Abstract
Background
To evaluate if the increase in chloride intake during a continuous infusion of 20% hypertonic saline solution (HSS) is associated with an increase in the incidence of acute kidney injury (AKI) compared to standard of care in traumatic brain injury patients.
Methods
In this post hoc analysis of the COBI trial, 370 patients admitted for a moderate-to-severe TBI in the 9 participating ICUs were enrolled. The intervention consisted in a continuous infusion of HSS to maintain a blood sodium level between 150 and 155 mmol/L for at least 48 h. Patients enrolled in the control arm were treated as recommended by the latest Brain Trauma foundation guidelines. The primary outcome of this study was the occurrence of AKI within 28 days after enrollment. AKI was defined by stages 2 or 3 according to KDIGO criteria.
Results
After exclusion of missing data, 322 patients were included in this post hoc analysis. The patients randomized in the intervention arm received a significantly higher amount of chloride during the first 4 days (intervention group: 97.3 ± 31.6 g vs. control group: 61.3 ± 38.1 g; p < 0.001) and had higher blood chloride levels at day 4 (117.9 ± 10.7 mmol/L vs. 111.6 ± 9 mmol/L, respectively, p < 0.001). The incidence of AKI was not statistically different between the intervention and the control group (24.5% vs. 28.9%, respectively; p = 0.45).
Conclusions
Despite a significant increase in chloride intake, a continuous infusion of HSS was not associated with AKI in moderate-to-severe TBI patients. Our study does not confirm the potentially detrimental effect of chloride load on kidney function in ICU patients.
Trial registration: The COBI trial was registered on clinicaltrial.gov (Trial registration number: NCT03143751, date of registration: 8 May 2017).
Similar content being viewed by others
Background
Traumatic brain injury (TBI) remains a worldwide health priority [1]. In 2016, 55 million patients suffered from TBI and it was responsible for 8.1 million years of life with disability [1]. At the initial phase of the insult, all medical and surgical interventions are focused on avoiding secondary cerebral insults [2]. In order to maintain an adequate cerebral perfusion pressure, intravenous fluid administration is one of the most common strategies prescribed by physicians [3, 4]. A recent consensus statement on fluid management for brain-injured patients suggested the use of crystalloids as the first-line choice for maintenance or resuscitation fluid [5]. The experts also stressed the poor level of proof to prefer buffered solution to normal saline solution (0.9% of NaCl) in brain-injured patients [5]. On the other hand, in case of refractory intracranial hypertension, hyperosmolar fluids (such as HSS) should be considered [5]. However, normal (0.9%) and hypertonic (3% or 20%) saline solutions are not devoid of side effects as saline solutions contain a “supra physiological” concentration of chloride that may induce metabolic disturbances [6]. Among them, hyperchloremia has been pointed out as an association has been reported between an increased concentration of extracellular chloride and an impairment of the vasotone of the afferent renal artery or a decrease in glomerular filtration rate [7,8,9]. These reports raised the question of the impact of hypertonic solutions on kidney function in neuro-intensive care patients.
Prospective and retrospective clinical studies have reported an association between chloride intake and AKI in critically ill patients [10,11,12,13,14]. However, these results remain controversial as a recent prospective multicenter study did not find any association between a “high dose” of chloride infusion and the renal prognosis in critically ill patients with septic shock [15, 16]. Moreover, the detrimental effect of saline solution on kidney function has not been consistently observed in the most recent randomized controlled trials [17,18,19,20,21]. Furthermore, the side effects of chloride-rich solution remain poorly studied in brain-injured patients, as it was underlined by a recent meta-analysis [22]. To our knowledge, only 3 small monocentric studies demonstrated metabolic disturbances induced by chloride-rich solution following brain injury, but they didn’t study the effect of HSS [23,24,25].
This post hoc analysis of the COBI trial aimed at determining whether high dose of chloride delivered by hypertonic saline solution infusion were associated with an increased acute kidney injury incidence within the 28 days after the infusion compared to standard care in TBI patients.
Methods
Design
This is a post hoc analysis of the COBI trial [26, 27]. Briefly, the COBI trial is a multicenter, randomized, open-label, controlled trial that has evaluated a continuous infusion of HSS (20% of NaCl) in a population of moderate-to-severe TBI [27]. Patients aged from 18 to 80 years old and admitted in one of the 9 participating ICU for moderate-to-severe TBI patients were eligible. Moderate-to-severe TBI was defined as the association of a Glasgow Coma Score (GCS) of 12 or lower and traumatic abnormal brain CT scan findings (such as: extradural hematoma, subdural hematoma, subarachnoid hemorrhage, brain contusion, brain hematoma, brain edema or skull fracture) [26]. In the interventional group, TBI patients received a 1-h bolus of 7.5 to 15 g of NaCl immediately after randomization. Then, a continuous infusion of HSS (1 g/h NaCl) was tapered to maintain a blood sodium level between 150 and 155 mmol/L for a minimum of 48 h. After 48 h, HSS (20%) was stopped in the absence of intracranial hypertension, and blood sodium level was maintained at a normal range (Na from 140 to 145 mmol/L). In case of a persistent intracranial hypertension, HSS infusion was maintained as long as necessary. In the control group, the latest Brain Trauma Foundation guidelines were applied as the standard of care [28]. The current post hoc analysis uses patient-level data focused on metabolic disturbances and kidney function to evaluate potential side effects associated with a continuous infusion of HSS (20%).
Ethical approval
The current study was approved by the Ethics Committee of Ile de France VIII in May 2017 and conducted in accordance with the principles of the Declaration of Helsinki. Written informed consent was provided to all eligible patients at inclusion or after they recovered the ability to consent. In the other cases, the next of kin provided informed consent. Consent to participate included a statement that the current study was carried out in accordance with the principles of the Declaration of Helsinki.
Data collection
We collected some physiological measurements to describe metabolic status. From the inclusion to day 4, the following parameters were collected daily: chloride, potassium, pH, bicarbonate, lactate, urea and creatinine. Blood sodium levels were collected from inclusion to Day 10. For these metabolic parameters, the worst value each day was collected. The highest weekly creatinine levels were collected from inclusion to Day 28. We calculated Glomerular Filtration Rate (GFR) according to the Modification of Diet in Renal Disease (MDRD)-175 equation. Estimated Glomerular Filtration Rate (eGFR) was calculated daily from inclusion to Day 4, and the worst eGFR each week from Week 1 to Week 4. The proportion of patients exposed to hyperchloremia (chloride level ≥ 110 mmol/L) was also reported. We defined metabolic acidosis using the following parameters: pH < 7.35 and bicarbonate level < 22 mmol/L. Urine outputs were collected from inclusion to Day 4. We also collected the amount of chloride received daily (g/day) during the first 4 days.
Primary outcome
The primary outcome was the proportion of patients developing an AKI from inclusion to Day 28. Kidney function was evaluated according to the KDIGO criteria such as: Serum creatinine (µmol/L) and Urine output (mL/kg/h) [29]. AKI was defined as a KDIGO stage 2 or 3 [29]. All data were analyzed, even those from patients who died before day 28. No censure was applied in the analysis.
Secondary outcomes
First, we planned to evaluate the association between the use of the continuous infusion of hypertonic saline solution and the occurrence of hyperchloremia and metabolic acidosis. Second, we studied the association between the continuous infusion of HSS and the need for renal replacement therapy (RRT) within 28 days after starting the infusion of HSS. Third, we studied the association of AKI from inclusion to Day 28 with chloride level and cumulative chloride dose infused. Finally, we tested the association between AKI and ICU length of stay, the need of RRT from inclusion to Day 28, and ICU mortality. As an exploratory analysis, we also compared eGFR and the highest creatinine levels between the two groups.
Statistical analysis
Continuous variables were expressed as mean (± SD) or median (IQR). Categorical variables were expressed as percentage. Missing data were identified, and multiple imputations (5 iterations) were used for variables with less than 20% missing data. Continuous variables normally distributed were compared with unpaired Student t-tests. A Wilcoxon test was used for other continuous variables. Categorical variables were compared with the Chi-Square test. In univariate analysis, we identified the unbalanced variables between the two groups (control vs. interventional arm) and any variables associated with AKI from inclusion to Day 28. For repeated measures comparison, we performed a mixed model with a “subject” random effect variable and a “time” fixed effect variable. Results of the mixed model are shown as coefficients and estimated p value according to Satterthwaite approximation method. Statistical significance was set at p < 0.05. All statistical analysis was performed using R statistical software (version 3.6).
Results
Patients
A total of 370 patients underwent randomization in the COBI study. Forty-eight (13%) patients were excluded from this post hoc analysis for missing data for the primary outcome. Compared to excluded patients (n = 48), patients included in this post hoc analysis were more frequently exposed to hypotension, hypoxemia or a drop of hemoglobin level < 9 g/dL before inclusion (Additional file 2: Table S2). Included patients had also more frequently a past medical history of chronic kidney disease. These results are summarized in Additional file 2: Table S2. Of the 322 remaining patients, 159 (49.4%) patients were randomized in the control group and 163 (50.6%) patients in the interventional group. The main patients’ characteristics at baseline are described in Table 1. At baseline, the chloride level was 106.5 in the control group versus 107.3 mmol/L in the intervention group (p = 0.18). The proportions of patients with chronic kidney disease (2.5% vs. 2.5%, respectively; p = 1.00) and baseline eGFR (109.6 vs. 111.5 mL/min/1.73 m2; p = 0.65) were also balanced between the two groups.
Chloride intake and metabolic parameters
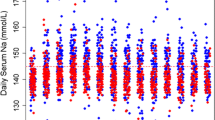
Patients randomized in the interventional arm received a continuous infusion of hypertonic saline solution for a mean of 2.6 (± 1.3) days. The cumulative chloride load received from ICU admission to Day 4 was 97.3 ± 38.1 g in the intervention group and 61.3 ± 31.6 g in the control group (p < 0.001). The time course of the recorded biological parameters is summarized in Table 2. From Day 1 to Day 4, the daily chloride levels were significantly higher for patients randomized in the interventional group (p < 0.001). This univariate analysis was confirmed by a mixed model which found a higher slope in chloride trend in the intervention arm from Day 1 to Day 4 with a regression coefficient of 5.8 (p < 0.001). From day 1 to day 4, the base deficit, arterial pH, and bicarbonate levels were not statistically different between the two groups. Repeated measures were analyzed using a mixed model. We did not find any between-group differences in terms of base deficit, arterial pH and bicarbonate level. These results are summarized in Table 2. The time course of chloride levels and arterial pH in the two study groups are shown in Fig. 1 with the results of the mixed model. The occurrence of metabolic acidosis from Day 1 to Day 4 was not statistically different between the two groups (Fig. 1).
Trend in main metabolic parameters measured from inclusion to Day 4 in the two groups. A Higher chloride level (mmol/L) from inclusion to Day 4. B Proportion of patients exposed to hyperchloremia (Cl > 109 mmol/L) at each time from inclusion to Day 4. C Lower pH level from inclusion to Day 4. D Proportion of patients exposed to metabolic acidosis (pH < 7.35 and bicarbonate level < 22 mmol/L) at each time from inclusion to Day 4. In each boxplot, dots represented outliers. *p < 0.05 for univariate analysis
Primary outcome
From inclusion to Day 28, AKI was recorded in 46 (28.9%) patients in the control group and 40 (24.5%) in the intervention group (p = 0.45, Table 3). The great majority of AKI event (96.5%) was observed before Day 7 without differences between the two groups: 46 (28.9%) in control group vs. 37 (22.7%) in intervention group (p = 0.25). Only 3 patients suffered from AKI after day 7, these patients were in the intervention arm.
Renal outcomes
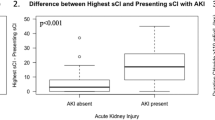
During the ICU stay, 3 (1.9%) patients in the control group and 3 (1.8%) in the intervention group received RRT (p = 1). The main reason for beginning RRT was: severe metabolic acidosis (3 patients), severe hyperkalemia (1 patient), elevated blood urea nitrogen (1 patient), and pulmonary edema (1 patient). The time course of the creatinine levels and eGFR during the first four days were not statistically different between the two groups from inclusion to Day 4. This was also the case for the highest weekly creatinine levels and lowest eGFR from inclusion to Day 28 (Fig. 2). The mixed model analysis confirmed the univariate analysis. In this random effect model, trends of creatinine level and eGFR were also comparable (p value > 0.05).
Trend in kidney parameters measured in the two groups. A Creatinine level (µmol/L) measured from inclusion to Day 4. B Higher creatinine level (µmol/L) measured each week from Week 1 to Week 4. C Daily eGFR (mL/min/1.73 m2) from inclusion to Day 4. D Worst eGFR (mL/min/1.73 m2) measured each week from Week 1 to Week 4. In each boxplot, dots represented outliers. eGFR estimated Glomerular Filtration Rate
Association of AKI with TBI patients’ outcomes
As an exploratory analysis, we studied the association between AKI onset, defined as a KDIGO stage 2 and 3, and several ICU outcomes. The length of ICU stay was 19.8 ± 17.4 days in TBI patients without AKI and 28.2 ± 25.4 days in patients with AKI (p = 0.001). ICU mortality was 19.5% for patients without AKI and 14% for patients with AKI (p = 0.33). There was no association between cumulative chloride infusion on Day 4 and the occurrence of AKI: 79.2 g versus 80.4 g (p = 0.81). There was no association between hyperchloremia exposure from inclusion to Day 4 and the onset of AKI within 28 days after randomization. These results are summarized in Additional file 1: Table S1.
Discussion
This post hoc analysis of the COBI trial evaluated the impact of a continuous infusion of HSS (20%) on renal function after moderate-to-severe TBI. No association between the continuous infusion of HSS (20%) and AKI (KDIGO stage 2 or 3) from inclusion to Day 28 was find. Creatinine levels and eGFR were also similar between the two groups. Chloremia was significantly higher in the HSS group. No difference in acid/base metabolic status was found between the two groups.
Fluid therapy solutions, administered in ICU, contain different concentrations of electrolytes [3]. Among these electrolytes, chloride has raised concern about its iniquity [3]. Actually, an acute administration of a large amount of chloride-rich solution may lead to hyperchloremic metabolic acidosis associated with pathophysiological consequences such as: coagulopathy, hypotension, or splanchnic disorders [30, 31]. Furthermore, chronic hyperchloremia is also theoretically associated with an excessive vasoconstriction of afferent renal arteries leading to a decrease in glomerular filtration rate [7,8,9].
In the past decade, three large RCTs compared buffered crystalloid to normal (0.9%) saline solution in various settings [17, 19,20,21]. These RCT’s did show a statistically significant difference between the groups regarding chloride intake or changes in chloremia and arterial acid–base balance [17, 19,20,21]. However, the clinical significance of these differences is questionable. Actually, in the SMART trial the difference of chloride intake between the two groups was of 0.5 g, and differences in terms of blood chloride levels were also small and probably not clinically relevant: 109 mmol/L (saline solution) versus 108 mmol/L (balanced crystalloid) [20]. These limitations are also found in the BaSICS and the PLUS trials [17, 19, 21]. Therefore, it is difficult to conclude about the kidney toxicity of high chloride intake in ICU patients with these clinical trials [32].
On the other hand, patients enrolled in the COBI Trial received a substantial and prolonged amount of chloride in the interventional arm. To our knowledge, this is the first time that the effect of such an important chloride intake has been reported. Our results are not in line with a potential toxicity of chloride intake on kidney function. To date, there was only one large RCT comparing HSS (3%) to normal saline solution (0.9%) and the proportion of patients who required RRT [33]. In this study, the needed of RRT between HSS and normal saline solution was not statistically different (38% vs. 33%, p = 0.32) [33].
The main strength of our study is the design, as it is a randomized controlled trial. Second, this is the first study reporting the effect of the infusion of a highly concentrated solution in chloride. Third, we report the amount of chloride infused and the difference in blood chloride levels which is rarely done in other studies.
Our study also has some limitations. First, this is a post hoc analysis, and this ancillary study was not planned in the original statistical analysis plan [27]. Second, patients in the control group also received a significant amount of chloride (61.3 ± 31.6 g in 4 days) and were also exposed to hyperchloremia from Day 1 to Day 4. This may have limited the statistical power of this study to demonstrate a difference between the two study groups. However, the amount of chloride infused in the control group is comparable to the estimated amount reported in the recent CENTER-TBI cohort [34]. Moreover, in our study the chloride intake is clinically significantly higher in the intervention arm. Third, patients included in the COBI trial were younger (mean age: 44 years old) and had a lower risk of AKI than patients included in previous studies [18, 20]. Moreover, the requirement of RRT (1.9%) was slightly lower compare to other studies [17, 20]. Thus, our results cannot be generalized to high risk patients. Fourth, AKI patients had more frequently a baseline chronic kidney disease but the proportion of patients who had a chronic kidney disease was well balanced between control and intervention group. Therefore, we believe that the analysis of the main results cannot be confounded by chronic kidney disease.
Conclusion
After moderate-to-severe TBI, the continuous infusion of HSS (20%) was not associated with an increased the risk of developing AKI from inclusion to Day 28. Our findings questioned the potentially detrimental effects of chloride-rich solution on kidney function. Further studies are warranted to better evaluate impact of fluid therapy in ICU patients, especially after TBI.
Availability of data and materials
The dataset supporting the conclusions of this article is fully available. To have an access on it, please contact the corresponding author (O.H.)
Abbreviations
- AKI:
-
Acute Kidney Injury
- eGFR:
-
Estimated Glomerular Filtration Rate
- GCS:
-
Glasgow Coma Scale
- GFR:
-
Glomerular Filtration Rate
- ICP:
-
Intracranial Pressure
- ICU:
-
Intensive Care Unit
- IQR:
-
Inter Quartile Range
- KDIGO:
-
Kidney Disease Improving Global Outcome
- MAP:
-
Mean Arterial Pressure
- MDRD:
-
Modification of Diet in Renal Disease
- RCT:
-
Randomized Controlled Trial
- RRT:
-
Renal Replacement Therapy
- SD:
-
Standard Deviation
- TBI:
-
Trauma Brain Injury
References
James SL, Theadom A, Ellenbogen RG, Bannick MS, Montjoy-Venning W, Lucchesi LR, et al. Global, regional, and national burden of traumatic brain injury and spinal cord injury, 1990–2016: a systematic analysis for the Global Burden of Disease Study 2016. Lancet Neurol. 2019;18:56–87.
Maas AIR, Menon DK, Adelson PD, Andelic N, Bell MJ, Belli A, et al. Traumatic brain injury: integrated approaches to improve prevention, clinical care, and research. Lancet Neurol. 2017;16:987–1048.
Finfer S, Myburgh J, Bellomo R. Intravenous fluid therapy in critically ill adults. Nat Rev Nephrol. 2018;14:541–57.
Cecconi M, Hofer C, Teboul J-L, Pettila V, Wilkman E, Molnar Z, et al. Fluid challenges in intensive care: the FENICE study: a global inception cohort study. Intensive Care Med. 2015;41:1529–37.
Oddo M, Poole D, Helbok R, Meyfroidt G, Stocchetti N, Bouzat P, et al. Fluid therapy in neurointensive care patients: ESICM consensus and clinical practice recommendations. Intensive Care Med. 2018;44:449–63.
Kaplan LJ, Frangos S. Clinical review: acid–base abnormalities in the intensive care unit—part II. Crit Care. 2005;9:198–203.
Imig JD, Passmore JC, Anderson GL, Jimenez AE. Chloride alters renal blood flow autoregulation in deoxycorticosterone-treated rats. J Lab Clin Med. 1993;121:608–13.
Wilcox CS. Regulation of renal blood flow by chloride. In: Laragh JH, Bühler FR, Seldin DW, editors. Frontiers in Hypertension Research. New York: Springer; 1981. p. 135–8.
Hansen PB, Jensen BL, Skøtt O. Chloride regulates afferent arteriolar contraction in response to depolarization. Hypertension. 1998;32:1066–70.
Shaw AD, Raghunathan K, Peyerl FW, Munson SH, Paluszkiewicz SM, Schermer CR. Association between intravenous chloride load during resuscitation and in-hospital mortality among patients with SIRS. Intensive Care Med. 2014;40:1897–905.
Chowdhury AH, Cox EF, Francis ST, Lobo DN. A randomized, controlled, double-blind crossover study on the effects of 2-L infusions of 0.9% saline and plasma-lyte® 148 on renal blood flow velocity and renal cortical tissue perfusion in healthy volunteers. Ann Surg. 2012;256:18–24.
Yunos NM, Bellomo R, Hegarty C, Story D, Ho L, Bailey M. Association between a chloride-liberal vs chloride-restrictive intravenous fluid administration strategy and kidney injury in critically ill adults. JAMA. 2012;308:1566–72.
Yunos NM, Bellomo R, Glassford N, Sutcliffe H, Lam Q, Bailey M. Chloride-liberal vs. chloride-restrictive intravenous fluid administration and acute kidney injury: an extended analysis. Intensive Care Med. 2015;41:257–64.
Suetrong B, Pisitsak C, Boyd JH, Russell JA, Walley KR. Hyperchloremia and moderate increase in serum chloride are associated with acute kidney injury in severe sepsis and septic shock patients. Crit Care. 2016;20:315.
Commereuc M, Nevoret C, Radermacher P, Katsahian S, Asfar P, Schortgen F, et al. Hyperchloremia is not associated with AKI or death in septic shock patients: results of a post hoc analysis of the “HYPER2S” trial. Ann Intensive Care. 2019;9:95.
Chapalain X, Huet O, Balzer T, Delbove A, Martino F, Jacquier S, et al. Does chloride intake at the early phase of septic shock resuscitation impact on renal outcome? Shock. 2021.
Young P, Bailey M, Beasley R, Henderson S, Mackle D, McArthur C, et al. Effect of a buffered crystalloid solution vs saline on acute kidney injury among patients in the intensive care unit: the SPLIT randomized clinical trial. JAMA. 2015;314:1701.
Self WH, Semler MW, Wanderer JP, Wang L, Byrne DW, Collins SP, et al. Balanced crystalloids versus saline in noncritically ill adults. N Engl J Med. 2018;378:819–28.
Zampieri FG, Machado FR, Biondi RS, Freitas FGR, Veiga VC, Figueiredo RC, et al. Effect of intravenous fluid treatment with a balanced solution vs 0.9% saline solution on mortality in critically ill patients: the BaSICS randomized clinical trial. JAMA. 2021 [cited 2021 Aug 26]; Available from: https://jamanetwork.com/journals/jama/fullarticle/2783039
Semler MW, Self WH, Wanderer JP, Ehrenfeld JM, Wang L, Byrne DW, et al. Balanced crystalloids versus saline in critically ill adults. N Engl J Med. 2018;378:829–39.
Finfer S, Micallef S, Hammond N, Navarra L, Bellomo R, Billot L, et al. Balanced multielectrolyte solution versus saline in critically ill adults. N Engl J Med. 2022;386:815–26.
Martín AMA, Mendoza JAB, Muriel A, Sáez I, Chico-Fernández M, Estrada-Lorenzo JM, et al. Buffered solutions versus 0.9% saline for resuscitation in critically ill adults and children. Cochrane Database Syst Rev. 2019. https://doi.org/10.1002/14651858.CD012247.pub2.
Roquilly A, Loutrel O, Cinotti R, Rosenczweig E, Flet L, Mahe PJ, et al. Balanced versus chloride-rich solutions for fluid resuscitation in brain-injured patients: a randomised double-blind pilot study. Crit Care. 2013;17:R77.
Lehmann L, Bendel S, Uehlinger DE, Takala J, Schafer M, Reinert M, et al. Randomized, double-blind trial of the effect of fluid composition on electrolyte, acid-base, and fluid homeostasis in patients early after subarachnoid hemorrhage. Neurocrit Care. 2013;18:5–12.
Hassan MH, Hassan WMNW, Zaini RHM, Shukeri WFWM, Abidin HZ, Eu CS. Balanced fluid versus saline-based fluid in post-operative severe traumatic brain injury patients: acid-base and electrolytes assessment. Malays J Med Sci. 2017;24:83–93.
Roquilly A, Moyer JD, Huet O, Lasocki S, Cohen B, Dahyot-Fizelier C, et al. Effect of continuous infusion of hypertonic saline vs standard care on 6-month neurological outcomes in patients with traumatic brain injury: the COBI randomized clinical trial. JAMA. 2021;325:2056–66.
Roquilly A, Lasocki S, Moyer JD, Huet O, Perrigault PF, Dahyot-fizelier C, et al. COBI (COntinuous hyperosmolar therapy for traumatic Brain-Injured patients) trial protocol: a multicentre randomised open-label trial with blinded adjudication of primary outcome. BMJ Open. 2017;7:e018035.
Carney N, Totten AM, O’Reilly C, Ullman JS, Hawryluk GWJ, Bell MJ, et al. Guidelines for the management of severe traumatic brain injury, fourth edition. Neurosurgery. 2017;80:6–15.
KDIGO. Clinical practice guideline update for the diagnosis, evaluation, prevention, and treatment of chronic kidney disease-mineral and bone disorder (CKD-MBD). Kidney Int Suppl. 2017;2017(7):1–59.
Kellum JA, Song M, Almasri E. Hyperchloremic acidosis increases circulating inflammatory molecules in experimental sepsis. Chest. 2006;130:962–7.
Tournadre JP, Allaouchiche B, Malbert CH, Chassard D. Metabolic acidosis and respiratory acidosis impair gastro-pyloric motility in anesthetized pigs. Anesth Analg. 2000;90:74–9.
Bellomo R, Kellum JA, Ronco C, Wald R, Martensson J, Maiden M, et al. Acute kidney injury in sepsis. Intensive Care Med. 2017;43:816–28.
Asfar P, Schortgen F, Boisramé-Helms J, Charpentier J, Guérot E, Megarbane B, et al. Hyperoxia and hypertonic saline in patients with septic shock (HYPERS2S): a two-by-two factorial, multicentre, randomised, clinical trial. Lancet Respir Med. 2017;5:180–90.
Wiegers EJA, Lingsma HF, Huijben JA, Cooper DJ, Citerio G, Frisvold S, et al. Fluid balance and outcome in critically ill patients with traumatic brain injury (CENTER-TBI and OzENTER-TBI): a prospective, multicentre, comparative effectiveness study. Lancet Neurol. 2021;20:627–38.
Acknowledgements
No acknowledgement.
Funding
This study is an investigator-initiated trial and was supported by a Grant from the French Ministry of Health—Programme Hospitalier de Recherche Clinique Inter-regional 2016 (PHRCI 2016, RC16_0474).
Author information
Authors and Affiliations
Consortia
Contributions
OH and XC were involved equally in the conception of the study, hypothesis generation, writing and revision of the article before submission. OH and XC contributed equally in data analysis. OH, XC, VV, J-DM, SL, BC, CD-F, KC, PS, YH, KA and AR contributed equally in data collection. OH and XC were involved in the writing of the manuscript before submission. OH, XC, VV and AR contributed equally in revision of manuscript before submission. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
The study protocol was approved by the Ethics Committee of Ile de France VIII in May 2017. Informed consent was given by the patients or their caregivers.
Consent for publication
A written inform consent for publication was obtained.
Competing interest
The authors declare that they have no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Additional file 1: Table S1
. Comparison between patients according to existence of AKI (KDIGO stage 2–3) from inclusion to Day 28.
Additional file 2: Table S2
. Comparison of baseline characteristics between included and non-included patients.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Huet, O., Chapalain, X., Vermeersch, V. et al. Impact of continuous hypertonic (NaCl 20%) saline solution on renal outcomes after traumatic brain injury (TBI): a post hoc analysis of the COBI trial. Crit Care 27, 42 (2023). https://doi.org/10.1186/s13054-023-04311-1
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s13054-023-04311-1