Abstract
Background
Inhaled nitric oxide (iNO) is used as rescue therapy in patients with refractory hypoxemia due to severe COVID-19 acute respiratory distress syndrome (ARDS) despite the recommendation against the use of this treatment. To date, the effect of iNO on the clinical outcomes of critically ill COVID-19 patients with moderate-to-severe ARDS remains arguable. Therefore, this study aimed to evaluate the use of iNO in critically ill COVID-19 patients with moderate-to-severe ARDS.
Methods
This multicenter, retrospective cohort study included critically ill adult patients with confirmed COVID-19 treated from March 01, 2020, until July 31, 2021. Eligible patients with moderate-to-severe ARDS were subsequently categorized into two groups based on inhaled nitric oxide (iNO) use throughout their ICU stay. The primary endpoint was the improvement in oxygenation parameters 24 h after iNO use. Other outcomes were considered secondary. Propensity score matching (1:2) was used based on the predefined criteria.
Results
A total of 1598 patients were screened, and 815 were included based on the eligibility criteria. Among them, 210 patients were matched based on predefined criteria. Oxygenation parameters (PaO2, FiO2 requirement, P/F ratio, oxygenation index) were significantly improved 24 h after iNO administration within a median of six days of ICU admission. However, the risk of 30-day and in-hospital mortality were found to be similar between the two groups (HR: 1.18; 95% CI: 0.77, 1.82; p = 0.45 and HR: 1.40; 95% CI: 0.94, 2.11; p= 0.10, respectively). On the other hand, ventilator-free days (VFDs) were significantly fewer, and ICU and hospital LOS were significantly longer in the iNO group. In addition, patients who received iNO had higher odds of acute kidney injury (AKI) (OR (95% CI): 2.35 (1.30, 4.26), p value = 0.005) and hospital/ventilator-acquired pneumonia (OR (95% CI): 3.2 (1.76, 5.83), p value = 0.001).
Conclusion
In critically ill COVID-19 patients with moderate-to-severe ARDS, iNO rescue therapy is associated with improved oxygenation parameters but no mortality benefits. Moreover, iNO use is associated with higher odds of AKI, pneumonia, longer LOS, and fewer VFDs.
Similar content being viewed by others
Introduction
One of the major complications of coronavirus disease (COVID-19) is acute hypoxemic respiratory failure requiring mechanical ventilation [1, 2]. This complication can increase mortality rates, particularly in patients who require mechanical ventilation [2,3,4,5]. It is estimated that the mortality rate in patients with COVID-19 and acute respiratory distress syndrome (ARDS) ranges between 26 and 88.3% [6,7,8,9]. ARDS involves an acute alveolar-capillary membrane inflammatory response that is characterized by poor oxygenation and pulmonary infiltrates, resulting in "stiffness" of the lungs leading to hypoxic failure [10, 11]. ARDS can cause an imbalance between ventilation and perfusion, resulting in intensified intrapulmonary shunting in nonventilated lung regions from pulmonary vasodilation and vasoconstriction in ventilated zones, as well as pulmonary hypertension [12].
There is no specific pharmacologic agent that treats ARDS, and the management mainly consists of supportive lung-protective ventilation to minimize lung injury [11]. Multiple rescue therapies, including neuromuscular blockade and inhaled pulmonary vasodilators such as inhaled nitric oxide (iNO), have been used in refractory hypoxemia ARDS [13]. Inhaled nitric oxide may improve ventilation and overcome perfusion imbalance and pulmonary vascular resistance, relieving hypoxemia caused by ARDS [14]. A previous meta-analysis including 14 studies reported that iNO was not associated with any mortality benefit in patients with ARDS [15]. Nonetheless, it had a transient positive effect on oxygenation, suggesting that there is a potential benefit of iNO in this patient population [15].
The evidence regarding the safety and efficacy of iNO in patients with ARDS due to COVID-19 is limited and contradictory [16]. In two observational studies, the use of iNO led to significant improvement in oxygenation and the PaO2/FiO2 ratio [17, 18]. In contrast, Tavazzi et al. did not find any significant benefit of oxygenation in patients with COVID-19 and refractory hypoxemia who received iNO as rescue therapy [19]. A subanalysis of a systematic review of inhaled pulmonary vasodilators, including four trials of iNO, did not find any improvement in oxygenation with the use of iNO as rescue therapy among patients with COVID-19 and refractory hypoxemia [20]. Therefore, several guidelines recommend against the routine use of iNO in mechanically ventilated patients with COVID-19 ARDS [21, 22]. However, in mechanically ventilated patients with refractory hypoxemia due to severe COVID-19 ARDS, iNO may be used as a rescue therapy.
Despite the limited evidence regarding the benefit of iNO, clinicians continue to use it as a last resort for mechanically ventilated patients with ARDS and COVID-19. To date, the effect of iNO on the clinical outcomes of critically ill patients with COVID-19 and moderate-to-severe ARDS remains arguable. Thus, the objective of this study was to evaluate the use of iNO in critically ill COVID-19 patients with moderate-to-severe ARDS.
Methods
Study design
A multicenter, retrospective cohort study all critically ill adult patients with confirmed COVID-19 who were admitted to intensive care units (ICUs) from March 01, 2020, until July 31, 2021. The patients were diagnosed with COVID-19 using reverse transcriptase-polymerase chain reaction (RT‒PCR) nasopharyngeal or throat swabs. The eligible patients were categorized into two groups based on iNO use during the ICU stay (control vs. iNO). All patients were followed until they were discharged from the hospital or died during their stay. The study was approved by King Abdullah International Medical Research Center (KAIMRC) in December 2020 (Ref.# RC20. 638.R). The IRB committee waived informed consent from the study patients due to the retrospective observational nature of the study. All methods were performed in accordance with relevant guidelines and regulations.
Study participants
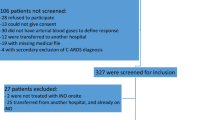
We included adult patients (age ≥ 18 years) admitted to the ICUs with confirmed COVID-19. Patients were excluded if they did not develop respiratory failure that required mechanical ventilation (MV) during the ICU stay, were not on MV at admission, had a PaO2/FiO2 ratio > 200 within 24 h of ICU admission, had serum creatinine > 2.5 mg/dL (221 mmol/l), had an ICU length of stay (LOS) ≤ one day, died within the first 24 h of ICU admission or were labeled "Do-Not-Resuscitate" (Fig. 1).
Study setting
The study took place at five hospitals in Saudi Arabia: King Abdulaziz Medical City (Riyadh & Jeddah), King Abdulaziz University Hospital (Jeddah), King Abdullah bin Abdulaziz University Hospital (KAAUH) (Riyadh), and King Salman Specialist Hospital (Hail). These centers were selected based on the geographic distribution, the availability of electronic records, and the center's willingness to participate in the national project. The primary site for this multicenter retrospective study was King Abdulaziz Medical City (Riyadh), a tertiary care center.
Inhaled nitric oxide administration
Nitric oxide emanates in gas form and is stored in cylinders. The gas regulator should be attached to the nitric oxide (NO) cylinder, and the cylinder should have sufficient pressure to initiate and maintain the therapy throughout the procedure. Calibration of the O2 analyzer is essential before connecting the mechanical ventilator inspiratory limb (to the patient) and then the NO and NO2 analyzer afterward. In this study, the gas sampling line was attached to the back of the analyzer, and then the gas sampling line was attached to the ventilator inspiratory limb just before the humidifier. The initial dose of iNO that is institutionally applied in clinical practice is 20 parts per million (PPM) following AARC clinical guidelines in severe ARDS adult patients, whereas 40 PPM is the maximum dose. However, observation of patient response to any complications related to iNO use and avoiding overdose is needed. Excess doses of iNO may cause methemoglobinemia (MetHb) and result in a drop in blood pressure [23,24,25].
Data collection
Each participant's data were collected and controlled using King Abdullah International Medical Research Center's Research Electronic Data Capture (REDCap®) software. We collected patients' demographic data, comorbidities, vital signs and laboratory tests, baseline severity scores (i.e., Acute Physiology and Chronic Health Evaluation II (APACHE II), Sequential Organ Failure Assessment (SOFA), Nutrition Risk in the Critically Ill (NUTRIC) and multiple organ dysfunction scores), Glasgow Coma Score (GCS), acute kidney injury status, use of prone positioning, receipt of mechanical ventilation (MV), MV parameters (e.g., PaO2/FiO2 ratio, FiO2 requirement) and oxygenation index (OI) within 24 h of ICU admission. Moreover, renal profile, liver function tests (LFTs), coagulation profile (i.e., INR, aPTT, fibrinogen, D-dimer), and inflammatory markers (ferritin, procalcitonin, and creatine phosphokinase (CPK)) within 24 h of ICU admission were collected. In addition, oxygenation parameters (i.e., PaO2, PaCO2, oxygenation index (OI), PaO2/FiO2 ratio, and FiO2 requirement) were collected pre- and 24 h post-iNO use. Last, the use of tocilizumab, corticosteroids, and inhaled nitric oxide (timing to initiation, dose, and duration) during the ICU stay was recorded for the eligible patients.
Outcomes
The primary endpoint was the improvement in oxygenation parameters 24 h after iNO use. The secondary endpoints were 30-day and in-hospital mortality, hospital LOS, ICU LOS, number of ventilator-free days (VFDs) at 30 days, and ICU-acquired complications (new-onset atrial fibrillation, thrombosis, acute kidney injury (AKI), liver injury, hospital/ventilator-acquired pneumonia, and secondary fungal infection). (Additional file 1) [26,27,28].
Statistical analysis
Continuous variables are presented as the mean with standard deviation (SD) or the median with lower and upper quartiles (Q1, Q3), and categorical variables are presented as a number with a percentage as appropriate. All numerical variables' normality assumptions were assessed using a statistical test (i.e., Shapiro–Wilk test) and graphical representation (i.e., histograms and Q–Q plots). In addition, model fit was assessed using the Hosmer‒Lemeshow goodness-of-fit test.
Baseline and outcome variables were compared between the two study groups. The Chi-square or Fisher exact test was used for categorical variables. Normally distributed continuous variables were compared using Student’s t test, and the Mann‒Whitney U test was used to compare non-normally distributed variables. Multivariable Cox proportional hazards regression analyses were performed for 30-day and in-hospital mortality. The proportionality assumption was assessed before fitting the Cox model. Visual assessment was performed to assess the assumption by plotting log(-log) plots and testing the correlation of scaled Schoenfeld residuals with rank-ordered time. Multivariable logistic regression and negative binomial regression analysis were used for the other outcomes considered in this study as appropriate. The odds ratios (OR) or estimates with the 95% confidence intervals (CI) were reported as appropriate. No imputation was made for missing data, as the cohort of patients in our study was not derived from random selection.
The propensity score matching procedure (Proc PS match) (SAS, Cary, NC) was used to match patients (1:2 ratio) who received inhaled nitric oxide therapy (active group) to patients who did not (control group). Regression analysis was performed for the study outcomes after considering PS scores as covariates in the model. These PS scores were generated through propensity score analysis after considering all relevant covariates, which included the patient's age, BMI, early use of dexamethasone and tocilizumab within 24 h of ICU admission, SOFA score, serum creatinine, PaO2/FiO2 ratio at admission, pulmonary hypertension, and right heart failure [29,30,31]. Patients were matched only if the difference in the logits of the propensity scores for pairs of patients from the two groups was less than or equal to 0.1 times the pooled estimate of the standard deviation. A greedy nearest neighbor matching method was used in which one patient who received inhaled nitric oxide (active) was matched with two patients who did not (control), which eventually produced the smallest within-pair difference among all available pairs with treated patients.
Results
During the study period, 1598 patients were screened. Based on the eligibility criteria, we included a total of 815 patients in our analysis (Fig. 1). Among them, 76 (9.3%) received inhaled nitric oxide (iNO), and of these, twenty-seven patients were considered iNO responders. After propensity score matching (1:2 ratio), we included 210 patients based on predefined criteria. Patients were given iNO with a median dose of 40.0 (32.5, 40.0) parts per million (PPM) for a median duration of 85.0 (37.0, 148.0) hours. The median time for iNO initiation from ICU admission was six days.
Demographic and clinical characteristics
Most patients in both arms (60.2%) were male, with a mean age of 62.5 (SD ± 14.3). Diabetes mellitus (DM) (58.2%), hypertension (HTN) (55.5%), and dyslipidemia (22%) were the most prevalent underlying comorbidities in our patients. Before propensity score (PS) matching, there was a notable difference between the groups, which demonstrated that patients in the iNO group received more inotrope support, nephrotoxic drugs/material, pharmacologic deep vein thrombosis (DVT) prophylaxis, and early use of tocilizumab and dexamethasone therapy. In addition, the patients who received iNO had a higher body mass index (BMI), and pulmonary HTN. Of interest, those who received iNO had more DVT and liver disease as a comorbid condition before PS matching. After PS matching, most of the patients' characteristics were found to be comparable between the two groups, except that patients who received iNO had a lower baseline C-reactive protein (CRP) and higher liver disease as comorbid conditions (Table 1).
Oxygenation parameters
After 24 h of receiving iNO, the FiO2 requirement significantly improved, with a median of 70 (IQR 35); p < 0.01. Moreover, the PaO2 and PaO2/FiO2 ratio were significantly improved, with a median of 72.8 (IQR 19.2); p < 0.001 and a median of 106.9 (IQR 58.9); p < 0.01, respectively. Additionally, the oxygenation index was significantly lower after using iNO, with a median of 18.9 (IQR 9.3); p < 0.001. Last, the PaCO2 was lower after receiving iNO; however, it did not reach statistical significance (p = 0.10) (Fig. 2).
Mortality, ventilator-free days, and length of stay
The multivariable Cox proportional hazards regression analyses showed higher 30-day mortality (HR 1.18; 95% CI 0.77, 1.82; p = 0.45) and in-hospital mortality in patients who received iNO (HR 1.40; 95% CI 0.94, 2.11; p = 0.10) than in patients who did not receive iNO. However, the difference did not reach statistical significance (Table 2).
The number of VFDs during the ICU stay at 30 days was significantly lower in the crude and regression analysis in patients who received iNO (beta coefficient (95% CI): − 1.17 (− 1.79, − 0.54), p < 0.001). Moreover, both ICU and hospital LOS were significantly longer in patients who received iNO than in the control patients, with beta coefficient (95% CI): 0.63 (0.32, 0.95) and beta coefficient (95% CI): 0.45 (0.04, 0.87), respectively (Table 2).
Complications during the ICU stay
Patients who received iNO demonstrated significantly higher odds of AKI (OR (95% CI): 2.35 (1.30, 4.26), p = 0.005) (Fig. 3). In addition, the mean delta serum creatinine was significantly increased 24 h post-iNO initiation compared to preiNO in the iNO group (19.6 mmol/l (± 74); p value = 0.001). Notably, after PS matching, there was no statistically significant difference in the number of nephrotoxic medications used during the ICU stay between the two groups at baseline (95.2% vs. 97.1%; p = 0.34) (Table 1).
The incidence of acute liver injury and pneumonia (HAP/VAP) was significantly higher among the patients who received iNO than control group (OR (95% CI): 3.32 (1.34, 8.22), p = 0.009, and OR (95% CI): 3.2 (1.76, 5.83), p < 0.001, respectively). At the baseline, patients who received iNO had a significantly higher rate of liver disease (Table 1). On the other hand, there was no statistically significant difference in the incidence of new-onset atrial fibrillation (NOAF), thrombosis events, or secondary fungal infection between the two groups (Fig. 3).
Discussion
There are limited studies investigating the role of iNO in the management of severe hypoxia due to COVID-19 [32,33,34]. In this retrospective cohort study, we aimed to assess the improvement in oxygenation parameters 24 h after using a median dose of 40 ppm of rescue iNO for a median duration of 85 h in critically ill COVID-19 patients with moderate to severe ARDS. A significant improvement was observed in our cohort in the PaO2, oxygenation index, PaO2/FiO2 ratio, and FiO2 requirement within 24 h after iNO initiation within a median of six days from ICU admission.
Chiara Robba et al., in a prospective observational study conducted to assess the effectiveness of ventilatory rescue strategies in ventilated patients with COVID-19 ARDS, demonstrated that the consumption of iNO improved PaO2 (from 65 [67–73] to 72 [67–73] mmHg, p = 0.015) and rSO2 (from 53 [51–56] to 57 [55–59] %, p = 0.007) [35]. This result was consistent with our findings of the improvement in PaO2 with a 10.1% difference post-iNO use.
In patients with COVID-19 and ARDS, significant vascular endothelial damage and a higher incidence of pulmonary microthrombi were previously noted [34]. Most critically ill patients require MV due to difficulty maintaining ventilation and oxygenation, which remains the main challenge in critically ill patients with COVID-19 [34]. In critical care settings, one goal during the COVID-19 pandemic is to increase ventilator-free days to minimize the requirement for respiratory support devices. Thus, enhanced oxygenation caused by smooth muscle vasodilation and increased blood flow to the alveoli is the primary justification for utilizing iNO [36]. In our study, patients with COVID-19 and ARDS who received iNO had significantly fewer VFDs and longer ICU stays. This result contradicts the previously reported finding that receiving iNO did not result in a reduction in the number of VFDs and duration of MV (MD (95% CI)− 0.57 (− 1.82–0.69); I2 = 0%) vs. (MD (95% CI) 1.02 (− 2.08–4.12); I2 = 76%), respectively.
Two observational studies evaluated the use of iNO in patients with COVID-19 and ARDS; both found a lower mortality rate in those patients. In the Parikh et al. study, the mortality rate was 23% of the total patients who received iNO [33]. Ferrari et al. reported that 80% of patients who received 20–30 ppm of iNO survived and were discharged from the hospital [32]. These results are difficult to interpret due to the lack of a comparator group. Our data showed no mortality benefit in patients who received iNO for ARDS secondary to COVID-19. The 30-day and in-hospital mortality hazard ratio were 1.18 (0.77, 1.82) (p = 0.45) and 1.40 (0.94, 2.11) (p = 0.10), respectively. The timing of initiating iNO therapy and the presence of other risk factors might justify this finding. Acute kidney injury, pneumonia, DM, or infections can influence the mortality rate [37, 38]; it is important to highlight that the SOFA and APACHE II scores were similar between the groups in our study. Our results are in accordance with the results derived from a randomized trial (2004) in ARDS patients, which found that iNO did not improve 28-day mortality [39]. Moreover, Angus et al. reported no difference in survival among the study population at hospital discharge and during the first year. None of the patients included in these studies were patients with COVID-19 and ARDS.
We report a longer ICU stay and hospital LOS in patients who receive iNO (p value = < 0.001). In our study, there was a trend toward an increased risk of AKI, and the rate of hospital/ventilator-acquired pneumonia was significantly higher in the iNO group. These two reasons may have affected the LOS in our study population. Nonetheless, our results agree with a randomized placebo-controlled trial performed by Taylor et al. published in 2004; the researchers restricted their study population to critically ill ARDS patients and found that treatment with iNO did not indicate a reduction in ICU or hospital LOS [39]. Conversely, no studies evaluated iNO effect on the ICU and hospital LOS in COVID-19 patients either with or without ARDS.
Our study demonstrated a possible correlation between the incidence of AKI and the administration of iNO in critically ill patients with COVID-19. This result is consistent with the findings of Wang et al., who identified an increased risk of AKI in ARDS patients with iNO therapy [40]. In our cohort, the occurrence of new-onset atrial fibrillation (NOAF) was not different between the groups. Similar to the results of Koyfman et al., only one patient out of 221 experienced NOAF during intrahospital transport [41]. Pulmonary embolism (PE), DVT, and systemic arterial embolism are common complications in critically ill patients with COVID-19 [42]. Inhaled NO inhibits platelet adhesion and aggregation by reducing fibrinogen binding, which prevents thrombus formation [43]. Our results suggest a possible correlation between the incidence of thrombosis and the administration of iNO. Venous/arterial thrombosis was lower in the iNO group, but the difference was not statistically significant. Notably, most of our patients (97.6%) were on pharmacological DVT prophylaxis.
Nitric oxide (NO) appears to have an immunopathological effect on a host's immunological response and has been shown to decrease type 1 helper T-cell-dependent immune responses. Beyond that, NO and oxygen radicals such as superoxide are essential mediators in the pathophysiology of several infectious illnesses. The biosynthesis of NO is found in a wide range of infectious diseases. Therefore, inducible nitric oxide synthase (NOS) is produced in a large amount over a long time, allowing for the formation of peroxynitrite, a highly reactive nitrogen oxide molecule, which induces oxidative tissue destruction [44]. In addition, patients with COVID-19 who are critically ill are at significant risk of hospital-acquired infections, which are usually caused by MDR bacteria [45]. On the other hand, in a randomized controlled trial, Taylor et al. reported more infection incidents in the low dose iNO group than in the placebo group (66% vs. 41%). Nevertheless, the incidence did not reach statistical significance. This contrasts with our findings, which showed a statistically significant difference in the incidence of ventilator-acquired pneumonia and hospital-acquired pneumonia [39]. Secondary fungal infection did not result in a statistically significant difference. Since most of our patients received steroids and tocilizumab during the first 24 h after admission, we could not completely rule out the effects of such drugs.
To our knowledge, our study is one of the first multicenter studies to investigate the efficacy, safety, ICU stay, and hospital LOS in critically ill patients using iNO for COVID-19–induced moderate-to-severe ARDS. In addition, a propensity matching score was used to decrease the risk of bias and to establish a balance between both groups. However, the limitations of our study include the following. First, this was a retrospective observational study; second, it had a small sample size; third, we did not assess the variety of nitric oxide dosing; last, a short follow-up period may have limited the capturing of other long-term complications. Accordingly, future randomized controlled trials are necessary to support our findings.
Conclusion
The use of iNO rescue therapy in critically ill COVID-19 patients with moderate-to-severe ARDS is significantly associated with an improvement in oxygenation parameters (PaO2, FiO2 requirement, P/F ratio, OI) with no mortality benefits. However, iNO use is associated with higher odds of AKI, pneumonia, and LOS, as well as fewer VFDs. Further randomized clinical and interventional studies are required to confirm our findings.
Availability of data and materials
The datasets used and/or analyzed during the current study are available from corresponding author on reasonable request.
References
Zhou F, Yu T, Du R, et al. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study [Internet]. Lancet. 2020;395:1054–62. https://doi.org/10.1016/S0140-6736(20)30566-3.
Wilcox SR. Management of respiratory failure due to covid-19. BMJ. 2020;369: m1786.
Lim ZJ, Subramaniam A, Ponnapa Reddy M, et al. Case fatality rates for patients with COVID-19 requiring invasive mechanical ventilation. a meta-analysis. Am J Respir Crit Care Med. 2021;203:54–66.
Oliveira E, Parikh A, Lopez-Ruiz A, et al. ICU outcomes and survival in patients with severe COVID-19 in the largest health care system in central Florida. PLoS ONE. 2021;16: e0249038.
Grasselli G, Cattaneo E, Florio G, et al. Mechanical ventilation parameters in critically ill COVID-19 patients: a scoping review. Crit Care. 2021;25:115.
Grasselli G, Zangrillo A, Zanella A, et al. Baseline characteristics and outcomes of 1591 patients infected with SARS-CoV-2 admitted to ICUs of the lombardy region. Italy JAMA. 2020;323:1574–81.
Wang Y, Lu X, Li Y, et al. Clinical course and outcomes of 344 intensive care patients with COVID-19. Am J Respir Crit Care Med. 2020;201:1430–4.
Yang X, Yu Y, Xu J, et al. Clinical course and outcomes of critically ill patients with SARS-CoV-2 pneumonia in Wuhan, China: a single-centered, retrospective, observational study. Lancet Respir Med. 2020;8:475–81.
Aslan A, Aslan C, Zolbanin NM, et al. Acute respiratory distress syndrome in COVID-19: possible mechanisms and therapeutic management. Pneumonia (Nathan Qld). 2021;13:14.
Jain R, DalNogare A. Pharmacological therapy for acute respiratory distress syndrome. Mayo Clin Proc. 2006;81:205–12.
Fan E, Brodie D, Slutsky AS. Acute respiratory distress syndrome: advances in diagnosis and treatment. JAMA. 2018;319:698–710.
Swenson KE, Swenson ER. Pathophysiology of acute Respiratory distress syndrome and COVID-19 lung injury. Crit Care Clin. 2021;37:749–76.
Welker C, Huang J, Gil IJN, et al. 2021 acute respiratory distress syndrome update, with coronavirus disease 2019 focus. J Cardiothorac Vasc Anesth. 2021;36:1188.
Dellinger RP, Zimmerman JL, Taylor RW, et al. Effects of inhaled nitric oxide in patients with acute respiratory distress syndrome: results of a randomized phase II trial. Inhaled nitric oxide in ARDS study group. Crit Care Med. 1998;26:15–23.
Gebistorf F, Karam O, Wetterslev J, et al. Inhaled nitric oxide for acute respiratory distress syndrome (ARDS) in children and adults. Cochrane Database Syst Rev. 2016;2016:CD002787.
Safaee Fakhr B, Di Fenza R, Gianni S, et al. Inhaled high dose nitric oxide is a safe and effective respiratory treatment in spontaneous breathing hospitalized patients with COVID-19 pneumonia. Nitric oxide Biol Chem. 2021;116:7–13.
Lotz C, Muellenbach RM, Meybohm P, et al. Effects of inhaled nitric oxide in COVID-19-induced ARDS - Is it worthwhile? Acta Anaesthesiol Scand. 2021;65:629–32.
Garfield B, McFadyen C, Briar C, et al. Potential for personalised application of inhaled nitric oxide in COVID-19 pneumonia. Br J Anaesth. 2021;126:e72–5.
Tavazzi G, Pozzi M, Mongodi S, et al. Inhaled nitric oxide in patients admitted to intensive care unit with COVID-19 pneumonia. Crit Care. 2020;24:508.
Beran A, Mhanna M, Srour O, et al. Inhaled pulmonary vasodilator treatment for COVID-19: a systematic review and meta-analysis. Chest. 2021;160:a558.
COVID-19 Treatment Guidelines Panel. Coronavirus Disease 2019 (COVID-19) Treatment Guidelines. National Institutes of Health. [Internet]. [cited 2022 Jan 21] Available from: https://www.covid19treatmentguidelines.nih.gov/management/critical-care/oxygenation-and-ventilation/
Alhazzani W, Møller MH, Arabi YM, et al. Surviving sepsis campaign: guidelines on the management of critically ill adults with Coronavirus Disease 2019 (COVID-19). Intensive Care Med. 2020;46:854–87.
Gentile MA. Inhaled medical gases: more to breathe than oxygen. Respir Care. 2011;56:1341–9.
DiBlasi RM, Myers TR, Hess DR. Evidence-based clinical practice guideline: inhaled nitric oxide for neonates with acute hypoxic respiratory failure. Respir Care. 2010;55:1717–45.
Weinberger B, Laskin DL, Heck DE, Laskin JD. The toxicology of inhaled nitric oxide. Toxicol Sci. 2001;59(1):5–16. https://doi.org/10.1093/toxsci/59.1.5.
Lin C-Y, Chen Y-C. Acute kidney injury classification: AKIN and RIFLE criteria in critical patients. World J Crit care Med. 2012;1:40–5.
Aleidan FAS, Alkhelaifi H, Alsenaid A, et al. Incidence and risk factors of carbapenem-resistant Enterobacteriaceae infection in intensive care units: a matched case-control study. Expert Rev Anti Infect Ther. 2021;19:393–8.
Rodriguez-Roisin R. Pulmonary gas exchange in acute respiratory failure. Eur J Anaesthesiol. 1994;11:5–13.
Hirsch JS, Ng JH, Ross DW, et al. Acute kidney injury in patients hospitalized with COVID-19. Kidney Int. 2020;98(1):209–18. https://doi.org/10.1016/j.kint.2020.05.006.
Al Sulaiman KA, Aljuhani O, Eljaaly K, et al. Clinical features and outcomes of critically ill patients with coronavirus disease 2019 (COVID-19): a multicenter cohort study. Int J Infect Dis. 2021;105:180–7. https://doi.org/10.1016/j.ijid.2021.02.037.
Norderfeldt J, Liliequist A, Frostell C, et al. Acute pulmonary hypertension and short-term outcomes in severe Covid-19 patients needing intensive care. Acta Anaesthesiol Scand. 2021;65(6):761–9. https://doi.org/10.1111/aas.13819.
Ferrari M, Santini A, Protti A, et al. Inhaled nitric oxide in mechanically ventilated patients with COVID-19. J Crit Care. 2020;60:159–60.
Parikh R, Wilson C, Weinberg J, et al. Inhaled nitric oxide treatment in spontaneously breathing COVID-19 patients. Ther Adv Respir Dis. 2020;14:1753466620933510.
Prakash A, Kaur S, Kaur C, et al. Efficacy and safety of inhaled nitric oxide in the treatment of severe/critical COVID-19 patients: a systematic review. Indian J Pharmacol. 2021;53:236–43.
Karam O, Gebistorf F, Wetterslev J, et al. The effect of inhaled nitric oxide in acute respiratory distress syndrome in children and adults: a cochrane systematic review with trial sequential analysis. Anaesthesia. 2017;72:106–17.
Longobardo A, Montanari C, Shulman R, et al. Inhaled nitric oxide minimally improves oxygenation in COVID-19 related acute respiratory distress syndrome. Br J Anaesth. 2021;126:e44–6.
Hoste EAJ, Bagshaw SM, Bellomo R, et al. Epidemiology of acute kidney injury in critically ill patients: the multinational AKI-EPI study. Intensive Care Med. 2015;41:1411–23.
Kumar A, Arora A, Sharma P, et al. Is diabetes mellitus associated with mortality and severity of COVID-19? Meta Anal Diabetes Metab Syndr. 2020;14:535–45.
Taylor RW, Zimmerman JL, Dellinger RP, et al. Low-dose inhaled nitric oxide in patients with acute lung injury: a randomized controlled trial. JAMA. 2004;291:1603–9.
Wang J, Cong X, Miao M, et al. Inhaled nitric oxide and acute kidney injury risk: a meta-analysis of randomized controlled trials. Ren Fail. 2021;43:281–90.
Koyfman L, Simchon O, Koyfman A, et al. Clinical outcomes of critically ill patients using inhaled nitric oxide (iNO) during intrahospital transport. Crit Care Res Pract. 2021;2021:6633210.
Page EM, Ariëns RAS. Mechanisms of thrombosis and cardiovascular complications in COVID-19. Thromb Res. 2021;200:1–8.
Bhat T, Neuman A, Tantary M, et al. Inhaled nitric oxide in acute pulmonary embolism: a systematic review. Rev Cardiovasc Med. 2015;16:1–8.
Akaike T, Maeda H. Nitric oxide and virus infection. Immunology. 2000;101:300–8.
Bateman RM, Sharpe MD, Jagger JE, et al. 36th International Symposium on Intensive Care and Emergency Medicine : Brussels, Belgium. 15–18 March 2016. Crit Care 2016; 20:94
Acknowledgements
We thank Princess Nourah bint Abdulrahman University Researchers Supporting Project number (PNURSP2022R78), Princess Nourah bint Abdulrahman University, Riyadh, Saudi Arabia. We would like to thank all the investigators who participated in this project from the Saudi critical care pharmacy research (SCAPE) platform.
Funding
This work ws supported by Princess Nourah bint Abdulrahman University Researchers Supporting Project (no. PNURSP2022R78), Princess Nourah bint Abdulrahman University, Riyadh, Saudi Arabia.
Author information
Authors and Affiliations
Contributions
All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas; took part in drafting, revising or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
The study was approved in December 2020 by King Abdullah International Medical Research Center Institutional Review Board, Riyadh, Saudi Arabia (Ref.# RC20.638.R). Participants’ confidentiality was strictly observed throughout the study by using anonymous unique serial number for each subject and restricting data only to the investigators. Informed consent was not required due to the research's method as per the policy of the governmental and local research center.
Consent for publication
Not applicable.
Competing interests
No author has a conflict of interest in this study.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Additional file 1.
Outcomes definition(s).
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Al Sulaiman, K., Korayem, G.B., Altebainawi, A.F. et al. Evaluation of inhaled nitric oxide (iNO) treatment for moderate-to-severe ARDS in critically ill patients with COVID-19: a multicenter cohort study. Crit Care 26, 304 (2022). https://doi.org/10.1186/s13054-022-04158-y
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s13054-022-04158-y