to it except for one additional outcome measure (rate of secondary infection) and two subgroup analyses (PCT protocol and patient population).
Eligibility criteria
Applying the PICO (Population, Intervention, Comparator, Outcome) framework, we included RCTs that were conducted in P: adult patients with known or suspected infection treated with antibiotics; I: PCT-guided AB therapy; C: SOC (without PCT use); and they provided data on either of the following, O: length of AB therapy, mortality, rate of recurrent infection (clinically confirmed infection in the same location caused by the same pathogen as the primary one), rate of secondary infection (clinically confirmed infection caused by an organism different from the primary one), length of ICU stay, length of hospital stay and healthcare costs. RCTs conducted in the ICU were included in the quantitative, those conducted in other clinical settings, were included in the qualitative analysis.
Information sources and search strategy
Our systematic search was conducted in three main databases—CENTRAL, Embase and Medline—on November 14, 2022. We used the following search key in all databases: (sepsis OR septic OR infection) AND (PCT OR procalcitonin) AND (antibiotic* OR antimicrobial OR anti-microbial). Conference papers were excluded.
Selection process and data extraction
Selection was performed by two independent review authors (M.P. and N.K.) using a reference management software (EndNote 20, Clarivate Analytics). After automatic and manual duplicate removal, reviewers screened titles and abstracts, then full texts against predefined eligibility criteria. Data were collected independently by two authors (M.P. and M.B.) on a standardized data extraction sheet. We used Google translate for an article in Chinese [16]. The following data were extracted in addition to the previously mentioned outcomes: digital object identifier, first author, publication year, countries, centers, study period, study population, sepsis definition, age, gender, PCT protocol, protocol adherence, appropriateness of AB therapy, and exclusion criteria.
Subgroup analysis
We planned to perform subgroup analyses to reduce heterogeneity according to the applied sepsis definitions (Sepsis-1 [13], 2 [12] and 3 [11]), PCT protocol (liberal—stop AB if PCT reduced > 80% of the peak value or < 0.5 ng/mL; and conservative—stop AB if PCT reduced > 90% of peak value or < 0.1–0.25 ng/mL or < 1 ng/mL for 3 days) and patient population (medical, surgical and mixed). We considered ventilator-associated pneumonia (VAP) as pulmonary sepsis.
Risk of bias assessment and evidence level
Synthesis methods
At least three studies had to be included to perform a meta-analysis. As we assumed considerable between-study heterogeneity in all cases, a random-effects model was used to pool effect sizes.
For dichotomous outcomes, odds ratio (OR) with 95% confidence interval (CI) was used to measure the effect size. Pooled OR based on raw data was calculated using the Mantel–Haenszel method [19, 20]. For continuous outcomes, the difference between means (MD) was used to measure the effect size. To calculate the pooled difference, the sample size, the mean and the corresponding standard deviation (SD) were extracted from each study. If the SD was not provided, but the standard error (SE) or confidence interval was available, we calculated the SD from it. The inverse variance weighting method was used to calculate the pooled MD.
We used a Hartung-Knapp adjustment if it resulted in a more conservative estimate than without adjustment [21, 22]. Results were considered statistically significant if the CI did not include the value zero. We summarized the findings for the meta-analysis in forest plots. Where appropriate, we reported the prediction intervals (i.e., the expected range of effects of future studies) of results. Heterogeneity was assessed using Higgins and Thompson I2 statistics [23].
All statistical analyses were performed with R (R Core Team 2023, v4.2.3) [24], using the meta (Schwarzer 2023, v6.2.1) [25] package for basic meta-analysis calculations and plots, and dmetar (Cuijpers, Furukawa, and Ebert 2023, v0.0.9000) [26] package for additional influential analysis calculations and plots.
When necessary and possible, model fitting parameters, and potential outlier publications were explored using different influence measures and plots (e.g., leave-one-out analysis for changes in fitted values, Bujat diagnostics values and plots) as recommended by Harrer et al. (2021) [27]. Small study publication bias was assessed by visual inspection of funnel plots and Egger's test (modified Egger’s test depends on the type of effect size measures) with 10% significance level [28].
For subgroup analysis, we used a fixed-effects “plural” model (aka. mixed-effects model). We assumed that subgroups had different τ2 values as we anticipated differences in the between-study heterogeneity in the subgroups, although for practical reasons, if any of the subgroup size was five or less, a common τ2 assumption was used [29].
Results
Search and selection
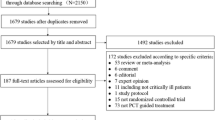
Our systematic search resulted in 15,788 eligible articles. After the selection process, 26 articles were included in the meta-analysis [10, 16, 30,31,32,33,34,35,36,37,38,39,40,41,42,43,44,45,46,47,48,49,50,51,52,53] and 23 articles in the systematic review. The latter included those patients who were treated outside the ICU [54,55,56,57,58,59,60,61,62,63,64,65,66,67,68,69,70,71,72,73,74,75,76]. Figure 1 shows the PRISMA 2020 Flow diagram of the search.
Basic characteristics of included studies
Baseline characteristics of the included studies are detailed in Table 1. Other relevant information is summarized in Additional file 1: Table S2.
We included mainly open-label, parallel group trials. One study had a factorial design [39]. Twenty studies recruited patients with suspected or confirmed infection/sepsis [10, 16, 30,31,32, 35, 37,38,39,40,41,42,43,44,45,46,47,48, 51,52,53], two studies included patients with ventilator-associated pneumonia (VAP) [34, 50]. We also included studies on acute exacerbation of COPD [36], aspiration pneumonia [33], pancreatitis [47] and one study on postoperative (cardiac surgery) patients [49]. PCT protocol was used to stop ABs [16, 30, 31, 34, 35, 37,38,39,40, 42, 43, 45, 50,51,52,53], to start ABs [41, 46, 49] or both [10, 32, 33, 36, 44, 47, 48]. Two studies [32, 43] used a predefined C-reactive protein (CRP) protocol in the control arm, all others used current AB guidelines.
Primary outcome: length of antibiotic therapy
A meta-analysis of 21 RCTs [10, 16, 30, 31, 33,34,35,36, 38,39,40, 42,43,44,45, 47, 48, 50,51,52,53] with a total of 6669 patients revealed that the duration of AB therapy was reduced in the PCT-guided group compared to the SOC group (MD − 1.79 days, 95% CI: − 2.65, − 0.92, p < 0.001) (see Additional file 1: Figure S1).
This significantly reduced AB length was observed in Sepsis-1 patients (MD − 2.58 days, 95% CI: − 3.87, − 1.29, p = 0.004) (Fig. 2A), whether a conservative or liberal PCT protocol was used (MD − 1.56 days, 95% CI: − 2.93, − 0.18, p = 0.03 vs. − 2.37 days, 95% CI: − 4.23, − 0.51, p = 0.02 (Fig. 2B) and in the 100% medical patient population (MD − 1.87 days, 95% CI: − 3.36, − 0.37, p = 0.019) (Fig. 2C). In the Sepsis-3 cohort, the difference was non-significant (− 3.01 days, 95% CI − 7.72, 1.69).
28-day, ICU and in-hospital mortality
The odds of 28-day mortality and in-hospital mortality was reduced in PCT guidance compared to SOC, the former being statistically significant (OR 0.84, 95% CI: 0.74, 0.95, p = 0.008 (Additional file 1: Figure S2) and OR 0.85, 95% CI: 0.66, 1.10 (Additional file 1: Figure S3), respectively). There was no difference in ICU mortality between the two groups (OR 1.00, 95% CI: 0.74, 1.36) (Additional file 1: Figure S4).
This significantly reduced 28-day mortality was observed in Sepsis-2 and Sepsis-3 patients (OR 0.86, 95% CI: 0.76, 0.97, p = 0.024 and OR 0.46, 95% CI: 0.27, 0.79, p = 0.026, respectively) (Fig. 3A), applying liberal PCT protocol (OR 0.75, 95% CI: 0.59, 0.95, p = 0.024) (Fig. 3B) and in medical patients (OR 0.76, 95% CI: 0.60, 0.97, p = 0.033) (Fig. 3C).
Recurrent and secondary infection
Infection recurrence was observed in 99 out of 2,070 patients in the PCT group and in 75 out of 2,080 patients in the SOC group, indicating a significant difference (OR 1.36, 95% CI: 1.10, 1.68, p = 0.008) (Fig. 4).
There was no significant difference in the rate of secondary infections (OR 0.81, 95% CI: 0.54, 1.21) (Fig. 5).
Length of ICU stay, length of hospital stay and healthcare costs
Length of ICU stay and length of hospital stay were non-significantly reduced in the PCT group compared to the SOC group (MD − 0.67 days 95% CI: − 1.76, 0.41 and MD − 1.23 days, 95% CI: − 3.13, 0.67, respectively) (Additional file 1: Figures S5 and S6). Due to the highly heterogeneous reporting of healthcare costs, we used a non-comprehensive method in the analysis, with results favoring PCT use (Additional file 1: Figure S7).
Risk of bias and GRADE assessment
Two trials had high overall ROB due to missing outcome data [34, 35], whereas 23 trials had some concerns about ROB assessment due to deviations from intended intervention (PCT protocol violations) or the lack of reporting it [10, 16, 30,31,32,33, 36,37,38,39,40,41, 43,44,45,46,47,48,49,50,51,52,53]. Only one trial had overall low ROB [42]. For assessing publication bias, funnel plots can be found in the supplementary material (Additional file 1: Figure S8 (a-f)).
Certainty of evidence proved to be high for length of AB therapy and 28-day mortality. Moderate results were observed for in-hospital mortality, ICU mortality, rate of recurrent infection and rate of secondary infection, while GRADE was low for length of ICU stay and length of hospital stay and very low for healthcare costs. ROB and GRADE results are shown in the respective forest plots.
Discussion
In our meta-analysis, we analyzed 26 RCTs [10, 16, 30,31,32,33,34,35,36,37,38,39,40,41,42,43,44,45,46,47,48,49,50,51,52,53] with a total of 9,048 patients, comparing the effects of PCT-guided AB therapy with standard of care on length of AB therapy, mortality, rate of recurrent and secondary infections, length of hospital and ICU stay and healthcare costs.
Length of AB therapy
Our study confirms the findings of previous meta-analyses [77, 78] that PCT-guided AB therapy, including AB cessation rules can significantly reduce the length of AB therapy in ICU patients. An interesting finding in our study was that the three different sepsis definitions had an impact on the results, with significantly shorter AB therapy in the PCT group in the Sepsis-1 cohort and non-significant results in Sepsis-2 and 3 cohorts. Although the mean difference was by far the largest in Sepsis-3 patients [30, 31, 34], the results lacked statistical significance. The relatively low sample size of Sepsis-3 patients compared to other sepsis cohorts could be an explanation for the lack of significant results. On the other hand, five out of nine trials in the Sepsis-2 cohort used conservative PCT protocols, two of them [43, 48] demonstrated even longer AB duration in the PCT group, which may also have contributed to the observed smaller effect on AB length in this patient population.
We further classified the trials into two subgroups (liberal and conservative) depending on the stopping rule in the PCT group except for three trials [39, 51, 52] that used a very unique protocol and studies using only starting rules that did not report this outcome [41, 46, 49]. Our analysis overtly suggests that a liberal PCT protocol may result in shorter AB duration compared with a conservative one. Furthermore, protocol adherence was very low (40–50%) in three trials of the group using the liberal protocol [10, 35, 40], so the difference could have been larger with fewer protocol violations.
Our results show that in mixed populations (the proportion of surgical patients is at least 25%), the length of AB therapy is slightly longer than in medical patients. Apart from one study with different PCT cut-offs for patients during the 48-h postoperative period [44], the trials included used the same protocol regardless of the population. PCT values can be elevated after surgery even in the absence of infection [79], and the use of absolute PCT stopping thresholds in these cases might result in AB overuse. Data on populations including only surgical patients were insufficient for meta-analysis, but pooling data from two surgical cohorts [51, 52] results in an even more pronounced reduction in the length of AB therapy. This may be explained by the high absolute stopping threshold (1 ng/mL) used in the study protocols.
28-day, in-hospital and ICU mortality
Our results suggest that 28-day and in-hospital mortality is lower in the PCT group than in the SOC group. However, results are conflicting, as some trials showed survival benefit [30, 40], and some others did not [10, 43, 48]. This contradiction may be partially resolved by our results, namely that mortality benefit is only observed in Sepsis-2 and Sepsis-3 patients, medical patients and trials using liberal PCT protocol, all of which are associated with shorter AB duration. Unfortunately, our results do not allow us to explain the relationship between AB therapy duration and mortality. Nevertheless, several studies have shown the potential harmful effects of ABs. These include direct toxic effects and organ injury [80], development of AB resistance and potentially higher chances of secondary infections, mostly caused by MDRO [1], mitochondrial dysfunction associated with ABs [81] and injury and collapse of the microbiome [82]. Moreover, an initial low PCT value can help the differential diagnosis, thereby optimizing patient care and reducing mortality.
Recurrent and secondary infections
Theoretically, too short course of ABs could risk infection recurrence, while overuse of ABs is a risk for secondary infections. Our data show significantly higher recurrence of infection in the PCT group, which contradicts the latest meta-analysis [77]; however, they included mostly non-ICU patients with respiratory tract infections. We share the view of the open-label SAPS trial group [40] that bias cannot be excluded, as clinicians might think of a reinfection sooner in the PCT group. The results on secondary infections are conflicting; hence, PCT guidance had uncertain effects on this outcome.
Length of ICU stay, length of hospital stay, healthcare costs
Higher infection recurrence rate did not result in excessive ICU and in-hospital stay in the PCT group, which is consistent with the previous meta-analysis in septic ICU patients [78]. Despite the high heterogeneity in cost-effectiveness reports, our results suggest that PCT guidance at least does not appear to be inferior to SOC, but further research is needed to draw firm conclusions about this outcome.
PCT-guided AB therapy outside the ICU
We included 23 RCTs [54,55,56,57,58,59,60,61,62,63,64,65,66,67,68,69,70,71,72,73,74,75,76] in our review. Eighteen studies recruited patients with respiratory tract infections treated in ED [56, 57, 62, 64, 68, 69, 72, 74,75,76], general ward [55, 61, 66, 67, 71] or primary care [54, 70, 73]. Two trials included patients with peritonitis [59, 60] and one study each included patients with fever [58], febrile neutropenia [63] and urinary tract infection (UTI) [65]. Some studies used additional diagnostics: thoracic ultrasound [54] or viral PCR [64]. In studies on respiratory tract infection, AB use was either reduced in the PCT group or similar between study arms with no difference in adverse outcomes. In patients with peritonitis, Mahmutaj et al. reported a significant reduction in AB use in the PCT arm without an elevated risk of infection recurrence [59]. Slieker et al. in a similar trial reported no adverse outcomes associated with non-significantly reduced AB treatment duration [60]. An approach based on PCT and pyuria in UTI patients [65] reduced AB exposure by 30% without adverse effects, whereas in febrile neutropenia, [63] PCT had no effect on AB use.
Strengths and limitations
To the best of our knowledge, this meta-analysis contains the largest number of studies to date, all of which are RCTs. We are also the first to perform subgroup analyses based on sepsis definitions, patient populations, and PCT protocols: our results provide some support that recruiting patients into studies according to the Sepsis-3 definition may have an impact on outcomes; that surgical and medical patients may require separate treatment protocols; and conservative guidance is not superior to a liberal strategy. Finally, we rigorously followed all Cochrane Collaboration guidelines, thereby ensuring maximum quality, transparency, and reproducibility of the results.
Our meta-analysis has certain limitations. First, in the control arm, SOC was not “standardized” as different AB guidelines were applied in different institutions that could potentially result in longer duration of AB therapy in some regions, thus overestimating the effect of PCT guidance. Second, “PCT guidance” does not mean a standard approach, as studies applied different PCT protocols: 16 out of 26 included studies used PCT protocol to stop ABs, 3 used PCT protocol to start ABs, while 7 used PCT guidance for both starting and stopping AB therapy. Furthermore, not all studies reported on all outcomes. The source of infection varied between the studies and the number of patients with septic shock ranged between 7 and 87%, indicating a huge variability in severity of patient populations on the one hand and, on the other hand, a possible impact on outcomes cannot be excluded according to the 15 studies reporting PCT protocol adherence, which ranged between 44 and 97%. Furthermore, AB appropriateness could have an important effect on outcome. However, we do not know whether patients received appropriate or inappropriate ABs in the same or similar proportion in the PCT-guided and control groups as this outcome was only reported in 5 studies in which the groups were well balanced in this regard [10, 30, 35, 45, 50], but we still cannot draw conclusions on this topic. Finally, almost all studies excluded immunocompromised patients in their medical history; therefore, the generalizability of our results is limited.
Implications for practice and research
The rapid application of scientific results is of utmost importance [83, 84]. Our results suggest that PCT-guided AB management could reduce the length of AB therapy in ICU patients, especially in countries and institutes where routine AB administration exceeds 7 days.
The current sepsis guideline [5] recommends against the use of PCT and clinical evaluation to decide when to start AB therapy in septic patients. However, we believe that further research is needed in this field, especially to evaluate PCT kinetics (i.e., changes in 12–24 h) compared to protocols based on a fix value (i.e., 0.5 ng/mL as cut off) [79, 85]. Furthermore, the increased rate of recurrent infections, the difference between medical and surgical patients and finally testing whether a liberal or a conservative regime is more beneficial should also deserve further investigations. We also suggest that in future trials, “organ support free days” should be used as the primary outcome [86] rather than mortality, which is affected by a number of confounding factors during the full course of a critical illness; therefore it may not necessarily reflect the efficacy of a particular intervention. Finally, we need data on immunocompromised patients who may also benefit from this approach.
Conclusion
PCT-guided AB therapy may be associated with reduced AB use, lower 28-day mortality but higher infection recurrence, with similar ICU and hospital length of stay. Our results render the need for better designed studies investigating the role of PCT-guided AB stewardship in critically ill patients.
Availability of data and materials
The datasets used in this study can be found in the full-text articles included in the systematic review and meta-analysis.
Abbreviations
- AB:
-
Antibiotic
- ABs:
-
Antibiotics
- AECOPD:
-
Acute exacerbation of chronic obstructive pulmonary disease
- CAP:
-
Community acquired pneumonia
- CI:
-
Confidence interval
- COPD:
-
Chronic obstructive pulmonary disease
- CRP:
-
C-reactive protein
- ED:
-
Emergency department
- GRADE:
-
Grading of Recommendations Assessment, Development, and Evaluation
- ICU:
-
Intensive care unit
- LOS:
-
Length of stay
- LRTI:
-
Lower respiratory tract infection
- MD:
-
Mean difference
- MDRO:
-
Multidrug-resistant organism
- OR:
-
Odds ratio
- PCR:
-
Polymerase chain reaction
- PCT:
-
Procalcitonin
- RCT:
-
Randomized controlled trial
- RCTs:
-
Randomized controlled trials
- ROB:
-
Risk of bias
- SD:
-
Standard deviation
- SE:
-
Standard error
- SOC:
-
Standard of care
- SOFA:
-
Sequential organ failure assessment or Sepsis-related organ failure assessment
- UTI:
-
Urinary tract infection
- VAP:
-
Ventilator-associated pneumonia
References
de Kraker MEA, Stewardson AJ, Harbarth S. Will 10 million people die a year due to antimicrobial resistance by 2050? PLoS Med. 2016;13: e1002184.
De Waele JJ, Boelens J, Leroux-Roels I. Multidrug-resistant bacteria in ICU: fact or myth. Curr Opin Anaesthesiol. 2020;33:156–61.
Vincent J-L, Rello J, Marshall J, Silva E, Anzueto A, Martin CD, et al. International study of the prevalence and outcomes of infection in intensive care units. JAMA. 2009;302:2323–9.
Klein Klouwenberg PMC, Cremer OL, van Vught LA, Ong DSY, Frencken JF, Schultz MJ, et al. Likelihood of infection in patients with presumed sepsis at the time of intensive care unit admission: a cohort study. Crit Care. 2015;19:319.
Evans L, Rhodes A, Alhazzani W, Antonelli M, Coopersmith CM, French C, et al. Surviving sepsis campaign: international guidelines for management of sepsis and septic shock 2021. Crit Care Med. 2021;49:e1063–143.
Reinhart K, Meisner M. Biomarkers in the critically ill patient: procalcitonin. Crit Care Clin. 2011;27:253–63.
Schwarz S, Bertram M, Schwab S, Andrassy K, Hacke W. Serum procalcitonin levels in bacterial and abacterial meningitis. Crit Care Med. 2000;28:1828–32.
Pfister R, Kochanek M, Leygeber T, Brun-Buisson C, Cuquemelle E, Machado MB, et al. Procalcitonin for diagnosis of bacterial pneumonia in critically ill patients during 2009 H1N1 influenza pandemic: a prospective cohort study, systematic review and individual patient data meta-analysis. Crit Care. 2014;18:R44.
Schuetz P, Wirz Y, Sager R, Christ-Crain M, Stolz D, Tamm M, et al. Procalcitonin to initiate or discontinue antibiotics in acute respiratory tract infections. Cochrane Database Syst Rev. 2017;10:CD007498.
Bouadma L, Luyt C-E, Tubach F, Cracco C, Alvarez A, Schwebel C, et al. Use of procalcitonin to reduce patients’ exposure to antibiotics in intensive care units (PRORATA trial): a multicentre randomised controlled trial. Lancet. 2010;375:463–74.
Singer M, Deutschman CS, Seymour CW, Shankar-Hari M, Annane D, Bauer M, et al. The third international consensus definitions for sepsis and septic shock (Sepsis-3). JAMA. 2016;315:801–10.
Levy MM, Fink MP, Marshall JC, Abraham E, Angus D, Cook D, et al. SCCM/ESICM/ACCP/ATS/SIS international sepsis definitions conference. Crit Care Med. 2003;31:1250–6.
Bone RC, Balk RA, Cerra FB, Dellinger RP, Fein AM, Knaus WA, et al. Definitions for sepsis and organ failure and guidelines for the use of innovative therapies in sepsis. The ACCP/SCCM consensus conference committee. american college of chest physicians/society of critical care medicine. Chest. 1992;101:1644–55.
Page MJ, McKenzie JE, Bossuyt PM, Boutron I, Hoffmann TC, Mulrow CD, et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. J Clin Epidemiol. 2021;134:178–89.
Cumpston MS, McKenzie JE, Welch VA, Brennan SE. Strengthening systematic reviews in public health: guidance in the cochrane handbook for systematic reviews of interventions 2nd edition. J Public Health. 2022;44:e588–92.
Xu XL, Yan FD, Yu JQ, Chen QH, Lin H. Zheng RQ [Efficacy and safety of procalcitonin guidance in reducing the duration of antibiotic treatment of sepsis patients]. Zhonghua Yi Xue Za Zhi. 2017;97:343–6.
Sterne JAC, Savović J, Page MJ, Elbers RG, Blencowe NS, Boutron I, et al. RoB 2: a revised tool for assessing risk of bias in randomised trials. BMJ. 2019;366:l4898.
GRADEpro. [cited 3 May 2023]. Available: http://gradepro.org
Mantel N, Haenszel W. Statistical aspects of the analysis of data from retrospective studies of disease. J Natl Cancer Inst. 1959;22:719–48.
Robins J, Greenland S, Breslow NE. A general estimator for the variance of the Mantel-Haenszel odds ratio. Am J Epidemiol. 1986;124:719–23.
Knapp G, Hartung J. Improved tests for a random effects meta-regression with a single covariate. Stat Med. 2003;22:2693–710.
IntHout J, Ioannidis JPA, Borm GF. The Hartung-Knapp-Sidik-Jonkman method for random effects meta-analysis is straightforward and considerably outperforms the standard DerSimonian-Laird method. BMC Med Res Methodol. 2014;14:25.
Higgins JPT, Thompson SG. Quantifying heterogeneity in a meta-analysis. Stat Med. 2002;21:1539–58.
The R Project for Statistical Computing. [cited 17 Jul 2023]. Available: https://www.R-project.org
GitHub - guido-s/meta: Official Git repository of R package meta. In: GitHub [Internet]. [cited 17 Jul 2023]. Available: https://github.com/guido-s/meta
Companion R package for the guide Doing Meta-Analysis in R. [cited 16 Jun 2023]. Available: https://dmetar.protectlab.org
Harrer M. Doing Meta-Analysis with R: A Hands-On Guide. CRC Press; 2021.
Harbord RM, Harris RJ, Sterne JAC. Updated tests for small-study effects in meta-analyses. Stata J. 2009;9:197–210.
Borenstein, Michael, Larry V. Hedges, Julian P. T. Higgins, and Hannah R. Rothstein. (2009) Introduction to Meta-Analysis, Wiley
Kyriazopoulou E, Liaskou-Antoniou L, Adamis G, Panagaki A, Melachroinopoulos N, Drakou E, et al. Procalcitonin to reduce long-term infection-associated adverse events in sepsis a randomized trial. Am J Respir Crit Care Med. 2021;203:202–10.
Vishalashi SG, Gupta P, Verma PK. Serum procalcitonin as a biomarker to determine the duration of antibiotic therapy in adult patients with sepsis and septic shock in intensive care units: a prospective study. Indian J Crit Care Med. 2021;25:507–11.
Ali WA, Bazan NS, Elberry AA, Hussein RRS. A randomized trial to compare procalcitonin and C-reactive protein in assessing severity of sepsis and in guiding antibacterial therapy in Egyptian critically ill patients. Ir J Med Sci. 2021;190:1487–95.
Labro G, Aptel F, Puyraveau M, Paillot J, Pili Floury S, Merdji H, et al. Impact on antimicrobial consumption of procalcitonin-guided antibiotic therapy for pneumonia/pneumonitis associated with aspiration in comatose mechanically ventilated patients: a multicenter, randomized controlled study. Ann Intensive Care. 2021;11:145.
Mazlan MZ, Ismail MA, Ali S, Salmuna ZN, Shukeri WFWM, Omar M. Efficacy and safety of the point-of-care procalcitonin test for determining the antibiotic treatment duration in patients with ventilator-associated pneumonia in the intensive care unit: a randomised controlled trial. Anaesthesiol Intensive Ther. 2021;53:207–14.
Jeon K, Suh JK, Jang EJ, Cho S, Ryu HG, Na S, et al. Procalcitonin-guided treatment on duration of antibiotic therapy and cost in septic patients (PRODA): a multi-center randomized controlled trial. J Korean Med Sci. 2019;34: e110.
Daubin C, Valette X, Thiollière F, Mira J-P, Hazera P, Annane D, et al. Procalcitonin algorithm to guide initial antibiotic therapy in acute exacerbations of COPD admitted to the ICU: a randomized multicenter study. Intensive Care Med. 2018;44:428–37.
Kip MMA, van Oers JA, Shajiei A, Beishuizen A, Berghuis AMS, Girbes AR, et al. Cost-effectiveness of procalcitonin testing to guide antibiotic treatment duration in critically ill patients: results from a randomised controlled multicentre trial in the Netherlands. Crit Care. 2018;22:293.
Liu Y, Yang W, Wei J. Guiding effect of serum procalcitonin (PCT) on the antibiotic application to patients with sepsis. Iran J Public Health. 2017;46:1535–9.
Bloos F, Trips E, Nierhaus A, Briegel J, Heyland DK, Jaschinski U, et al. Effect of sodium selenite administration and procalcitonin-guided therapy on mortality in patients with severe sepsis or septic shock: a randomized clinical trial. JAMA Intern Med. 2016;176:1266–76.
de Jong E, van Oers JA, Beishuizen A, Vos P, Vermeijden WJ, Haas LE, et al. Efficacy and safety of procalcitonin guidance in reducing the duration of antibiotic treatment in critically ill patients: a randomised, controlled, open-label trial. Lancet Infect Dis. 2016;16:819–27.
Najafi A, Khodadadian A, Sanatkar M, Shariat Moharari R, Etezadi F, Ahmadi A, et al. The comparison of procalcitonin guidance administer antibiotics with empiric antibiotic therapy in critically ill patients admitted in intensive care unit. Acta Med Iran. 2015;53:562–7.
Shehabi Y, Sterba M, Garrett PM, Rachakonda KS, Stephens D, Harrigan P, et al. Procalcitonin algorithm in critically ill adults with undifferentiated infection or suspected sepsis. A randomized controlled trial. Am J Respir Crit Care Med. 2014;190:1102–10.
Oliveira CF, Botoni FA, Oliveira CRA, Silva CB, Pereira HA, Serufo JC, et al. Procalcitonin versus C-reactive protein for guiding antibiotic therapy in sepsis: a randomized trial. Crit Care Med. 2013;41:2336–43.
Annane D, Maxime V, Faller JP, Mezher C, Clec’h C, Martel P, et al. Procalcitonin levels to guide antibiotic therapy in adults with non-microbiologically proven apparent severe sepsis: a randomised controlled trial. BMJ Open. 2013. https://doi.org/10.1136/bmjopen-2012-002186.
Deliberato RO, Marra AR, Sanches PR, Martino MDV, dos Ferreira CE, S, Pasternak J, et al. Clinical and economic impact of procalcitonin to shorten antimicrobial therapy in septic patients with proven bacterial infection in an intensive care setting. Diagn Microbiol Infect Dis. 2013;76:266–71.
Layios N, Lambermont B, Canivet J-L, Morimont P, Preiser J-C, Garweg C, et al. Procalcitonin usefulness for the initiation of antibiotic treatment in intensive care unit patients. Crit Care Med. 2012;40:2304–9.
Qu R, Ji Y, Ling Y, Ye C-Y, Yang S-M, Liu Y-Y, et al. Procalcitonin is a good tool to guide duration of antibiotic therapy in patients with severe acute pancreatitis. A randomized prospective single-center controlled trial. Saudi Med J. 2012;33:382–7.
Jensen JU, Hein L, Lundgren B, Bestle MH, Mohr TT, Andersen MH, et al. Procalcitonin-guided interventions against infections to increase early appropriate antibiotics and improve survival in the intensive care unit: a randomized trial. Crit Care Med. 2011;39:2048–58.
Maravić-Stojković V, Lausević-Vuk L, Jović M, Ranković A, Borzanović M, Marinković J. Procalcitonin-based therapeutic strategy to reduce antibiotic use in patients after cardiac surgery: a randomized controlled trial. Srp Arh Celok Lek. 2011;139:736–42.
Stolz D, Smyrnios N, Eggimann P, Pargger H, Thakkar N, Siegemund M, et al. Procalcitonin for reduced antibiotic exposure in ventilator-associated pneumonia: a randomised study. Eur Respir J. 2009;34:1364–75.
Hochreiter M, Köhler T, Schweiger AM, Keck FS, Bein B, von Spiegel T, et al. Procalcitonin to guide duration of antibiotic therapy in intensive care patients: a randomized prospective controlled trial. Crit Care. 2009;13:R83.
Schroeder S, Hochreiter M, Koehler T, Schweiger A-M, Bein B, Keck FS, et al. Procalcitonin (PCT)-guided algorithm reduces length of antibiotic treatment in surgical intensive care patients with severe sepsis: results of a prospective randomized study. Langenbecks Arch Surg. 2009;394:221–6.
Nobre V, Harbarth S, Graf J-D, Rohner P, Pugin J. Use of procalcitonin to shorten antibiotic treatment duration in septic patients: a randomized trial. Am J Respir Crit Care Med. 2008;177:498–505.
Lhopitallier L, Kronenberg A, Meuwly J-Y, Locatelli I, Mueller Y, Senn N, et al. Procalcitonin and lung ultrasonography point-of-care testing to determine antibiotic prescription in patients with lower respiratory tract infection in primary care: pragmatic cluster randomised trial. BMJ. 2021;374: n2132.
O’Riordan F, Shiely F, Byrne S, O’Brien D, Palmer B, Dahly D, et al. An investigation of the effects of procalcitonin testing on antimicrobial prescribing in respiratory tract infections in an Irish university hospital setting: a feasibility study. J Antimicrob Chemother. 2019;74:3352–61.
Montassier E, Javaudin F, Moustafa F, Nandjou D, Maignan M, Hardouin J-B, et al. Guideline-based clinical assessment versus procalcitonin-guided antibiotic use in pneumonia: a pragmatic randomized trial. Ann Emerg Med. 2019;74:580–91.
Huang DT, Yealy DM, Filbin MR, Brown AM, Chang C-CH, Doi Y, et al. Procalcitonin-guided use of antibiotics for lower respiratory tract infection. N Engl J Med. 2018;379:236–49.
van der Does Y, Limper M, Jie KE, Schuit SCE, Jansen H, Pernot N, et al. Procalcitonin-guided antibiotic therapy in patients with fever in a general emergency department population: a multicentre non-inferiority randomized clinical trial (HiTEMP study). Clin Microbiol Infect. 2018;24:1282–9.
Mahmutaj D, Krasniqi S, Braha B, Limani D, Neziri B. The Predictive role of procalcitonin on the treatment of intra-abdominal infections. Open Access Maced J Med Sci. 2017;5:909–14.
Slieker JC, Aellen S, Eggimann P, Guarnero V, Schäfer M, Demartines N. Procalcitonin-guided antibiotics after surgery for peritonitis: a randomized controlled study. Gastroenterol Res Pract. 2017;2017:3457614.
Ulm L, Hoffmann S, Nabavi D, Hermans M, Mackert B-M, Hamilton F, et al. The randomized controlled STRAWINSKI trial: procalcitonin-guided antibiotic therapy after stroke. Front Neurol. 2017;8:153.
Corti C, Fally M, Fabricius-Bjerre A, Mortensen K, Jensen BN, Andreassen HF, et al. Point-of-care procalcitonin test to reduce antibiotic exposure in patients hospitalized with acute exacerbation of COPD. Int J Chron Obstruct Pulmon Dis. 2016;11:1381–9.
Lima SSS, Nobre V, de Castro Romanelli RM, Clemente WT, da Silva Bittencourt HN, Melo ACM, et al. Procalcitonin-guided protocol is not useful to manage antibiotic therapy in febrile neutropenia: a randomized controlled trial. Ann Hematol. 2016;95:1169–76.
Branche AR, Walsh EE, Vargas R, Hulbert B, Formica MA, Baran A, et al. Serum procalcitonin measurement and viral testing to guide antibiotic use for respiratory infections in hospitalized adults: a randomized controlled trial. J Infect Dis. 2015;212:1692–700.
Drozdov D, Schwarz S, Kutz A, Grolimund E, Rast AC, Steiner D, et al. Procalcitonin and pyuria-based algorithm reduces antibiotic use in urinary tract infections: a randomized controlled trial. BMC Med. 2015;13:104.
Verduri A, Luppi F, D’Amico R, Balduzzi S, Vicini R, Liverani A, et al. Antibiotic treatment of severe exacerbations of chronic obstructive pulmonary disease with procalcitonin: a randomized noninferiority trial. PLoS ONE. 2015;10: e0118241.
Ogasawara T, Umezawa H, Naito Y, Takeuchi T, Kato S, Yano T, et al. Procalcitonin-guided antibiotic therapy in aspiration pneumonia and an assessment of the continuation of oral intake. Respir Investig. 2014;52:107–13.
Tang J, Long W, Yan L, Zhang Y, Xie J, Lu G, et al. Procalcitonin guided antibiotic therapy of acute exacerbations of asthma: a randomized controlled trial. BMC Infect Dis. 2013;13:596.
Long W, Deng X, Zhang Y, Lu G, Xie J, Tang J. Procalcitonin guidance for reduction of antibiotic use in low-risk outpatients with community-acquired pneumonia. Respirology. 2011;16:819–24.
Burkhardt O, Ewig S, Haagen U, Giersdorf S, Hartmann O, Wegscheider K, et al. Procalcitonin guidance and reduction of antibiotic use in acute respiratory tract infection. Eur Respir J. 2010;36:601–7.
Kristoffersen KB, Søgaard OS, Wejse C, Black FT, Greve T, Tarp B, et al. Antibiotic treatment interruption of suspected lower respiratory tract infections based on a single procalcitonin measurement at hospital admission–a randomized trial. Clin Microbiol Infect. 2009;15:481–7.
Schuetz P, Christ-Crain M, Thomann R, Falconnier C, Wolbers M, Widmer I, et al. Effect of procalcitonin-based guidelines vs standard guidelines on antibiotic use in lower respiratory tract infections: the ProHOSP randomized controlled trial. JAMA. 2009;302:1059–66.
Briel M, Schuetz P, Mueller B, Young J, Schild U, Nusbaumer C, et al. Procalcitonin-guided antibiotic use vs a standard approach for acute respiratory tract infections in primary care. Arch Intern Med. 2008;168:2000–7.
Stolz D, Christ-Crain M, Bingisser R, Leuppi J, Miedinger D, Müller C, et al. Antibiotic treatment of exacerbations of COPD: a randomized, controlled trial comparing procalcitonin-guidance with standard therapy. Chest. 2007;131:9–19.
Christ-Crain M, Stolz D, Bingisser R, Müller C, Miedinger D, Huber PR, et al. Procalcitonin guidance of antibiotic therapy in community-acquired pneumonia: a randomized trial. Am J Respir Crit Care Med. 2006;174:84–93.
Christ-Crain M, Jaccard-Stolz D, Bingisser R, Gencay MM, Huber PR, Tamm M, et al. Effect of procalcitonin-guided treatment on antibiotic use and outcome in lower respiratory tract infections: cluster-randomised, single-blinded intervention trial. Lancet. 2004;363:600–7.
Elnajdy D, El-Dahiyat F. Antibiotics duration guided by biomarkers in hospitalized adult patients; a systematic review and meta-analysis. Infect Dis. 2022;54:387–402.
Gutiérrez-Pizarraya A, León-García MDC, De Juan-Idígoras R, Garnacho-Montero J. Clinical impact of procalcitonin-based algorithms for duration of antibiotic treatment in critically ill adult patients with sepsis: a meta-analysis of randomized clinical trials. Expert Rev Anti Infect Ther. 2022;20:103–12.
Trásy D, Tánczos K, Németh M, Hankovszky P, Lovas A, Mikor A, et al. Early procalcitonin kinetics and appropriateness of empirical antimicrobial therapy in critically ill patients: A prospective observational study. J Crit Care. 2016;34:50–5.
Wright J, Paauw DS. Complications of antibiotic therapy. Med Clin North Am. 2013;97(667–79):xi.
Singh R, Sripada L, Singh R. Side effects of antibiotics during bacterial infection: mitochondria, the main target in host cell. Mitochondrion. 2014;16:50–4.
Alverdy JC, Krezalek MA. Collapse of the microbiome, emergence of the pathobiome, and the immunopathology of sepsis. Crit Care Med. 2017;45:337–47.
Hegyi P, Erőss B, Izbéki F, Párniczky A, Szentesi A. Accelerating the translational medicine cycle: the Academia Europaea pilot. Nat Med. 2021;27:1317–9.
Hegyi P, Petersen OH, Holgate S, Erőss B, Garami A, Szakács Z, et al. Academia Europaea position paper on translational medicine: the cycle model for translating scientific results into community benefits. J Clin Med Res. 2020. https://doi.org/10.3390/jcm9051532.
Trásy D, Tánczos K, Németh M, Hankovszky P, Lovas A, Mikor A, et al. Delta procalcitonin is a better indicator of infection than absolute procalcitonin values in critically ill patients: a prospective observational study. J Immunol Res. 2016;2016:3530752.
Laterre P-F, Berry SM, Blemings A, Carlsen JE, François B, Graves T, et al. Effect of selepressin vs placebo on ventilator- and vasopressor-free days in patients with septic shock: the SEPSIS-ACT randomized clinical trial. JAMA. 2019;322:1476–85.
Acknowledgments
Not applicable.
Funding
Open access funding provided by Semmelweis University. The project was partially supported by the Hungarian National Research, Development and Innovation Office (K 138816). No other sponsors had a role in the design, data collection, analysis, interpretation, and manuscript preparation.
Author information
Authors and Affiliations
Contributions
MP contributed to conceptualization, investigation, writing—original draft; MB contributed to investigation, writing—review and editing; NK contributed to investigation, writing—review and editing; DT contributed to conceptualization, supervision, investigation, writing—review and editing; LZ contributed to conceptualization, supervision, writing—review and editing; FP contributed to conceptualization, statistical analysis review and editing; HA contributed to statistical methodology, writing—review and editing; CT contributed to methodology, supervision, writing—review and editing; PH contributed to supervision, project administration, funding acquisition, writing—review and editing; ZM contributed to conceptualization, project administration, supervision, writing—original draft.
All authors certify that they have participated sufficiently in the work to take public responsibility for the content, including participation in the concept, design, analysis, writing, or revision of the manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
No ethics approval was required for this systematic review with meta-analysis, as all data were already published in peer-reviewed journals. No patients were involved in the design, conduct or interpretation of our study.
Competing interests
The authors declare no competing interests.
Consent for publication
Not applicable.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Additional file1
: Table S1 Prisma Checklist 2020. Table S2 Other characteristics of included studies. Figure S1 Length of AB therapy. Figure S2 28-day mortality. Figure S3 In-hospital mortality. Figure S4 ICU mortality. Figure S5 Length of ICU stay. Figure S6 Length of hospital stay. Figure S7 Healthcare costs Figure S8 (a-f) Funnel plots
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Papp, M., Kiss, N., Baka, M. et al. Procalcitonin-guided antibiotic therapy may shorten length of treatment and may improve survival—a systematic review and meta-analysis. Crit Care 27, 394 (2023). https://doi.org/10.1186/s13054-023-04677-2
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s13054-023-04677-2