Abstract
Background
Understanding the pathophysiology of fluid distribution in acute high-risk abdominal (AHA) surgery is essential in optimizing fluid management. There is currently no data on the time course and haemodynamic implications of fluid distribution in the perioperative period and the differences between the surgical pathologies.
Methods
Seventy-three patients undergoing surgery for intestinal obstruction, perforated viscus, and anastomotic leakage within a well-defined perioperative regime, including intraoperative goal-directed therapy, were included in this prospective, observational study. From 0 to 120 h, we measured body fluid volumes and hydration status by bioimpedance spectroscopy (BIA), fluid balance (input vs. output), preload dependency defined as a > 10% increase in stroke volume after preoperative fluid challenge, and post-operatively evaluated by passive leg raise.
Results
We observed a progressive increase in fluid balance and extracellular volume throughout the study, irrespective of surgical diagnosis. BIA measured variables indicated post-operative overhydration in 36% of the patients, increasing to 50% on the 5th post-operative day, coinciding with a progressive increase of preload dependency, from 12% immediately post-operatively to 58% on the 5th post-operative day and irrespective of surgical diagnosis. Patients with overhydration were less haemodynamically stable than those with normo- or dehydration.
Conclusion
Despite increased fluid balance and extracellular volumes, preload dependency increased progressively during the post-operative period. Our observations indicate a post-operative physiological incoherence between changes in the extracellular volume compartment and inadequate physiological preload control in patients undergoing AHA surgery. Considering the increasing overhydration during the observational period, our findings show that an indiscriminate correction of preload dependency with intravenous fluid bolus could lead to overhydration.
Trial registration clinicaltrials.gov. (NCT03997721), Registered 23 May 2019, first participant enrolled 01 June 2019.
Similar content being viewed by others
Introduction
Inadequate fluid management after initial haemodynamic resuscitation can have detrimental consequences [1]. Several studies have demonstrated a positive correlation between overhydration and adverse outcomes in critically ill patients [1,2,3]. On the other hand, intravascular volume depletion leads to an increased risk of acute kidney injury [4]. However, there are currently no studies focusing on post-operative fluid treatment beyond the immediate post-operative period [5, 6], and the optimal therapeutic target parameters for volume control and fluid therapy after the acute stage of post-operative critical illness remain unclear.
There is some evidence [7] that the use of cardiac output monitoring or goal-directed therapy approach to guide intravenous fluid administration as part of a haemodynamic therapy algorithm modifies inflammatory pathways [8], improves tissue perfusion and oxygenation [9], and reduces post-operative complication rates and hospital stay [10] when applied to elective surgery. However, there is still an ongoing debate on whether this treatment applies to all types of surgical populations [11].
The term acute high-risk abdominal (AHA) surgery [12, 13] encompasses a surgical exploration of the acute abdomen for several underlying pathologies, with intestinal obstruction, perforation, and peritonitis being the most frequent. These patients often suffer from hypovolaemia, dehydration, and sepsis, which may result in extravascular fluid accumulation and post-operative organ dysfunctions, complicating fluid management and influencing patient outcomes [10, 14, 15]. Despite the suggestion of benefit in elective surgery, cardiac output-guided resuscitation may not be generalizable to patients undergoing AHA surgery, where similarities with critically ill patients are many and in whom the evidence for fluid resuscitation based on cardiac output is uncertain [4, 10, 16, 17].
As such, the assessment of hydration status in AHA surgery and consequent fluid treatment are still complex and require an in-depth knowledge of body fluid homeostasis to establish a strategy that optimizes tissue perfusion and identifies the transition from necessary fluid resuscitation to harmful fluid volume accumulation [18, 19]. Additionally, it is essential to consider the diversity in the pathophysiology of patients undergoing AHA surgery, as an association between overhydration and the negative outcome may be dependent on the surgical diagnosis [20]
The present study aimed to assess the perioperative fluid changes and haemodynamics in intestinal obstruction, perforated viscus, and anastomotic leakage following elective surgery within a goal-directed therapy approach.
Methods
This was a single-centre, prospective observational cohort study from 01–06-2019 to 25–02-2021 at the department of Anaesthesiology and Intensive Care and the department of Gastrointestinal Surgery at Hvidovre University Hospital. The ethics committee approved the study (H-19010653), The Danish Data Protection Agency (VD-2019-121) and registered at clinicaltrials.gov. (NCT03997721). We followed Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) guidelines.
Patient population
After verbal and written consent, we included all adults (18 y/o or over) undergoing AHA surgery for primary intestinal obstruction (small and large intestine), perforated viscus (defined as either perforated ulcer, small or large intestine), and anastomotic leakage following elective surgery.
Intestinal ischemia, abdominal bleeding, reoperations not including anastomotic leakage following elective surgery, and subacute surgeries (scheduled within 48H after initial diagnosis) were excluded. Elective surgeries converted intraoperatively to acute were also excluded. Patients with a negative find (no acute abdominal pathology discovered during surgery) missing data on fluid administration, vasopressor and inotropes administration, plasma electrolyte levels, and patients transferred to another hospital immediately following surgery were also excluded (Fig. 1).
Outcome
The primary outcome was to describe the hydration status and fluid distribution (including the association with registered volume administration) measured by bioimpedance spectroscopy analysis (BIA) during the early perioperative period in patients with intestinal obstruction vs. perforated viscus vs. anastomotic leakage following elective surgery. Secondarily, we wanted to explore the association between perioperative haemodynamics, and hydration status measured by BIA.
Perioperative management
At this surgical centre, a well-established multimodal standardized protocol is applied to patients undergoing AHA surgery [12], following recent National Emergency Laparotomy Audit guidelines [13], including haemodynamic monitoring with invasive arterial pressure intraoperatively as well as during Post-Anaesthesia Care Unit (PACU) or Intensive Care (ICU) stay, preoperative stroke volume (SV)-guided fluid and vasopressor management (LiDCOrapidTM; LiDCO, London, UK), neuraxial analgesia and anaesthesia.
Study protocol
All study-related measurements were performed by the primary study investigator, and attending physicians were unaware of the results. After initial patient triage and diagnostic workup leading to a decision to operate, the study protocol was initiated. Figure 2 summarizes the steps:
Timeline of data collection. *As per institutional protocol for acute high-risk abdominal surgery. **Fluid challenge w/250 mL human albumin; ***Passive leg raise; BIA: bioimpedance spectroscopy analysis; CNAP: The LiDCO continuous non-invasive arterial pressure haemodynamic monitoring; LiDCO: Lithium Dilution Cardiac Output haemodynamic monitoring; OR: operating room; PACU: Post-Anaesthesia Care Unit
Bioimpedance spectroscopy analysis (BIA) hydration status and definition of overhydration
BIA assesses body composition and estimates total body water (TBW) and extracellular water (ECW) volumes based on the tissue’s capacity to conduct electrical impulses [21].
The BIA device displays absolute fluid overload/overhydration (AFO), the difference between normal, expected ECW and the measured ECW, expressed in litres, as well as relative fluid overload/overhydration (RFO), absolute fluid overload/extracellular water ratio (AFO/ECW), expressed in percentages. A negative AFO indicates underhydration, while a positive one indicates overhydration. Based on RFO values, overhydration was defined as RFO > 15% [22, 23]. BIA was assessed using the Body Composition Monitor (BCM, Fresenius Medical Care, Germany) as proposed by the manufacturer.
Preload dependencyPreoperative preload dependency was assessed by the initial fluid challenge, performed routinely during the resuscitation phase before general anaesthesia and neuraxial analgesia, with 250 mL human albumin solution as a bolus over 5 min. Preload dependency was defined as a > 10% increase in stroke volume [24].
post-operative preload dependency was assessed by the passive leg raise manoeuvre [25], and the monitoring (when the patient was admitted to the surgical ward) was done with continuous non-invasive arterial pressure (CNAP) [26] (Non-invasive LiDCOrapidTM; LiDCO, London, UK). Passive leg raise was performed with the patients placed in a semi-recumbent position. The trunk was then lowered to a supine position, while the legs were elevated up to 45 degrees. After 2 min, the patients were placed back in a semi-recumbent position.
Stroke volume-guided fluid management or passive leg raise measurements were not a part of standard post-operative routine and were regarded purely as a research parameter. No treating physician or nurse were at any time aware of the results, thereby not influencing the post-operative fluid management. It is important to underline that no fluid was administered as a direct consequence of the result of the PLR manoeuvre. Post-operative fluid administration was done at the discretion of the treating physician with no interference from the primary investigator.
Fluid balance
Daily fluid balance was defined as the difference between total input (all fluids, nutrition, blood products, medications) and total output (losses through urinary, gastrointestinal, or other drainage tubes), not including insensible losses. Cumulated fluid balance from 06.00 to 06.00 the following morning was calculated as the algebraic sum of daily fluid balance during the first five post-operative days. Fluid balance was determined from six o’clock in the morning from the previous day.
Exposures
Haemodynamic and bioimpedance variables were collected at; baseline (before surgery) and 6 h after surgery. The same procedure was applied on the 1st, 3rd, and 5th post-operative day.
The following variables were recorded upon inclusion: demographic, clinical history (comorbidities), American Society of Anaesthesiology classification, ECOG performance score [27], Carlson comorbidity score, and qSOFA from electronic patient records. Further data collection included perioperative fluid and vasopressor administration, weight, perioperative haemodynamic parameters (SV, CO, HR, MAP); bioimpedance spectroscopy variables (AFO, RFO, BIA FO, TBW, Intracellular water (ICW), ECW), plasma lactate, C-reactive protein, sodium, potassium, albumin, and pro-brain natriuretic peptide (BNP). Body temperature, urine output, Mannheim peritonitis index (MPI) [28], use of epidural analgesia, length of hospital stay, ICU admission. Thirty-day major post-operative complications were registered according to Clavien–Dindo classification [29], specifically: pulmonary (pulmonary oedema, ultrasound-guided pleural drainage, admission to the ICU due to respiratory failure), gastrointestinal (emergency reoperations for intestinal obstruction, perforated viscus, anastomotic leakage or surgical wound infection, emergency endoscopy for bleeding ulcer, ultrasound-guided drainage of intraabdominal abscess, ICU stay due to septic shock) and renal (acute kidney injury, need for renal replacement therapy) where we applied Rifle criteria for acute kidney disease [30].
Statistical analysis
Based on a recent study [20], we estimated an incidence of patients with overhydration on the 5th post-operative day at 50%. To estimate the assumed incidence and intending an equal inclusion, with the confidence interval (38–62), we needed to include at least 70 patients.
Data are presented by descriptive statistics (nonparametric distribution: medians with 25th–75th inter-quartile ranges (IQR) and range, normal distribution means with 95% CI and range). Normal distribution was assessed from Q–Q plots and histograms and Kolmogorov–Smirnoff's test. Categorical data were analysed using the chi-square test. Continuous data were analysed with Kruskal–Wallis test. The unpaired t test or Mann–Whitney test was used to compare data between the groups. Univariate analysis for association with outcome was applied.
All statistical assessments were done by a two-sided test using a p value at a 0.05 level of significance. All analyses were performed using R statistical software. (www.r-project.org).
Results
From 01-06-2019 to 25-02-2021, 275 patients were assessed for eligibility, and 202 patients did not meet the inclusion criteria, where 34 were not included due to logistic reasons (detailed description: Fig. 1). There was a pause in inclusion due to COVID-19 pandemic—from March 2020–September 2020. Seventy-three patients were included in the study; 27 underwent AHA surgery for intestinal obstruction, 26 for perforated viscus, and 20 for anastomotic leakage. Data entry was complete for the cohort with no variables exceeding 10% of missing data.
Descriptive data are shown in Table 1. Median (IQR) values for 5-day cumulative fluid balance were 3.4 L (1.1–11.4), 6.9 L (3.0–11.5), and 1.3 L (− 3.1–6.8) for intestinal obstruction, perforated viscus, and anastomotic leakage, respectively (p = 0.014) (Table 1). During the first 5 days after surgery, patients received crystalloids primarily, with cumulated 5-day administration highest in patients with anastomotic leakage 8.6 L (5.7–11.2), compared to intestinal obstruction, 6.6 L (4.5–10.1) and perforated viscus, 5.1 L (4.2–9.9), though the difference was not statistically significant.
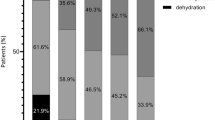
Before surgery, 16% of the population had BIA measured overhydration (RFO > 15%) (Fig. 3A), and the majority of these were patients had perforated viscus. By the 5th post-operative day, 50% of all patients were overhydrated, with no statistical difference between groups.
Preload dependency and overhydration in the perioperative period for acute high-risk abdominal surgery: A Overhydration, defined as relative fluid overload (RFO) > 15%, measured by bioimpedance spectroscopy; B preload dependency defined as stroke volume increase during fluid challenge or passive leg raise manoeuvre, *p < 0.05
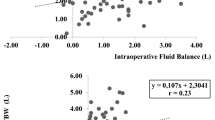
Table 2 shows intraoperative fluid administration and changes in volume status. Six hours after surgery, the number of patients with BIA measured overhydration was 26 (36%), significantly higher (p < 0.001) than before surgery. This was consistent for all 3 groups (p < 0.01, p < 0.001, p = 0.024, respectively). Absolute overhydration increased significantly by 1.77 ± 1.4 L (p < 0.0001), from 0.68 ± 2.5 L preoperatively to 2.4 L ± 2.7 post-operatively, equivalent to a significant 10% rise in ECW. Total body water and intracellular water increased without reaching statistical significance (p = 0.430 and 0.876, respectively).
Figure 4 shows absolute changes in fluid compartments and inflammatory markers throughout the perioperative period. Extracellular water increased across the cohort, most pronounced in patients with perforated viscus (ΔECW 5.91 ± 5.83L). Intracellular water decreased in patients with intestinal obstruction (ΔICW − 2.15 ± 2.71), while it remained unchanged or slightly increased throughout the perioperative course for patients with perforated viscus and anastomotic leakage (ΔICW 0.66 ± 2.62L and ΔICW 1.79 ± 4.30L, respectively). A post-operative fall in plasma albumin occurred in the whole cohort, although most profound in IO, Δalbumin -6.7 ± 5.5 g/L, p = 0.001. Inflammatory response peaked at 1st POD in both intestinal obstruction and perforated viscus IO and PV, with ΔCRP increase 80 ± 104 mmol/L and ΔCRP 119 ± 119 mmol/L (p < 0.001), respectively. The C-reactive protein remained unchanged (ΔCRP 1 ± 104 mmol/L) in patients with anastomotic leakage, beginning a downward trajectory on 3rd POD (Fig. 4).
Dynamic changes between compartments in the perioperative period and inflammatory markers for acute high-risk abdominal surgery. A relationship between registered cumulative fluid balance and BIA measured fluid shifts and B inflammatory markers during the perioperative period in acute high-risk abdominal surgery
Preoperative preload response to a fluid bolus was seen in 34% of all patients, with no statistical differences between groups (Fig. 3B). There was a significant reduction of preload dependency 6 h after surgery (12%), but a progressive post-operative preload dependency assessed by PLR increased progressively throughout the cohort, peaking on the 5th post-operative day. Thus, 75% of the patients with AL responded to passive leg raise, significantly higher than IO (59%) and PV (50%). Before surgery, 50% of the patients with overhydration were preload dependent (Additional file 1: Appendix 1), and the number increased throughout the observational period, irrespective of hydration status.
Patients with overhydration were less haemodynamically stable, with significantly higher cardiac biomarker proBNP during the entire observational period as well as incidence of septic shock, with no difference in preoperative cardiac pathology (Additional file 2: Appendix 2).
The median length of epidural analgesia in our cohort was 3 days, and 70% of the patients had their epidural discontinued by day 3. We found no difference in the incidence of preload dependence when stratified according to the presence of epidural analgesia (Additional file 3: Appendix 3).
The incidence of post-operative major complications including death was significantly higher in patients with perforated viscus (65%), than in patients with intestinal obstruction (26%) and anastomotic leakage (35%), p = 0.003 (Table 3), as was the need for ICU admission immediately after surgery with p = 0.026. Although there was no statistically significant difference in the overall incidence of pulmonary complications between groups (p = 0.208), patients with perforated viscus had a significantly higher need for ultrasound-guided pleural drainage (39%) than intestinal obstruction (4%) and anastomotic leakage (10%), p = 0.048. Also, 35% of the patients with anastomotic leakage were admitted to the ICU due to respiratory failure, compared to intestinal obstruction (4%) and perforated viscus (15%), without reaching statistical significance (p = 0.105).
Discussion
During the first five days after AHA surgery, we found progressive overhydration, measured by bioimpedance spectroscopy, irrespective of surgical diagnosis. Simultaneously, we observed a progressive increase in preload dependency, as evaluated by passive leg raise, again irrespective of surgical diagnosis.
Overhydration was most prevalent in patients with perforated viscus compared to intestinal obstruction and anastomotic leakage, with a persistent increase in extracellular volume coinciding with fluid administration. The increase in extracellular volume was present irrespective of diagnosis, although most pronounced in patients with perforated viscus. Interestingly, intracellular volume decreased for patients with intestinal obstruction but remained unchanged or slightly increased for perforated viscus and anastomotic leakage.
There is extensive literature on the perioperative fluid status and the impact of overhydration on mortality in critically ill patients [1,2,3]. However, limited data exist [17] on the perioperative fluid status beyond the immediate post-operative period in patients undergoing AHA surgery. These patients, with a high degree of acute inflammation, sepsis, and fluid disturbances, established even before surgery, share similarities with critically ill patients.
Since patients undergoing AHA surgery may have prolonged derangement of cardiovascular, pulmonary, and gastrointestinal function for days to weeks after primary surgery, judicious fluid replacement is needed to prevent multi-organ failure. In this study, we found that patients with overhydration were less haemodynamically stable, with significantly higher cardiac biomarker proBNP as well as incidence of septic shock, with no difference in preoperative cardiac pathology (Additional file 2: Appendix 2). As such, the haemodynamic instability could indicate either cardiac failure, septic shock, or both. Previously, several studies have found association between elevated proBNP and inflammation, and as such the causality is elusive [31, 32].
There is a consensus that applying GDT in managing perioperative fluid administration in elective surgery could reduce post-operative complications [7, 33]. In contrast, there are conflicting results regarding critical care patients [10, 16, 17], though they do have one thing in common: they focus on the immediate perioperative period with no current data on the potential application of GDT principles to guide fluid therapy beyond this period.
However, while the importance of correct late fluid management cannot be overstated [3, 34], the strategies and monitoring needed are unknown and more complex than elective surgery [35]. Studies in critically ill patients indicate that overhydration is not a problem confined to the early period. Resuscitation fluids comprise less than 10% of overall fluid intake during a whole ICU stay, about 25% are maintenance/replacement fluids, and nearly one-third of the fluid intake consists of “hidden” fluids associated with drug administration, etc. [36].
We found 16% of all patients undergoing AHA surgery to be significantly overhydrated before the application of a GDT protocol, but 50% of those still responded with a significant increase in SV during the preoperative fluid challenge. There seems to be a physiological incoherence between overhydration and preload dependency throughout the observational period, suggesting vasoplegia and endothelial dysfunction rather than absolute intravascular hypovolaemia as a driver of preload dependency.
The effect of the dyshydration might be diagnosis-specific, with patients with IO presenting with a higher degree of post-operative fluid shifts (Fig. 4). Sepsis-induced vasoplegia and endothelial dysfunction are the expected primary drivers of an increase in ECW [37], whereas loss of ICW is relatively unexplored.
Although patients with IO presenting with overhydration did have a higher degree of inflammation, as expected [20], they suffer from water depletion, resulting in hypertonicity in the extracellular space and leading to intracellular dehydration [38] and secondary protein loss [39]. This could explain the rather steep curve of protein loss in patients with intestinal obstruction compared to patients with perforated viscus and anastomotic leakage.
General and neuraxial (epidural) anaesthesia suppress the sympathetic tone, reducing preload and afterload, potentially inducing or amplifying preload dependency. The effect of an epidural is greatest in the initial post-operative period, where we found no or very low incidence of preload dependency (Fig. 3).
Still, post-operative vasoplegia due to continuous neuraxial blockage and opioid therapy, combined with post-operative inflammatory response, should be considered when assessing preload dependency in a surgical ward. Considering the increasing overhydration during the observational period, our findings show that if we were to apply correction of preload dependency with a fluid bolus as a primary basis for post-operative fluid therapy, as practiced in GDT protocols, we might create unnecessary overhydration.
The inflammatory response was on the downward trajectory by 5th POD (Fig. 4), but there was still progressive increase in preload dependence and overhydration. However, C-reactive protein did not reach baseline levels in patients with intestinal obstruction and perforated viscus and was still at a median value > 100 mg/L in patients with anastomotic leakage. Simultaneously, we observed continuous decline in plasma albumin, indicating that the inflammatory reaction is still present. These findings suggest a need for more extended studies to better understand the trajectory of preload dependency and overhydration and to determine when they start to decline.
During the post-operative period, preload dependency was assessed by passive leg raise, which is a way of challenging preload without administering fluid and thereby avoiding unnecessary fluid administration, provided cardiac output monitoring [40]. Several studies have confirmed the reliability of the passive leg raise with exceptional consistency, and passive leg raise is frequently applied in the ICU departments [40]. A recent meta-analysis found a pooled sensitivity of 85% and a pooled specificity of 91% for detecting fluid responsiveness. We measured stroke volume before and after passive leg raise when the patient has been moved back to the semi-recumbent position to check that it returns to its baseline (data not shown).
Our study has several strengths. This was the first study to assess the fluid status and fluid shifts in patients undergoing AHA surgery, a group equated to the international term emergency laparotomy, beyond the immediate intra- and post-operative period while considering the fundamental pathophysiological differences of diagnosis AHA surgery. This study suggests that specific fluid resuscitation strategies should depend on the diagnosis and highlight the discussion about the place of vasoconstriction therapy in the context of GDT protocols [41, 42].
Limitations of the study include a single-centre study and thus prone to inclusion bias. However, it was a prospective study, and the patient enrolment was unselected. Our results indicate a significant variation in fluid administration, compared to elective surgery, and a considerable amount of fluid is administered. However, there were no data on the indications for post-operative fluid management, which would have been important.
Bioimpedance spectroscopy fluid analysis has been validated in several studies evaluating different patient populations, both elective and emergent [23, 43, 44]. This analysis attempts to measure intra- and extracellular fluid volume and provides absolute and relative fluid overload, but it can be affected by absolute sodium content and thereby overestimate the volume. However, a recent study did find a correlation between the absolute fluid overload measured by bioimpedance spectroscopy and registered weight changes and fluid balance [45].
In conclusion, despite progressive overhydration throughout the perioperative period, post-operative preload dependency assessed by PLR increased steadily in patients undergoing AHA surgery, indicating a physiological incoherence between fluid status and preload dependence, where patients appeared to be volume deficient but still overhydrated. Considering the increasing overhydration during the observational period, our findings show that an indiscriminate correction of preload dependency with intravenous fluid bolus could lead to overhydration.
Availability of data and materials
All authors had full access to all data in the study and take responsibility for the integrity of the data. The data that support the findings of this study are available from the corresponding author, MC, upon reasonable request.
Abbreviations
- AFO:
-
Absolute fluid overload = overhydration (L)
- AHA:
-
Acute high-risk abdominal surgery
- AL:
-
Anastomotic leakage
- CNAP:
-
Continuous non-invasive arterial pressure
- CO:
-
Cardiac output
- ECOG:
-
Eastern Cooperative Oncology Group
- ECW:
-
Extracellular water
- ICU:
-
Intensive care unit
- ICW:
-
Intracellular Water
- IO:
-
Intestinal obstruction
- LiDCO:
-
Lithium dilution cardiac output measurement
- MPI:
-
Mannheim peritonitis index
- NELA:
-
National Emergency Laparotomy Audit
- PACU:
-
Post-Anaesthesia Care Unit
- PLR:
-
Passive leg raise
- PV:
-
Perforated viscus
- RFO:
-
Relative fluid overload = overhydration (% ECW)
- SV:
-
Stroke volume
- TBW:
-
Total body water
References
Boyd JH, Forbes J, Nakada T, Walley KR, Russell JA. Fluid resuscitation in septic shock: a positive fluid balance and elevated central venous pressure are associated with increased mortality. Crit Care Med. 2011;39:259–65.
Wang N, Jiang L, Zhu B, Wen Y, Xi X-M, Beijing Acute Kidney Injury Trial (BAKIT) Workgroup. Fluid balance and mortality in critically ill patients with acute kidney injury: a multicenter prospective epidemiological study. Crit Care. 2015;19:371.
Malbrain MLNG, Marik PE, Witters I, Cordemans C, Kirkpatrick AW, Roberts DJ, et al. Fluid overload, de-resuscitation, and outcomes in critically ill or injured patients: a systematic review with suggestions for clinical practice. Anaesthesiol Intensive Ther. 2014;46:361–80.
Myles PS, Bellomo R, Corcoran T, Forbes A, Peyton P, Story D, et al. Restrictive versus liberal fluid therapy for major abdominal surgery. N Engl J Med. 2018;378:2263–74. https://doi.org/10.1056/NEJMoa1801601.
Edwards MR, Forbes G, MacDonald N, Berdunov V, Mihaylova B, Dias P, et al. Optimisation of perioperative cardiovascular management to improve surgical outcome II (OPTIMISE II) trial: study protocol for a multicentre international trial of cardiac output-guided fluid therapy with low-dose inotrope infusion compared with usual car. BMJ Open. 2019;9:e023455.
Edwards M. FLO-ELA trial, Fluid Optimization in Emergency Laparotomy. 2021. p. https://floela.org/downloads/FLO-ELA%20protocol%20.
Jessen MK, Vallentin MF, Holmberg MJ, Bolther M, Hansen FB, Holst JM, et al. Goal-directed haemodynamic therapy during general anaesthesia for noncardiac surgery: a systematic review and meta-analysis. Br J Anaesth. 2022;128:416–33.
Wodack KH, Poppe AM, Lena T, Bachmann KA, Strobel CM, Bonk S, et al. Individualized early goal-directed therapy in systemic inflammation. Crit Care Med. 2014;42:e741–51.
Jhanji S, Vivian-Smith A, Lucena-Amaro S, Watson D, Hinds CJ, Pearse RM. Haemodynamic optimisation improves tissue microvascular flow and oxygenation after major surgery: a randomised controlled trial. Crit Care. 2010. https://doi.org/10.1186/cc9220.
Pearse RM, Harrison DA, MacDonald N, Gillies MA, Blunt M, Ackland G, et al. Effect of a perioperative, cardiac output-guided hemodynamic therapy algorithm on outcomes following major gastrointestinal surgery: a randomized clinical trial and systematic review. JAMA. 2014;311:2181–90.
Wrzosek A, Jakowicka-Wordliczek J, Zajaczkowska R, Serednicki WT, Jankowski M, Bala MM, et al. Perioperative restrictive versus goal-directed fluid therapy for adults undergoing major non-cardiac surgery. Cochrane Database Syst Rev. 2019. https://doi.org/10.1002/14651858.CD012767.pub2.
Tengberg LT, Bay-Nielsen M, Bisgaard T, Cihoric M, Lauritsen ML, Foss NB, et al. Multidisciplinary perioperative protocol in patients undergoing acute high-risk abdominal surgery. Br J Surg. 2017;104:463–71.
NELA Project Team. Fifth Patient Report of the National Emergency Laparotomy Audit 2019. https://www.nela.org.uk/downloads/The%20Fifth%20Patient%20Report%20of%20the%20NELA%202019%20-%20Full%20Patient%20Report.pdf.
Cannesson M, Pestel G, Ricks C, Hoeft A, Perel A. Hemodynamic monitoring and management in patients undergoing high risk surgery: a survey among North American and European anesthesiologists. Crit Care. 2011. https://doi.org/10.1186/cc10364.
Bailey M, Bellomo R, Peter A, Cooper DJ, Higgins AM, Hold- A, et al. Goal-directed resuscitation for patients with early septic shock. N Engl J Med. 2014;371:1496–506. https://doi.org/10.1056/NEJMoa1404380.
Yealy DM, Kellum JA, Huang DT, Barnato AE, Weissfeld LA, Pike F, Terndrup T, Wang HE, Hou PC, LoVecchio F, Filbin MR, Shapiro NIAD, ProCESS Investigators. A randomized trial of protocol-based care for early septic shock. N Engl J Med. 2014;370:1683–93.
Aaen AA, Voldby AW, Storm N, Kildsig J, Hansen EG, Zimmermann-Nielsen E, et al. Goal-directed fluid therapy in emergency abdominal surgery: a randomised multicentre trial. Br J Anaesth. 2021;127:521–31.
Malbrain MLNG, Van Regenmortel N, Saugel B, De Tavernier B, Van Gaal P-J, Joannes-Boyau O, et al. Principles of fluid management and stewardship in septic shock: it is time to consider the four D’s and the four phases of fluid therapy. Ann Intensive Care. 2018;8:66. https://doi.org/10.1186/s13613-018-0402-x.
Kattan E, Castro R, Miralles-Aguiar F, Hernández G, Rola P. The emerging concept of fluid tolerance: a position paper. J Crit Care. 2022;71:154070.
Cihoric M, Kehlet H, Lauritsen ML, Højlund J, Kanstrup K, Foss NB. Inflammatory response, fluid balance and outcome in emergency high-risk abdominal surgery. Acta Anaesthesiol Scand. 2021. https://doi.org/10.1111/aas.13792.
Chamney PW, Wabel P, Moissl UM, Müller MJ, Bosy-Westphal A, Korth O, et al. A whole-body model to distinguish excess fluid from the hydration of major body tissues. Am J Clin Nutr. 2007;85:80–9.
Wang Y, Gu Z. Effect of bioimpedance-defined overhydration parameters on mortality and cardiovascular events in patients undergoing dialysis: a systematic review and meta-analysis. J Int Med Res. 2021;49:030006052110310. https://doi.org/10.1177/03000605211031063.
Tabinor M, Elphick E, Dudson M, Kwok CS, Lambie M, Davies SJ. Bioimpedance-defined overhydration predicts survival in end stage kidney failure (ESKF): systematic review and subgroup meta-analysis. Sci Rep. 2018;8:4441.
Cecconi M, De Backer D, Antonelli M, Beale R, Bakker J, Hofer C, et al. Consensus on circulatory shock and hemodynamic monitoring. Task force of the European Society of Intensive Care Medicine. Intensive Care Med. 2014;40:1795–815. https://doi.org/10.1007/s00134-014-3525-z.
Monnet X, Teboul J-L. Passive leg raising: five rules, not a drop of fluid! Crit Care. 2015;19:18.
Gratz I, Deal E, Spitz F, Baruch M, Allen IE, Seaman JE, et al. Continuous Non-invasive finger cuff CareTaker® comparable to invasive intra-arterial pressure in patients undergoing major intra-abdominal surgery. BMC Anesthesiol. 2017;17:48.
Oken MM, Creech RH, Tormey DC, Horton J, Davis TE, McFadden ETCP. Toxicity and response criteria of the Eastern Cooperative Oncology Group. Am J Clin Oncol. 1982;5:649–55.
Linder MM, Wacha H, Feldmann U, Wesch G, Streifensand RA, Gundlach E. The Mannheim peritonitis index. An instrument for the intraoperative prognosis of peritonitis. Chirurg. 1987;58:84–92.
Clavien PA, Barkun J, de Oliveira ML, Vauthey JN, Dindo D, Schulick RD, et al. The Clavien-Dindo classification of surgical complications: five-year experience. Ann Surg. 2009;250:187–96.
Bellomo R, Ronco C, Kellum JA, Mehta RL, Palevsky P, Acute Dialysis Quality Initiative workgroup. Acute renal failure—definition, outcome measures, animal models, fluid therapy and information technology needs: the second international consensus conference of the acute dialysis quality initiative (ADQI) group. Crit Care. 2004;8:204–12.
Fish-Trotter H, Ferguson JF, Patel N, Arora P, Allen NB, Bachmann KN, et al. Inflammation and circulating natriuretic peptide levels. Circ Hear Fail. 2020. https://doi.org/10.1161/CIRCHEARTFAILURE.119.006570.
Jensen J, Ma L-P, Fu MLX, Svaninger D, Lundberg P-A, Hammarsten O. Inflammation increases NT-proBNP and the NT-proBNP/BNP ratio. Clin Res Cardiol. 2010;99:445–52. https://doi.org/10.1007/s00392-010-0140-z.
Michard F, Biais M, Lobo SM, Futier E. Perioperative hemodynamic management 4.0. Best Pract Res Clin Anaesthesiol. 2019. https://doi.org/10.1016/j.bpa.2019.04.002.
Rivers E, Nguyen B, Havstad S, Ressler J, Muzzin A, Knoblich B, et al. Early goal-directed therapy in the treatment of severe sepsis and septic shock. N Engl J Med. 2001;345:1368–77.
Miller TE, Myles PS. Perioperative fluid therapy for major surgery. Anesthesiology. 2019;130:825–32.
Van Regenmortel N, Verbrugghe W, Roelant E, Van den Wyngaert T, Jorens PG. Maintenance fluid therapy and fluid creep impose more significant fluid, sodium, and chloride burdens than resuscitation fluids in critically ill patients: a retrospective study in a tertiary mixed ICU population. Intensive Care Med. 2018;44:409–17. https://doi.org/10.1007/s00134-018-5147-3.
Burgdorff A-M, Bucher M, Schumann J. Vasoplegia in patients with sepsis and septic shock: pathways and mechanisms. J Int Med Res. 2018;46:1303–10. https://doi.org/10.1177/0300060517743836.
le Quesne LP, Horsey PJ. Discussion on fluid and electrolyte problems in abdominal emergencies. Proc R Soc Med. 1961;54:269–74.
Finn P, Plank L, Clark M, Connolly A, Hill G. Progressive cellular dehydration and proteolysis in critically ill patients. Lancet. 1996;347:654–6.
Monnet X, Marik P, Teboul J-L. Passive leg raising for predicting fluid responsiveness: a systematic review and meta-analysis. Intensive Care Med. 2016;42:1935–47. https://doi.org/10.1007/s00134-015-4134-1.
Foss NB, Kehlet H. Perioperative haemodynamics and vasoconstriction: time for reconsideration? Br J Anaesth. 2019;123:100–3.
Michard F, Futier E, Joosten A. Goal-directed haemodynamic therapy: what else? Comment on Br J Anaesth. Br J Anaesth. 2022;128:416–33.
Samoni S, Vigo V, Reséndiz LIB, Villa G, De Rosa S, Nalesso F, et al. Impact of hyperhydration on the mortality risk in critically ill patients admitted in intensive care units: comparison between bioelectrical impedance vector analysis and cumulative fluid balance recording. Crit Care. 2016. https://doi.org/10.1186/s13054-016-1269-6.
Rochwerg B, Lalji F, Cheung JH, Ribic CM, Meade MO, Cook DJ, et al. Using bioimpedance analysis to assess intensive care unit patients with sepsis in the post-resuscitation period: a prospective multicentre observational study. Can J Anesth Can d’anesthésie. 2020;67:437–44.
Cihoric M, Kehlet H, Højlund J, Lauritsen ML, Kanstrup KFN. Bioimpedance spectroscopy fluid analysis in acute high-risk abdominal surgery, a prospective clinician-blinded observational feasibility study. J Clin Monit Comput. 2022. https://doi.org/10.1007/s10877-022-00934-x.
Funding
This study is a part of a PhD project and was funded by Capital Region of Denmark.
Author information
Authors and Affiliations
Contributions
MC, HK, and NBF contributed to study concept and design and drafting of the manuscript. MC, HK, NBF, JH, MLL, and KK were involved in analysis and interpretation of data and critical revision of the manuscript for important intellectual content. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
This regional ethics committee approved the study (H-19010653). Written informed consent was obtained from each patient prior to inclusion. The Declaration of Helsinki and its subsequent revisions were followed. The study was also approved by the Danish Data Protection Agency (VD-2019-121) and registered at clinicaltrials.gov. (NCT03997721) on 23 May 2019, with first participant enrolled 01 June 2019.
Consent for publication
Not applicable.
Competing interests
All authors declare no competing interests related to the submitted work.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Additional file 1
. Appendix 1: Preload dependency and hydration status in acute high-risk abdominal surgery. RFO: relative fluid overload (measured by Bioimpedance spectroscopy)—the absolute fluid overload/extracellular water ratio (AFO/ECW), expressed in percentages; normo- or dehydration: RFO < 15%; overhydration: RFO > 15%; Preload dependency defined as stroke volume increase during fluid challenge or passive leg raise manoeuvre.
Additional file 2
. Appendix 2: Changes in haemodynamic variables during the observational period stratified according to hydration status. RFO: relative fluid overload (measured by Bioimpedance spectroscopy)—the absolute fluid overload / extracellular water ratio (AFO/ECW), expressed in percentages; normo- or dehydration: RFO < 15%; overhydration: RFO > 15%; POD: post-operative day; *p<0.05.
Additional file 3
. Appendix 3: Postoperative preload dependency stratified according to the days with epidural analgesia.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Cihoric, M., Kehlet, H., Højlund, J. et al. Perioperative changes in fluid distribution and haemodynamics in acute high-risk abdominal surgery. Crit Care 27, 20 (2023). https://doi.org/10.1186/s13054-023-04309-9
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s13054-023-04309-9