Abstract
Background
In-hospital acute resuscitation in trauma has evolved toward early and balanced transfusion resuscitation with red blood cells (RBC) and plasma being transfused in equal ratios. Being able to deliver this ratio in prehospital environments is a challenge. A combined component, like leukocyte-depleted red cell and plasma (RCP), could facilitate early prehospital resuscitation with RBC and plasma, while at the same time improving logistics for the team. However, there is limited evidence on the clinical benefits of RCP.
Objective
To compare prehospital transfusion of combined RCP versus RBC alone or RBC and plasma separately (RBC + P) on mortality in trauma bleeding patients.
Methods
Data were collected prospectively on patients who received prehospital transfusion (RBC + thawed plasma/Lyoplas or RCP) for traumatic hemorrhage from six prehospital services in England (2018–2020). Retrospective data on patients who transfused RBC from 2015 to 2018 were included for comparison. The association between transfusion arms and 24-h and 30-day mortality, adjusting for age, injury mechanism, age, prehospital heart rate and blood pressure, was evaluated using generalized estimating equations.
Results
Out of 970 recruited patients, 909 fulfilled the study criteria (RBC + P = 391, RCP = 295, RBC = 223). RBC + P patients were older (mean age 42 vs 35 years for RCP and RBC), and 80% had a blunt injury (RCP = 52%, RBC = 56%). RCP and RBC + P were associated with lower odds of death at 24-h, compared to RBC alone (adjusted odds ratio [aOR] 0.69 [95%CI: 0.52; 0.92] and 0.60 [95%CI: 0.32; 1.13], respectively). The lower odds of death for RBC + P and RCP vs RBC were driven by penetrating injury (aOR 0.22 [95%CI: 0.10; 0.53] and 0.39 [95%CI: 0.20; 0.76], respectively). There was no association between RCP or RBC + P with 30-day survival vs RBC.
Conclusion
Prehospital plasma transfusion for penetrating injury was associated with lower odds of death at 24-h compared to RBC alone. Large trials are needed to confirm these findings.
Similar content being viewed by others
Background
The majority of deaths from traumatic hemorrhage occur within the first three hours of injury [1]. This is often prehospital, especially in the more rural, remote or austere settings. While in-hospital acute resuscitation in trauma has evolved toward early and balanced resuscitation with red blood cells (RBC), plasma, and platelets being transfused in equal ratios [2, 3, 4], this is challenging to deliver prehospital. In prehospital environments, the logistics of delivering several blood components could be challenging as this would necessitate carrying additional blood storage boxes for the prehospital personnel and increase the complexity of resuscitating patients due to several bags needing to be administered to patients (who may not have enough intravenous access) and delay their transfer to hospital.
Several military and civilian observational studies have reported survival benefits with prehospital RBC transfusion, with the effect being greater if transfusion was started within 15 min [1, 5, 6]. However, we need to acknowledge the risk of selection bias from these study designs. In contrast, a recent randomized controlled trial (RCT) showed that RBC transfusion plus lyophilized plasma in the prehospital setting was not superior to saline resuscitation for improving tissue perfusion or reducing episode mortality; however, at 3-h, the adjusted average difference in mortality was 7% lower (95% CI: 15% lower to 1% higher, p = 0.08) in the blood components arm [7]. Two RCTs (PAMPer and COMBAT) evaluated the role of plasma transfusion in prehospital resuscitation and showed conflicting results [8, 9], although their combined analysis indicated that the survival benefit is greatest in patients who received both RBC and plasma, had blunt injuries [10], and longer prehospital transport times (> 20 min) [10, 11, 12, 13]. The evidence on the effect of prehospital plasma transfusion in addition to RBC transfusion versus RBC transfusion alone is limited.
The drive to deliver an early 1:1 ratio of RBC and plasma transfusion early has led to an increasing interest in the use of a whole blood (WB) component based on military experience [14], even though evidence on its benefits and risks is limited [15, 16, 17]. In the United Kingdom (UK), most prehospital services carry RBC and thawed plasma. Due to storage requirements, it is not possible to carry platelets in the prehospital setting. Currently, in the UK, the leukocyte depletion process introduced in the 1990s to reduce the risk of variant CJD transmission via blood transfusion removes 80% of platelets in whole blood donations, and therefore, the remaining component contains red cells and plasma in one bag (herein referred to as RCP) [18]. The prehospital use of the RCP component in traumatic hemorrhage may offer logistical and clinical benefits compared to the use of separate blood components; however, this has not been evaluated before.
The overall objective of this multicenter observational cohort study was to evaluate the effect of prehospital RCP transfusion (RCP arm) on 24-h mortality and 30-day mortality in traumatically injured bleeding patients, when compared with prehospital RBC transfusion only (RBC arm) and prehospital RBC plus plasma transfusion (RBC + P arm).
Methods
The study received ethical approval from the UK Health Research Authority (IRAS reference: 236783). Data were collected from six Helicopter Emergency Medical Services (HEMS) in England. All patients underwent their normal course of treatment as directed by their HEMS and hospital protocols and at no point was their care altered for the purpose of this study. Data for the RCP and RBC + P arms were collected prospectively between October 2018 and October 2020, while data for the RBC arm were collected retrospectively between October 2015 and October 2018.
Study setting
The participating HEMS in this study all operate within their regional major trauma systems (MTC). The HEMS teams can transfer traumatically injured bleeding patients directly from scene to the regional MTC. A total of nine MTCs participated in this study, four within the London region and five outside. HEMS that work across MTCs may transfer patients to more than one MTC; conversely, each MTC may receive trauma patients from more than one HEMS. The HEMS operate across rural and urban settings with access to both air and road transport modalities dependent on time of day, weather and operational constraints. Operational models were doctor-paramedic, or doctor-doctor-paramedic in 5 services, with one service running a two critical care paramedic model for 20% of operational hours, during which blood transfusion was authorized by an on-call consultant.
Study population
Patients were included if they had suffered traumatic injury, were attended by a HEMS team and had started receiving or received at least one type of blood component or blood products (i.e., Lyophilized plasma or Lyoplas) prehospital, according to local protocols. All age groups were included. Patients were excluded from the main analysis if they were transfused for non-traumatic indications and/or if they were admitted to hospital from another hospital rather than HEMS (or secondary transfer). All patients were followed up for 30 days or until death, whichever occurred first.
Interventions
The RBC arm carried four units of RBC (each ~ 250 mL) and data for this arm were from one service (London Air Ambulance) only. The RCP arm carried two units of RCP (each 470 mL). A maximum of up to four RCP units could be given to any patient. The RCP units were derived from whole blood donations that were leucocyte-depleted and collected into 66.5 mL CPD following standard operating procedures. RCP units are stored in the same way as the red blood cells [18]. The RBC + P arm transfused two units of RBC and two units of thawed plasma (average of 250 mL per bag) or two units of RBC and two units of Lyoplas (200mls when reconstituted). All sites carried a Golden Hour BoxTM (Pelican BioThermal, MN, USA) prehospital that maintains a steady-state temperature of 4 °C (± 2 °C) for 48–72 h.
All services recommended that patients were transfused blood if there was: a) clinical suspicion of (or confirmed) hemorrhage and b) systolic blood pressure was < 90 mmHg (at any time). In cases without documented non-invasive blood pressure, patients with central pulse only were assumed to have a systolic blood pressure of 40 mmHg, a femoral pulse of 60 mmHg, and a radial pulse of 80 mmHg. Patients in cardiac arrest were described as having no central pulse or heart rate. Pediatric patients with central pulse only were considered to fulfill the criterion for hypotension. The HEMS and in-hospitals major hemorrhage protocols from all participating sites were reviewed for consistency of practice. In hospital, transfusion protocols followed national guidelines [19]. No site used a viscoelastic guided transfusion protocol prehospital.
Data collection
Patients for all arms were identified by the research and laboratory teams at each site based on blood components issued and transfused prehospital. Once a case was identified, the research team completed a case report form (CRF) using a unique identification number, which was a sequential number for each site. The CRFs contained two parts: Part-1 which collected clinical data for up to 24 h, and Part-2 which collected clinical information from 24 h to 30 days, discharge or death, whichever was first.
Clinical data included information on demographics, incident characteristics prior to hospital arrival (scene arrival time, hospital arrival time) abbreviated injury score (AIS) and injury severity scores (ISS), resuscitative parameters and laboratory test results within the first 24 h of emergency department (ED) arrival, in-hospital transfusion requirement at 24 h, morbidity (ventilator days, hospital length of stay, adverse events), and 24-h and 30-day mortality. Cause of death was collected in a standardized way from all hospitals using death certificate, coroner’s report, or clinical notes (either prehospital or in-hospital). Patients were included even if data points were missing, provided mortality outcome and transfusion data were available.
Analysis
Counts and percentages were used to summarize the distribution of categorical variables. Mean and standard deviation, or median and interquartile range (IQR), were used for continuous data as appropriate. To account for the dependence between HEMS/hospital and outcomes (clustering), generalized estimating equations (robust standard errors and exchangeable correlation structure) were used to evaluate the association between the different blood transfusion arms and 24-h and 30-day mortality, adjusting for age, patient’s prehospital co-variates (i.e., mechanism of injury, prehospital heart rate, and blood pressure, time from injury to scene arrival, time from injury to hospital arrival). Variable selection for the final model was based on those significantly associated (p < 0.05) with outcomes in univariable analysis. Effect modification by mechanism of injury (penetrating vs blunt) was investigated. All tests were two-tailed at the 5% level of significance. Statistical analysis was performed using Stata version 16.1 (StataCorp LLC, Texas, USA).
Results
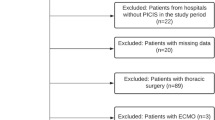
A total of 970 patients were recruited to the study: 258 patients in the RBC arm, 318 in the RCP and 394 in the RBC + P arm. Of these, 61 patients were excluded (see Fig. 1 for reasons for exclusion), leaving a total of 909 patients for analysis. The baseline characteristics for each study arm are summarized in Table 1. Patients in the RBC + P arm were older, had a higher incidence of blunt injury, and had slightly longer median times to scene and hospital. All other covariables, including the injury severity scores (ISS) and prehospital physiology markers, were not significantly different between the three arms.
Prehospital, patients received a median of two RBC units in the RBC arm (Interquartile Range [IQR] 2;4), one RBC (IQR 0;1) and one plasma (IQR 0;1) in the RBC + P arm, and 2 units of RCP in the RCP (IQR 1;2) arm. Overall, of those that survived to hospital, those in the two plasma arms had a lower incidence for sepsis (chi-square, p = 0.041) and received fewer blood components in the first 24 h and after arrival in ED when compared with the RBC arm. Most of the transfusion occurred in the first 24 h across the three arms, with very few transfusions (mainly red cells) occurring beyond 24 h. The median [IQR] of red cells transfused after 24 h was 2 (0.0; 4.0) for RBC arm, 1.00 [0.0;3.0) for RBC + P and 0 [0.0;2.0] for RCP. Survival at 24 h and 30 days for the three arms is provided in Table 2. There was no significant difference in other complications between the three arms (Table 2). The overall causes of death are provided in Table 2, with the breakdown of cause of death at 24 h and 30 days provided under Additional file 1.
Multivariate regression analysis using generalized estimating equations (adjusting for age, mechanism of injury, prehospital heart rate and blood pressure) showed that compared to RBC, the RCP arm and RBC + P arm were associated with lower odds of death at 24 h (adjusted odds ratio [OR] 0.69 [95%CI 0.52;0.92] and 0.60 [95%CI; 0.32; 1.13], respectively). Investigating effect modification by type of injury showed that lower odds of death at 24 h for RCP and RBC + P arms were driven by penetrating injury (OR 0.39 [95%CI: 0.20; 0.76] and 0.22 [95%CI 0.10;0.53], respectively) and not blunt injury (OR 1.03 [95%CI: 0.75; 1.41] and 0.94 [95%CI 0.48;1.86], respectively). Additionally adjusting for time to scene and time to hospital arrival did not materially alter results and thus was omitted from the final model. (Table 3). For 30-day mortality, the multivariate regression analysis which adjusted for the same co-variates as the 24-h mortality analysis showed no association between RCP (OR 0.84 [95%CI; 0.59; 1.21]) or RBC + P (OR 1.00 [95%CI: 0.72; 1.38]) with survival, compared with RBC alone.
Discussion
The main objectives of this study were to evaluate the effect of prehospital RCP transfusion (RCP arm) on 24-h mortality and 30-day mortality compared to prehospital RBC transfusion only (RBC arm) and prehospital RBC plus plasma transfusion (RBC + P arm). Our results showed that prehospital plasma transfusion was associated with lower odds of death at 24 h compared to RBC alone (after adjusting for type of injury, age and prehospital observational markers) and that addition of plasma to RBC resuscitation (versus RBC only) brought greater benefit for penetrating injury than blunt injury. At 30 days, there was no difference in survival between the three arms, indicating that the effect of plasma transfusion is greatest in the short term, but there is potential for residual confounding in longer-term outcomes.
The concept of providing damage control resuscitation to trauma bleeding patients shifted to the prehospital environment following publication of the PROPR trial [2] and several observational studies showing survival benefits in patients who received transfusion at the scene of injury [1, 6]. This body of evidence changed prehospital resuscitation practice in the UK, and by the time we started this study, most services had moved to providing RBC and plasma prehospital, meaning that we could not include a prospective RBC arm in our study (a limitation). During the same period, several RCTs were published in this field, aiming to establish the role of RBC and plasma transfusion in prehospital resuscitation. Currently, the role of plasma transfusion in addition to RBC in prehospital hemostatic resuscitation (versus RBC) is poorly understood and remains an ongoing discussion [12].
Two RCTs (PAMPer and COMBAT) that evaluated the effect of prehospital plasma transfusion on 24-h and 30-day mortality provided conflicting results, with PAMPer showing overall survival benefits with prehospital plasma transfusion [9], while COMBAT showed no difference in survival [8]. In this study, we saw that addition of plasma to RBC transfusion prehospital was associated with lower odds of death at 24 h (compared to RBC alone), which is similar to the PAMPer result [9]; however, unlike PAMPer, we saw no association with addition of prehospital plasma to RBC transfusion on 30-day mortality. Previous studies [6] that have evaluated the impact of RBC only transfusion versus saline resuscitation in prehospital setting and the REPHILL trial [7] have also shown a similar pattern of survival benefits with transfusion improving early survival, but having no impact on overall survival. A systematic review on the use of prehospital blood product resuscitation in trauma also showed no overall survival benefit, but there was some evidence for improved survival at 24 h [20]. These results may not be surprising, as from the recent analysis of RCTs, we know that approximately 75% of deaths from bleeding occur within 6 h of injury or hospital admission [21], and beyond 24 h traumatic brain injury or multiorgan failure with sepsis is the main causes of deaths [22].
The post hoc analysis of PAMPer and COMBAT trials showed that the benefit from plasma is primarily seen in blunt injury patients who had a transport time of > 20 min [13]. The results of this study appear contrasting, although caution is needed when comparing results, as the fundamental differences in study designs, population and methods make comparisons difficult. Firstly the ‘standard of care’ arm in the COMBAT trial was 0.9% saline [8], while in PAMPer trial, it was either RBC transfusion (13 of the 27 air medical services), or crystalloid-based resuscitation only [9]. The PAMPer trial had higher rates of blunt injury (73% control vs 81% intervention), which is similar to the RBC + P (80%) arm, whereas penetrating injury was predominant in the COMBAT trial (54% intervention vs. 47% control), comparable to the RBC (45%) and RCP (48%) arms of this study. The typical transport time in the COMBAT study was 16–19 min, while in the PAMPer trial, it was 39–52 min. In this study, the transport time was longer for all three arms with the median time ranging between 80 and 97 min. The injury severity scores (ISS) of patients in PAMPer (median 22) and COMBAT (median 27) trials are lower than in this study (median 30 to 33), and indeed, this reflected the overall differences in baseline mortality at both 24-h and 30-day between studies. These sources of variation could explain the differences in results between studies.
One major area of future prehospital research should be to better identify the group of patients that are most likely to benefit from prehospital plasma transfusion. Understanding the coagulopathy of different types of injuries and the mechanism whereby plasma transfusion corrects these coagulopathies is key to addressing this important topic [12]. In addition to providing clotting factor replacement, plasma is also an ideal volume expander in the intravascular space and several in vitro studies have now shown that plasma also has a homeostatic effect on endothelial function and innate immune system activation, leading to an improved inflammatory response to injury compared with crystalloid resuscitation [23,24,25,26]. Further analysis of the COMBAT study showed that prehospital plasma resuscitation reduces the incidence of hyperfibrinolysis [27]. We were not able to measure this in our study, but it is a possible mechanism by which early plasma transfusion corrects the coagulopathy of penetrating injury patients and better diagnostic tests are required to prove this.
While these data suggest that plasma has benefits on coagulation and endothelium, there remain logistical challenges with providing prehospital blood transfusion. The main advantages of administering a combined component like RCP or whole blood, versus separate components, are the additional logistical benefits on scene, such as shorter transport time to hospital and the reduced number of processes, personnel and resources required to administer blood prehospital. Based on the results of this study, these advantages were not translated into clinical benefits for the RCP arm, and this is likely due to the same transfusion content of prospective arms in this study (i.e., RCP and RBC + P) and any potential benefits (if these exist) from shortening the prehospital transport time might be too small to detect given the current sample. Either way, our results show that the clinical benefit from prehospital plasma transfusion appears to be consistent, irrespective of the mode of transfusion (separately or combined), which may give reassurance to services deciding on the best plasma component/product to use prehospital [28]. If the results of upcoming whole blood trials show no benefits with a whole blood component in prehospital environments, then the RCP component could become the frontrunner for prehospital services in the UK to treat traumatically injured bleeding patients, considering its logistical benefits and equivalent effect on outcomes with current standard practice. In this scenario, extending its shelf life to beyond 14 days would be a priority for reducing hospital blood wastage [18, 29].
Strengths and limitations
A key strength of the study is that the data for plasma arms were prospectively collected by several air ambulance services in England, with information being directly retrieved from patients’ case notes and not from administrative databases. Further, the design of the study did not rely on patient consent for data collection, a potential source of selection bias. Some limitations, however, should be recognized. Firstly, this was an observational study, and its conclusions are not as definitive as those from a randomized controlled trial for evaluating a strategy. Secondly, the sample size for the RBC arm was smaller than the other two arms, due to the data being collected from a single site, and furthermore, the data for the RBC arm were collected at a different period compared to plasma arms and it could be argued that overall treatment of bleeding patients would have changed over time, influencing the survival independent of transfusion. However, the similarities in patients’ characteristics and their outcomes between RBC and RCP arms mitigate these concerns. Further, the implementation of RCP transfusion in London happened the day after cessation of RBC transfusion, and during the study period, the London region did not implement any other major intervention in prehospital environments for treatment of bleeding that could have materially impacted survival. Thirdly, there were some demographic differences between arms, with patients in the RBC and RCP arms being younger than the RBC + P arm and having a higher incidence of penetrating injury and a shorter prehospital transit time, all of which could have contributed to survival outcomes independently of the intervention. The analysis adjusted for these confounding factors as well as differences between services and sites, and evidence for the existence of overall benefit from RCP, compared to RBC alone, remained. Lastly, given the long transport times and the high ISS seen in our cohort, it would have been interesting to evaluate the impact of operative versus non-operative management, but this information was not collected.
Conclusions
In conclusion, prehospital plasma transfusion in addition to red blood cell transfusion (RBC) is associated with lower odds of death at 24 h compared to RBC transfusion alone, while at 30 days, there was no difference in survival, indicating that the effect of prehospital plasma may be greatest in the short term. There is evidence that the addition of prehospital plasma to RBC transfusion (versus RBC only) was associated with greater benefit for penetrating injury than blunt injury, but future randomized controlled trials are needed to confirm these results.
Availability of data and materials
Data supporting the results are presented in the article. The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Abbreviations
- AIS:
-
Abbreviated injury score
- CI:
-
Confidence interval
- CRF:
-
Case report form
- ED:
-
Emergency department
- HEMS:
-
Helicopter emergency medical services
- IQR:
-
Interquartile range
- ISS:
-
Injury severity score
- Lyoplas:
-
Lyophilized plasma
- MTC:
-
Major trauma center
- OR:
-
Odds ratio
- RBC:
-
Red blood cells
- RBC + P:
-
Red blood cells and plasma separately
- RCP:
-
Red cell and plasma combined (in one bag)
- WB:
-
Whole blood
- UK:
-
United Kingdom
References
Shackelford SA, Del Junco DJ, Powell-Dunford N, Mazuchowski EL, Howard JT, Kotwal RS, et al. Association of prehospital blood product transfusion during medical evacuation of combat casualties in Afghanistan with acute and 30-day survival. JAMA. 2017;318(16):1581–91.
Holcomb JB, Tilley BC, Baraniuk S, Fox EE, Wade CE, Podbielski JM, et al. Transfusion of plasma, platelets, and red blood cells in a 1:1:1 vs a 1:1:2 ratio and mortality in patients with severe trauma: the PROPPR randomized clinical trial. JAMA. 2015;313(5):471–82.
Hunt BJ, Allard S, Keeling D, Norfolk D, Stanworth SJ, Pendry K, et al. A practical guideline for the haematological management of major haemorrhage. Br J Haematol. 2015;170(6):788–803.
Spahn DR, Bouillon B, Cerny V, Duranteau J, Filipescu D, Hunt BJ, et al. The European guideline on management of major bleeding and coagulopathy following trauma: fifth edition. Crit Care. 2019;23(1):98.
Griggs JE, Jeyanathan J, Joy M, Russell MQ, Durge N, Bootland D, et al. Mortality of civilian patients with suspected traumatic haemorrhage receiving prehospital transfusion of packed red blood cells compared to prehospital crystalloid. Scand J Trauma Resusc Emerg Med. 2018;26(1):100.
Rehn M, Weaver A, Brohi K, Eshelby S, Green L, Roislien J, et al. Effect of prehospital red blood cell transfusion on mortality and time of death in civilian trauma patients. Shock. 2019;51(3):284–8.
Crombie N, Doughty HA, Bishop JRB, Desai A, Dixon EF, Hancox JM, et al. Resuscitation with blood products in patients with trauma-related haemorrhagic shock receiving prehospital care (RePHILL): a multicentre, open-label, randomised, controlled, phase 3 trial. Lancet Haematol. 2022. https://doi.org/10.1016/S2352-3026(22)00040-0.
Moore HB, Moore EE, Chapman MP, McVaney K, Bryskiewicz G, Blechar R, et al. Plasma-first resuscitation to treat haemorrhagic shock during emergency ground transportation in an urban area: a randomised trial. Lancet. 2018;392(10144):283–91.
Sperry JL, Guyette FX, Brown JB, Yazer MH, Triulzi DJ, Early-Young BJ, et al. Prehospital plasma during air medical transport in trauma patients at risk for hemorrhagic shock. N Engl J Med. 2018;379(4):315–26.
Reitz KM, Moore HB, Guyette FX, Sauaia A, Pusateri AE, Moore EE, et al. Prehospital plasma in injured patients is associated with survival principally in blunt injury: results from two randomized prehospital plasma trials. J Trauma Acute Care Surg. 2020;88(1):33–41.
Guyette FX, Sperry JL, Peitzman AB, Billiar TR, Daley BJ, Miller RS, et al. Prehospital blood product and crystalloid resuscitation in the severely injured patient: a secondary analysis of the prehospital air medical plasma trial. Ann Surg. 2021;273(2):358–64.
Tucker H, Davenport R, Green L. The role of plasma transfusion in prehospital haemostatic resuscitation. Transfus Med Rev. 2021;35(4):91–5.
Pusateri AE, Moore EE, Moore HB, Le TD, Guyette FX, Chapman MP, et al. Association of prehospital plasma transfusion with survival in trauma patients with hemorrhagic shock when transport times are longer than 20 minutes: a post hoc analysis of the PAMPer and COMBAT clinical trials. JAMA Surg. 2019; e195085.
Murdock AD, Berseus O, Hervig T, Strandenes G, Lunde TH. Whole blood: the future of traumatic hemorrhagic shock resuscitation. Shock. 2014;41(Suppl 1):62–9.
Avery P, Morton S, Tucker H, Green L, Weaver A, Davenport R. Whole blood transfusion versus component therapy in adult trauma patients with acute major haemorrhage. Emerg Med J; 2020.
Malkin M, Nevo A, Brundage SI, Schreiber M. Effectiveness and safety of whole blood compared to balanced blood components in resuscitation of hemorrhaging trauma patients–a systematic review. Injury. 2021;52(2):182–8.
Geneen LJ, Brunskill SJ, Doree C, Estcourt LJ, Green L. The difference in potential harms between whole blood and component blood transfusion in major bleeding: a rapid systematic review and meta-analysis of RCTs. Transfus Med Rev. 2021.
Huish S, Green L, Curnow E, Wiltshire M, Cardigan R. Effect of storage of plasma in the presence of red blood cells and platelets: re-evaluating the shelf life of whole blood. Transfusion. 2019;59(11):3468–77.
Stanworth SJ, Dowling K, Curry N, et al. Haematological management of major haemorrhage: a British Society for Haematology Guideline. Br J Haematol. 2022;198(4):654–667.
Smith IM, James RH, Dretzke J, Midwinter MJ. Prehospital blood product resuscitation for trauma: a systematic review. Shock. 2016;46(1):3–16.
Holcomb JB, Moore EE, Sperry JL, Jansen JO, Schreiber MA, Del Junco DJ, et al. Evidence-based and clinically relevant outcomes for hemorrhage control trauma trials. Ann Surg. 2021;273(3):395–401.
Oyeniyi BT, Fox EE, Scerbo M, Tomasek JS, Wade CE, Holcomb JB. Trends in 1029 trauma deaths at a level 1 trauma center: impact of a bleeding control bundle of care. Injury. 2017;48(1):5–12.
Pati S, Matijevic N, Doursout MF, Ko T, Cao Y, Deng X, et al. Protective effects of fresh frozen plasma on vascular endothelial permeability, coagulation, and resuscitation after hemorrhagic shock are time dependent and diminish between days 0 and 5 after thaw. J Trauma. 2010;69(Suppl 1):S55–63.
Chang R, Holcomb JB, Johansson PI, Pati S, Schreiber MA, Wade CE. Plasma resuscitation improved survival in a Cecal ligation and puncture rat model of sepsis. Shock. 2018;49(1):53–61.
Straat M, Muller MC, Meijers JC, Arbous MS, Spoelstra-de Man AM, Beurskens CJ, et al. Effect of transfusion of fresh frozen plasma on parameters of endothelial condition and inflammatory status in non-bleeding critically ill patients: a prospective substudy of a randomized trial. Crit Care. 2015;19:163.
Shah S, Coppolino K, Menocha S, Beceiro S, Nateri J, Spinella PC, et al. Immunomodulatory effects of plasma products on monocyte function in vitro. J Trauma Acute Care Surg. 2018;84(6S Suppl 1):S47–53.
Chapman MP, Moore EE, Moore HB, Sauaia A. Plasma-first resuscitation to treat haemorrhagic shock in urban areas–authors’ reply. Lancet. 2020;395(10224):562–3.
Cardigan R, Green L. Thawed and liquid plasma–what do we know? Vox Sang. 2015;109(1):1–10.
McCullagh J, Proudlove N, Tucker H, Davies J, Edmondson D, Lancut J, et al. Making every drop count: reducing wastage of a novel blood component for transfusion of trauma patients. BMJ Open Qual. 2021. https://doi.org/10.1136/bmjoq-2021-001396.
Funding
The study was funded jointly by the London Air Ambulance charity, the Barts and the London Charity and NHS Blood and Transplant. LG and AW obtained funding for the study.
Author information
Authors and Affiliations
Contributions
LG, KB, RD, AW, RC and SS designed the study, reviewed the manuscript and made all subsequent revisions. HT collected the data, performed the analysis and drafted the manuscript. JTan provided statistical advice, performed the analysis and contributed to the writing of the manuscript. CA, RB, ED, PG, RH, RL, JM, JThompson, JU contributed to the data collection and writing of the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Study received ethical approval from the Health Research Authority (IRAS reference: 236783). Due to the study design, consent from patients was not required.
Consent for publication
Not applicable.
Competing interests
All authors declare the following: LG, RC, SS are employees of NHSBT and report obtaining research funds for multiple clinical studies, but they report no direct relevant financial disclosures. All other authors declare no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Additional file 1.
Table A. Cause of Death at 24 hours and 30 days.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Tucker, H., Brohi, K., Tan, J. et al. Association of red blood cells and plasma transfusion versus red blood cell transfusion only with survival for treatment of major traumatic hemorrhage in prehospital setting in England: a multicenter study. Crit Care 27, 25 (2023). https://doi.org/10.1186/s13054-022-04279-4
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s13054-022-04279-4