Abstract
Background
Non-invasive ventilation (NIV) with bi-level positive pressure ventilation is a first-line intervention for selected patients with acute hypercapnic respiratory failure. Compared to conventional oxygen therapy, NIV may reduce endotracheal intubation, death, and intensive care unit length of stay (LOS), but its use is often limited by patient tolerance and treatment failure. High-flow nasal cannula (HFNC) is a potential alternative treatment in this patient population and may be better tolerated.
Research question
For patients presenting with acute hypercapnic respiratory failure, is HFNC an effective alternative to NIV in reducing the need for intubation?
Methods
We searched EMBASE, MEDLINE, and the Cochrane library from database inception through to October 2021 for randomized clinical trials (RCT) of adults with acute hypercapnic respiratory failure assigned to receive HFNC or NIV. The Cochrane risk-of-bias tool for randomized trials was used to assess risk of bias. We calculated pooled relative risks (RR) for dichotomous outcomes and mean differences (MD) for continuous outcomes, with corresponding 95% confidence intervals (CI) using a random-effects model.
Results
We included eight RCTs (n = 528) in the final analysis. The use of HFNC compared to NIV did not reduce the risk of our primary outcome of mortality (RR 0.86, 95% CI 0.48–1.56, low certainty), or our secondary outcomes including endotracheal intubation (RR 0.80, 95% CI 0.46–1.39, low certainty), or hospital LOS (MD − 0.82 days, 95% CI − 1.83–0.20, high certainty). There was no difference in change in partial pressure of carbon dioxide between groups (MD − 1.87 mmHg, 95% CI − 5.34–1.60, moderate certainty).
Interpretation
The current body of evidence is limited in determining whether HFNC may be either superior, inferior, or equivalent to NIV for patients with acute hypercapnic respiratory failure given imprecision and study heterogeneity. Further studies are needed to better understand the effect of HFNC on this population.
Similar content being viewed by others
Background
Non-invasive positive pressure ventilation (NIV) delivers two levels of pressure during the respiratory cycle—a lower pressure during the expiratory phase and a higher pressure during the inspiratory phase. The pressure differential assists with the washout of accumulated carbon dioxide (CO2) and supports respiratory muscles to reduce work of breathing [1]. As such, NIV has been found to reduce mortality and need for intubation in patients with acute hypercapnic respiratory failure secondary to acute exacerbation of chronic obstructive pulmonary disease (AECOPD) [2, 3], NIV is also suggested for use in acute respiratory failure in immunocompromised and postoperative patients, and for prevention of post-extubation respiratory failure in high-risk patients [2].
Despite wide potential for application, NIV use can be limited due to patient intolerance of the interface or positive pressure. NIV requires a tight-fitting mask or helmet, delivery of high pressures to an awake patient, is associated with skin breakdown after prolonged use, causes gastric insufflation with increased risk of aspiration, can be associated with patient-ventilator asynchrony, and limits both secretion management and nutritional intake [4, 5]. Patients who cannot tolerate NIV will often require invasive mechanical ventilation [6,7,8].
High-flow nasal cannula (HFNC) is an oxygen delivery device which utilizes high inspiratory flows of up to 60L/min through a nasal cannula to deliver up to 100% fraction of inspired oxygen (FiO2). HFNC has been studied in the hypoxemic population and is recommended in the setting of hypoxemic respiratory failure, post-extubation in selected patients, and in the postoperative setting for high-risk patients after cardiac or thoracic surgery [5, 9]. While the majority of evidence for HFNC is in the setting of acute hypoxemic respiratory failure, it is of increasing interest as an alternative to NIV in hypercapnic respiratory failure. Physiological studies suggest that the high gas flows of HFNC may improve ventilation by increasing mean airway pressure and washout of dead space, all while being more comfortable and tolerable by the patient [10,11,12]. Initial observational studies have demonstrated improvement in hypercapnia with the use of HFNC [13, 14].
Hence, our objective was to conduct a systematic review and meta-analysis to determine the efficacy and safety of HFNC compared to NIV for adults with acute hypercapnic respiratory failure. While previous systematic reviews have compared HFNC to NIV for the treatment of hypercapnia, they have important limitations, such as including heterogeneous patient populations [15, 16]. Additionally, these systematic reviews do not include several recently published randomized clinical trials (RCTs) [17, 18]. We hypothesized that there would be no increased risk of mortality when HFNC is used compared to NIV, but potentially an increased risk of intubation.
Methods
Study selection
We included parallel-group and crossover RCTs that enrolled adults ≥ 18 years old presenting with acute hypercapnic respiratory failure, defined as a pH < 7.35 or partial pressure of carbon dioxide (PaCO2) > 45 mmHg, regardless of the etiology. Eligible studies compared HFNC (any setting or duration) to NIV (defined as those with bi-level positive airway pressure, regardless of setting, interface or duration). Studies reporting on at least one of the following outcomes were included: the primary outcome of mortality at longest follow-up, or secondary outcomes of endotracheal intubation and invasive mechanical ventilation, hospital length of stay (LOS), Intensive Care Unit (ICU) LOS, change in PaCO2, change in partial pressure of oxygen (PaO2), respiratory rate (measured at the end of treatment), comfort (measured on a 10-point analog scale at the longest duration of treatment), or dyspnea (defined by the Borg scale taken at longest follow up). In addition to study inclusion criteria, collected characteristics were patient age, patient sex, Acute Physiologic Assessment and Chronic Health Evaluation II (APACHE II) score, and characteristics of the intervention and control group. We excluded pseudo- or quasi-randomized trials, and studies including patients with tracheostomy or were immediately post-extubation. Ethics approval was not obtained as no patient-level data was used in this systematic review.
Electronic search strategy
We searched EMBASE, MEDLINE, and the Cochrane library from inception to October 2021 (Additional file 1: Tables S1 and S2), without limits on publication status or language. Existing systematic reviews and meta-analyses were cross-referenced for potentially eligible studies. Retrieved references were uploaded to Covidence for data management and screening (Covidence systematic review software, Veritas Health Innovation, Melbourne, Australia).
Data collection and analysis
Two independent pairs of reviewers (SO, EH; and NO, KL) screened titles and abstracts in duplicate, and any potentially relevant study was advanced to full-text review. Full-text review was also performed in duplication, with disagreements resolved through discussion. Reviewers (NO and KL) extracted relevant data from eligible trials independently and in duplicate using a pre-designed and piloted data extraction form.
Risk of bias
Two reviewers (NO and KL) independently assessed the studies for risk of bias (RoB) using the original Cochrane risk-of-bias tool (RoB) for randomized trials [19]. RoB was assessed in each study by outcome with reference to: random sequence generation, allocation concealment, blinding of participants and personnel, blinding of outcome assessors, incomplete outcome data, selective reporting, and other biases. RoB was judged to be low if all domains had low risk of bias. High risk of bias in any domain resulted in a high-risk categorization for that outcome. Disagreements were resolved by discussion between the two reviewers, or with arbitration with senior authors (KL and SO) if needed.
Analysis
Measurement of treatment effect
We uploaded extracted data into RevMan (Review Manager, version 5.3. Copenhagen: The Nordic Cochrane Centre, The Cochrane Collaboration, 2014) for meta-analysis. We used the DerSimonian and Laird random-effects model to pool the weighted effect of estimates across all studies [20]. The Mantel–Haenszel method was used to estimate study weights for dichotomous outcomes and inverse variance for continuous outcomes. Pooled relative risks (RRs), mean differences (MDs) or standardized mean differences (SMDs) were calculated for dichotomous and continuous outcomes (respectively), with corresponding 95% confidence intervals (CIs). When required, medians and interquartile ranges were converted to means and standard deviations for the purpose of the meta-analysis [21]. Funnel plots were inspected to assess for any publication bias if ten or more studies existed for that outcome [22].
Unit of analysis
For all main outcomes, only one pair-wise comparison was conducted so the same groups of participants were only included once in the meta-analysis. For crossover trials, data was extracted only from the first phase to avoid the potential of carry-over effects.
Heterogeneity and subgroup analysis
Statistical heterogeneity was assessed using Chi2 and I2 statistics. A Chi2 P value of < 0.1 or an I2 > 50% was pre-determined to meet the criteria of significant heterogeneity [23]. Significant heterogeneity between studies was explored through predefined subgroup analyses to investigate whether certain baseline factors influenced treatment effects. We had two planned subgroup analyses: etiology of hypercapnic respiratory failure (AECOPD vs non-AECOPD diagnoses, hypothesizing a larger treatment effect in AECOPD subgroup), and severity of acidosis (7.30–7.34 vs < 7.30, hypothesizing larger treatment effect in the 7.30–7.34 subgroup).
Sensitivity analysis
We conducted a pre-specified sensitivity analysis restricted to studies without concerns for risk of bias. We hypothesized that the treatment effect would be smaller after excluding studies with some or high concerns of bias. Additionally, we conducted a post hoc analysis excluding one study (Wang et al.) which was only available as an abstract [15, 24].
Assessing the certainty of evidence
Certainty of evidence for all major outcomes was assessed using the Grading of Recommendations Assessment, Development and Evaluation (GRADE) approach [25]. GRADE considers individual study risk of bias, inconsistency, indirectness, imprecision, and publication bias. This was performed by two reviewers (NO and KL) independently and in duplicate for each outcome. Certainty of evidence was ranked as very low, low, moderate, or high.
GRADEpro software [GRADEpro GDT: GRADEpro Guideline Development Tool (Software), McMaster University, 2020] was used to prepare the Summary of findings (SoF) table (Table 1) [26]. Justification of all decisions are presented in the footnotes. We used minimal important differences to assist in judgements of imprecision. The minimal important differences can be found in the SoF table footnotes and all values were based on clinical judgements post hoc.
Trial sequential analysis
We used trial sequential analysis (TSA) to determine if the required sample size to reach the threshold for statistical significance was met for the important outcomes of morality, intubation and ICU LOS. We performed these analyses using TSA software v. 0.9.5.10 Beta (Copenhagen Trial Unit, Center for Clinical Intervention Research, Rigshospitalet, Copenhagen, Denmark available at http://ctu.dk/tsa/). We constructed cumulative z-scores and the required information sizes (RIS) to definitively accept or refute the effect size of interest. We conducted primary TSA using an alpha of 0.05, power of 0.90 (beta 0.10), estimated diversity, unweighted control event proportions for binary outcomes and variances as estimated in the included trials for continuous outcomes. We defined relative risk reduction (RRR) of 15% as a clinically important difference for the outcomes of mortality and intubation and a mean difference (MD) of 24 h for the outcome of ICU LOS. Of note, the TSA was performed post hoc at the request of the journal.
Results
Screening
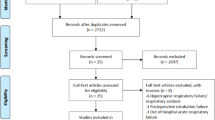
Following the electronic search, 7735 studies were imported for screening and 4915 were screened by title and abstract after removal of duplicates (Fig. 1). Full-text review was completed for 273 studies and eight were included in the analysis [17, 18, 24, 27,28,29,30,31]. All studies except for one were published as full manuscripts [24]. Excluded studies and reasons for exclusion are available in the supplement (Additional file 1: Table S3).
Characteristics of included studies
The eight studies included a total of 528 patients (Table 2) [17, 18, 24, 27,28,29,30,31]. The mean age of participants was 65.9 ± 11.8 years, with 43% being females. The mean APACHE II score was 21.0 ± 7.6. The mean pH of patients on presentation was 7.32 ± 0.04 and the mean PaCO2 was 64.33 ± 7.25 mmHg. All studies were limited to patients with acute hypercapnic respiratory failure. Six studies were parallel group RCTs [17, 18, 24, 27, 28, 32], and two were crossover trials [29, 31].
Five studies assessed the outcomes of HFNC vs. NIV in patients with AECOPD [18, 24, 27,28,29]. One study studied patients with cystic fibrosis [31] and two studies enrolled patients with any cause of hypercapnic respiratory failure [17, 32]. Two studies included patients in the emergency department (ED) [17, 30] and one limited to ICU patients [28]. Four studies had broad inclusion criteria of inpatients or admissions to the ED, ICU, or respiratory unit [18, 27, 29, 31]. Location of admission was not available for one study [24].
Inclusion criteria for pH and PaCO2 varied. Three studies set a limit of a pH ranging from 7.25 to 7.35 [18, 27, 29], whereas another required patients to have a pH > 7.20 [17]. One study’s inclusion criteria for hypercapnic respiratory acidosis was based on pH alone (< 7.35) and another was based on PaCO2 alone [31, 32]. Two studies did not set specific pH or CO2 cutoffs in their inclusion criteria [24, 28].
Risk of bias
Risk of bias varied significantly based on the type of outcome measure (Additional file 1: Table S4). Risk was overall low for objective measures (mortality, intubation, hospital LOS, ICU LOS, respiratory rate, PaO2, and PaCO2) with the exception of one study which had a high loss to follow-up rate resulting in high risk of bias [27]. Two studies were deemed to be at potentially high risk of bias due to their funding [30, 31]. One study had high risk of bias due to selective reporting, with the addition of outcomes measured following trial registration [29]. Risk of bias was rated as high in all studies for the subjective outcomes of dyspnea and comfort in all studies due to lack of blinding.
Outcomes
Mortality
Four studies (n = 250) reported on mortality at the longest follow-up [17, 18, 24, 27]. The use of HFNC compared to NIV did not demonstrate a difference (RR 0.86, 95% CI 0.48–1.56, I2 = 0%, low certainty) (Fig. 2). The absolute risk difference was − 2% (95% CI – 9–10) (Table 1).
Endotracheal intubation
Four studies (n = 275) reported on endotracheal intubation outcomes [18, 24, 27, 30]. The confidence interval was imprecise, indicating no difference in outcome (RR 0.80, 95% CI 0.46–1.39, I2 = 0%, low certainty) (Fig. 3). This translates into an absolute risk difference of − 3% (95% CI – 9–7) (Table 1).
ICU length of stay
The pooled point estimate from two studies (n = 67) demonstrated no statistically significant reduction in duration of ICU LOS when HFNC was used compared to NIV (MD 0.08 days, 95% CI − 1.16–1.32, I2 = 56%, low certainty) (Fig. 4) [24, 30].
Hospital length of stay
Four studies (n = 352) measured hospital LOS [17, 18, 28, 30]. HFNC did not change the duration of hospital LOS compared to NIV (MD − 0.82 days, 95% CI − 1.83–0.20, I2 = 0%, high certainty) (Fig. 4).
Comfort
Two studies (n = 101) measured comfort at the longest duration of treatment [18, 31]. The comfort of patients on HFNC did not differ from those receiving NIV (SMD − 0.32 points, 95% CI − 1.78–1.13, I2 = 91%, very low certainty) (Fig. 4) [18, 31].
Dyspnea
Four studies (n = 191) reported on dyspnea using a Borg scale or equivalent [33, 34]. The pooled estimate showed no clinically important difference in dyspnea scores after treatment when HFNC was used compared to NIV (MD − 0.04 points, 95% CI − 0.54–0.45, I2= 18%, very low certainty) (Fig. 4) [18, 29,30,31].
Respiratory rate
Five studies (n = 234) reported on respiratory rate [17, 18, 29,30,31]. There was no statistical difference in the respiratory rate between the two interventions (MD − 0.85 breaths/min, 95% CI − 1.88–0.18, I2 = 0%, low certainty).
PaO2 and PaCO2
Five studies (n = 427) measured change in PaO2, and no difference in PaO2 level was observed (MD − 0.78 mmHg, 95% CI − 4.18–2.62, I2 = 0%, high certainty) (Additional file 1: Fig. S1) [17, 18, 27, 28, 30].
Pooling the results across seven studies (n = 487) showed no difference in change in PaCO2 between those treated with HFNC versus NIV (MD − 1.87 mmHg, 95% CI − 5.34–1.60 mmHg, I2 = 47%, moderate certainty) (Additional file 1: Fig. S2) [17, 18, 27,28,29,30,31].
Subgroup and sensitivity analyses
Subgroup analysis by AECOPD category for the comfort outcome demonstrated a subgroup effect favoring HFNC in AECOPD (P-interaction = 0.001, I2 = 90.6%; Additional file 1: Fig. S3), however this analysis only included two studies. There was no subgroup effect for the remaining outcomes (Additional file 1: Figs. S4–S6). We were unable to conduct subgroup analyses by severity of acidosis.
Sensitivity analyses excluding high risk of bias trials or excluding the only study published as an abstract [24] did not alter the results of analyzed outcomes (Additional file 1: Figs. S7–S16).
The TSA for all outcomes was inconclusive, as they did not meet the RIS and the boundaries for benefit, harm, or futility were not crossed (Additional file 1: Figs. S17–S19).
Discussion
In this systematic review and meta-analysis of eight RCTs (n = 528 patients), there was no difference in the need for endotracheal intubation (low certainty), mortality at longest follow-up (low certainty), ICU LOS (low certainty), hospital LOS (high certainty), or change in PaCO2 (moderate certainty) or PaO2 (high certainty) when HFNC was compared to NIV in patients with hypercapnic respiratory failure.
While NIV use may reduce risks of death and endotracheal intubation in patients with hypercapnic respiratory failure compared to conventional oxygen therapy, it is not tolerated by all patients, leaving physicians with few options other than proceeding with endotracheal intubation. HFNC is increasingly used in acute hypoxic respiratory failure, but theoretically may also assist in ventilation, potentially with increased comfort and tolerance compared to NIV. Recent ERS guidelines made a conditional recommendation for a trial of NIV prior to use of HFNC in patients with COPD and acute hypercapnic respiratory failure, noting that there is high certainty that NIV reduces intubation, and that more evidence was needed before HFNC could be considered equivalent or superior to NIV. It was noted that there was limited evidence outside of COPD, and that more information was needed to identify patient populations where HFNC could be trialed prior to NIV.
Overall, our results are similar to those of previous systematic reviews, even accounting for the differences in trial selection [15, 16]. Specifically, previous systematic reviews included post-extubation studies. This population is excluded in the current analysis as they may have reasons other than hypercapnic respiratory failure for requiring reintubation, including post-extubation stridor, ineffective cough, and secretion management [35].
The study has a number of strengths, including use of a peer-reviewed electronic search strategy, with iterative searches up to October 2021. Screening, risk of bias, and certainty of evidence assessment were done in duplicate. We considered a priori subgroups of patient populations, hypothesizing that effect of HFNC may be different in patients with AECOPD.
The interpretation of these results is limited by the relatively small number of studies and patients, which resulted in imprecision of the results. As an emerging clinical entity, many studies evaluated physiologic variables rather than the patient-important outcomes of mortality and intubation. Additionally, patient goals of care (whether or not they would be candidates for intubation) were not reported and would be valuable for assessment of the mortality and intubation outcomes. Although a lack of significance may be seen as a limitation, this simply means that we have identified a knowledge gap and there needs to be a call to action by critical care researchers to expand on this important topic. This is further supported with the TSA. Some subgroup analyses may be underpowered due to small number of included studies. Moreover, we hypothesized that patients with more severe respiratory acidosis treated with HFNC may require intubations more frequently than those treated with NIV. Unfortunately, we were unable to complete an analysis based on degree of acidosis due to a complete lack of subgroup data. Study populations were also heterogenous, without consistent stratification between AECOPD and non-AECOPD causes of hypercapnic respiratory failure, thereby limiting conclusions on this specific question. Lastly, we were unable to examine funnel plots to detect publication bias given the small number of available studies. We attempted to minimize publication bias through extensive searches of databases, employing no language restrictions, and discussing the findings with experts in the field. Although, this systematic review protocol was not registered or published, this study was a sub-study of an ongoing clinical practice guideline that follows pre-specified methodology. As indicated above, the only post hoc analysis was a sensitivity analysis where we excluded abstracts. All other decisions were made a priori.
Conclusions
In summary, emerging evidence is inconclusive in identifying whether HFNC may be an alternative to NIV for patients with hypercapnic respiratory failure. Further trials, such as an upcoming randomized non-inferiority trial [36], may improve the precision of the estimates.
Availability of data and materials
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Abbreviations
- AECOPD:
-
Acute exacerbation of chronic obstructive pulmonary disease
- APACHE:
-
Acute physiologic assessment and chronic health evaluation
- ARR:
-
Absolute risk reduction
- BIPAP:
-
Bi-level positive airway pressure
- CO2 :
-
Carbon dioxide
- CI:
-
Confidence interval
- ED:
-
Emergency department
- EPAP:
-
Expiratory positive airway pressure
- FiO2 :
-
Fraction of inspired oxygen
- GRADE:
-
Grading of recommendations assessment, development and evaluation
- HFNC:
-
High-flow nasal cannula
- ICU:
-
Intensive care unit
- IPAP:
-
Inspiratory positive airway pressure
- LOS:
-
Length of stay
- MD:
-
Mean difference
- NIV:
-
Non-invasive ventilation
- PaCO2 :
-
Partial pressure of carbon dioxide
- PaO2 :
-
Partial pressure of oxygen
- PICO:
-
Population, intervention, comparator, outcome
- RR:
-
Relative risk
- RCT:
-
Randomized controlled trial
- SMD:
-
Standardized mean difference
- ROB:
-
Risk of bias
- RIS:
-
Required information size
- RRR:
-
Relative risk reduction
- SoF:
-
Summary of findings
- TSA:
-
Trial sequential analysis
References
Pisani L, Corcione N, Nava S. Management of acute hypercapnic respiratory failure. Curr Opin Crit Care. 2016;22(1):45–52.
Rochwerg B, Brochard L, Elliott MW, Hess D, Hill NS, Nava S, et al. Official ERS/ATS clinical practice guidelines: noninvasive ventilation for acute respiratory failure. Eur Respir J. 2017. https://doi.org/10.1183/13993003.02426-2016.
Davidson AC, Banham S, Elliott M, Kennedy D, Gelder C, Glossop A, et al. BTS/ICS guideline for the ventilatory management of acute hypercapnic respiratory failure in adults. Thorax. 2016;71(Suppl 2):1–35. https://doi.org/10.1136/thoraxjnl-2015-208209.
Carron M, Freo U, Bahammam AS, Dellweg D, Guarracino F, Cosentini R, et al. Complications of non-invasive ventilation techniques: a comprehensive qualitative review of randomized trials. Br J Anaesth. 2013;110(6):896–914.
Oczkowski S, Ergan B, Bos L, Chatwin M, Ferrer M, Gregoretti C, et al. ERS clinical practice guidelines: high-flow nasal cannula in acute respiratory failure. Eur Respir J. 2021;59(4):2101574.
Bourke SC, Piraino T, Pisani L, Brochard L, Elliott MW. Beyond the guidelines for non-invasive ventilation in acute respiratory failure: implications for practice. Lancet Respir Med. 2018;6(12):935–47. https://doi.org/10.1016/S2213-2600(18)30388-6.
Mehta AB, Douglas IS, Walkey AJ. Hospital non-invasive ventilation case-volume and outcomes for acute exacerbations of chronic obstructive pulmonary disease. Ann Am Thorac Soc. 2016. https://doi.org/10.1513/AnnalsATS.201603-209OC.
Stefan MS, Nathanson BH, Higgins TL, Steingrub JS, Lagu T, Rothberg MB, et al. Comparative effectiveness of noninvasive and invasive ventilation in critically ill patients with acute exacerbation of chronic obstructive pulmonary disease. Crit Care Med. 2015;43(7):1386–94.
Rochwerg B, Einav S, Chaudhuri D, Mancebo J, Mauri T, Helviz Y, et al. The role for high flow nasal cannula as a respiratory support strategy in adults: a clinical practice guideline. Intensive Care Med. 2020;46(12):2226–37.
Bräunlich J, Köhler M, Wirtz H. Nasal highflow improves ventilation in patients with COPD. Int J Chron Obstruct Pulmon Dis. 2016;13:1077. Available from: https://www-ncbi-nlm-nih-gov.bvs.clas.cineca.it/pmc/articles/PMC4887061/pdf/copd-11-1077.pdf.
Mündel T, Feng S, Tatkov S, Schneider H. Mechanisms of nasal high flow on ventilation during wakefulness and sleep. J Appl Physiol. 2013;114(8):1058–65.
Fricke K, Tatkov S, Domanski U, Franke K-J, Nilius G, Schneider H. Nasal high flow reduces hypercapnia by clearance of anatomical dead space in a COPD patient. Respir Med Case Reports. 2016;19:115–7. https://doi.org/10.1016/j.rmcr.2016.08.010.
Kim ES, Lee H, Kim SJ, Park J, Lee YJ, Park JS, et al. Effectiveness of high-flow nasal cannula oxygen therapy for acute respiratory failure with hypercapnia. J Thorac Dis. 2018;10(2):882–8.
Bräunlich J, Wirtz H. Nasal high-flow in acute hypercapnic exacerbation of COPD. Int J COPD. 2018;13:3895–7.
Huang Y, Lei W, Zhang W, Huang JA. High-flow nasal cannula in hypercapnic respiratory failure: a systematic review and meta-analysis. Can Respir J. 2020;7406457.
Yang PL, Yu JQ, Chen HB. High-flow nasal cannula for acute exacerbation of chronic obstructive pulmonary disease: a systematic review and meta-analysis. Hear Lung. 2021;50(2):252–61. https://doi.org/10.1016/j.hrtlng.2020.12.010.
Papachatzakis Y, Nikolaidis PT, Kontogiannis S, Trakada G. High-flow oxygen through nasal cannula vs. non-invasive ventilation in hypercapnic respiratory failure: a randomized clinical trial. Int J Environ Res Public Health. 2020;17(16):1–8.
Cortegiani A, Longhini F, Madotto F, Groff P, Scala R, Crimi C, et al. High flow nasal therapy versus noninvasive ventilation as initial ventilatory strategy in COPD exacerbation: a multicenter non-inferiority randomized trial. Crit Care. 2020;24(1):692.
Higgins JPT, Altman DG, Gotzsche PC, Juni P, Moher D, Oxman AD, et al. The cochrane collaboration’s tool for assessing risk of bias in randomised trials. BMJ. 2011;343(oct 18 2):d5928–d5928. https://doi.org/10.1136/bmj.d5928.
DerSimonian R, Laird N. Meta-analysis in clinical trials revisited. Contemp Clin Trials. 2015;45(Pt A):139–45. Available from: http://www.ncbi.nlm.nih.gov/pubmed/26343745.
Higgins JPT, Li TDJ (editors). Cochrane handbook section 7.7.3.5 medians and interquartile ranges. In: Higgins JPT, Thomas J, Chandler J, Cumpston M, Li T, Page MJ WV, editors. Cochrane handbook for systematic reviews of interventions version 62 (updated February 2021). Cochrane; 2021. Available from: www.training.cochrane.org/handbook.
Page MJ, Higgins JPT SJ. 13.3.5.2 Funnel plots. In: Higgins JPT, Thomas J, Chandler J, Cumpston M, Li T, Page MJ WV, editors. Cochrane handbook for systematic reviews of interventions version 62 (updated February 2021). Cochrane; 2021. Available from: https://training.cochrane.org/handbook/current/chapter-13#section-13-3-5-2.
Higgins JPT. Measuring inconsistency in meta-analyses. BMJ. 2003;327(7414):557–60. https://doi.org/10.1007/s10844-006-2974-4.
Wang J, Hong-ying J, Qing L. Randomized controlled study of HFNC and NPPV in the treatment of AECOPD combined with type II respiratory failure. Chin J Crit care Med. 2019;39(10):945–8.
Guyatt GH, Oxman AD, Vist GE, Kunz R, Falck-Ytter Y, Alonso-Coello P, et al. GRADE: an emerging consensus on rating quality of evidence and strength of recommendations. BMJ. 2008;336(7650):924–6. https://doi.org/10.1136/bmj.39489.470347.AD.
McMaster University. GradePRO guideline development tool. 2021 [cited 2021 Jul 2]. Available from: https://gdt.gradepro.org/app/
Lee MK, Choi J, Park B, Kim B, Lee SJ, Kim S-H, et al. High flow nasal cannulae oxygen therapy in acute-moderate hypercapnic respiratory failure. Clin Respir J. 2018;12(6):2046–56. https://doi.org/10.1111/crj.12772.
Cong L, Zhou L, Liu H, Wang J. Outcomes of high-flow nasal cannula versus non-invasive positive pressure ventilation for patients with acute exacerbations of chronic obstructive pulmonary disease. Int J Clin Exp Med. 2019;12(8):10863–7.
Rezaei A, Fakharian A, Ghorbani F, Idani E, Abedini A, Jamaati H. Comparison of high-flow oxygenation with noninvasive ventilation in COPD exacerbation: a crossover clinical trial. Clin Respir J. 2020;15(4):420–9. https://doi.org/10.1111/crj.13315.
Doshi PB, Whittle JS, Dungan G, Volakis LI, Bublewicz M, Kearney J, et al. The ventilatory effect of high velocity nasal insufflation compared to non-invasive positive-pressure ventilation in the treatment of hypercapneic respiratory failure: a subgroup analysis. Hear Lung. 2020;49(5):610–5. https://doi.org/10.1016/j.hrtlng.2020.03.008.
Sklar MC, Dres M, Rittayamai N, West B, Grieco DL, Telias I, et al. High-flow nasal oxygen versus noninvasive ventilation in adult patients with cystic fibrosis: a randomized crossover physiological study. Ann Intensive Care. 2018;8(1):1–9. https://doi.org/10.1186/s13613-018-0432-4.
Doshi P, Whittle JS, Bublewicz M, Kearney J, Ashe T, Graham R, et al. High-velocity nasal insufflation in the treatment of respiratory failure: a randomized clinical trial. Ann Emerg Med. 2018;72(1):73-83.e5. https://doi.org/10.1016/j.annemergmed.2017.12.006.
Kendrick KR, Baxi SC, Smith RM. Usefulness of the modified 0–10 Borg scale in assessing the degree of dyspnea in patients with COPD and asthma. J Emerg Nurs. 2000;26(3):a107012. Available from: http://www1.mosby.com/scripts/om.dll/serve?action=searchDB&searchDBfor=art&artType=abs&id=a107012.
Meek PM, Schwartzstein RM, Adams L, Altose MD, Breslin EH, Carrieri-Kohlman V, et al. Dyspnea: mechanisms, assessment, and management: a consensus statement. Am J Respir Crit Care Med Am Lung Assoc. 1999;159:321–40.
Thille AW, Richard J-CM, Brochard L. The decision to extubate in the intensive care unit. Am J Respir Crit Care Med. 2013;187(12):1294–302. https://doi.org/10.1164/rccm.201208-1523CI.
Bräunlich J, Köppe-Bauernfeind N, Petroff D, Franke A, Wirtz H (2022) Nasal high-flow compared to non-invasive ventilation in treatment of acute acidotic hypercapnic exacerbation of chronic obstructive pulmonary disease—protocol for a randomized controlled noninferiority trial (ELVIS). Trials 23(1):28
Acknowledgements
Thank you to all the collaborators and to the European Respiratory Society who allowed us to update their guideline research. Thank you to our librarian, Kaitryn Campbell, for developing and running our database searches and updates. All data available upon request.
Funding
There is no funding to declare.
Author information
Authors and Affiliations
Contributions
SO, KL, and NO contributed to the study conception and design. NO, KL, EH, DC, and SO completed data extraction and analysis. NO and KL prepared the initial manuscript. WA, AC, BE, RS, GS, DC, and SO had a significant role in manuscript drafting and editing. KL is the guarantor of this paper. All authors approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Additional file 1: Table S1.
Embase and Medline Search Results. Table S2. Cochrane Central Search Results. Table S3. Excluded Studies. Table S4. Risk of Bias Table. Fig. S1. Forest plot of mortality—subgroup analysis by risk of bias. Fig. S2. Forest plot of mortality—subgroup analysis excluding Wang et al. Fig. S3. Forest plot of intubation—subgroup analysis by risk of bias. Fig. S4. Forest plot of intubation—subgroup analysis excluding Wang et al. Fig. S5. Forest plot of ICU Length of Stay—subgroup analysis by risk of bias. Fig. S6. Forest plot of ICU Length of Stay—subgroup analysis excluding Wang et al. Fig. S7. Forest plot of Hospital Length of Stay—subgroup analysis by risk of bias. Fig. S8. Forest plot of change in comfort—subgroup analysis by AECOPD studies alone. Fig. S9. Forest plot of change in dyspnea—subgroup analysis by AECOPD studies alone. Fig. S10. Forest plot of change in respiratory rate—subgroup analysis by AECOPD studies alone. Fig. S11. Forest plot of respiratory rate—subgroup analysis by risk of bias. Fig. S12. Forest plot of change in PO2. Fig. S13. Forest plot of change in PO2—subgroup analysis by risk of bias. Fig. S14. Forest plot of change in PCO2. Fig. S15. Forest plot of change in PCO2—subgroup analysis by AECOPD studies alone. Fig. S16. Forest plot of change in PCO2—subgroup analysis by risk of bias. Fig. S17. Trial sequential analysis for mortality. Fig. S18. Trial sequential analysis for intubation. Fig. S19. Trial sequential analysis for ICU length of stay.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Ovtcharenko, N., Ho, E., Alhazzani, W. et al. High-flow nasal cannula versus non-invasive ventilation for acute hypercapnic respiratory failure in adults: a systematic review and meta-analysis of randomized trials. Crit Care 26, 348 (2022). https://doi.org/10.1186/s13054-022-04218-3
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s13054-022-04218-3