Abstract
Background
The study aimed to describe the epidemiology and outcomes of hospital-acquired bloodstream infections (HABSIs) between COVID-19 and non-COVID-19 critically ill patients.
Methods
We used data from the Eurobact II study, a prospective observational multicontinental cohort study on HABSI treated in ICU. For the current analysis, we selected centers that included both COVID-19 and non-COVID-19 critically ill patients. We performed descriptive statistics between COVID-19 and non-COVID-19 in terms of patients’ characteristics, source of infection and microorganism distribution. We studied the association between COVID-19 status and mortality using multivariable fragility Cox models.
Results
A total of 53 centers from 19 countries over the 5 continents were eligible. Overall, 829 patients (median age 65 years [IQR 55; 74]; male, n = 538 [64.9%]) were treated for a HABSI. Included patients comprised 252 (30.4%) COVID-19 and 577 (69.6%) non-COVID-19 patients. The time interval between hospital admission and HABSI was similar between both groups. Respiratory sources (40.1 vs. 26.0%, p < 0.0001) and primary HABSI (25.4% vs. 17.2%, p = 0.006) were more frequent in COVID-19 patients. COVID-19 patients had more often enterococcal (20.5% vs. 9%) and Acinetobacter spp. (18.8% vs. 13.6%) HABSIs. Bacteremic COVID-19 patients had an increased mortality hazard ratio (HR) versus non-COVID-19 patients (HR 1.91, 95% CI 1.49–2.45).
Conclusions
We showed that the epidemiology of HABSI differed between COVID-19 and non-COVID-19 patients. Enterococcal HABSI predominated in COVID-19 patients. COVID-19 patients with HABSI had elevated risk of mortality.
Trial registration ClinicalTrials.org number NCT03937245. Registered 3 May 2019.
Similar content being viewed by others
Background
Hospital-acquired bloodstream infections (HABSI) are a frequent event in critically ill patients and are associated with increased morbidity and mortality [1, 2]. Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) virus emerged in 2019 and its disease (COVID-19) caused millions of deaths worldwide. Probably due to various reasons, critically ill patients infected with SARS-CoV-2 were more prone to hospital-acquired infections [3], more specifically bloodstream infections (BSIs) [4]. A recent systematic review showed a pooled estimated occurrence of BSIs of almost 30% in patients admitted to intensive care unit (ICU) [5]. Several epidemiological studies suggested that HABSI acquired in the ICU occurred more often during the different COVID-19 waves [6, 7]. Multicentric analyses illustrated that ICU-BSI in COVID-19 patients were associated with prolonged length of ICU stay and increased mortality [8].
Most of the literature has focused on COVID-19 patients and little is known about differences in the pathogen distribution between COVID-19 and non-COVID-19 patients. In August 2019, we started the Eurobact II study which included critically ill ICU patients with HABSIs, regardless of their status with respect to COVID-19 infection. The data collection was continued during the different COVID-19 waves, thus allowing an accurate evaluation of the epidemiology of HABSIs in ICU patients during the study period. The primary objective of this study was to describe the epidemiology of HABSI between COVID-19 and non-COVID-19 critically ill patients in terms of patients’ characteristics, source of infection, microorganism distribution and mortality.
Material and methods
Eurobact II study design
The Eurobact II study was a prospective observational multicontinental cohort study conducted between August 2019 and June 2021 [9]. This observational study was registered within ClinicalTrials.org (NCT03937245) and was reported in accordance with the STrengthening the Reporting of OBservational studies in Epidemiology (STROBE) guidelines [10].
Setting
Endorsement, financial and logistical support was obtained from the European Society of Intensive Care Medicine (ESICM), the Infectious Diseases (ESCMID) study Group for Infections in Critically Ill Patients (ESGCIP) and the European Society of Clinical Microbiology. An operational committee (AT, NB, FB, SR and JFT) was constituted to oversee all study operations. Study oversight and logistics were provided through a non-profit research organization, the OUTCOMEREA® study group (Paris, France). A Scientific Committee and National coordinators (NCs) were recruited for each participating country by the operational committee with assistance from the endorsing societies. Main responsibilities of NCs included recruiting participating ICUs, applying for ethical and regulatory approvals at national level where applicable, and facilitating communication with ICUs within their countries.
ICU and patient recruitment
ICUs eligible to participate were defined as a department specifically designed to manage patients with organ failures within a health-care facility and able to provide invasive mechanical ventilation for a duration of at least 24 h. For this observational study, among all Eurobact II participating ICUs, we selected only those that recruited both COVID-19 and non-COVID-19 patients.
Patients ≥ 18 years old with a first episode of HABSI treated in ICU were included. HABSI was defined as a positive blood culture sampled 48 h after hospital admission. Both patients with blood cultures sampled in ICU (i.e., ICU-acquired HABSI) and patients transferred (i.e., in 48 h) to the ICU for the treatment of the HABSI were enrolled.
Blood cultures with typical skin contaminants (e.g., coagulase-negative staphylococci, Corynebacterium species, Bacillus species, Propionibacterium species, Micrococcus species) were included if at least two blood cultures with the same antimicrobial susceptibility profile were observed or strong clinical grounds that the blood culture was not a contaminant (e.g., infected material proven as a source for the HABSI). All HABSIs including typical skin contaminants were carefully reviewed by at least one member of the scientific committee (AT, FB or NB). Only the first bloodstream infection during the eligibility period was included for the current analysis. We excluded community-acquired bloodstream infections, typical skin contaminants that did not fulfill inclusions criteria, cases with missing core outcome data (i.e., dates of bloodstream infections and hospital/ICU admission, dates of discharge and/or death as applicable, pathogen and treatment inclusive of antimicrobials and source control as applicable) and retrospective inclusions.
The Eurobact II study recruited centers with HABSI from 1st August 2019 to 30th January 2021. The minimal recruitment period was 3 months or 10 consecutive HABSIs (whichever came first) and could be extended on request from the local investigator for the whole duration of the study. Of note, a flexible start of the inclusion period was allowed for each ICU to facilitate participation in the study.
Data collection
The Eurobact II was an observational study, pre-specifying that all data had to be collected from the patients’ chart without additional diagnostic tests or interventions.
The study website and case report form (CRF) comprised a center form that collected data which described the ICU, antimicrobial stewardship features and microbiology laboratory specifics. For each patient, we collected demographic data and the main diagnosis at ICU admission, including ICU admission for SARS-CoV-2 infection. Comorbidities were assessed using the five markers of the Chronic Health Evaluation of the APACHE II score and the Charlson index [11, 12]. Severity of illness was defined at ICU admission by the Simplified Acute Physiology Score II (SAPS II) [13], and at HABSI diagnosis using the Sequential Organ Failure Assessment (SOFA) score. Data on antimicrobial exposure from one week prior to the study infection were routinely collected. Further information on definitions is illustrated in the electronic supplementary material (ESM).
For each microorganism, we routinely collected: date and time of blood culture sampling, category according to Gram-stain, phenotypical resistance and, when available, genotypical resistance mechanisms. Carbapenem resistance for Enterobacterales was defined according to the U.S. Centers for Diseases Control and Prevention (CDC) as resistant to at least one carbapenem [14]. Difficult-to-treat resistance (DTR) in Gram-negative bacteria was defined as resistance to all first-line antimicrobials (carbapenems, fluoroquinolone, cephalosporins). It was assessed for Enterobacterales, Pseudomonas spp., and Acinetobacter spp., and required all three categories reported plus an assessment of sensitivities to piperacillin-tazobactam or aztreonam if available as outlined by Kadri et al. [15]. Our primary outcome was the distribution of microorganism. Our secondary outcome was mortality. Patients were followed for up to 28 days or until hospital discharge, for further HABSI, duration of organ support, length of ICU and hospital stay, and vital status. Data quality and processes were detailed in the ESM.
Statistical analyses
Characteristics of centers and patients were described as count (percent) or median (interquartile range) for qualitative and quantitative variables, respectively. Only first episodes of HABSI were analyzed. First, we described the differences between patients using chi-square (or Fisher) and Wilcoxon tests for categorical and numeric variables, respectively. Second, we described the difference in sources of infection and microorganisms’ distribution. In order to mitigate the bias of time-to-HABSI, we performed a sensitivity analysis including only ICU-acquired HABSI, thus excluding patients transferred to the ICU for HABSI management. Third, we performed an explanatory analysis that compared COVID-19 and non-COVID-19 with HABSIs due only to enterococci or DTR Gram-negative microorganisms. Fourth, a graphical representation with Kaplan–Meier curves (with log-rank test) using mortality as an outcome was performed. Finally, we tested the association between COVID-19 status and mortality using a multivariable fragility Cox model. A random effect for center was included. For the multivariable analysis, we imputed the solely missing value (i.e., BMI) among the included covariates at the median. Further details on methods were illustrated in the ESM.
All statistical analyses were performed with SAS (version 9.4) and R (Version 3.5.3).
Ethics
This study was approved by the ethics Committee from the Royal Brisbane & Women's Hospital Human Research (LNR/2019/QRBW/48376); further details were illustrated in the ESM.
Results
Centers
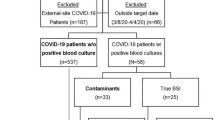
Among the 333 centers recruited in the Eurobact II study, we excluded 278 centers that did not include HABSI in COVID-19 patients (Fig. 1). In addition, two centers included only HABSI in COVID-19 patients and were therefore excluded.
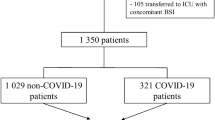
Finally, 53 centers from 19 countries were included (Additional file 1: Fig. S1, Additional file 1: Table S1). Centers were mostly located in Europe and central Asia (n = 42, 79%) and were mostly from high-income countries (n = 33, 62%). We recruited in median 10 (IQR 7; 20) patients per center, with 25% (IQR 14; 46) of them being COVID-19 patients.
Patients, HABSIs characteristics and microorganisms
We included 829 patients with a HABSI. Their median age was 65 years old (IQR 55; 74) and 538 (64.9%) were male (Table 1).
The most frequently observed comorbidities were the metabolic ones (n = 326, 39.3%), followed by cardio-vascular (n = 191, 23%) and respiratory (n = 147, 17.7%), and malignancies (n = 143, 17.2%). More than 80% of patients were admitted to ICU for a medical reason, with a median SAPS II on admission at 47 (IQR 37; 58); 617 (74.4%) were receiving invasive mechanical ventilation at HABSI onset.
The most frequently observed sources of infection were an intravascular catheter (n = 257, 31%) and the respiratory tract (n = 251, 30.3%, Table 2).
We identified 939 microorganisms: Klebsiella spp. (n = 147, 15.7%), Acinetobacter spp (n = 143, 15.2%) and enterococci (n = 118, 12.6%) were the most frequently detected microorganisms. The rate of DTR Gram-negative microorganisms was 15%. S. aureus was identified only in 79 HABSIs (8.4%).
Differences between COVID-19 and non-COVID-19 patients
We included 252 COVID-19 and 577 non-COVID-19 patients. COVID-19 patients had fewer comorbidities (Table 1). On ICU admission, COVID-19 patients had lower SAPS II scores (median 42 [IQR 33; 50] vs. 49 [IQR 38;62], p < 0.0001) and were frequently receiving high-flow oxygen nasal cannula (13.9% vs. 7.1%) and non-invasive mechanical ventilation (15.1% vs. 9%, p < 0.0001) compared to non-COVID-19 patients.
The time between hospital admission and HABSI was similar between COVID-19 (14 [IQR 9; 23] days) and non-COVID-19 patients (15 [IQR 8; 29] days, p = 0.69). However, ICU-acquired HABSI in COVID-19 patients (10 days, IQR 6; 16) occurred later compared to non-COVID-19 patients (8, [IQR 2; 17], p = 0.02). COVID-19 patients were more frequently exposed to antimicrobials in the week before the occurrence of HABSI (81.0% vs. 72.4% in non-COVID-19 patients, p = 0.009). Ceftriaxone was most frequently administered in COVID-19 patients (9.0% vs. 6.4% in non-COVID-19 patients, p = 0.089, Additional file 1: Table S2). No significant differences in other antimicrobials were observed (Additional file 1: Table S2).
HABSIs in COVID-19 patients were most often from respiratory sources (40.1% vs. 26.0%, p < 0.0001) and primary HABSI (25.4% vs. 17.2%, p = 0.006), whereas HABSIs in non-COVID-19 patients were most often related to intraabdominal (12.5% vs. 1.2%, p < 0.0001) and bone/soft tissues (6.6% vs. 2.0%) infections (Table 2). Gram-positive bacteria were most often involved in COVID-19 patients HABSIs (39.7% vs. 32.1%, p = 0.033). Interestingly, HABSI due to DTR Gram-negative were more often observed in COVID-19 patients. Of note, a sensitivity analysis including only ICU-acquired HABSI showed similar sources of infection and microorganism distribution compared to the main analysis between COVID-19 and non-COVID-19 patients (Additional file 1: Tables S3–S4).
Figure 2 shows the distribution of microorganisms between COVID-19 and non-COVID-19 patients. HABSIs in COVID-19 patients were most frequently caused by enterococci (20.5% vs. 9.0%) and Acinetobacter spp. (18.8% vs. 13.6%), whereas those in non-COVID-19 patients were most frequently caused by Klebsiella spp. (17.5% vs. 11.6%, p < 0.0001). Distribution of microorganism during the ICU length-of-stay is illustrated in Additional file 1: Fig. S2.
Enterococcal HABSIs
E. faecalis accounted for 45% (n = 27) and 34.5% (n = 20) of enterococcal HABSIs in the COVID-19 and non-COVID-19 group, respectively. E. faecium proportion were similar in COVID-19 (n = 32, 53.3%) and non-COVID-19 (n = 33, 56.9%) patients. Similar proportions of vancomycin-resistant E. faecium (VRE) were observed in both groups. COVID-19 patients with enterococcal HABSI were less often immunosuppressed (3.4% vs. 29.3% in non-COVID-19; p = 0.0002) and had less often malignancy (5.2% vs. 25.9%, p = 0.002, Additional file 1: Table S5). Primary (n = 26, 44.8%) enterococcal HABSIs were more frequent in COVID-19 patients compared to non-COVID-19 patients (n = 11, 19.0%, p = 0.0028). HABSI were frequently assigned to an abdominal source in non-COVID-19 patients (n = 13, 22.4%). Polymicrobial enterococcal HABSI were more frequently but non-statistically significantly observed in non-COVID-19 patients (36.2% vs. 25.6%, p = 0.23).
Gram-negative DTR HABSIs
HABSIs due to DTR Gram-negative pathogens occurred a median 11 days (IQR 8;18) after hospital admission in COVID-19 patients, whereas in non-COVID-19 were observed after 20 days (IQR 10; 40, p = 0.001, Additional file 1: Table S6).
Acinetobacter spp. accounted for 60.3% (n = 35) of Gram-negative DTR in COVID-19 patients and Klebsiella spp. accounted for 40.2% (n = 35) of Gram-negative DTR in non-COVID-19 patients (Additional file 1: Table S7).
Mortality
Overall, the day-28 mortality rate was 45.7% (n = 379), reaching 58.7% in COVID-19 patients, versus 40.0% for non-COVID-19 patients (p < 0.0001, Fig. 3). In patients with Gram-negative DTR HABSIs, the day-28 mortality was also higher for COVID-19 (83.7% vs. 65.3% in non-COVID-19, p = 0.025, Fig. 3).
Using a multivariable fragility Cox model, we observed a significant association between COVID-19 status and mortality (hazard ratio 1.91, 95% CI 1.49–2.45, p < 0.0001, Additional file 1: Table S8).
Discussion
Using a large multicontinental prospective cohort, we showed that the epidemiology of HABSI in critically ill patients was different between COVID-19 and non-COVID-19 patients. Enterococcal HABSIs were more frequently observed in COVID-19 patients.
Several studies showed that enterococcal HABSIs were frequent in critically ill COVID-19 patients, ranging from 25% to almost 50% of HABSI [16, 17]. Interestingly, only few studies have reported the differences between COVID-19 and non-COVID-19 critically ill patients with HABSI. The first study matched critically ill COVID-19 patients with similar non-COVID-19 patients and showed a higher rate of enterococcal HABSI among COVID-19 patients [3]. However, this study (1) included a limited number of patients; (2) reported a relatively small number of all-causes HABSI without enterococcal HABSI in non-COVID-19 patients; and (3) included non-COVID-19 patients prior the COVID-19 pandemic. The second study was a small monocentric retrospective cohort study that compared SARS-CoV-2 or influenza patients with inpatients without positive SARS-CoV-2 or influenza tests during the study period. Enterococci were detected in 6 of 20 bacteremic COVID-19 patients, whereas in critically ill influenza patients no enterococcal HABSI was observed [18]. Both studies, due to the small numbers of patients included, showed only a trend towards an increased proportion of enterococcal HABSI in COVID-19 ICU patients. Using high-quality data from a large multicontinental prospective cohort, we found that enterococcal HABSIs were more frequently observed in critically ill COVID-19 patients. A subgroup analysis including only ICU-acquired HABSI illustrated that the enterococcal frequency was increased in this subpopulation. This finding could have several explanations. First, enterococci colonized the gastrointestinal tract [19]. Even if Eurobact II investigators rarely allocated HABSIs to the abdominal source in COVID-19 patients, it is conceivable that more abdominal translocations could occur in COVID-19 patients and were allocated by our investigators to primary HABSI. Critically ill COVID-19 were at a particularly high risk for developing gastrointestinal complications ranging from acute cholecystitis or pancreatitis to ileus or mesenteric ischemia [20,21,22,23,24]. Indeed, SARS-CoV-2 has been detected in the gastrointestinal tract and it may enter gastrointestinal cells via angiotensin-converting enzyme 2 receptors, which are highly expressed in the gastrointestinal tract, to cause direct damage to gastrointestinal organs [25,26,27,28,29]. The microvascular inflammatory coagulopathy of COVID-19 leading to higher incidence of deep vein thrombosis may be another pathophysiological mechanism possibly leading to bacterial translocation. An inflammatory coagulopathy may be associated with deep vein thrombosis or cerebrovascular accidents [30,31,32]: it is conceivable that a similar mechanism may lead to mesenteric ischemia and, therefore, may increase the proportion of detected enterococcal BSI. Second, our study suggested that COVID-19 patients were more frequently exposed to antimicrobials. Cephalosporins are often ineffective against enterococcal species and their prior use was demonstrated to be a major risk factor for the acquisition of enterococcal infections [33, 34]. Previous exposure to antibiotics is unlikely to be the sole explanation for our findings but it can be an instrumental concomitant factor leading to increased proportion of enterococcal HABSI in critically ill patients with COVID-19. Third, enterococci, in particular VRE, may be a marker for poor infection prevention and control (IPC) measures and hand hygiene compliance [35]. The COVID-19 pandemic produced many challenges for IPC, including unit over-crowding, fatigue and sessional use of PPE. These factors likely reduced compliance with IPC measures and contributed to a rise in nosocomial infections. In this context, it is possible that intravascular catheters were more frequently contaminated and subsequently infected with enterococci. However, HABSI patients with and without COVID-19 were recruited for this study during the same period and we did not observe a predominance of VRE or a specific enterococcal species (faecalis vs. faecium) in COVID-19 patients, thus suggesting this was not the dominant cause of the effects seen. Interestingly, a tendency towards more HABSI due to Acinetobacter spp. [36] in COVID-19 patients was observed. This result remains intriguing: several outbreaks of Acinetobacter spp. were observed during the COVID-19 pandemic and, therefore, a possible impact of reduced IPC measures in the solely COVID-19 population might be hypothesized [37].
Our results have several clinical implications. Whether empirical therapy with glycopeptides or oxazolidinones should be administered in septic in critically ill patients with abdominal sepsis is still debated [38]. A recent multicentric study showed that an initial antibiotic treatment which did not cover enterococci was associated with an increased mortality in critically ill patients with a microbiologically confirmed intra-abdominal infection with Enterococcus spp. [39]. In light of these considerations, for septic critically ill COVID-19 patients, an empirical therapy covering all enterococcal species should be considered, especially when a third-generation cephalosporin was previously used. Due to the less pronounced results for resistant Gram-negative microorganisms, no firm conclusions on empirical antibiotic therapy for Gram-negative can be provided.
Our study has several limitations. First, on one hand, the Eurobact II was designed prior to the COVID-19 pandemic. Therefore, several important SARS-CoV-2 variables (e.g., SARS-CoV-2 specific therapies [corticosteroids, tocilizumab] that could affect bacterial infectious risk, SARS-CoV-2 genotype and ICU overcrowding data) were not routinely collected and could not be analyzed. Immunosuppressive drugs administered for COVID-19 may impact on HABSI epidemiology even if large randomized controlled trials did [40, 41] not show a substantial impact on subsequent infections. Moreover, several HABSI patients in our cohort did not receive immunosuppressive drugs according to our definitions, thus highlighting a COVID-19 population during the pre-tocilizumab era. On the other hand, the Eurobact II study, thanks to huge efforts from the local investigators despite the pandemic crisis, allowed an analysis in ICU that prospectively recruited HABSI in both COVID-19 and non-COVID-19 patients, thus mitigating this selection bias. Second, centers recruited patients during different periods, and COVID-19 were not matched with non-COVID-19 patients. Third, four countries (Turkey, France, United Kingdom, and Italy) recruited 50% of patients, thus potentially limiting the generalizability of our results. However, at least one country of all five continents recruited patients for this study. Fourth, centers were allowed to extend the number of inclusions, thus leading to an imbalance of the total number of HABSI recruited between the various centers. Finally, denominator data (i.e., ICU admissions) were not provided by all centers, thus limiting the interpretation of our results.
Conclusions
Using a large multicontinental prospective cohort, we showed that the epidemiology of HABSI differed between COVID-19 and non-COVID-19 patients, with enterococcal HABSI being disproportionately more common in COVID-19 patients. Despite less comorbidities and lower severity scores on admission, COVID-19 patients with HABSI had significantly higher mortality than patients with HABSI but without COVID-19.
Availability of data and materials
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Abbreviations
- BMI:
-
Body Mass Index
- BSI:
-
Bloodstream infections
- CDC:
-
Centers for Diseases Control and Prevention
- CI:
-
Confidence interval
- CRF:
-
Case report form
- DTR:
-
Difficult-to-treat resistance
- ESICM:
-
European Society of Intensive Care Medicine
- ESGCIP:
-
Infectious Diseases (ESCMID) study Group for Infections in Critically Ill Patients
- ESM:
-
Electronic supplementary material
- HABSI:
-
Hospital-acquired bloodstream infection
- HR:
-
Hazard ratio
- ICU:
-
Intensive Care Unit
- IPC:
-
Infection prevention and control
- IQR:
-
Interquartile range
- NCs:
-
National coordinators
- SAPS:
-
Simplified Acute Physiology Score
- SOFA:
-
Sequential organ failure assessment
- SARS-CoV-2:
-
Severe acute respiratory syndrome coronavirus 2
References
Tabah A, Koulenti D, Laupland K, Misset B, Valles J, Bruzzi de Carvalho F, et al. Characteristics and determinants of outcome of hospital-acquired bloodstream infections in intensive care units: the EUROBACT International Cohort Study. Intensive Care Med. 2012;38(12):1930–45.
Vincent JL, Sakr Y, Singer M, Martin-Loeches I, Machado FR, Marshall JC, et al. Prevalence and outcomes of infection among patients in intensive care units in 2017. JAMA. 2020;323(15):1478–87.
Buetti N, Ruckly S, de Montmollin E, Reignier J, Terzi N, Cohen Y, et al. COVID-19 increased the risk of ICU-acquired bloodstream infections: a case-cohort study from the multicentric OUTCOMEREA network. Intensive Care Med. 2021;47(2):180–7.
Langford BJ, So M, Leung V, Raybardhan S, Lo J, Kan T, et al. Predictors and microbiology of respiratory and bloodstream bacterial infection in patients with COVID-19: living rapid review update and meta-regression. Clin Microbiol Infect Off Publ Eur Soc Clin Microbiol Infect Dis. 2022;28(4):491–501.
Ippolito M, Simone B, Filisina C, Catalanotto FR, Catalisano G, Marino C, et al. Bloodstream infections in hospitalized patients with COVID-19: a systematic review and meta-analysis. Microorganisms. 2021;9(10):2016.
Damonti L, Kronenberg A, Marschall J, Jent P, Sommerstein R, De Kraker MEA, et al. The effect of the COVID-19 pandemic on the epidemiology of positive blood cultures in Swiss intensive care units: a nationwide surveillance study. Crit Care. 2021;25(1):403.
Zhu N, Rawson TM, Mookerjee S, Price JR, Davies F, Otter J, et al. Changing patterns of bloodstream infections in the community and acute care across two COVID-19 epidemic waves: a retrospective analysis using data linkage. Clin Infect Dis Off Publ Infect Dis Soc Am. 2021;75:e1082–91.
Massart N, Maxime V, Fillatre P, Razazi K, Ferre A, Moine P, et al. Characteristics and prognosis of bloodstream infection in patients with COVID-19 admitted in the ICU: an ancillary study of the COVID-ICU study. Ann Intensive Care. 2021;11(1):183.
Tabah A, Buetti N, Staiquly Q, Ruckly S, et al. Epidemiology and determinants of outcome of hospital-acquired bloodstream infections in intensive care unit patients: the EUROBACT-2 international cohort study. Intensive Care Med. 2022. (Under review).
von Elm E, Altman DG, Egger M, Pocock SJ, Gotzsche PC, Vandenbroucke JP, et al. The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement: guidelines for reporting observational studies. J Clin Epidemiol. 2008;61(4):344–9.
Knaus WA, Draper EA, Wagner DP, Zimmerman JE. APACHE II: a severity of disease classification system. Crit Care Med. 1985;13(10):818–29.
Charlson ME, Pompei P, Ales KL, MacKenzie CR. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis. 1987;40(5):373–83.
Le Gall JR, Lemeshow S, Saulnier F. A new Simplified Acute Physiology Score (SAPS II) based on a European/North American multicenter study. JAMA. 1993;270(24):2957–63.
Prevention Cfdca. Carbapenem-resistant Enterobacterales (CRE): CRE Technical Information 2019. Available from https://www.cdc.gov/hai/organisms/cre/technical-info.html#Definition. 2019.
Kadri SS, Adjemian J, Lai YL, Spaulding AB, Ricotta E, Prevots DR, et al. Difficult-to-treat resistance in Gram-negative Bacteremia at 173 US hospitals: retrospective cohort analysis of prevalence, predictors, and outcome of resistance to all first-line agents. Clin Infect Dis Off Publ Infect Dis Soc Am. 2018;67(12):1803–14.
Grasselli G, Scaravilli V, Mangioni D, Scudeller L, Alagna L, Bartoletti M, et al. Hospital-acquired infections in critically ill patients with COVID-19. Chest. 2021;160(2):454–65.
Bonazzetti C, Morena V, Giacomelli A, Oreni L, Casalini G, Galimberti LR, et al. Unexpectedly high frequency of enterococcal bloodstream infections in coronavirus disease 2019 patients admitted to an Italian ICU: an observational study. Crit Care Med. 2021;49(1):e31–40.
DeVoe C, Segal MR, Wang L, Stanley K, Madera S, Fan J, et al. Increased rates of secondary bacterial infections, including Enterococcus bacteremia, in patients hospitalized with coronavirus disease 2019 (COVID-19). Infect Control Hosp Epidemiol. 2021;1–8. https://doi.org/10.1017/ice.2021.391. Online ahead of print.
Krawczyk B, Wityk P, Galecka M, Michalik M. The many faces of Enterococcus spp.—commensal, probiotic and opportunistic pathogen. Microorganisms. 2021;9(9):1900.
Kaafarani HMA, El Moheb M, Hwabejire JO, Naar L, Christensen MA, Breen K, et al. Gastrointestinal complications in critically ill patients with COVID-19. Ann Surg. 2020;272(2):e61–2.
Sun JK, Liu Y, Zou L, Zhang WH, Li JJ, Wang Y, et al. Acute gastrointestinal injury in critically ill patients with COVID-19 in Wuhan, China. World J Gastroenterol. 2020;26(39):6087–97.
Akkus C, Yilmaz H, Mizrak S, Adibelli Z, Akdas O, Duran C. Development of pancreatic injuries in the course of COVID-19. Acta Gastroenterol Belg. 2020;83(4):585–92.
Rasch S, Herner A, Schmid RM, Huber W, Lahmer T. High lipasemia is frequent in Covid-19 associated acute respiratory distress syndrome. Pancreatology. 2021;21(1):306–11.
El Moheb M, Christensen MA, Naar L, Gaitanidis A, Breen K, Alser O, et al. Comment on “Gastrointestinal complications in critically ill patients with COVID-19”: an update. Ann Surg. 2021;274(6):e821–3.
Lin L, Jiang X, Zhang Z, Huang S, Zhang Z, Fang Z, et al. Gastrointestinal symptoms of 95 cases with SARS-CoV-2 infection. Gut. 2020;69(6):997–1001.
Balaphas A, Gkoufa K, Meyer J, Peloso A, Bornand A, McKee TA, et al. COVID-19 can mimic acute cholecystitis and is associated with the presence of viral RNA in the gallbladder wall. J Hepatol. 2020;73(6):1566–8.
Liao Y, Wang B, Wang J, Shu J, Zhou W, Zhang H. SARS-CoV-2 in the bile of a patient with COVID-19-associated gallbladder disease. Endoscopy. 2020;52(12):1148.
Schepis T, Larghi A, Papa A, Miele L, Panzuto F, De Biase L, et al. SARS-CoV2 RNA detection in a pancreatic pseudocyst sample. Pancreatology. 2020;20(5):1011–2.
Zhang H, Li HB, Lyu JR, Lei XM, Li W, Wu G, et al. Specific ACE2 expression in small intestinal enterocytes may cause gastrointestinal symptoms and injury after 2019-nCoV infection. Int J Infect Dis. 2020;96:19–24.
Bilaloglu S, Aphinyanaphongs Y, Jones S, Iturrate E, Hochman J, Berger JS. Thrombosis in hospitalized patients with COVID-19 in a New York City Health System. JAMA. 2020;324(8):799–801.
Hill JB, Garcia D, Crowther M, Savage B, Peress S, Chang K, et al. Frequency of venous thromboembolism in 6513 patients with COVID-19: a retrospective study. Blood Adv. 2020;4(21):5373–7.
Moll M, Zon RL, Sylvester KW, Chen EC, Cheng V, Connell NT, et al. VTE in ICU patients with COVID-19. Chest. 2020;158(5):2130–5.
Shepard BD, Gilmore MS. Antibiotic-resistant enterococci: the mechanisms and dynamics of drug introduction and resistance. Microbes Infect. 2002;4(2):215–24.
Kristich CJ, Rice LB, Arias CA. Enterococcal infection-treatment and antibiotic resistance. In: Gilmore MS, Clewell DB, Ike Y, Shankar N, editors. Enterococci: from commensals to leading causes of drug resistant infection. Boston: Massachusetts Eye and Ear Infirmary; 2014.
De Angelis G, Cataldo MA, De Waure C, Venturiello S, La Torre G, Cauda R, et al. Infection control and prevention measures to reduce the spread of vancomycin-resistant enterococci in hospitalized patients: a systematic review and meta-analysis. J Antimicrob Chemother. 2014;69(5):1185–92.
O’Toole RF. The interface between COVID-19 and bacterial healthcare-associated infections. Clin Microbiol Infect Off Publ Eur Soc Clin Microbiol Infect Dis. 2021;27(12):1772–6.
Thoma R, Seneghini M, Seiffert SN, Vuichard Gysin D, Scanferla G, Haller S, et al. The challenge of preventing and containing outbreaks of multidrug-resistant organisms and Candida auris during the coronavirus disease 2019 pandemic: report of a carbapenem-resistant Acinetobacter baumannii outbreak and a systematic review of the literature. Antimicrob Resist Infect Control. 2022;11(1):12.
Niederman MS, Baron RM, Bouadma L, Calandra T, Daneman N, DeWaele J, et al. Initial antimicrobial management of sepsis. Crit Care. 2021;25(1):307.
Morvan AC, Hengy B, Garrouste-Orgeas M, Ruckly S, Forel JM, Argaud L, et al. Impact of species and antibiotic therapy of enterococcal peritonitis on 30-day mortality in critical care-an analysis of the OUTCOMEREA database. Crit Care. 2019;23(1):307.
Hermine O, Mariette X, Tharaux PL, Resche-Rigon M, Porcher R, Ravaud P, et al. Effect of tocilizumab versus usual care in adults hospitalized with COVID-19 and moderate or severe pneumonia: a randomized clinical trial. JAMA Intern Med. 2021;181(1):32–40.
Group WHOREAfC-TW, Sterne JAC, Murthy S, Diaz JV, Slutsky AS, Villar J, et al. Association between administration of systemic corticosteroids and mortality among critically ill patients with COVID-19: a meta-analysis. JAMA. 2020;324(13):1330–41.
Acknowledgements
The Eurobact 2 study group, National coordinators, scientific committee and participating intensive care units: East Asia and Pacific: Australia—National Coordinator: A/Prof. Alexis Tabah; Scientific Committee: Prof. Jeffrey Lipman; Participating ICUs: The Prince Charles Hospital, Adult Intensive Care Services: Dr. Mahesh Ramanan. Fiona Stanley Hospital, Intensive Care Unit: Dr. Edward Litton, Ms Anna Maria Palermo, Mr Timothy Yap, Mr Ege Eroglu. Japan—National Coordinator: Dr. Yoshiro Hayashi; Participating ICUs: Hiroshima University Hospital, ICU: Dr. Koji Hosokawa. St. Marianna University School of Medicine Hospital, Mixed ICU: Dr. Hideki Yoshida, Prof. Shigeki Fujitani. Middle East and North Africa: Iran—National Coordinator: Prof. Farid Zand; Participating ICUs: Imam-Reza, General Icu: Prof Ata Mahmoodpoor. Zahedan University of Medical Sciences, Clinical Immunology Research Center: Dr. Seyed Mohammad Nasirodin (S.M.N.) Tabatabaei. Saudi Arabia—Participating ICUs: Prince Sultan Medical Military Center, Intensive Care Unit: Dr. Omar Elrabi, Dr. Ghaleb A Almekhlafi. Latin America and The Caribbean: Argentina—National Coordinator: Dr. Gabriela Vidal; Participating ICUs: Hospital Zatti, Ucia: Dra Marta Aparicio, Microbiologa Irene Alonzo. Mexico—National Coordinator: Dr. Silvio A. Namendys-Silva; Participating ICUs: Centenario Hospital Miguel Hidalgo: Dr. Mariana Hermosillo, Dr. Roberto Alejandro Castillo. Europe And Central Asia: Belgium—National Coordinator: Dr. Liesbet De Bus; Scientific Committee: Jan De Waele; Participating ICUs: A.S.Z., Iz: Dr. Isabelle Hollevoet. Clinique Saint-Pierre, Intensive Care Unit: Dr. Nicolas De Schryver, Dr. Nicolas Serck. Bosnia And Herzegovina—National Coordinator: Dr. Pedja Kovacevic; Participating ICUs: University Clinical Centre of The Republic Of Srpska, Medical Intensive Care Unit: Dr. Pedja Kovacevic, Dr. Biljana Zlojutro. France—National Coordinator: Prof. Marc Leone; Scientific Committee: Prof. Jean-François Timsit, Prof. Etienne Ruppe, Mr. Stephane Ruckly, Prof. Philippe Montravers; Participating ICUs: Centre Hospitalier De Bigorre, Service De Réanimation Polyvalente: Dr. Thierry Dulac, Dr. Jérémy Castanera. Centre Hospitalier De Pau, Réanimation Polyvalente: Dr. Alexandre Massri, Dr. Charlotte Guesdon. Ghef Site De Marne-La-Vallée, Réanimation Polyvalente: Dr. Pierre Garcon, Dr. Matthieu Duprey. Groupe Hospitalier Paris Saint Joseph, Médecine Intensive et Réanimation: Dr. François Philippart, Dr. Marc Tran, Dr. Cédric Bruel. Hôpital De La Source, Centre Hospitalier Régional D'orléans, Médecine Intensive & Réanimation (Medical Icu): Dr. François Barbier. Hôpital Louis Pasteur, Réanimation: Dr. Pierre Kalfon, Mr Gaëtan Badre. Sorbonne Universite Pitie Salpetriere, Médecine Intensive Et Réanimation Neurologique: Dr. Sophie Demeret, Dr. Loïc Le Guennec. Italy—National Coordinator: Prof. Matteo Bassetti and Dr. Daniele Giacobbe; Participating ICUs: Città Della Salute E Della Scienza - Molinette, Anestesia E Rianimazione Universitaria: Dr. Giorgia Montrucchio, Dr. Gabriele Sales. Irccs Sacro Cuore Don Calabria, Terapia Intensiva: Dr. Ivan Daroui, Dr. Giovanni Lodi. Policlino Paolo Giaccone, Università Degli Studi Di Palermo, Terapia Intensiva Polivalente: Dr. Andrea Cortegiani, Dr. Mariachiara Ippolito, Dr. Davide Bellina, Dr. Andrea Di Guardo. Sant'andrea Hospital Sapienza University of Rome, Department of Medical And Surgical Science And Translational Medicine Intensive Care Unit: Dr. Monica Rocco, Dr. Silvia Fiorelli. Poland—National Coordinator: Dr. Adam Mikstacki; Participating ICUs: Wss Im. Wl. Bieganskiego, Oddzial Anestezjologii I Intensywnej Terapii - Osrodek Pozaustrojowych Technik Wspomagania Czynnosci Nerek I Wątroby: Prof Assoc Mariusz Peichota, Dr. Iwona Pietraszek-Grzywaczewska. Portugal—National Coordinator: Prof. José-Artur Paiva; Scientific Committee: Prof. Pedro Póvoa; Participating ICUs: CHUA Faro, Smi-1: Dr. Andriy Krystopchuk, Dr. Ana Teresa. Hospital De Cascais Dr Jose De Almeida, Unidade de Cuidados Intensivos: Dr. António Manuel Pereira de Figueiredo, Dr. Isabel Botelho. Hospital Sao Francisco Xavier, CHLO, Unidade De Cuidados Intensivos Polivalente: Dr. Vasco Costa, Dr. Rui Pedro Cunha. Russian Federation—National Coordinator: Prof Alexey Gritsan; Participating ICUs: Privolzhskiy District Medical Center, Department Anesthesiology and Intensive Care: Dr. Vladislav Belskiy, Dr. Mikhail Furman. Spain—National Coordinator: Dr. Ricard Ferrer; Participating ICUs: Vall D'herbon, Intensive Care Medicine: Dr. Ricard Ferrer, Dr. Maria Martinez, Dr. Vanessa Casares. Hospital Del Mar, Critical Care Unit: Dr. Maria Pilar Gracia Arnillas, Dr. Rosana Munoz Bermudez. Hospital Punta De Europa, Intensive Care Unit: Dr. Alejandro Ubeda, Dra Maria Salgado. Hospital Universitario La Paz, Surgical Critical Care Unit: Dr. Emilio Maseda, Dr. Alejandro Suarez De La Rica. University Hospital Severo Ochoa, Intensive Care Unit: Dr. Miguel Angel Blasco-Navalpotro, Dr. Alberto Orejas Gallego. Switzerland—National Coordinator: Dr. Josef Prazak; Scientific Committee: Dr. Niccolò Buetti; Participating ICUs: Chuv, Service De Médecine Intensive Adulte: Dr. Jl Pagani, Mrs S Abed-Maillard. Turkey—National Coordinator: Prof. Akova Murat, Dr. Abdullah Tarık Aslan; Participating ICUs: Hacettepe University of Faculty of Medicine, Intensive Care Unit(ICU): Dr. Akova Murat, Dr. Abdullah Tarik Aslan, Dr. Arzu Topeli Iskit. Acibadem Kadikoy Hospital, ICU: Dr. Selcuk Mehtap, Dr. Solakoğlu Ceyhun. Ankara Yildirim Beyazit University, Ankara City Hospital, Infectious Diseases and Clinical Microbiology: Dr. Bircan Kayaaslan, Dr. Ayşe Kaya Kalem. Aydin Adnan Menderes University Research Hospital, Anesthesia and Reanimation ICU: Prof. Dr. Ibrahim Kurt, Dr. (Professor) Murat Telli, Dr. (Associate Professor) Barcin Ozturk. Hitit University Erol Olcok Education and Research Hospital, Infectious Diseases and Clinical Microbiology: Prof. Dr. Nurcan (N) Baykam, Assistant Prof. Dr. Özlem (O) Akdoğan. Istanbul University-Cerrahpasa, Cerrahpasa Medical Faculty, Sadi Sun ICU: Prof.Dr. Nese Saltoglu, Ass Prof.Dr. Ridvan Karaali. Karadeniz Technical University Faculty of Medicine, Infectious Disease and Clinical Microbiology: Prof Dr. Iftihar Koksal, Assist. Prof. Firdevs Aksoy. Kartal Dr. Lutfi Kirdar Training and Research Hospital, ICU: Dr. Kemal Tolga Saracoglu, Dr. Yeliz Bilir. Kayseri City Hospital, ICU: Dr. Seda Guzeldag. Mersin University Hospital, Department of Infectious Diseases and Clinical Microbiology: Dr. Gulden Ersoz, Dr. Guliz Evik. School Of Medicine, Medipol Mega University Hospitals Complex, Department of Anesthesiology and Reanimation: Dr. Cem Erdogan. Turgut Ozal Medical Center, Department of Infectious Diseases and Clinical Microbiology: Dr. Yasar Bayindir, Dr. Yasemin Ersoy. The United Kingdom—National Coordinator: Dr. Andrew Conway Morris; Participating ICUs: Addenbrookes Hospital, John V Farman Intensive Care Unit: Dr. Andrew Conway Morris, Dr. Matthew Routledge. Addenbrookes Hospital, Neurocritical Care Unit (NCCU): Dr. Andrew Conway Morris, Dr. Ari Ercole. Croydon University Hospital, Critical Care Unit: Dr. Ashok Raj, Dr. Artemis Zormpa, Dr. George Tinaslanidis, Mrs Reena Khade. Queen Elizabeth Hospital, Lewisham and Greenwich NHS Trust, Critical Care Unit: Dr. Ashraf Roshdy Sandwell And West Birmingham Hospitals NHS Trust, Intensive Care Unit: Dr. Santhana Kannan, Dr. Supriya Antrolikar, Dr. Nicholas Marsden. Warwick Hospital, Intensive Care Unit: Dr. Ben Attwood, Dr. Jamie Patel. South Asia: India—National Coordinator: Prof. Mohan Gurjar; Participating ICUs: St Johns Medical College Hospital, Department of Critical Care Medicine, Micu: Dr. Carol Dsilva, Dr. Jagadish Chandran. Sub-Saharan Africa: Sudan—National Coordinator: Dr. Bashir El Sanousi; Participating ICUs: East Nile Hospital, Intensive Care Unit: Dr. Elfayadh Saidahmed, Dr. Hytham K.S. Hamid.
Funding
Research grants were obtained from the European society of Intensive Care Medicine (ESICM) and the European Society of Clinical Microbiology and Infectious Diseases (ESCMID) study Group for Infections in Critically Ill Patients (ESGCIP), the Norva Dahlia foundation and the Redcliffe Hospital Private Practice Trust Fund. Dr. Buetti received a grant from the Swiss National Science Foundation (Grant Number: P4P4PM_194449). The study was endorsed by the critically ill group of the ESCMID (ESGCIP) and by the infection group of the ESICM with scientific input of the OUTCOMEREA network.
Author information
Authors and Affiliations
Consortia
Contributions
NB, AT and JFT designed the study. All authors acquired the data. NB, AL, SR and JFT did the statistical analysis. NB, AL, SR, AT and JFT analyzed and interpreted the data. NB and JFT drafted the manuscript. All authors critically reviewed the manuscript and approved the final report. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards. The study was approved by the ethics Committee from the Royal Brisbane & Women's Hospital Human Research (LNR/2019/QRBW/48376). Waiver of informed consent was granted in the initial ethical approval and for most participating ICUs. Informed consent was obtained from all individual participants included in the study recruited from ICUs whose regional/national ethics committees required informed consent.
Consent for publication
Not applicable.
Competing interests
The authors have disclosed that they do not have conflict of interest. Dr. Buetti received a grant from the Swiss National Science Foundation (Grant Number: P4P4PM_194449). Prof. Timsit received fees for lectures to 3M, MSD, Pfizer, and BioMérieux; he received research grants from Astellas, 3M, MSD, and Pfizer; and he participated to advisory boards of 3M, MSD, Bayer Pharma, Nabriva, and Pfizer. Dr. Barbier received consulting and lecture fees from MSD and BioMérieux. Prof. Cortegiani received fees for lectures from Gilead, MSD, Pfizer; and he participated to advisory boards of MSD, Gilead, Pfizer. Dr. Montrucchio received fees for lectures from Gilead, Pfizer, Thermofisher; and she participated to advisory boards of Gilead. Dr. Conway Morris sits on the scientific advisory board of Cambridge Infection Diagnostics. Prof. Akova received grants from Pfizer and Gilead, had lecture fees paid to the institution by Pfizer and Sanofi. Dr. Ramanan acknowledges support from the Metro North Hospital and Health Services Clinician-Researcher Fellowship. Dr. Conway Morris sits on the scientific advisory board of Cambridge Infection Diagnostics. Dr. Conway Morris is supported by a Clinician Scientist Fellowship from the Medical Research Council (MR/V006118/1). Prof. José-Artur Paiva received fees for consulting, advisory boards or lectures from MSD, Pfizer, Astra-Zeneca, Gilead, Jansen, Cepheid, AOP Orphan Pharmaceuticals.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Additional file 1.
Additional methods (definitions, additional methods, statistical analyses and ethics), Additional tables (Tables S1–S8) and Additional figures (Figs. S1–S2).
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Buetti, N., Tabah, A., Loiodice, A. et al. Different epidemiology of bloodstream infections in COVID-19 compared to non-COVID-19 critically ill patients: a descriptive analysis of the Eurobact II study. Crit Care 26, 319 (2022). https://doi.org/10.1186/s13054-022-04166-y
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s13054-022-04166-y