Abstract
Background
Several clinical guidelines recommend monitoring blood lactate levels and central venous oxygen saturation for hemodynamic management of patients with sepsis. We hypothesized that carbon dioxide production (VCO2) and oxygen extraction (VO2) evaluated using indirect calorimetry (IC) might provide additional information to understand the dynamic metabolic changes in sepsis.
Methods
Adult patients with sepsis who required mechanical ventilation in the intensive care unit (ICU) of our hospital between September 2019 and March 2020 were prospectively enrolled. Sepsis was diagnosed according to Sepsis-3. Continuous measurement of VCO2 and VO2 using IC for 2 h was conducted within 24 h after tracheal intubation, and the changes in VCO2 and VO2 over 2 h were calculated as the slopes by linear regression analysis. Furthermore, temporal lactate changes were evaluated. The primary outcome was 28-day survival.
Results
Thirty-four patients with sepsis were enrolled, 26 of whom survived 76%. Significant differences in the slope of VCO2 (− 1.412 vs. − 0.446) (p = 0.012) and VO2 (− 2.098 vs. − 0.851) (p = 0.023) changes were observed between non-survivors and survivors. Of note, all eight non-survivors and 17 of the 26 survivors showed negative slopes of VCO2 and VO2 changes. For these patients, 17 survivors had a median lactate of − 2.4% changes per hour (%/h), whereas non-survivors had a median lactate of 2.6%/hr (p = 0.023).
Conclusions
The non-survivors in this study showed temporal decreases in both VCO2 and VO2 along with lactate elevation. Monitoring the temporal changes in VCO2 and VO2 along with blood lactate levels may be useful in predicting the prognosis of sepsis.
Similar content being viewed by others
Introduction
Sepsis is a leading cause of death in intensive care units (ICUs) [1, 2]. Hemodynamic management in patients with sepsis is important for providing a sufficient amount of oxygen to the organs and preventing the development of multiple organ dysfunction. Several therapeutic strategies for sepsis, such as early goal-directed therapy [3] and Hour-1 Bundle [4], include hemodynamic management. Measuring blood lactate level and its temporal changes (lactate clearance) and monitoring central venous oxygen saturation (SCVO2) improved the outcomes of patients with sepsis [5,6,7,8]. However, these indicators have some limitations. Some studies have reported that interventions with SCVO2 monitoring failed to show better outcomes [9]. Blood lactate levels will be affected by liver dysfunction [10]. Therefore, some other monitoring indicators are needed for clinically managing sepsis.
Sepsis is characterized by altered cellular metabolism and impaired oxygen usage despite adequate oxygen delivery (DO2) [11]. Recently, mitochondrial impairment termed “cytopathic hypoxia” has been recognized as the mechanism of organ dysfunctions in sepsis [12]. Under the decrease in oxygen extraction, the mitochondria cannot generate energy via oxidative phosphorylation, and energy metabolism will become dependent on anaerobic glycolysis under hypoxic state [13]. Monitoring carbon dioxide production (VCO2) and oxygen extraction (VO2) is expected to help detect the progression of sepsis exacerbation, especially impaired oxygen usage in the mitochondria. Indirect calorimetry (IC) can simultaneously and noninvasively measure VCO2 and VO2. It has already been used in ICUs for measuring energy expenditure and oxygen consumption [14, 15]. IC will provide information not only for estimated nutritional requirements but also for tissue metabolism [16].
This study was designed to explore the possible role of IC in monitoring cellular oxygen metabolism in patients with sepsis. We measured the temporal changes in VCO2 and VO2 in mechanically ventilated patients with sepsis and evaluated whether these parameters are associated with 28-day survival.
Method
Study design and participants
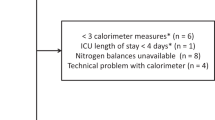
This study was a single-center prospective observational study, which has been registered on the UMIN Clinical Trials Registry (registry number: UMIN 000045966). Adult patients (> 18 years old), who were diagnosed with sepsis and orally intubated in the ICU of the University of Tokyo Hospital between September 2019 and March 2020, were included. Sepsis was diagnosed according to Sepsis-3 [17]. We excluded the following patients because of the inaccuracy of IC measurement: patients complicated with pneumothorax, those treated with extracorporeal membrane oxygenation (ECMO) therapy, those ventilated with more than 85% fraction of inspired oxygen (FIO2), those with changes in ventilator settings including FIO2 during IC measurement, those intubated from nasal or tracheostomy, and those isolated for high risk of airborne infection. Moreover, we excluded patients who declared do not attempt resuscitation and those without informed consent. During the study period, 66 patients with sepsis were mechanically ventilated in our ICU, and 34 patients were finally enrolled in this study (Fig. 1). IC measurement was initiated within 24 h after oral tracheal intubation. VCO2 and VO2 values were measured for 2 h continuously.
This study was conducted according to the amended Declaration of Helsinki, and the Institutional Review Board of the University of Tokyo approved this study (2018094NI). Informed consent was obtained from all participants or their legal representatives.
Indirect calorimetry
For IC, CCM Express (MGC Diagnostics, Saint Paul, Minnesota) [18] was used. Warm-up and calibration were conducted according to the specifications of the manufacturer. IC measures the difference between inspiratory and expiratory VCO2 and VO2 using the breath-by-breath analysis method. It uses a pneumotach flowmeter connected near the endotracheal tube. Inspiratory and expiratory gases were collected through a sampling line connected to this flowmeter. VCO2 is measured using an infrared analyzer, while VO2 is measured using a galvanic fuel cell. Patient ventilation is measured at the endotracheal tube. Therefore, considering any bias flow provided by the ventilator is not needed [19, 20].
Data collection
The following patient characteristics and clinical data were collected from the medical records: age, sex, past medical history, height, weight, catecholamine use, continuous renal replacement therapy use, induction medication, sedation, Richmond Agitation-Sedation Scale (RASS), source of infection, thiamine administration, and positive results of the culture. Blood gas analysis, including blood lactate levels, was performed during intubation and after IC measurement. Furthermore, ventilator settings and vital signs during IC measurement were obtained. The Acute Physiology and Chronic Health Evaluation (APACHE) II score, Sequential Organ Failure Assessment (SOFA) score, and catecholamine index were calculated.
VCO2, VO2, and respiratory quotient (RQ) were measured using IC. Before the analysis, VCO2, VO2, and RQ data obtained using IC were modified to minimize the possible artifacts in the following process (Additional file 1: Fig. S1). First, the values considered out of the physiological range (VCO2 < 70 mL/min or > 800 mL/min; VO2 < 100 mL/min or > 1000 mL/min; and RQ < 0.67 or > 1.3) were excluded [21,22,23]. Second, the average values every 5 min (24 points for 2 h) in each of VCO2, VO2, and RQ were obtained. The outlier values outside the mean ± 2 standard deviation of 24 points and points before and after the outlier values were excluded. Finally, a linear regression line was obtained from the remaining points, and each slope was defined as VCO2, VO2 and RQ slopes.
For temporal changes in lactate levels, the percentage of change was measured hourly (%/hr); the percentage of changes in blood lactate level was obtained by dividing the hours from two time points (during intubation and the end of IC measurement).
Outcomes
The primary outcome was 28-day survival, and its association with VCO2 and VO2 slopes was evaluated. We further evaluated the additional information provided by VCO2 and VO2 slopes along with lactate temporal changes.
Statistical analysis
Continuous variables were presented as median (interquartile range), and categorical variables were presented as percentages. Categorical data were compared using the chi-square test or Fisher’s exact test as appropriate. Multivariate logistic regression analysis was performed to examine the associations of VCO2 and VO2 slopes with 28-day mortality adjusted from the predefined confounding factors of APACHE II score and lactate temporal changes. Predictive performance of each parameter for 28-day mortality was evaluated by receiver operating characteristic (ROC) analysis, and the cutoff values were determined with Youden’s index. All statistical analyses were performed using JMP Pro (version 15.1.0; SAS Institute Inc., Cary, NC, US). Two-tailed p values of less than 0.05 were used to denote statistical significance for all tests.
Results
Patient characteristics
Among the 34 enrolled patients, 26 survived and eight died within 28 days after ICU admission. The characteristics and clinical parameters are shown in Table 1. Age and APACHE II scores in non-survivors were significantly higher than those in survivors. Moreover, a significant difference in lactate temporal changes was observed between non-survivors and survivors. No significant differences in other characteristics and parameters were observed between these groups. Average values of VCO2, VO2, RQ, and REE during 2 h IC measurement in each patient are shown in Additional file 2: Table S1.
Relationship between 28-day survival and VCO2, VO2 and RQ slopes
Temporal changes of VCO2 and VO2 are shown in Fig. 2. Median values of VCO2 and VO2 slopes in survivors and non-survivors showed negative values, indicating temporal reduction of VCO2 and VO2. Compared with survivors, the absolute values of VCO2 and VO2 slopes in non-survivors were significantly greater (VCO2 slope; − 1.412 vs. − 0.446, p = 0.012; VO2 slope; − 2.098 vs. − 0.851, p = 0.023). However, the slopes of RQ between non-survivors and survivors were not significantly different (RQ slope; 0.001 vs. 0.003, p = 0.180) (Additional file 3: Fig. S2). Multiple regression analysis revealed that 28-day mortality was significantly associated with decreases in VCO2 slope (adjusted OR (Odds Ratio), 0.349; 95% confidence interval (CI), 0.128–0.953) adjusted of APACHE II score and lactate temporal changes (Table 2). The predictive value of VCO2 and VO2 slopes for 28-day mortality was evaluated by ROC analysis. We observed significant predictive performance of VCO2 and VO2 slopes (Additional file 2: Table S2).
Combination of VCO2 and VO2 slopes with lactate temporal change
The patients were categorized into four groups according to combinations of VCO2 and VO2 slopes (Fig. 3). All non-survivors and 17 of the 26 survivors showed negative VCO2 and VO2 slopes (category C). No patients were classified into category D. Among the patients in category C, lactate temporal changes in the non-survivors were significantly higher than those in the survivors (i.e., lower lactate clearance) (2.6 vs. − 2.4, respectively) (p = 0.023) (Fig. 4). Among the survivors, those in category C had significantly lower lactate temporal changes than those in categories A and B (− 2.4 vs. 1.7, respectively) (p = 0.024).
Discussion
Principal findings
Among the intubated patients with sepsis, all the non-survivors showed temporal declines in both VCO2 and VO2. Temporal changes in blood lactate levels provided additional information for discriminating non-survivors from survivors when patients showed temporal decreases in VCO2 and VO2. In contrast, survivors with temporal elevations in VCO2 or VO2 showed significantly lower lactate clearance than other survivors. Taken together, declines in VCO2 and VO2 and lower lactate clearance seemed to be risk factors for poor outcomes. These findings suggest that temporal changes in VCO2 and VO2 with lactate clearance will enable us to monitor the severity of dysregulation of carbon dioxide production and oxygen consumption in sepsis and predict the outcome of death.
Comparison with other studies
Because one of the major aspects of the pathophysiology of sepsis-induced organ dysfunction is failure of carbon dioxide production and oxygen use in cellular mitochondria [11], VCO2 or VO2 measurements are expected to provide physiological information on the severity of sepsis. Several studies have reported that VCO2 or VO2 can be measured using IC in patients with sepsis. Hoeyer-Nielsen et al. recently have shown a higher VO2 lactate ratio in survivors and a significant difference in temporal VCO2 changes between survivors and non-survivors [16]. This study used three parameters (i.e., VCO2, VO2, and lactate clearance) and found possible additive values by combining these three parameters. These two studies suggested the importance of temporal change in physiological parameters such as VCO2 and VO2. Evaluation only with absolute values will be hampered by individual differences, which will be frequently observed in severe sepsis patients. Of note, previous studies reported the superiority of temporal changes compared with absolute value of lactate in sepsis patients [8, 24]. Temporal evaluation can overcome the problem of individual variation and may contribute to personalized medicine. It should be noted that indirect calorimetry used in this study enabled continuous monitoring of VCO2 and VO2.
SCVO2 and lactate clearance are predictors of mortality in patients with sepsis [5,6,7,8]. However, these clinical parameters have several limitations to be considered. In septic conditions, microcirculatory heterogeneity that generates capillary shunting frequently elevates SCVO2 values [9]. Hyperlactatemia does not necessarily reflect sepsis progression as other diseases, such as liver dysfunction, seizure, and diabetic ketoacidosis, could cause hyperlactatemia [10].
Study strength
Figure 5 shows a speculation regarding temporal changes in VCO2 and VO2 along with DO2 in patients with sepsis. VO2 reaches a plateau at a higher level of DO2 (critical oxygen delivery point) [25]. Due to cytopathic hypoxia, sepsis reduces the slope of the VO2/DO2 ratio. Patients with sepsis require high VO2 [26]. This change will also increase VCO2 (Pathway 1 in Fig. 5). When DO2 is below the critical oxygen delivery point, VO2 will also decrease (Pathway 2 in Fig. 5). Further reduction in DO2 will induce anaerobic metabolism, and VCO2 will decrease (Pathway 3 in Fig. 5). When some therapeutic interventions successfully increase VCO2, VO2 will increase (Pathway 4 in Fig. 5). A decrease in VCO2 will be observed during the recovery phase in which VO2 is decreased (Pathways 5 and 6 in Fig. 5). All non-survivors and some survivors in this study showed a dual reduction in VCO2 and VO2 (Pathways 3, 5, and 6), and we assumed that non-survivors and survivors with VCO2 and VO2 reductions correspond to Pathways 3 and 5 or 6, respectively. Continuous monitoring of VCO2 and VO2 could provide valuable information for determining management of sepsis and be expected to be a useful indicator for therapeutic interventions to sepsis patients. However, evaluating temporal changes in VCO2 and VO2 alone could not discriminate non-survivors from survivors. Additional information, especially lactate temporal changes, will provide a better prediction performance. In contrast, patients with increased VCO2 or VO2 had a good prognosis despite lactate elevation. Although this speculation must be confirmed with a larger population, the combination of VCO2 and VO2 measurement using IC with lactate temporal changes may be expected to be a good predictor of prognosis in sepsis.
Limitations
This study has several limitations. First, we focused on the temporal changes in VCO2 and VO2 values instead of their absolute values for eliminating individual variations among patients and the measurement procedure. However, obtaining the temporal data and evaluating relative changes are time-consuming. Second, the obtained results in this study should be confirmed using other IC models. No changes in mechanical ventilation for FIO2 were required because a change in supplementary oxygen during IC measurement can lead to inaccurate data [27]. This will be a barrier for clinical application. Third, all data were obtained, while no nutritional support was provided. Additional VCO2 production by nutrition should be considered in the later phase of ICU stay. Fourth, severe metabolic acidosis induces excess carbon dioxide excretion from the lungs as compensation. Other factors that may affect changes in VCO2 and VO2 during IC measurement, such as sedation level, body temperature, cardiac function including cardiac output, hemoglobin, and SCVO2, should also be evaluated. Fifth, factors affecting lactate levels, such as hepatic function and lack of thiamine, were not examined. Finally, the sample size was small because this is a single-center cohort study. We used only APACHE II score and temporal lactate changes as variables for multivariate analysis to avoid overfitting. Further studies involving larger cohorts and analysis considering sufficient possible confounding factors are necessary to confirm our conclusions. Because the results of this study are preliminary, our approach cannot be immediately clinically applied. Clinical application will be possible when the profound technological improvement on IC will allow us to monitor VO2 and VCO2 more easily as end-tidal carbon dioxide monitoring, which is widely used in mechanical ventilated patients.
Conclusion
Among mechanically ventilated patients with sepsis, non-survivors showed temporal decreases in VCO2 and VO2 with lactate elevation. Monitoring changes in VCO2 and VO2 along with lactate changes will provide information on tissue metabolism in sepsis and may be useful in predicting the prognosis of patients with sepsis.
Availability of data and materials
The datasets used and/or analyzed in this study are available from the corresponding author on reasonable request.
Abbreviations
- APACHE:
-
Acute Physiology and Chronic Health Evaluation
- AUC:
-
Area under the curve
- CI:
-
Confidence interval
- DO2 :
-
Oxygen delivery
- ECMO:
-
Extracorporeal membrane oxygenation
- FIO2 :
-
Fraction of inspired oxygen
- IC:
-
Indirect calorimetry
- ICUs:
-
Intensive care units
- OR:
-
Odds ratio
- RASS:
-
Richmond Agitation-Sedation Scale
- ROC:
-
Receiver operating characteristic
- RQ:
-
Respiratory quotient
- SCVO2 :
-
Central venous oxygen saturation
- SOFA:
-
Sequential Organ Failure Assessment
- VCO2 :
-
Carbon dioxide production
- VO2 :
-
Oxygen extraction
References
Vincent JL, Rello J, Marshall J, Silva E, Anzueto A, Martin CD, Moreno R, Lipman J, Gomersall C, Sakr Y, et al. International study of the prevalence and outcomes of infection in intensive care units. JAMA. 2009;302(21):2323–9.
Vincent JL, Marshall JC, Namendys-Silva SA, François B, Martin-Loeches I, Lipman J, Reinhart K, Antonelli M, Pickkers P, Njimi H, et al. Assessment of the worldwide burden of critical illness: the intensive care over nations (ICON) audit. Lancet Respir Med. 2014;2(5):380–6.
Rhodes A, Evans LE, Alhazzani W, Levy MM, Antonelli M, Ferrer R, Kumar A, Sevransky JE, Sprung CL, Nunnally ME, et al. Surviving sepsis campaign: international guidelines for management of sepsis and septic shock: 2016. Intensive Care Med. 2017;43(3):304–77.
Levy MM, Evans LE, Rhodes A. The surviving sepsis campaign bundle: 2018 update. Intensive Care Med. 2018;44(6):925–8.
Jansen TC, van Bommel J, Schoonderbeek FJ, Sleeswijk Visser SJ, van der Klooster JM, Lima AP, Willemsen SP, Bakker J. Early lactate-guided therapy in intensive care unit patients: a multicenter, open-label, randomized controlled trial. Am J Respir Crit Care Med. 2010;182(6):752–61.
Textoris J, Fouché L, Wiramus S, Antonini F, Tho S, Martin C, Leone M. High central venous oxygen saturation in the latter stages of septic shock is associated with increased mortality. Crit Care. 2011;15(4):R176.
Nichol A, Bailey M, Egi M, Pettila V, French C, Stachowski E, Reade MC, Cooper DJ, Bellomo R. Dynamic lactate indices as predictors of outcome in critically ill patients. Crit Care. 2011;15(5):R242.
Nguyen HB, Rivers EP, Knoblich BP, Jacobsen G, Muzzin A, Ressler JA, Tomlanovich MC. Early lactate clearance is associated with improved outcome in severe sepsis and septic shock. Crit Care Med. 2004;32(8):1637–42.
Pope JV, Jones AE, Gaieski DF, Arnold RC, Trzeciak S, Shapiro NI. Multicenter study of central venous oxygen saturation (ScvO(2)) as a predictor of mortality in patients with sepsis. Ann Emerg Med. 2010;55(1):40-46.e41.
Andersen LW, Mackenhauer J, Roberts JC, Berg KM, Cocchi MN, Donnino MW. Etiology and therapeutic approach to elevated lactate levels. Mayo Clin Proc. 2013;88(10):1127–40.
Fink MP. Bench-to-bedside review: cytopathic hypoxia. Crit Care. 2002;6(6):491–9.
Crouser ED. Mitochondrial dysfunction in septic shock and multiple organ dysfunction syndrome. Mitochondrion. 2004;4(5–6):729–41.
Loiacono LA, Shapiro DS. Detection of hypoxia at the cellular level. Crit Care Clin. 2010;26(2):409–21.
Li A, Mukhopadhyay A. Substrate utilization and energy expenditure pattern in sepsis by indirect calorimetry. Crit Care. 2020;24(1):535.
Uber A, Grossestreuer AV, Ross CE, Patel PV, Trehan A, Donnino MW, Berg KM. Preliminary observations in systemic oxygen consumption during targeted temperature management after cardiac arrest. Resuscitation. 2018;127:89–94.
Hoeyer-Nielsen AK, Holmberg MJ, Grossestreuer AV, Yankama T, Branton JP, Donnino MW, Berg KM. Association between the oxygen consumption: lactate ratio and survival in critically ill patients with sepsis. Shock. 2021;55(6):775–81.
Singer M, Deutschman CS, Seymour CW, Shankar-Hari M, Annane D, Bauer M, Bellomo R, Bernard GR, Chiche JD, Coopersmith CM, et al. The third international consensus definitions for sepsis and septic shock (sepsis-3). JAMA. 2016;315(8):801–10.
Smallwood CD, Martinez EE, Mehta NM. A comparison of carbon dioxide elimination measurements between a portable indirect calorimeter and volumetric capnography monitor: an in vitro simulation. Respir Care. 2016;61(3):354–8.
Sundström M, Tjäder I, Rooyackers O, Wernerman J. Indirect calorimetry in mechanically ventilated patients. A systematic comparison of three instruments. Clin Nutr. 2013;32(1):118–21.
Graf S, Karsegard VL, Viatte V, Heidegger CP, Fleury Y, Pichard C, Genton L. Evaluation of three indirect calorimetry devices in mechanically ventilated patients: which device compares best with the Deltatrac II(®)? A prospective observational study. Clin Nutr. 2015;34(1):60–5.
Rivers EP, Rady MY, Martin GB, Fenn NM, Smithline HA, Alexander ME, Nowak RM. Venous hyperoxia after cardiac arrest. Characterization of a defect in systemic oxygen utilization. Chest. 1992;102(6):1787–93.
Hayes MA, Timmins AC, Yau EH, Palazzo M, Hinds CJ, Watson D. Elevation of systemic oxygen delivery in the treatment of critically ill patients. N Engl J Med. 1994;330(24):1717–22.
Takemae A, Takazawa T, Kamiyama J, Kanamoto M, Tobe M, Hinohara H, Kunimoto F, Saito S. A novel prediction equation of resting energy expenditure for Japanese septic patients. J Crit Care. 2020;56:236–42.
Okorie ON, Dellinger P. Lactate: biomarker and potential therapeutic target. Crit Care Clin. 2011;27(2):299–326.
Squara P. Matching total body oxygen consumption and delivery: a crucial objective? Intensive Care Med. 2004;30(12):2170–9.
Englert JA, Rogers AJ. Metabolism, metabolomics, and nutritional support of patients with sepsis. Clin Chest Med. 2016;37(2):321–31.
Shinozaki K, Becker LB, Saeki K, Kim J, Yin T, Da T, Lampe JW. Dissociated oxygen consumption and carbon dioxide production in the post-cardiac arrest rat: a novel metabolic phenotype. J Am Heart Assoc. 2018;7(13):e007721.
Acknowledgements
We are grateful to Dr. Kurato Tokunaga for helpful discussions.
Funding
There was no funding source for this study.
Author information
Authors and Affiliations
Contributions
IH, TA, and KD designed the analysis plan. IH performed all statistical analyses. IH wrote the first draft of the study. MY, NH, TH, and KD critically reviewed the manuscript. All authors contributed to the study design, interpretation of results, and critical revision of the article for intellectually important content. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
The study was approved by the Institutional Review Board of the University of Tokyo (2018094NI). Written informed consent was obtained from each participant.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Additional file 1. Figure S1.
Data processing method.
Additional file 2. Table S1.
Average values of VCO2, VO2, RQ, and REE during 2 h IC measurement. Table S2. ROC analysis for 28-day survival.
Additional file 3. Figure S2.
Temporal change of RQ.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Hirayama, I., Asada, T., Yamamoto, M. et al. Changes in carbon dioxide production and oxygen uptake evaluated using indirect calorimetry in mechanically ventilated patients with sepsis. Crit Care 25, 416 (2021). https://doi.org/10.1186/s13054-021-03830-z
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s13054-021-03830-z