Abstract
Gut microbiota plays an essential role in health and disease. It is constantly evolving and in permanent communication with its host. The gut microbiota is increasingly seen as an organ, and its failure, reflected by dysbiosis, is seen as an organ failure associated with poor outcomes. Critically ill patients may have an altered gut microbiota, namely dysbiosis, with a severe reduction in “health-promoting” commensal intestinal bacteria (such as Firmicutes or Bacteroidetes) and an increase in potentially pathogenic bacteria (e.g. Proteobacteria). Many factors that occur in critically ill patients favour dysbiosis, such as medications or changes in nutrition patterns. Dysbiosis leads to several important effects, including changes in gut integrity and in the production of metabolites such as short-chain fatty acids and trimethylamine N-oxide. There is increasing evidence that gut microbiota and its alteration interact with other organs, highlighting the concept of the gut–organ axis. Thus, dysbiosis will affect other organs and could have an impact on the progression of critical diseases. Current knowledge is only a small part of what remains to be discovered. The precise role and contribution of the gut microbiota and its interactions with various organs is an intense and challenging research area that offers exciting opportunities for disease prevention, management and therapy, particularly in critical care where multi-organ failure is often the focus. This narrative review provides an overview of the normal composition of the gut microbiota, its functions, the mechanisms leading to dysbiosis, its consequences in an intensive care setting, and highlights the concept of the gut–organ axis.
Similar content being viewed by others
Background: why focus on the gut microbiota in intensive care patients?
The digestive tract contains a considerable number of microorganisms that are in constant communication and symbiosis with their host. They play a major role both in health and in the pathogenesis of many diseases such as inflammatory, cardiovascular or metabolic diseases when dysbiosis occurs [1,2,3], i.e. when the composition of the gut microbiota is altered.
Critically ill patients are often instable with multi-organ damage. They undergo a major state of stress mediated by endocrine, immunological, neuronal and inflammatory mechanisms [4]. In addition, the gut microbiota is under tremendous pressure due to various factors such as medications, critical illness or the discontinuation of the normal diet [5]. More recently, the gut microbiota is more considered as a dynamic organ and its failure, reflected by dysbiosis, as an organ failure, associated with poor outcomes [5,6,7,8]. It is therefore urgent to understand the mechanisms of its evolution and its involvement in critical illnesses.
Actual evidence on the gut microbiota comes either from animal models or from human studies. Murine models have different gut physiology from that of large mammalian models. This must be taken into account when extrapolating results from murine models to humans [9,10,11]. However, these data allow us to better understand the gut microbiota and its dynamic changes.
Our aim is to provide an overview of the normal composition of the gut microbiota, its functions, the concept of the gut–organ axis, the mechanisms leading to dysbiosis and its consequences in the intensive care units (ICU).
Normal composition of the gut microbiota and its evolution in intensive care
Normal composition of the gut microbiota
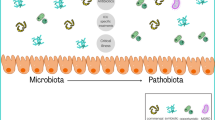
While there is currently no definition of a “normal” microbiota [12, 13], many factors, such as diet, age or lifestyle habits, influence its composition [3, 13,14,15,16]. In the colon, the phyla Firmicutes and Bacteroidetes compose 90% of the gut microbiota (60–75% and 30–40%, respectively), followed by the phyla Actinobacteria, Proteobacteria and Verrucomicrobia [16,17,18]. The Firmicutes phylum contains predominantly Gram-positive obligate or facultative anaerobic bacteria and includes, for example, Lactobacillus spp., Clostridium spp. or Enterococcus spp. [5, 16, 17]. The Bacteroidetes phylum contains less genera and predominantly Gram-negative anaerobic bacteria, such as Bacteroides spp. or Prevotella spp. [5, 16]. The majority of the normal gut microbiota consists of obligate anaerobic bacteria. The latter play a role in inhibiting the growth of other potentially pathogenic bacteria (referred to as pathobionts), mostly composed of aerobic bacteria or facultative anaerobic bacteria such as Escherichia coli [19].
Functions of the gut microbiota
The intestinal microbiota has many functions. First, anaerobic bacteria degrade food polysaccharides, that are fermented into various metabolites including short-chain fatty acids (SCFAs) such as butyrate, acetate and propionate, which are necessary substrates for enterocyte function [20]. It also plays a role in the defence against infections of the digestive tract by a competitive effect between commensal and pathogenic bacteria and in building the local immune defence. In addition, the gut microbiota is closely linked to all our organs and contributes to their normal functioning [21, 22]. This last point, which led to the concept of gut–organ axis, will be detailed below.
Assessment of the gut microbiota
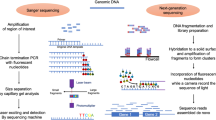
Gut microbiota can be examined using various methods, the two most commonly used in clinical practice are described below.
16S ribosomal RNA (rRNA) profiling (metataxonomics) [23] provides a taxonomic overview of the bacteria present in a sample and, among others, gives information on microbial richness and diversity [5]. This is a simple, fast and low-cost technique. Limitations include that it gives no information on gene functions and that two organisms with the same 16S rRNA gene sequence could be misclassified [23,24,25].
A more complete microbial composition can be assessed through unbiased sequencing of all DNA (shotgun metagenomics) present in a sample [23]. This higher resolution approach, although more expensive, allows the identification of bacteria up to species level and provides information on microbial richness, diversity and gene functions [23, 24, 26]. These approaches can be further informed by integrating them with proteins (metaproteomics) and small molecules (metabolomics) profiling.
Finally, these methods produce complex results whose interpretation must be related to a specific research question [27].
Critical illnesses and the gut microbiota
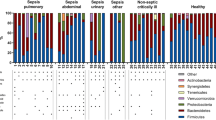
Critical diseases are associated with a loss of commensal intestinal bacteria such as Firmicutes or Bacteroidetes and an increase in potentially pathogenic bacteria (pathobionts) such as Proteobacteria [12, 28]. This dysbiosis is determined both by the decrease in diversity and by the change in the ratio of pathogenic bacteria to the detriment of “health-promoting” commensal bacteria (Fig. 1). In some cases, an overgrowth (> 50% relative abundance) of potentially pathogenic genera such as Enterococcus spp., Clostridium difficile, Staphylococcus spp., can be highlighted [28]. Several indexes exist to identify and define dysbiosis [29]. These changes in microbiota and intestinal homeostasis may occur within the first 48 h following a critical illness and seem to vary according to the patient’s age [12, 30, 31]. A study of 115 critically ill patients comparing the microbiota on ICU admission with that at discharge showed a decrease in Firmicutes and Bacteroidetes phyla, a significant increase in Proteobacteria and an increase in taxa with pathogenic bacteria such as Enterobacter spp. and Staphylococcus spp. [12]. Another study of mechanically ventilated ICU patients found that the proportion of Bacteroidetes and Firmicutes varies from patient to patient during their stay. This last study also suggested that the Bacteroidetes/Firmicutes ratio could be a predictor of mortality [7]. Intestinal dysbiosis has been shown to be associated with patient susceptibility to nosocomial infections, sepsis, organ failure and even COVID-19 disease severity [32,33,34,35,36,37].
Intestinal dysbiosis: how does it work?
Colonic mucus changes
The intestinal wall is covered with hydrophobic mucus, which is continuously produced by the goblet cells of the mucosa. This mucus protects the enterocytes and colonocytes from digestive enzymes and acts as a barrier against the passage of bacteria and toxins into the bloodstream [28, 32]. In critically ill patients with splanchnic hypoperfusion, mucus production and mucus hydrophobicity decrease, leading to enterocytes injury that promotes cell apoptosis and pathogen translocation [28, 38]. This leads to reduced absorption of nutrients and reduced production of SCFAs and favours diarrhoea [28].
Intestinal integrity changes and the role of short-chain fatty acids (SCFAs)
The vast majority of knowledge about SCFAs comes from in vitro bench work on human or mice faeces and conclusions from interventional studies with prebiotics [39,40,41,42]. The intestinal anaerobic microbiota ferments dietary fibres and produces metabolites such as SCFAs, which help maintain the integrity of the gut barrier and promote the host’s immune response [43]. SCFAs are the primary source of energy for the colonic epithelium and contribute to maintaining functional intercellular junctions. Mostly studied in rodent models, they also play a role in intestinal immunity by controlling the production of T-helper cells, regulatory T cells (Treg), antibodies and cytokines with mainly anti-inflammatory effects [44,45,46]. SCFAs have also been shown to induce cytoprotective proteins in epithelial cells that help maintain cell viability under stress conditions [32, 47]. Critically ill patients exhibit dysbiosis with a reduction in anaerobic bacteria leading to a decrease in SCFAs concentration, which has been associated with cellular apoptosis, malabsorption, diarrhoea and bacterial translocation [44, 48,49,50].
Changes in trimethylamine N-oxide (TMAO) production
TMAO is an important metabolite produced jointly by the intestinal microbiota and the liver [51]. First, trimethylamine (TMA) is produced by the gut microbiota from choline, lecithin and carnitine which are found in food precursors such as meat, fish and eggs [52]. Second, TMA is absorbed and translocated to the liver through portal circulation [51], where TMAO is converted from TMA directly [52]. As the production of TMAO depends on the diversity and composition of the gut microbiota, TMAO levels can change with dysbiosis, resulting often in higher levels [52, 53]. A study in humans showed that broad-spectrum antibiotics suppressed the production of TMAO, which reappeared after the discontinuation of the antibiotics [54], supporting the importance of the gut microbiota in TMAO production. High levels of TMAO have been recognized to be associated with heart failure, atherosclerosis and thrombosis formation [52, 55,56,57,58,59].
Immune mucosal changes
The gut microbiota plays a crucial role in the development of the immune system and is in constant communication with it [60]. On the one hand, the microbiota promotes the immune system and adapts it to certain conditions; on the other hand, it is tolerated by this adaptive immunity. This occurs through the involvement and recognition of microbe-associated molecular patterns via the toll-like receptor system [61] and through the release of pro-inflammatory cytokines [62], mucus secretion and the formation of SCFAs that activate Treg [20, 63]. This barrier plays an important role in preventing colonization by pathogens and appears to be compromised by antibiotic administration [64].
In order to control its relationship with the microbiota, the immune system limits the contact between the microbiota and epithelial cells, thus limiting the possible translocation of bacteria. This “mucosal firewall” consists of epithelial cells, IgA secretion, antimicrobial peptides and immune cells [65, 66]. Alteration of the microbiota can lead to dysregulation of the immune system, including a decrease in IgA and T cell levels, favouring bacterial infection [5, 67].
Intestinal dysbiosis in critically ill patients: pathophysiological concepts
Multiple environmental changes take place during critical illness, during which there is selective pressure due to splanchnic hypoperfusion in the context of shock, inflammation, impaired immunity, change in diet, medications and decreased intestinal motility [27, 33, 68]. All these conditions could contribute to the development of intestinal dysbiosis.
Factors favouring dysbiosis in an intensive care setting
Several factors influence the change in microbiota and its virulence. First, during critical illness, transit time is prolonged, leading to a reduction in bacterial excretion, which is known to be associated with bacterial overgrowth [6, 69]. The slowing down of intestinal transit time may be due to electrolyte fluctuations and the frequent use of sedatives and opiates in the ICU [6].
Second, many drugs commonly administered in the ICU can affect the composition of the gut microbiota, such as antibiotics but also non-steroidal anti-inflammatory drugs, beta-blockers, amines, or proton pump inhibitors [70,71,72,73]. A possible explanation for this last drug family is that the gut pH exerts selection pressures on bacteria, which cannot all grow in the same acidic environment [74, 75]. The dysbiosis induced by proton pump inhibitor has been associated with an increased risk of Clostridium difficile infection [71, 76, 77].
The effects of antibiotics on microbiota depend on many factors, including the class of antibiotic therapy and its route of elimination. In general, antibiotics alter the commensal flora and its diversity and could select and/or promote the growth of resistant microorganisms [5, 78].
Finally, another important factor is the change in nutrition patterns. Critically ill patients are often starving and are fed with enteral nutrition (EN) or parenteral nutrition (PN). Little is known about the effects of EN and PN on the human gut microbiota. However, a study in children in ICU confirmed the findings of murine models, that exclusive PN was associated with significant dysbiosis [79, 80]. In contrast, an in vitro study on human faecal samples has shown that EN promotes the growth of commensal microbiota, with intraindividual differences depending on the enteral formula [81]. Nutritional therapy seems to have significant impacts on the gut microbiota. NE appears to be a protective factor for the gut microbiota, whereas periods of starvation or total PN should be avoided as they may affect the integrity of the gut microbiota [28, 82,83,84].
When the normal gut flora becomes pathogenic
It is assumed that bacteria are able to sense their environment including the density and diversity of other bacteria [32]. In fact, depending on the intestinal lumen environment, intestinal bacteria either continue colonizing or become pathogenic. Many bacteria express virulence genes through a system called quorum sensing [32]. This system causes the bacteria's virulence genes to be expressed only when a certain bacterial density is reached that can overwhelm the host, and only when a negative environmental change is perceived, such as nutrient deficiency or specific treatment with opiates [6, 32, 85]. Indeed, a study showed that in patients with long ICU stays, “normal” microbiota was replaced by ultra-low-diversity communities of resistant pathogens whose virulence varied depending on the local environment, such as exposure to opiates [85]. Another study has shown that during acute stress associated with intestinal ischaemia/reperfusion, the production of dynorphin, a natural human opioid, was increased. In this study, exposure of Pseudomonas aeruginosa to dynorphin activated the quorum sensing system, which enables bacteria to recognize stress in the host, become pathogenic and take advantage of the host weaknesses [86].
Furthermore, the electrolytes levels also seem to influence gut microbiota. For example, local phosphate levels have been suggested to influence gut microbiota virulence [87, 88]. In this context, a study on mice models has shown that Pseudomonas aeruginosa and other pathogens can develop a lethal phenotype in the case of hypophosphatemia [87,88,89].
The main factors that influence the microbiota in critical illness are as follows: the critical illness itself, the host status, the drugs and the nutrition administered [27].
Sepsis and microbiota
Numerous ICU patients have severe infections. Although the specific mechanisms are not yet fully identified, the gut microbiota appears to play a role in the pathophysiology of sepsis [90, 91]. This is partly due to the fact that critically ill patients often receive a wide range of medications, which affect gut microbiota diversity [90], and partly because of the patients’ precarious condition, which can lead to hypoxic lesions, inflammation, disruption of epithelial integrity, dysmotility, changes in intraluminal pH or impaired immune function in the gut [92]. There are some characteristic patterns of gut microbiota associated with sepsis. In a multicentre study, the microbiota of ICU patients with sepsis showed an increased abundance of microbes closely associated with inflammation, such as Parabacteroides, Fusobacterium and Bilophila species [93]. Other studies showed that the gut loses important bacterial genera, including Faecalibacterium spp., Prevotella spp., Blautia spp. and Ruminococcaceae spp. [7, 85, 94], which are known to produce SCFAs [20]. Furthermore, it has been shown that certain antibiotic-resistant species prevalent in sepsis, such as Enterococcus spp. or Clostridia spp., are associated with unfavourable outcomes [85, 93, 95]. The gut microbiota is thought to influence sepsis not only through bacterial translocation [14, 96] and through the prevention of colonization by multi-resistant pathogens [64], but also by regulating the immune system [97, 98]. Laboratory data show greater bacterial spread, higher levels of inflammation and organ failure, and higher mortality in germ-free mice during sepsis compared to healthy mice, likely due to a less pronounced immunomodulatory response [97].
Modulation of the gut microbiota
Prebiotics, probiotics, synbiotics and faecal microbiota transplantation (FMT) are the most studied specific treatments for modulating gut microbiota.
Prebiotics are defined as undigested food substrates, such as fibres, inulin or oligosaccharides, that are used by the commensal gut microbiota after ingestion and provide health benefits [99]. A few studies on prebiotics showed that administration of fibre in ICU patients could improve dysbiosis, increase SCFAs production and reduce hospital length of stay [100, 101], while other studies showed contrasting results [102, 103].
Probiotics are living microorganisms that help maintain the balance of gut microbiota and improve the health of the host. Synbiotics is the concomitant administration of prebiotics and probiotics [99]. Previous studies have shown a possible effect of probiotics in reducing the incidence of ventilator-associated pneumonia (VAP) [104, 105]. However, subsequent randomized controlled trials (RCTs) yielded conflicting results [106, 107]. The results of these studies cannot be generalized because the probiotics used and their dosage varied from study to study, which is a recurrent problem in studies comparing probiotics. Other studies using other genera, species, strains or doses are expected to clarify this issue [107]. Although the use of probiotics is an attractive microbiota-targeted therapy, they are not without risk, particularly in ICU patients, where Lactobacillus bacteraemia has been described following probiotic administration [108].
Recently, there has been increasing interest on FMT, which consists of transplanting an autologous or donor stool through colonoscopy, oral capsules or enteral feeding tube to restore a healthy microbiota. FMT has for example been proposed as an alternative treatment for severe or recurrent Clostridium difficile colitis [109, 110]. In the ICU, there are case reports in septic patients with multiple organ failures and suspected dysbiosis highlighting successful FMT in these patients [111, 112]. The physiopathological hypotheses are that FMT increases SCFA-producing bacteria, which could help restore the systemic immune response and allow the clearance of the sepsis pathogen [113]. However, FMT is not without risk in ICU patients and is still an experimental treatment.
As knowledge about gut microbiota keeps growing at an impressive rate, we can anticipate further definitions of the value and use of specific treatments modulating gut microbiota.
Interaction of the gut microbiota with key organs: the concept of the gut–organ axis
As gut microbiota interacts with other organs, the concept of gut–organ axis is explored in this section. Figure 2 illustrates the different gut–organ axes and provides examples of diseases associated with an alteration of the gut microbiota.
Gut–brain axis
Gut–brain axis is an important, constant bidirectional communication system [114,115,116], taking place via immunological, endocrine, neural and metabolic pathways [117].
Immune signalling is mediated by cytokines (IL-1, IL-6), that are produced in the gut, travel through the bloodstream and cross the blood–brain barrier [118, 119]. These cytokines then influence one of the most powerful activators of the stress system, the hypothalamic–pituitary–adrenal axis [118, 119].
The gut microbiota has been shown to interact with the brain via neurotransmitters and the vagus nerve. The neurotransmitters produced and consumed by the gut include dopamine, norepinephrine, GABA and serotonin [120]. Some bacteria have been shown to express more neurotransmitters, such as Lactobacillus rhamnosus, which is associated with neurological GABA secretion. Interestingly, the vagus nerve appears to recognize metabolites of the gut microbiota and responds through a cholinergic pathway that appears to reduce intestinal inflammation and intestinal permeability, thus modulating the gut microbiota [120,121,122]. Recent studies also suggested that alterations of these neurotransmitters by the microbiota have an impact on the onset and development of neurological diseases such as ischaemic stroke or neuroimmune diseases [123]. The vagus nerve also seems to be activated by SCFAs [124,125,126].
Metabolic components also serve as communication pathways between the brain and the gut microbiota. For example, it has been shown that colonization with Bifidobacterium infantis leads to higher plasma tryptophan levels and secondarily to higher central serotonin levels [127, 128].
The gut–brain interaction has been demonstrated in neurocritically ill patients. Indeed, their gut microbiota appears different from that of healthy subjects and dysbiosis increases with ICU length of stay [129]. Furthermore, an increased abundance of Enterobacteriales and Enterobacteriaceae in the first week after ICU admission was associated with 180-day mortality in these patients [129]. Another well-studied clinical example is acute ischaemic stroke, which leads to intestinal ischaemia and dysbiosis, which in turn exacerbate cerebral infarction by enhancing systemic inflammation [130, 131]. In addition, dysbiosis is associated with poor outcomes following acute ischaemic stroke as it interacts with the brain through all of the above mechanisms [56, 132, 133].
Different stroke dysbiosis indexes are being explored to characterize gut microbiota in these patients and correlate them with patient outcomes [134].
Gut–lung axis
The gut microbiota constantly interacts with the lung microbiota [135]. In fact, the lung microbiota has been shown to change when a newborn's diet is altered [136].
Since the microbiota is known to have an effect on local immunity, it is thought to play a role in pulmonary immunity as well. The immune response of the lungs can probably be modulated in the following way. Besides portal circulation, drainage of the gastrointestinal tract also occurs via lymph nodes, which drain into the thoracic duct and then into the subclavian vein. The first capillary bed that then filters the chyle is the pulmonary capillary bed [6].
SCFAs seem to play a role in immunity by reducing lung inflammation through induction of Treg [137]. Furthermore, dysbiosis with an increased ratio of Firmicutes/Bacteroidetes species is also associated with increased IL-17 and IL-22 responses in the lung, which could lead to airway hyperreactivity [137].
In mice models, the gut microbiota appears to have a protective effect in severe lung infections, as several studies showed that germ-free mice had increased mortality after lung infection with Klebsiella pneumoniae, Streptococcus pneumoniae or Pseudomonas aeruginosa [138, 139]. One of the possible mechanisms is that the phagocytic capacity of macrophages decreases in germ-free mice [97].
The importance of the gut–lung axis and its therapeutic potential is also supported by a few interventional studies in humans. For example, some studies have shown that the use of probiotics could reduce the risk of VAP in the ICU [48, 140].
Gut–heart axis
Gut microbiota and the cardiovascular (CV) system also interact bidirectionally [141].
Dysbiosis has recently been associated with CV risk factors and diseases such as atherosclerosis, obesity, diabetes, hypertension or coronary artery disease [142]. On the one hand, CV diseases lead to dysbiosis, while on the other hand, the gut microbiota affects the CV system through various metabolites, including TMAO and SCFAs [142].
High TMAO levels have been shown to be associated with CV disease [143, 144] and with an increased risk of serious CV events (e.g. death, myocardial infarction and stroke) and heart failure [54, 145,146,147]. The gut microbiota has also been shown to influence platelet hyperresponsiveness and blood clot formation through the production of TMAO [56].
SCFAs may play a role in blood pressure regulation by influencing renin secretion via the G-protein-coupled receptor pathway [148], and different studies suggest a link between gut microbiota and hypertension [148, 149]. Moreover, dysbiosis is associated with lower butyrate production, leading to increased intestinal permeability and systemic inflammation, promoting atherosclerosis and heart failure [141].
Patients with heart failure also experience relative splanchnic hypoperfusion, leading to oedema of the intestinal wall and impaired function and permeability of the intestinal epithelium, which could lead to dysbiosis [150, 151]. This dysbiosis is thought to be associated with increased inflammation, which can exacerbate acute heart failure [150].
In CV surgery patients, a small longitudinal study has shown marked changes in gut microbiota in patients admitted to the ICU, with more complications in patients with the most pronounced dysbiosis [30].
In summary, an imbalance of the gut microbiota metabolites seems to contribute to the development or exacerbation of CV diseases. This has led to new research and clinical opportunities, with a focus on the use of TMAO as a potential biomarker.
Gut–kidney axis
So far, several mechanisms have been identified (e.g. SCFAs, TMAO) that could explain how the gut microbiota interacts with the kidney, but knowledge in humans and in critical care situations remains scarce [152]. First, regarding the SCFAs mechanism, Andrade-Oliveira et al. [153] showed that mice treated with acetate-producing bacteria had better outcomes after acute kidney injury (AKI) by regulating inflammation. Second, high levels of TMAO have been recognized as a risk factor for chronic kidney disease (CKD). In murine models, dysbiosis can lead to an increase in circulating TMAO, which in turn can cause kidney interstitial fibrosis [154]. TMAO levels have also been shown to be higher in patients with CKD compared to healthy subjects and associated with poor prognosis [155].
Intestinal bacteria are known to affect dendritic cell activity on intestinal T cells as well as on peripheral Treg differentiation. It has been shown that the amount of CD4 T-helper cells producing pro-inflammatory IL-17 is higher in patients with autoimmune kidney disease [156].
Increased inflammation also affects kidney function. In sepsis and subsequent dysbiosis, there is an increased intestinal permeability and silent translocation of bacteria and toxins into the bloodstream. This increases inflammation and promotes the switch to renal aerobic glycolysis, leading to a decrease in ATP stores and ultimately to mitochondrial and cellular damage in the kidney [157].
Finally, urea works both ways. On the one hand, it accumulates in AKI and promotes intestinal damage [157]. On the other hand, dysbiosis produces more uremic toxins that can lead to tubular dysfunction [156].
Gut–liver axis
Bidirectional interactions between gut microbiota and the liver occur through continuous exchange via the portal circulation as well as the biliary enterohepatic cycle [158].
Via the portal circulation, the liver is directly exposed to molecules absorbed through the intestinal mucosa. A study [159] indicated an inverse correlation between SCFA levels and the severity of portal hypertension, the degree of endotoxemia and systemic inflammation, emphasizing the role of gut microbiota in gut–liver interactions and in the progression of liver pathologies such as cirrhosis [160]. The gut–liver interaction was also confirmed in another study [161] which demonstrated a negative correlation between the abundance of endogenous bacteria and inflammatory markers in patients with alcohol use disorders.
Moreover, non-alcoholic fatty liver disease and its severity have also been associated with TMAO levels [53, 59]. TMAO may affect triglycerides levels in the liver and influence their metabolism [162].
The biliary enterohepatic cycle is another central protagonist allowing the liver to communicate with the gut by releasing bile acids (BAs) and other bioactive mediators through the biliary tract. Furthermore, almost 5% of the BAs are metabolized into secondary BAs which exert direct control on microbiota by inhibiting microbial overgrowth [163]. Indeed, dysbiosis is thought to lead to an imbalance between primary and secondary BAs which causes an additional metabolic burden on the liver.
The role of the microbiota has led to the development of a cirrhosis–dysbiosis ratio (CDR) to classify the severity of dysbiosis in cirrhotic patients, as reported by Bajaj et al. [160, 164]. The latter pointed out that low CDR (i.e. more severe dysbiosis) was associated with decompensated cirrhosis, organ failure and death [160].
Gut–liver interplays are of interest in the ICU, especially in the context of liver transplantation and hepatic encephalopathy (HE). Liver transplantation seems to improve dysbiosis in cirrhotic patients and establish better cognitive status [165]. Moreover, a phase I RCT highlighted that FMT in cirrhotic patients with HE could improve dysbiosis and cognitive state [166].
Conclusion
The gut microbiota is in constant communication with key organs of our organism and strongly influences them. According to the latest evidence, gut microbiota could be considered as an organ and its failure, manifested by dysbiosis, as an organ failure, which is possibly associated with poor clinical outcomes. The exact roles and contributions of the gut microbiota and its interactions with the various organs are an intense and challenging area of research, and much remains to be discovered. Another aspect that should not be neglected is that the composition of the gut microbiota is influenced by genetic and non-genetic factors such as lifestyle, diet, but also by diseases and their treatments. Further research on the gut microbiota is needed to better understand these processes, and to offer new opportunities for disease prevention, management and therapy, especially in critical care where multi-organ failure is often the focus.
Availability of data and materials
Not applicable.
Abbreviations
- SCFA:
-
Short-chain fatty acid
- ICU:
-
Intensive care unit
- TMAO:
-
Trimethylamine N-oxide
- TMA:
-
Trimethylamine
- EN:
-
Enteral nutrition
- PN:
-
Parenteral nutrition
- CV:
-
Cardiovascular
- RCT:
-
Randomized controlled trial
- VAP:
-
Ventilator-associated pneumonia
- FMT:
-
Faecal microbiota transplant
- CKD:
-
Chronic kidney disease
- BA:
-
Bile acid
- CDR:
-
Cirrhosis–dysbiosis ratio
References
Thursby E, Juge N. Introduction to the human gut microbiota. Biochem J. 2017;474(11):1823–36.
Wischmeyer PE, McDonald D, Knight R. Role of the microbiome, probiotics, and “dysbiosis therapy” in critical illness. Curr Opin Crit Care. 2016;22(4):347–53.
Manor O, Dai CL, Kornilov SA, Smith B, Price ND, Lovejoy JC, et al. Health and disease markers correlate with gut microbiome composition across thousands of people. Nat Commun. 2020;11(1):1–12.
Agudelo-Ochoa GM, Valdés-Duque BE, Giraldo-Giraldo NA, Jaillier-Ramírez AM, Giraldo-Villa A, Acevedo-Castaño I, et al. Gut microbiota profiles in critically ill patients, potential biomarkers and risk variables for sepsis. Gut Microbes. 2020;12(1):1–16.
Szychowiak P, Villageois-Tran K, Patrier J, Timsit JF, Ruppé É. The role of the microbiota in the management of intensive care patients. Ann Intensive Care. 2022;12(1).
Dickson RP, Arbor A. The microbiome and critical illness. Lancet Respir Med. 2017;4(1):59–72.
Ojima M, Motooka D, Shimizu K, Gotoh K, Shintani A, Yoshiya K, et al. Metagenomic analysis reveals dynamic changes of whole gut microbiota in the acute phase of intensive care unit patients. Dig Dis Sci. 2016;61(6):1628–34.
Lankelma JM, van Vught LA, Belzer C, Schultz MJ, van der Poll T, de Vos WM, et al. Critically ill patients demonstrate large interpersonal variation in intestinal microbiota dysregulation: a pilot study. Intensive Care Med. 2017;43(1):59–68.
Gonzalez LM, Moeser AJ, Blikslager AT. Porcine models of digestive disease: the future of large animal translational research. Transl Res. 2015;166(1):12–27.
Nejdfors P, Ekelund M, Jeppsson B, Weström BR. Mucosal in vitro permeability in the intestinal tract of the pig, the rat, and man: species- and region-related differences. Scand J Gastroenterol. 2000;35(5):501–7.
Buchman AL, Mestecky J, Moukarzel A, Anient ME. Intestinal Immune function is unaffected by parenteral nutrition in man. J Am Coll Nutr. 1995;14(6):656–61.
Mcdonald D, Ackermann G, Khailova L, Baird C, Heyland D, Kozar R, et al. Critical Illness. 1(4):1–6.
King CH, Desai H, Sylvetsky AC, LoTempio J, Ayanyan S, Carrie J, et al. Baseline human gut microbiota profile in healthy people and standard reporting template. PLoS ONE. 2019;14(9):1–25.
Klingensmith NJ, Coopersmith CM. The gut ad the Motor of multiple organ dysfunction in critical illness. Crit Care Clin. 2016;32(2):203–12.
Claesson MJ, Cusack S, O’Sullivan O, Greene-Diniz R, De Weerd H, Flannery E, et al. Composition, variability, and temporal stability of the intestinal microbiota of the elderly. Proc Natl Acad Sci U S A. 2011;108(SUPPL. 1):4586–91.
Rinninella E, Raoul P, Cintoni M, Franceschi F, Miggiano GAD, Gasbarrini A, et al. What is the healthy gut microbiota composition? A changing ecosystem across age, environment, diet, and diseases. Microorganisms. 2019;7(1):14.
Jacobs MC, Haak BW, Hugenholtz F, Wiersinga WJ. Gut microbiota and host defense in critical illness. Curr Opin Crit Care. 2017;23(4):257–63.
Arumugam M, Raes J, Pelletier E, Le Paslier D, Yamada T, Mende DR, Fernandes GR, Tap J, Bruls T, Batto JM, Bertalan M, Borruel N, Casellas F, Fernandez L, Gautier L, Hansen T, Hattori M, Hayashi T, Kleerebezem M, Kurokawa K, Leclerc M, Levenez F, Manichan BP. Enterotypes of the human gut microbiome. Nature. 2011;473(11):174–80.
Lorian V. Colonization resistance. Antimicrob Agents Chemother. 1994;38(7):1693.
Rajilić-Stojanović M, de Vos WM. The first 1000 cultured species of the human gastrointestinal microbiota. FEMS Microbiol Rev. 2014;38(5):996–1047.
D’Argenio V, Salvatore F. The role of the gut microbiome in the healthy adult status. Clin Chim Acta. 2015;451:97–102.
Sekirov I, Russell SL, Antunes LCM, Finlay BB. Gut microbiota in health and disease. Am Physiol Soc. 2010;90:859–904.
Durazzi F, Sala C, Castellani G, Manfreda G, Remondini D, De Cesare A. Comparison between 16S rRNA and shotgun sequencing data for the taxonomic characterization of the gut microbiota. Sci Rep [Internet]. 2021;11(1):1–10. https://doi.org/10.1038/s41598-021-82726-y.
Ashe EC, Comeau AM, Zejdlik K, O’Connell SP. Characterization of bacterial community dynamics of the human mouth throughout decomposition via metagenomic, metatranscriptomic, and culturing techniques. Front Microbiol. 2021;12(June):1389.
Hilton SK, Castro-Nallar E, Pérez-Losada M, Toma I, McCaffrey TA, Hoffman EP, et al. Metataxonomic and metagenomic approaches versus culture-based techniques for clinical pathology. Front Microbiol. 2016;7(APR):1–12.
Quince C, Walker AW, Simpson JT, Loman NJ, Segata N. Shotgun metagenomics, from sampling to analysis. Nat Biotechnol. 2017;35(9):833–44.
Wolff NS, Hugenholtz F, Wiersinga WJ. The emerging role of the microbiota in the ICU. Crit Care. 2018;22(1):1–7.
Moron R, Galvez J, Colmenero M, Anderson P, Cabeza J, Rodriguez-Cabezas ME. The importance of the microbiome in critically ill patients: Role of nutrition. Nutrients. 2019;11(12):1–17.
Wei S, Bahl MI, Baunwall SMD, Hvas CL, Licht TR. Determining gut microbial dysbiosis: a review of applied indexes for assessment of intestinal microbiota imbalances. Appl Environ Microbiol. 2021;87(11):1–13.
Aardema H, Lisotto P, Kurilshikov A, Diepeveen JRJ, Friedrich AW, Sinha B, et al. Marked changes in gut microbiota in cardio-surgical intensive care patients: a longitudinal cohort study. Front Cell Infect Microbiol. 2020;9(January):1–10.
Victoria M, Elena VDB, Amparo GGN, María JRA, Adriana GV, Irene AC, et al. Gut microbiota alterations in critically ill older patients: a multicenter study. BMC Geriatr. 2022;22(1):1–12.
Alverdy JC, Chang EB. The re-emerging role of the intestinal microflora in critical illness and inflammation: why the gut hypothesis of sepsis syndrome will not go away. J Leukoc Biol. 2008;83(3):461–6.
Latorre M, Krishnareddy S, Freedberg DE. Microbiome as mediator: do systemic infections start in the gut? World J Gastroenterol. 2015;21(37):10487–92.
Zuo T, Zhang F, Lui GCY, Yeoh YK, Li AYL, Zhan H, et al. Alterations in gut microbiota of patients with COVID-19 during time of hospitalization. Gastroenterology. 2020;159(January):944–55.
Lynch SV, Pedersen O. The human intestinal microbiome in health and disease. N Engl J Med. 2016;375(24):2369–79.
Albrich WC, Ghosh TS, Ahearn-Ford S, Mikaeloff F, Lunjani N, Forde B, et al. A high-risk gut microbiota configuration associates with fatal hyperinflammatory immune and metabolic responses to SARS-CoV-2. Gut Microbes. 2022;14(1):1–16.
Yeoh YK, Zuo T, Lui GCY, Zhang F, Liu Q, Li AYL, et al. Gut microbiota composition reflects disease severity and dysfunctional immune responses in patients with COVID-19. Gut. 2021;70(4):698–706.
Qin X, Caputo FJ, Xu DZ, Deitch EA. Hydrophobicity of mucosal surface and its relationship to gut barrier function. Shock. 2008;29(3):372–6.
Bourriaud C, Robins RJ, Martin L, Kozlowski F, Tenailleau E, Cherbut C, et al. Lactate is mainly fermented to butyrate by human intestinal microfloras but inter-individual variation is evident. J Appl Microbiol. 2005;99(1):201–12.
El KA, Armougom F, Gordon JI, Raoult D, Henrissat B. The abundance and variety of carbohydrate-active enzymes in the human gut microbiota. Nat Rev Microbiol. 2013;11(7):497–504.
Belenguer A, Holtrop G, Duncan SH, Anderson SE, Calder AG, Flint HJ, et al. Rates of productionand utilization of lactate by microbial communities fromthe human colon. FEMS Microbiol Ecol. 2011;77(1):107–19.
Morrison DJ, Mackay WG, Edwards CA, Preston T, Dodson B, Weaver LT. Butyrate production from oligofructose fermentation by the human faecal flora: what is the contribution of extracellular acetate and lactate? Br J Nutr. 2006;96(3):570–7.
Hamer HM, Jonkers D, Venema K, Vanhoutvin S, Troost FJ, Brummer RJ. Review article: the role of butyrate on colonic function. Aliment Pharmacol Ther. 2008;27(2):104–19.
Valdés-Duque BE, Giraldo-Giraldo NA, Jaillier-Ramírez AM, Giraldo-Villa A, Acevedo-Castaño I, Yepes-Molina MA, Barbosa-Barbosa J, Barrera-Causil CJ, Agudelo-Ochoa GM. Stool short chain fatty acids in critically Ill patients with sepsis. J Am Coll Nutr. 2020;39(8):706–12.
Arpaia N, Campbell C, Fan X, Dikiy S, van der Veeken J, deRoos P, Liu H, Cross JR, Pfeffer K, Coffer PJ. Rudensky AY (2013) Metabolites produced by commensal bacteria promote peripheral regulatory T cell generation. Nature. 2013;504(7480):451–5.
Smith PM, Howitt MR, Panikov N, Michaud M, Gallini CA, Bohlooly-Y M, Glickman JN, Garrett WS. The microbial metabolites, short-chain fatty acids, regulate colonic Treg cell homeostasis. Science (80-). 2013;30(1):569–73.
Shimizu K, Ogura H, Goto M, Asahara T, Nomoto K, Morotomi M, et al. Altered gut flora and environment in patients with severe SIRS. J Trauma: Inj Infect Crit Care. 2006;60(1):126–33.
Shimizu K, Ogura H, Asahara T, Nomoto K, Morotomi M, Tasaki O, et al. Probiotic/synbiotic therapy for treating critically Ill patients from a gut microbiota perspective. Dig Dis Sci. 2013;58(1):23–32.
Osuka A, Shimizu K, Ogura H, Tasaki O, Hamasaki T, Asahara T, et al. Prognostic impact of fecal pH in critically ill patients. Crit Care [Internet]. 2012;16(4):R119.
Yamada T, Shimizu K, Ogura H, Asahara T, Nomoto K, Yamakawa K, et al. Rapid and sustained long-term decrease of fecal short-chain fatty acids in critically ill patients with systemic inflammatory response syndrome. J Parent Enter Nutr. 2015;39(5):569–77.
Coutinho-Wolino KS, Ludmila LFM, de Oliveira LV, Mafra D, Stockler-Pinto MB. Can diet modulate trimethylamine N-oxide (TMAO) production? What do we know so far? Eur J Nutr. 2021;60(7):3567–84.
Liu Y, Dai M. Trimethylamine N-oxide generated by the gut microbiota is associated with vascular inflammation: new insights into atherosclerosis. Mediators Inflamm. 2020;2020.
Velasquez MT, Ramezani A, Manal A, Raj DS. Trimethylamine N-oxide: the good, the bad and the unknown. Toxins (Basel). 2016;8(11):326.
Tang WH, Wang Z, Levison BS, Koeth RA, Britt EB, Fu X, Wu Y, Hazen SL. Intestinal microbial metabolism of phosphatidylcholine and cardiovascular risk. NEJM. 2013;368(17):1575–84.
Seldin MM, Meng Y, Qi H, Zhu WF, Wang Z, Hazen SL, et al. Trimethylamine N-oxide promotes vascular inflammation through signaling of mitogen-activated protein kinase and nuclear factor-κb. J Am Heart Assoc. 2016;5(2):1–12.
Zhu W, Gregory JC, Org E, Buffa JA, Gupta N, Wang Z, Li L, Fu X, Wu Y, Mehrabian M, Sartor RB, McIntyre TM, Silverstein RL, Tang WHW, DiDonato JA, Brown JM, Lusis AJHS. Gut microbial metabolite TMAO enhances platelet hyperreactivity and thrombosis risk. Cell. 2016;176(12):139–48.
Koeth RA, Wang Z, Levison BS, Buffa JA, Org E, Sheehy BT, et al. Intestinal microbiota metabolism of l-carnitine, a nutrient in red meat, promotes atherosclerosis. Nat Med. 2013;19(5):576–85.
Bu J, Wang Z. Cross-talk between gut microbiota and heart via the routes of metabolite and immunity. Gastroenterol Res Pract. 2018;2018.
Chen YM, Liu Y, Zhou RF, Chen XL, Wang C, Tan XY, et al. Associations of gut-flora-dependent metabolite trimethylamine-N-oxide, betaine and choline with non-alcoholic fatty liver disease in adults. Sci Rep. 2015;2016(6):1–9.
Garrett MGR, WS. Gut microbiota, metabolites and host immunity. Nat Rev Immunol. 2016;16(6):341–52.
Pamer EG. Resistant Pathogens. Science (80-). 2016;352(6285):535–8.
Kamada N, Seo SU, Chen GY, Núñez G. Role of the gut microbiota in immunity and inflammatory disease. Nat Rev Immunol. 2013;13(5):321–35.
Berger MM, Appelberg O, Reintam-Blaser A, Ichai C, Joannes-Boyau O, Casaer M, et al. Prevalence of hypophosphatemia in the ICU: results of an international one-day point prevalence survey. Clin Nutr. 2021;40(5):3615–21.
Blaser M. Antibiotic overuse: stop the killing of beneficial bacteria. Nature. 2011;476(7361):393–4.
Belkaid Y, TH,. Role of the microbiota in immunity and inflammation yasmine. Cell. 2015;157(1):121–41.
Macpherson AJ, Uhr T. Induction of protective IgA by intestinal dendritic cells carrying commensal bacteria. Science (80-). 2004;303(5664):1662–5.
Honda K, Littman DR. The microbiota in adaptive immune homeostasis and disease. Nature. 2016;535(7610):75–84.
Francino MP. Antibiotics and the human gut microbiome: dysbioses and accumulation of resistances. Front Microbiol. 2016;6(JAN):1–11.
Ladopoulos T, Giannaki M, Alexopoulou C, Proklou A, Pediaditis E, Kondili E. Gastrointestinal dysmotility in critically ill patients. Ann Gastroenterol. 2018;31(3):273–81.
Zhernakova A, Kurilshikov A, Bonder MJ, Tigchelaar EF, Schirmer M, Vatanen T, Mujagic Z, Vila AV, Falony G, Vieira-Silva S, Wang J, Imhann F, Brandsma E, Jankipersadsing SA, Joossens M, Cenit MC, Deelen P, Swertz MA; LifeLines cohort study, Weersma RK, Fe FJ. Population-based metagenomics analysis reveals markers for gut microbiome composition and diversity. Science (80- ). 2016;2(74):565–9
Imhann F, Bonder MJ, Vila AV, Fu J, Mujagic Z, Vork L, et al. Proton pump inhibitors affect the gut microbiome. Gut. 2016;65(5):740–8.
Rogers MAMAD. The influence of non-steroidal anti-inflammatory drugs on the gut microbiome. Clin Microbiol Infect. 2016;176(12):139–48.
Habes QLM, Van Ede L, Gerretsen J, Kox M, Pickkers P. Norepinephrine contributes to enterocyte damage in septic shock patients: a prospective cohort study. Shock. 2018;49(2):137–43.
Ilhan ZE, Marcus AK, Kang D-W, Rittmann BE, Krajmalnik-Brown R. pH-mediated microbial and metabolic interactions in fecal enrichment cultures. mSphere. 2017;2(3):1–12.
Jackson MA, Goodrich JK, Maxan ME, Freedberg DE, Abrams JA, Poole AC, et al. Proton pump inhibitors alter the composition of the gut microbiota. Gut. 2016;65(5):749–56.
Naito Y, Kashiwagi K, Takagi T, Andoh A, Inoue R. Intestinal dysbiosis secondary to proton-pump inhibitor use. Digestion. 2018;97(2):195–204.
Pérez-Cobas AE, Artacho A, Ott SJ, Moya A, Gosalbes MJ, Latorre A. Structural and functional changes in the gut microbiota associated to clostridium difficile infection. Front Microbiol. 2014;5(JULY):1–15.
Ramirez J, Guarner F, Bustos Fernandez L, Maruy A, Sdepanian VL, Cohen H. Antibiotics as major disruptors of gut microbiota. Front Cell Infect Microbiol. 2020;10(November):1–10.
Miyasaka EA, Feng Y, Poroyko V, Falkowski NR, Erb-Downward J, Gillilland MG, et al. Total parenteral nutrition-associated lamina propria inflammation in mice is mediated by a MyD88-dependent mechanism. J Immunol. 2013;190(12):6607–15.
Dahlgren AF, Pan A, Lam V, Gouthro KC, Simpson PM, Salzman NH, et al. Longitudinal changes in the gut microbiome of infants on total parenteral nutrition. Pediatr Res. 2019;86(1):107–14.
Modrackova N, Copova I, Stovicek A, Makovska M, Schierova D, Mrazek J, et al. Microbial shifts of faecal microbiota using enteral nutrition in vitro. J Funct Foods. 2021;77:104330.
Schörghuber M, Fruhwald S. Effects of enteral nutrition on gastrointestinal function in patients who are critically ill. Lancet Gastroenterol Hepatol. 2018;3(4):281–7.
Zaher S. Nutrition and the gut microbiome during critical illness: a new insight of nutritional therapy. Saudi J Gastroenterol. 2020;26(6):290–8.
Ralls MW, Demehri FR, Feng Y, Ignatoski KMW. Teitelbaum DH enteral nutrient deprivation in patients leads to a loss of intestinal epithelial barrier function. Surgery. 2015;157(4):732–42.
Zaborin A, Smith D, Garfield K, Quensen J, Shakhsheer B, Kade M, et al. Membership and behavior of ultra-low-diversity pathogen critical illness. MBio. 2014;5(5):1–14.
Zaborina O, Lepine F, Xiao G, Valuckaite V, Chen Y, Li T, et al. Dynorphin activates quorum sensing quinolone signaling in pseudomonas aeruginosa. PLoS Pathog. 2007;3(3):e35.
Krezalek MA, DeFazio J, Zaborina O, Zaborin A, Alverdy JC. The shift of an intestinal “microbiome” to a “pathobiome” governs the course and outcome of sepsis following surgical injury. Shock. 2017;45(5):475–82.
Zaborin A, Gerdes S, Holbrook C, Liu DC, Zaborina OY, Alverdy JC. Pseudomonas aeruginosa overrides the virulence inducing effect of opioids when it senses an abundance of phosphate. PLoS One. 2012;7(4):e34883.
Babrowski T, Holbrook C, Moss J, Gottlieb L, Valuckaite V, Zaborin A, et al. Pseudomonas aeruginosa virulence expression is directly activated by morphine and is capable of causing lethal gut-derived sepsis in mice during chronic morphine administration. Ann Surg. 2012;255(2):386.
Haak BW, Wiersinga WJ. The role of the gut microbiota in sepsis. Lancet Gastroenterol Hepatol. 2017;2(2):135–43.
Kullberg RFJ, Wiersinga WJ, Haak BW. Gut microbiota and sepsis: from pathogenesis to novel treatments. Curr Opin Gastroenterol. 2021;37(6):578–85.
Rohit Mittal and CMC. Redefining the gut as the motor of critical illness. NIH. 2014;20(1):214–23.
Agudelo-Ochoa GM, Valdés-Duque BE, Giraldo-Giraldo NA, Jaillier-Ramírez AM, Giraldo-Villa A, Acevedo-Castaño I, et al. Gut microbiota profiles in critically ill patients, potential biomarkers and risk variables for sepsis. Gut Microbes [Internet]. 2020;12(1):1707610. https://doi.org/10.1080/19490976.2019.1707610.
Wan YD, Zhu RX, Wu ZQ, Lyu SY, Zhao LX, Du ZJ, et al. Gut microbiota disruption in septic shock patients: a pilot study. Med Sci Monit. 2018;24:8639–46.
Shimizu K, Ogura H, Hamasaki T, Goto M, Tasaki O, Asahara T, et al. Altered gut flora are associated with septic complications and death in critically ill patients with systemic inflammatory response syndrome. Dig Dis Sci. 2011;56(4):1171–7.
C. James Carrico, Jonathan L Meakins, J.C. Marshall, Donald Fry RVM. Multiple organ failure syndrome. Crit Care Nurse. 1986;14(2).
Schuijt TJ, Lankelma JM, Scicluna BP, De Sousa E, Melo F, Roelofs JJTH, De Boer JD, et al. The gut microbiota plays a protective role in the host defence against pneumococcal pneumonia. Gut. 2016;65(4):575–83.
Clarke TB, Davis KM, Lysenko ES, Zhou AY, Yu Y, Weiser JN. Recognition of peptidoglycan from the Microbiota by nod1 enhances systemic innate immunity. Natur Med. 2010;16(2):228–31.
Gibson GR, Scott KP, Rastall RA, Tuohy KM, Hotchkiss A, Dubert-Ferrandon A, et al. Dietary prebiotics: current status and new definition. Food Sci Technol Bull Funct Foods. 2010;7(1):1–19.
O’Keefe SJ. Effect of fiber supplementation on the microbiota in critically ill patients. World J Gastrointest Pathophysiol. 2011;2(6):138.
Schneider SM, Girard-Pipau F, Anty R, van der Linde EGM, Philipsen-Geerling BJ, Knol J, et al. Effects of total enteral nutrition supplemented with a multi-fibre mix on faecal short-chain fatty acids and microbiota. Clin Nutr. 2006;25(1):82–90.
Yang G, Wu XT, Zhou Y, Wang YL. Application of dietary fiber in clinical enteral nutrition: a meta-analysis of randomized controlled trials. World J Gastroenterol. 2005;11(25):3935–8.
Majid HA, Cole J, Emery PW, Whelan K. Additional oligofructose/inulin does not increase faecal bifidobacteria in critically ill patients receiving enteral nutrition: a randomised controlled trial. Clin Nutr. 2014;33(6):966–72.
Manzanares W, Lemieux M, Langlois PL, Wischmeyer PE. Probiotic and synbiotic therapy in critical illness: a systematic review and meta-analysis. Crit Care [Internet]. 2016. https://doi.org/10.1186/s13054-016-1434-y.
Batra P, Soni KD, Mathur P. Efficacy of probiotics in the prevention of VAP in critically ill ICU patients: an updated systematic review and meta-analysis of randomized control trials. J Intensive Care. 2020;9(1):1–14.
Shimizu K, Yamada T, Ogura H, Mohri T, Kiguchi T, Fujimi S, et al. Synbiotics modulate gut microbiota and reduce enteritis and ventilator-associated pneumonia in patients with sepsis: a randomized controlled trial. Crit Care. 2018;22(1):1–9.
Johnstone J, Meade M, Lauzier F, Marshall J, Duan E, Dionne J, et al. Effect of probiotics on incident ventilator-associated pneumonia in critically ill patients: a randomized clinical trial. JAMA: J Am Med Assoc. 2021;326(11):1024–33.
Yelin I, Flett KB, Merakou C, Mehrotra P, Stam J, Snesrud E, et al. Genomic and epidemiological evidence of bacterial transmission from probiotic capsule to blood in ICU patients. Nat Med. 2019;25(11):1728–32.
Hocquart M, Lagier JC, Cassir N, Saidani N, Eldin C, Kerbaj J, et al. Early fecal microbiota transplantation improves survival in severe clostridium difficile infections. Clin Infect Dis. 2018;66(5):645–50.
Tixier EN, Verheyen E, Luo Y, Grinspan LT, Du CH, Ungaro RC, et al. Systematic review with meta-analysis: fecal microbiota transplantation for severe or fulminant clostridioides difficile. Dig Dis Sci. 2022;67(3):978–88.
Li Q, Wang C, Tang C, He Q, Zhao X, Li N, et al. Successful treatment of severe sepsis and diarrhea after vagotomy utilizing fecal microbiota transplantation: a case report. Crit Care. 2015;19(1):1–12.
Wei Y, Yang J, Wang J, Yang Y, Huang J, Gong H, et al. Successful treatment with fecal microbiota transplantation in patients with multiple organ dysfunction syndrome and diarrhea following severe sepsis. Crit Care. 2016;20(1):1–9.
Keskey R, Cone JT, DeFazio JRAJ. The use of fecal microbiota transplant in sepsis. Transl Res. 2020;226(3):12–25.
Dinan TG, Cryan JF. Gut instincts: microbiota as a key regulator of brain development, ageing and neurodegeneration. J Physiol. 2017;595(2):489–503.
Dinan TG, Cryan JF. Mood by microbe: towards clinical translation. Genome Med. 2016;8(1):36–8.
Vuong HE, Hsiao EY. Emerging roles for the gut microbiome in autism spectrum disorder. Acta Psychiatr Scand. 2017;81(5):411–23.
Moloney RD, Desbonnet L, Clarke G, Dinan TG, Cryan JF. The microbiome: stress, health and disease. Mamm Genome. 2014;25(1–2):49–74.
Powell N, Walker MM, Talley NJ. The mucosal immune system: master regulator of bidirectional gut–brain communications. Nat Rev Gastroenterol Hepatol. 2017;14(3):143–59.
El AS, Dinan TG, Cryan JF. Immune modulation of the brain-gut-microbe axis. Front Microbiol. 2014;5(APR):3–6.
Bonaz B, Bazin T, Pellissier S. The vagus nerve at the interface of the microbiota-gut–brain axis. Front Neurosci. 2018;12(FEB):1–9.
Bravo JA, Forsythe P, Chew MV, Escaravage E, Savignac HM, Dinan TG, et al. Ingestion of Lactobacillus strain regulates emotional behavior and central GABA receptor expression in a mouse via the vagus nerve. Proc Natl Acad Sci U S A. 2011;108(38):16050–5.
Svensson E, Horváth-Puhó E, Thomsen RW, Djurhuus JC, Pedersen L, Borghammer P, et al. Vagotomy and subsequent risk of Parkinson’s disease. Ann Neurol. 2015;78(4):522–9.
Strandwitz P. Neurotransmitter modulation by the gut microbiota. Brain Res. 2018;1693(Pt B):128–33.
Lal S, Kirkup AJ, Brunsden AM, Thompson DG, Grundy D. Vagal afferent responses to fatty acids of different chain length in the rat. Am J Physiol: Gastrointest Liver Physiol. 2001;281(4):907–15.
Stilling RM, Dinan TG, Cryan JF. Microbial genes, brain & behaviour: epigenetic regulation of the gut–brain axis. Genes, Brain Behav. 2014;13(1):69–86.
Galland L. The gut microbiome and the brain. J Med Food. 2014;17(12):1261–72.
Jenkins TA, Nguyen JCD, Polglaze KE, Bertrand PP. Influence of tryptophan and serotonin on mood and cognition with a possible role of the gut–brain axis. Nutrients. 2016;8(1):1–15.
Tian P, Zou R, Song L, Zhang X, Jiang B, Wang G, et al. Ingestion of: bifidobacterium longum subspecies infantis strain CCFM687 regulated emotional behavior and the central BDNF pathway in chronic stress-induced depressive mice through reshaping the gut microbiota. Food Funct. 2019;10(11):7588–98.
Xu R, Tan C, Zhu J, Zeng X, Gao X, Wu Q, et al. Dysbiosis of the intestinal microbiota in neurocritically ill patients and the risk for death. Crit Care. 2019;23(1):1–13.
Xu K, Gao X, Xia G, Chen M, Zeng N, Wang S, You C, Tian X, Di H, Tang W, Li P, Wang H, Zeng X, Tan C, Meng F, Li H, He Y, Zhou HYJ. Rapid gut dysbiosis induced by stroke exacerbates brain infarction in turn. Gut. 2021;70(8):1486–94.
Yin J, Liao SX, He Y, Wang S, Xia GH, Liu FT, et al. Dysbiosis of gut microbiota with reduced trimethylamine-n-oxide level in patients with large-artery atherosclerotic stroke or transient ischemic attack. J Am Heart Assoc. 2015;4(11):1–13.
Sinagra E, Pellegatta G, Guarnotta V, Maida M, Rossi F, Conoscenti G, et al. Microbiota gut–brain axis in ischemic stroke: a narrative review with a focus about the relationship with inflammatory bowel disease. Life. 2021;11(7):715.
Battaglini D, Pimentel-Coelho PM, Robba C, dos Santos CC, Cruz FF, Pelosi P, et al. Gut microbiota in acute ischemic stroke: from pathophysiology to therapeutic implications. Front Neurol. 2020;11(June):1–13.
Xia GH, You C, Gao XX, Zeng XL, Zhu JJ, Xu KY, et al. Stroke dysbiosis index (SDI) in gut microbiome are associated with brain injury and prognosis of stroke. Front Neurol. 2019;10(APR):1–13.
Enaud R, Prevel R, Ciarlo E, Beaufils F, Wieërs G, Guery B, et al. The gut-lung axis in health and respiratory diseases: a place for inter-organ and inter-kingdom crosstalks. Front Cell Infect Microbiol. 2020;10(February):1–11.
Rogers GB, Bruce KD. Exploring the parallel development of microbial systems in neonates with cystic fibrosis. MBio. 2012;3(6):e00408-12.
McAleer JP, Kolls JK. Contributions of the intestinal microbiome in lung immunity. Eur J Immunol. 2018;48(1):39–49.
Fagundes CT, Amaral FA, Vieira AT, Soares AC, Pinho V, Nicoli JR, et al. Transient TLR activation restores inflammatory response and ability to control pulmonary bacterial infection in germfree mice. J Immunol. 2012;188(3):1411–20.
Enaud R, Prevel R, Ciarlo E, Beaufils F, Wieërs G, Guery B, et al. The gut-lung axis in health and respiratory diseases: a place for inter-organ and inter-kingdom crosstalks. Front Cell Infect Microbiol. 2020;10:9.
Morrow LE, Kollef MH, Casale TB. Probiotic prophylaxis of ventilator-associated pneumonia: a blinded, randomized, controlled trial. Am J Respir Crit Care Med. 2010;182(8):1058–64.
Trøseid M, Andersen GØ, Broch K, Hov JR. The gut microbiome in coronary artery disease and heart failure: current knowledge and future directions. EBioMedicine. 2020;52:102649.
Tang WHW, Kitai T, Hazen SL, Clinic C, Clinic C, Clinic C. Gut microbiota in cardiovascular health and disease. Circ Res. 2018;120(7):1183–96.
Wang Z, Klipfell E, Bennett BJ, Koeth R, Levison BS, Dugar B, et al. Gut flora metabolism of phosphatidylcholine promotes cardiovascular disease. Nature. 2011;472(7341):57–65.
Tang WH, Wang Z, Shrestha K, Borowski AG, Wu Y, Troughton RW, Klein ALHS. Intestinal microbiota-dependent phosphatidylcholine metabolites, diastolic dysfunction, and adverse clinical outcomes in chronic systolic heart failure. J Card Fail. 2015;2(21):91–6.
Li XS, Obeid S, Klingenberg R, Gencer B, Mach F, Räber L, et al. Gutmicrobiota-dependent trimethylamine N-oxide in acute coronary syndromes: a prognostic marker for incident cardiovascular events beyond traditional risk factors. Eur Heart J. 2017;38(11):814–24.
Organ CL, Otsuka H, Bhushan S, Wang Z, Bradley J, Trivedi R, Polhemus DJ, Tang WH, Wu Y, Hazen SLLD. Choline diet and its gut microbe derived metabolite, trimethylamine N-oxide (TMAO), Exacerbate pressure overloadinduced heart failure. Circ Hear Fail. 2016;176(12):139–48.
Zhang Y, Wang Y, Ke B, Du J. TMAO: how gut microbiota contributes to heart failure. Transl Res. 2021;228:109–25.
Pluznick JL, Protzko RJ, Gevorgyan H, Peterlin Z, Sipos A, Han J, et al. Olfactory receptor responding to gut microbiotaderived signals plays a role in renin secretion and blood pressure regulation. Proc Natl Acad Sci U S A. 2013;110(11):4410–5.
Yang T, Santisteban MM, Rodriguez V, Li E, Ahmari N, Sahay B, et al. Gut microbiota dysbiosis is linked to hypertension. Hypertension. 2015;65(6):1331–40.
Kamo T, Akazawa H, Suzuki JI, Komuro I. Novel concept of a heart-gut axis in the pathophysiology of heart failure. Korean Circ J. 2017;47(5):663–9.
Sandek A, Bauditz J, Swidsinski A, Buhner S, Weber-Eibel J, von Haehling S, et al. Altered intestinal function in patients with chronic heart failure. J Am Coll Cardiol. 2007;50(16):1561–9.
Gong J, Noel S, Pluznick JL, Hamad ARA, Rabb H. Gut Microbiota-kidney cross-talk in acute kidney injury. Semin Nephrol. 2019;39(1):107–16.
Andrade-Oliveira V, Amano MT, Correa-Costa M, Castoldi A, Felizardo RJF, De Almeida DC, et al. Gut bacteria products prevent AKI induced by ischemia-reperfusion. J Am Soc Nephrol. 2015;26(8):1877–88.
Sun G, Yin Z, Liu N, Bian X, Yu R, Su X, et al. Gut microbial metabolite TMAO contributes to renal dysfunction in a mouse model of diet-induced obesity. Biochem Biophys Res Commun. 2017;493(2):964–70.
Tang WH, Wang Z, Kennedy DJ, Wu Y, Buffa JA, Agatisa-Boyle B, Li XS, Levison BSHS. Gut microbiota-dependent trimethylamine N-oxide (TMAO) pathway contributes to both development of renal insufficiency and mortality risk in chronic kidney disease. Circ Res. 2015;116(3):448–55.
Velden J, Paust HJ, Hoxha E, Turner JE, Steinmetz OM, Wolf G, et al. Renal IL-17 expression in human ANCA-associated glomerulonephritis. Am J Physiol: Ren Physiol. 2012;302(12):1663–73.
Zhang J, Ankawi G, Sun J, Digvijay K, Yin Y, Rosner MH, et al. Gut-kidney crosstalk in septic acute kidney injury. Crit Care. 2018;22(1):1–8.
Tripathi A, Debelius J, Brenner DA, Karin M, Loomba R, Schnabl B, Knight R. The gut-liver axis and the intersection with the microbiome. Nat Rev Gastroenterol Hepatol. 2018;15(7):397–411.
Juanola O, Ferrusquía-Acosta J, García-Villalba R, Zapater P, Magaz M, Marín A, et al. Circulating levels of butyrate are inversely related to portal hypertension, endotoxemia, and systemic inflammation in patients with cirrhosis. FASEB J. 2019;33(10):11595–605.
Bajaj JS, Heuman DM, Hylemon PB, Sanyal AJ, White MB, Monteith P, et al. Altered profile of human gut microbiome is associated with cirrhosis and its complications. J Hepatol. 2014;60(5):940–7.
Addolorato G, Ponziani FR, Dionisi T, Mosoni C, Vassallo GA, Sestito L, et al. Gut microbiota compositional and functional fingerprint in patients with alcohol use disorder and alcohol-associated liver disease. Liver Int. 2020;40(4):878–88.
Chen Y, Ji F, Guo J, Shi D, Fang D, Li L. Dysbiosis of small intestinal microbiota in liver cirrhosis and its association with etiology. Sci Rep. 2016;6(August):1–9.
Inagaki T, Moschetta A, Lee YK, Peng L, Zhao G, Downes M, et al. Regulation of antibacterial defense in the small intestine by the nuclear bile acid receptor. Proc Natl Acad Sci U S A. 2006;103(10):3920–5.
Bajaj JS. Altered microbiota in cirrhosis and its relationship to the development of infection. Clin Liver Dis. 2019;14(3):107–11.
Bajaj JS, Fagan A, Sikaroodi M, White MB, Sterling RK, Gilles HC, et al. Liver transplant modulates gut microbial dysbiosis and cognitive function in cirrhosis. Liver Transplant. 2017;23(7):907–14.
Bajaj JS, Salzman NH, Acharya C, Sterling RK, White MB, Gavis EA, et al. Fecal microbial transplant capsules are safe in hepatic encephalopathy: a phase 1, randomized. Placebo-Controll Trial Hepatol. 2019;70(5):1690–703.
Acknowledgements
Not applicable.
Funding
The authors declare that they have no funding.
Author information
Authors and Affiliations
Contributions
HW, CPH and JS contributed to conceptualization and draft preparation. HW, TSB, LF, TS, CLT, AW, JS and CPH contributed to original draft preparation and reviewing and editing. All authors have read and approved the manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Wozniak, H., Beckmann, T.S., Fröhlich, L. et al. The central and biodynamic role of gut microbiota in critically ill patients. Crit Care 26, 250 (2022). https://doi.org/10.1186/s13054-022-04127-5
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s13054-022-04127-5