Abstract
Background
Zinc is a trace element that plays a role in stimulating innate and acquired immunity. The role of zinc in critically ill patients with COVID-19 remains unclear. This study aims to evaluate the efficacy and safety of zinc sulfate as adjunctive therapy in critically ill patients with COVID-19.
Methods
Patients aged ≥ 18 years with COVID-19 who were admitted to the intensive care unit (ICU) in two tertiary hospitals in Saudi Arabia were retrospectively assessed for zinc use from March 1, 2020 until March 31, 2021. After propensity score matching (1:1 ratio) based on the selected criteria, we assessed the association of zinc used as adjunctive therapy with the 30-day mortality. Secondary outcomes included the in-hospital mortality, ventilator free days, ICU length of stay (LOS), hospital LOS, and complication (s) during ICU stay.
Results
A total of 164 patients were included, 82 patients received zinc. Patients who received zinc sulfate as adjunctive therapy have a lower 30-day mortality (HR 0.52, CI 0.29, 0.92; p = 0.03). On the other hand, the in-hospital mortality was not statistically significant between the two groups (HR 0.64, CI 0.37–1.10; p = 0.11). Zinc sulfate use was associated with a lower odds of acute kidney injury development during ICU stay (OR 0.46 CI 0.19–1.06; p = 0.07); however, it did not reach statistical significance.
Conclusion
The use of zinc sulfate as an additional treatment in critically ill COVID-19 patients may improve survival. Furthermore, zinc supplementation may have a protective effect on the kidneys.
Similar content being viewed by others
Background
The coronavirus disease 2019 (COVID-19) caused by the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), has emerged as a threat to public health worldwide [1]. Symptoms of COVID-19 manifest as a cluster of mild to severe respiratory symptoms [1]. Severe COVID-19 amplifies the overall systematic inflammatory response in critically ill patients, increasing the risk of multi-organ dysfunctions, acute respiratory disease syndrome (ARDS), and mortality [1,2,3,4]. This inflammatory response is caused by the hyperactivation of chemokines and cytokines, prominently interleukin-6 (IL-6) [5]. Therefore, the dysregulation of cytokines was one of the targets for many treatment strategies in treating patients with severe COVID-19 [3].
To date, no specific pharmacological agents have proven efficacy against SARS-CoV; instead, host-directed therapies and supportive therapy are used [5]. The current treatment options for critically ill patients with COVID-19 include anti-viral agents, immunosuppressive agents, and immunomodulators [6]. However, the evidence about these treatment options' mortality benefit in critically ill patients with COVID-19 is conflicting [7,8,9,10]. Moreover, some mineral supplements and vitamins with immunomodulatory activity and antioxidant effects such as thiamine, vitamins C and D, zinc, and selenium use in patients with COVID-19 are investigated [11,12,13,14]
Zinc is a trace element that plays a role in the development and function of the immune system exhibiting direct or indirect anti-viral properties [13, 14]. High intracellular zinc levels were reported to stall the replication of coronavirus (SARS-CoV-2) and influenza virus that caused the severe acute respiratory syndrome [14,15,16]. Some reports demonstrate the synergistic effect of zinc with anti-viral therapy in SARS-CoV-2 [17]. The benefit of zinc supplementation on the immune system function has been previously observed in non-COVID-19 patients [17, 18]. However, there is inadequate evidence to support the use of zinc for COVID-19 treatment in critically ill patients [19, 20]. Yet, providers continue to use it as adjunctive therapy in this patient population. Therefore, we aim to evaluate the efficacy and safety of zinc supplementation as adjunctive therapy in treating critically ill patients with COVID-19.
Methods
Study design
This was a two-center, retrospective study of all critically ill patients with COVID-19 who were admitted to the Intensive Care Unit (ICU) from March 1, 2020 until March 31, 2021. We aimed to enroll as many patients as possible, with no predefined sample size. The COVID-19 was confirmed using a reverse transcriptase-polymerase chain reaction (RT-PCR) obtained from nasopharyngeal or throat swabs. The study was approved by King Abdullah International Medical Research Center (KAIMRC)-Institutional Review Board, Riyadh, Saudi Arabia (Study Number: NRC21R/287/07).
Participants
Patients aged ≥ 18 years and admitted to ICU for more than 24 h with a confirmed COVID-19 were eligible for inclusion. Patients were excluded if they died within 24 h of ICU admission, labeled as "Do-Not-Resuscitate" within 24 h of ICU admission, or received zinc before ICU admission (Fig. 1). Enrolled patients were classified into two groups based on the administration of zinc sulfate as adjunctive therapy during ICU stay. Patients who received new initiation of zinc sulfate during ICU stay were included in the active group. The decision on whether to use zinc sulfate with a daily dose of 220 mg (50 mg of elemental zinc) enteral tablets for patients was solely based on physician clinical judgment. Patients were followed during their hospital stay, until discharge or in-hospital death, whichever occurred first.
Setting
This study was conducted in two large, tertiary governmental hospitals; King Abdulaziz Medical City (KAMC), Riyadh, and King Abdulaziz University Hospital (KAUH), Jeddah. The ICUs admit medical, surgical, trauma, burn, and transplant patients and operate as a closed unit with 24/7 onsite coverage by critical care board-certified intensivists and clinical pharmacists. The primary site for this study was King Abdulaziz Medical City.
Data collection
Each patients’ data were collected and managed using Research Electronic Data Capture (REDCap®) software hosted by King Abdullah International Medical Research Center (KAIMRC). Patient's demographic data, comorbidities, vital signs, and laboratory tests were extracted from electronic medical records. The laboratory tests included the renal profile (Estimated glomerular filtration rate (eGFR), AKI status), liver profile (Aspartate transaminase (AST), Alanine transaminase (ALT), and total bilirubin), coagulation profile (international normalized ratio (INR), activated Partial Thromboplastin Time (aPTT)), and albumin levels within 24 h of ICU admission. In addition, surrogate biomarkers of the COVID-19-associated inflammation (ferritin, D-dimer, fibrinogen and C-reactive protein (CRP)) were collected and reported with their baseline and peak values during the ICU stay [30]. Peak levels were defined to be the highest level of the surrogate marker checked at any time during their ICU length of stay. The aim of investigating these biomarkers was to establish a reasonable correlation between zinc administration and disease progression that might be captured via an increase in the inflammatory surrogate markers. The selected biomarkers have been associated with poor outcomes in COVID-19 patients [4, 30]. The severity baseline scores (i.e., Acute Physiology and Chronic Health Evaluation II (APACHE II), Sequential Organ Failure Assessment (SOFA)), and Nutrition Risk in Critically ill (NUTRIC)) were calculated using the MDCalc website for each patient [20]. In addition, we collected the needs for mechanical ventilation (MV), and MV parameters (e.g., PaO2/FiO2 ratio, FiO2 requirement) within 24 h of ICU admission. Lastly, tocilizumab and corticosteroids use within 24 h and the concomitant use of nephrotoxic medications (i.e. Vancomycin IV, Gentamicin IV, Amikacin IV, Colistin IV, Furosemide, Sulfamethoxazole/trimethoprim and Contrast,) during ICU stay were recorded for the eligible patients.
Aim of study and outcomes
This study aimed to evaluate the efficacy and safety of zinc sulfate as adjunctive therapy in critically ill patients with COVID-19. The primary endpoint was the 30-day mortality in critically ill patients who received zinc sulfate as adjunctive therapy. The secondary endpoint included the in-hospital mortality, ICU length of stay (LOS), hospital LOS, ventilator free days (VFDs), and evaluation of complications during ICU stay after zinc initiation, including acute kidney injury (AKI), liver injury, and thrombosis/infraction during ICU stay.
Definition(s)
-
The 30-day mortality was defined as the in-hospital death occurring for any cause within 30 days of the admission date during the hospital stay.
-
Ventilator-free days (VFDs) at 30 days were calculated as the following: if the patients die within 30 days of MV, the VFDs = 0, VFDs = 30-days after MV initiation (if the patient survived and was successfully liberated from MV), and VFDs = 0 if the patient is on MV for > 30 days.
-
Acute kidney injury (AKI) was defined using the Acute Kidney Injury Network (AKIN) definition [21].
-
Thrombosis/infraction was defined using the International Statistical Classification of Diseases (ICD) 10-CM code (i.e., Myocardial infarction (MI), ischemic stroke, pulmonary embolism, deep vein thrombosis) [22]
-
Respiratory failure was defined as either hypoxemic respiratory failure (PaO2 < 60 mm Hg with a normal or low arterial carbon dioxide tension (PaCO2) or hypercapnic respiratory failure (PaCO2 > 50 mm Hg) that requires invasive mechanical ventilation.
-
Liver injury is defined as alanine aminotransferase (ALT) exceeding three times the upper limit of normal or double in patients with elevated baseline ALT.
Data management and statistical analysis
Categorical variables were reported using numbers and percentages, whereas continuous variables were reported using mean with standard deviation (SD) or median with interquartile range (IQR) when appropriate. We compared categorical variables using the Chi-square or Fisher exact test. Continuous variables were compared using numerical student t test (for the normally distributed variables) or other quantitative variables with the Mann–Whitney U test (for the non-normally distributed variables). The normality assumptions were assessed for all numerical variables using statistical tests (i.e., Shapiro–Wilk test) and graphical representation (i.e., histograms and Q–Q plots). Model fit assessed using the Hosmer–Lemeshow goodness-of-fit test.
Propensity score matching procedure (Proc PS match) (SAS, Cary, NC) was used to match patients who newly received zinc sulfate (active group) to patients who did not (control group) based on patient’s APACHE II score, acute kidney injury, and systemic use of corticosteroids within 24 h of ICU admission. A greedy nearest neighbor matching method was used in which one patient who newly received zinc sulfate (active) group matched with one patient who did not (control), which eventually produced the smallest within-pair difference among all available pairs with treated patients. Patients were matched only if the difference in the logits of the propensity scores for pairs of patients from the two groups was less than or equal to 0.5 times the pooled estimate of the standard deviation.
Multivariable Cox proportional hazards regression analysis were performed for the 30-day and in-hospital mortality. Additionally, Kaplan–Meier (KM) plots were generated for these outcomes. Multivariable regression analysis and negative binomial regression were used as appropriate for the other outcomes considered in this study. Regression analysis was done by consider PS score as one of the covariates in the model. The odds ratios (OR), hazard ratio (HR), or estimates with the 95% confidence intervals (CI) were reported as appropriate. No imputation was made for missing data as the cohort of patients in our study was not derived from random selection. We considered a P value of < 0.05 statistically significant and used SAS version 9.4 for all statistical analysis.
Results
A total of 756 eligible critically ill patients with COVID-19 were admitted to the ICU during the study period in the two study centers. Zinc sulfate 220 mg (50 mg of elemental zinc) enteral tablets once daily was newly initiated in the ICU to 90 patients, whereas 666 patients did not receive zinc as adjunctive therapy. We matched 164 patients using propensity score (1:1) according to the selected criteria. A total of 38 patients (46.3%) have received zinc sulfate within 24 h of ICU admission. The median (Q1, Q3) duration of zinc sulfate was 11 days (6, 15).
Study population
Before PS matching, most of the patients in both arms were men (70.9%), and the average age was 60.8 ± 14.6 years in the whole cohort. The most common comorbidities were diabetes mellitus (60.1%), followed by hypertension (56.8%), and dyslipidemia (20.8%) (Additional file 1: Table e1). Moreover, there was a significant difference in the baseline characteristics between the two groups before PS. The baseline severity scores (i.e., APACHE II and SOFA scores), the nutritional status based on the NUTRIC score stratification, total WBCs, procalcitonin levels, AKI status, and INR were higher in the control group. Conversely, patients in the zinc group have a higher eGFR baseline and received more pharmacological deep vein thrombosis prophylaxis. However, after using PS matching based on the selected criteria, most of the baseline characteristics and comorbidities were balanced between the two groups except for lactic acid baseline, which was significantly higher in the control group. Moreover, the tocilizumab, corticosteroids use within 24 h of ICU admission, and the concomitant use of nephrotoxic medications during ICU stay were similar between the groups (Additional file 1: Table e1).
30-Day and in-hospital mortality
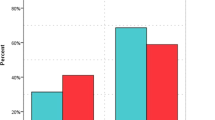
In crude analysis, 19 patients (23.2%) died within 30 days among the zinc group, compared with 31 patients (38.8%) in the control group (P = 0.03). At multivariable Cox proportional hazards regression analyses, the 30-day mortality was lower in patients who received zinc sulfate (HR 0.52 CI 0.29, 0.92; p = 0.03). On the other hand, the in-hospital mortality was similar between the two groups (HR 0.64 CI 0.37, 1.10; p = 0.11) (Table 1). The overall survival probabilities using Kaplan–Meier (KM) plots were not statistically different during hospital stay among patients who received zinc after propensity score-matched (P = 0.0979) (Fig. 2).
Ventilator free days (VFDs) and length of stay (LOS)
Patients who received zinc have a longer median of VFDs than patients who did not (twenty versus zero days; P = 0.02). However, it was not statistically significant in the regression analysis (Beta coefficient 0.33 CI − 0.21, 0.87; p = 0.22). There were no statistically significant differences in ICU and hospital LOS between the two groups after using PS matching (Table 1).
Complications during ICU stay
The acute kidney injury occurred in 10 patients who received zinc, compared with 25 patients in the control group (15.4% vs. 31.3%; P = 0.02). However, in the logistic regression analysis, it did not reach the statistical significance (OR (95%CI) 0.46 (0.19, 1.06), p = 0.07). The concomitant use of nephrotoxic medications was assessed and not statistically significant before and after PS matching between the two groups. Moreover, among the zinc group, only two patients (2.5%) have an acute liver injury versus seven patients (8.8%) in the control group. However, it was not statistically significant (OR (95%CI) 0.24 (0.05, 1.26), p = 0.09). No significant differences were observed in thrombosis/infraction (OR (95%CI) 0.46 (0.11, 1.98), p = 0.29) or respiratory failure requiring MV (OR (95%CI) 0.98 (0.31, 3.14), p = 0.98) (Table 2).
Follow-up inflammatory surrogate markers
Comparing the effect of zinc on the COVID-19 inflammatory markers, patients who received zinc as new initiation in the ICU stay have a lower d-dimer (Beta coefficient − 0.80 CI − 1.28, − 0.32; p = < 0.001) and fibrinogen levels (Beta coefficient − 0.99 CI − 1.51, − 0.48; p = 0.0002) as a follow-up surrogate marker than the control group as shown in Table 3.
Discussion
In this two-center, retrospective, propensity score matching study, zinc use as adjunctive therapy was associated with significantly lower 30-day mortality in critically ill patients with COVID-19; but the in-hospital mortality did not reach to a statistically significant difference. The overall survival probabilities were not statistically different during hospital stay between the two groups after propensity score. In addition, there was no significant difference in the MV duration, ICU and hospital LOS between the patients who used zinc and those who did not. Instead, patients who received zinc had lower odds of developing AKI; however. this finding did not reach statistical significance.
We have observed survival benefits with zinc supplementation within 30 days of hospital stay in critically ill COVID-19 patients. A retrospective study have shown that using zinc in combination with hydroxychloroquine and azithromycin in hospitalized patients with COVID-19 reduces mortality, ICU admission, and MV needs than patients who did not receive zinc[23]. However, a subgroup analysis in the same study of severely ill patients with COVID-19 found that zinc was not associated with a significant reduction in terms of in-hospital mortality [23]. A recent meta-analysis compared the outcomes of hospitalized patients receiving zinc supplementation with standard care [24]. This meta-analysis concluded that zinc supplementation did not have any beneficial impact on hospital mortality in COVID-19 patients [24]. It is noteworthy that the included studies in the meta-analysis were not critically ill patients, where the disease severity and low pre-admission nutritional status could justify the survival benefit observed in our analysis.
The pre-admission nutritional status is an important indicator for disease severity and might impact the COVID-19 patient's survival. A higher risk of mortality and longer stay in hospital was reported in critically ill COVID-19 patients with higher Nutritional Risk Screening (NRS) 2002 [25]. We assessed our patients' nutritional status using the NUTRIC score, which was not significantly different among the groups after propensity score matching. It worth to mention, that Saudi population has a low intake of some micronutrients (such as zinc and selenium) which might have an impact on the preadmission nutritional status and the disease progression [26]. In agreement with our explanation, a recent study has evaluated the impact of preadmission zinc levels in non-critically ill COVID-19 patients and showed that patients with hypozincemia have a higher hospitalization rate due to respiratory complications within ten days [27]. Thus, the impact of hypozincemia on the disease progression and the clinical outcomes still worth further investigation in critically ill COVID-19 patients.
Several reports showed that elevated inflammatory surrogate markers such as d-dimer and fibrinogen levels had been linked with a higher mortality rate in critically ill COVID-19 patients [4, 28, 30]. Zinc effect on COVID-19 surrogate markers was not well studied. In our study, patients who received zinc supplementation had lower follow-up d-dimer and fibrinogen levels during their stay. This may play a role in COVID-19 disease progression, which might be translated to the mortality benefit seen in our study.
There were no statistically significant differences in the ICU LOS and hospital LOS between the two groups after using PS matching. Our results are consistent with previous retrospective studies and randomized controlled trials (RCTs) showing that the concomitant use of zinc with hydroxychloroquine did not affect the ICU LOS, hospital LOS, or duration of MV [5, 22]. A systematic review of four RCTs conducted in non-COVID-19 critically ill patients found that zinc supplementation was not associated with significant differences in the duration of MV, ICU LOS, and hospital stay [18]. It is worth mentioning that most of these studies included patients with mild, and moderate COVID-19 but not critically ill patients [5, 22].
In terms of the complications during ICU stay, zinc use was associated with a lower odd of acute kidney injury; however, did not reach statistical significance. We included the AKI status within 24 h of ICU admission in PS analysis; additionally, the concomitant use of nephrotoxic medications was assessed and it was not statistically significant after PS matching between the two groups. The exact mechanism and explanation for the reduced AKI risk observed in our cohort are unknown; zinc was thought to reduce renal injury incidence via its antioxidant effect in pre-clinical studies [25]. The zinc renal protective effect and the precise impact of zinc supplementation on renal function in critically ill COVID-19 patients are worth further investigation.
Currently, the available evidence about the use of zinc in critically ill COVID-19 patients is limited. Many of the previous reports investigated zinc use in either non-COVID-19 or non-critically ill patients. As we are writing this manuscript, there is an ongoing double-blinded RCT study investigating the use of high-dose zinc in critically ill patients with SARS-COV2 [29]. Compared to the published data, our study is one of the few studies that assessed the use of zinc in critically ill patients with COVID-19 with a propensity-score-matched group of patients.
Nonetheless, this study still has several limitations, such as the observational nature, small sample size, and the possibility of residual confounding may remain despite using propensity score matching. Additionally, our findings might be limited due to the administration of zinc via the enteral route, which might reduce the absorption in critically ill patients due to gastrointestinal ischemia and impaired intestinal flora. Even though zinc levels are challenging to measure accurately, this level might be valuable in determining the amount of zinc absorbed. The unavailability of zinc levels in this study might also limit our results. Furthermore, there was no defined treatment regimen, and the choice to initiate zinc was left to the discretion of the clinician. Given these limitations, the study's findings should not be used to guide clinical practice but to support the need for future RCTs to investigate the potential impact of zinc supplementation in critically ill patients with COVID-19.
Conclusion
The use of zinc sulfate as adjunctive therapy in critically ill patients with COVID-19 may have survival benefits. Additionally, zinc use may have a protective effect on the kidneys. Further randomized clinical and interventional studies are needed to confirm our findings.
Availability of data and materials
The datasets used and/or analyzed during the current study are available from corresponding author on reasonable request.
Abbreviations
- ICUs:
-
Intensive care units
- COVID-19:
-
Coronavirus disease
- MV:
-
Mechanical ventilation
- LOS:
-
Length of Stay
- APACHE II:
-
Acute Physiology and Chronic Health Evaluation II
- SOFA:
-
Sequential Organ Failure Assessment
- NUTRIC:
-
Nutrition Risk in Critically ill
- Q1, Q3:
-
1St interquartile and 3rd interquartile
- eGFR:
-
Glomerular filtration rate
- AKI:
-
Acute Kidney Injury
- MV:
-
Mechanical Ventilation
- INR:
-
International normalized ratio
- aPTT:
-
Activated partial thromboplastin time
- C-RP:
-
C-reactive protein
- CPK:
-
Creatine phosphokinase
- PaO2/FiO2 :
-
Arterial oxygen tension/fraction of inspired oxygen
References
Huang C, Wang Y, Li X, Ren L, Zhao J, Hu Y, et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. The Lancet. 2020;395:497–506.
Wang D, Hu B, Hu C, Zhu F, Liu X, Zhang J, et al. Clinical characteristics of 138 hospitalized patients with 2019 novel coronavirus-infected pneumonia in Wuhan, China. JAMA. 2020;323:1061–9.
Coomes EA, Haghbayan H. Interleukin-6 in COVID-19: a systematic review and meta-analysis. Rev Med Virol. 2020;30:1–9.
al Sulaiman KA, Aljuhani O, Eljaaly K, Alharbi AA, al Shabasy AM, Alsaeedi AS, et al. Clinical features and outcomes of critically ill patients with coronavirus disease 2019 (COVID-19): a multicenter cohort study. Int J Infect Dis. 2021;105:180–7.
Abd-Elsalam S, Soliman S, Esmail ES, Khalaf M, Mostafa EF, Medhat MA, et al. Do zinc supplements enhance the clinical efficacy of hydroxychloroquine? A randomized multicenter trial. Biol Trace Elem Res. 2020;25:1–5.
Alhazzani W, Møller MH, Arabi YM, Loeb M, Gong MN, Fan E, et al. Surviving Sepsis Campaign: guidelines on the management of critically ill adults with Coronavirus Disease 2019 (COVID-19). Intensive Care Med. 2020;46:854–87. https://doi.org/10.1007/s00134-020-06022-5.
Alhazzani W, Evans L, Alshamsi F, Møller MH, Ostermann M, Prescott HC, et al. Surviving sepsis campaign guidelines on the management of adults with coronavirus disease 2019 (COVID-19) in the ICU: first update. Critical Care Med. 2019;2021:E219–34.
The RECOVERY Collaborative Group. Dexamethasone in hospitalized patients with Covid-19. N Engl J Med. 2021;384:693–704. https://doi.org/10.1056/NEJMoa2021436.
Gupta S, Wang W, Hayek SS, Chan L, Mathews KS, Melamed ML, et al. Association between early treatment with tocilizumab and mortality among critically ill patients with COVID-19. JAMA Intern Med. 2021;181:41–51.
Veiga VC, Prats JAGG, Farias DLC, Rosa RG, Dourado LK, Zampieri FG, et al. Effect of tocilizumab on clinical outcomes at 15 days in patients with severe or critical coronavirus disease 2019: randomised controlled trial. BMJ. 2019;2021:372.
Aleissa MM, Silverman EA, Paredes Acosta LM, Nutt CT, Richterman A, Marty FM. New perspectives on antimicrobial agents: remdesivir treatment for COVID-19. Antimicrob Agents Chemother. 2021;65:e01814.
Shakoor H, Feehan J, al Dhaheri AS, Ali HI, Platat C, Ismail LC, et al. Immune-boosting role of vitamins D, C, E, zinc, selenium and omega-3 fatty acids: could they help against COVID-19? Maturitas. 2021;143:1–9.
Read SA, Obeid S, Ahlenstiel C, Ahlenstiel G. The role of zinc in antiviral immunity. Adv Nutr. 2019;10:696–710.
Barnard DL, Wong M-H, Bailey K, Day CW, Sidwell RW, Hickok SS, et al. Effect of oral gavage treatment with ZnAL42 and other metallo-ion formulations on influenza A H5N1 and H1N1 virus infections in mice. Antiviral Chem Chemother. 2007;18:125–32.
te Velthuis AJW, van den Worml SHE, Sims AC, Baric RS, Snijder EJ, van Hemert MJ. Zn2+ inhibits coronavirus and arterivirus RNA polymerase activity in vitro and zinc ionophores block the replication of these viruses in cell culture. PLoS Pathog. 2010;6:e1001176.
Pormohammad A, Monych NK, Turner RJ, Pormohammad A. Zinc and SARS-CoV-2: a molecular modeling study of Zn interactions with RNA-dependent RNA-polymerase and 3C-like proteinase enzymes. Int J Mol Med. 2021;47:326–34.
Kumar A, Kubota Y, Chernov M, Kasuya H. Potential role of zinc supplementation in prophylaxis and treatment of COVID-19. Med Hypotheses. 2020;144:109848.
Derwand R, Scholz M, Zelenko V. COVID-19 outpatients: early risk-stratified treatment with zinc plus low-dose hydroxychloroquine and azithromycin: a retrospective case series study. Int J Antimicrob Agents. 2020;56:106214.
Heyland DK, Jones N, Cvijanovich NZ, Wong H. Review: zinc supplementation in critically ill patients: a key pharmaconutrient? J Parenter Enter Nutr. 2008;32:509–19.
MDcalc. MDcalc-Medical calculators, equations, scores, and guidelines. [Internet]. [cited 2021 May 28]. Available from: MDcalc.com
Kidney Disease: Improving Global Outcomes (KDIGO). Section 2: AKI Definition. Kidney International Supplements. 2012;2:19–36.
ICD - ICD-10-CM: International Classification of Diseases, Tenth Revision, Clinical Modification. 2021.
Carlucci PM, Ahuja T, Petrilli C, Rajagopalan H, Jones S, Rahimian J. Hydroxychloroquine and azithromycin plus zinc vs. hydroxychloroquine and azithromycin alone: outcomes in hospitalized COVID-19 patients. J Medical Microbiol. 2020;1228–34.
Szarpak L, Pruc M, Gasecka A, Jaguszewski MJ, Michalski T, Peacock FW, et al. Should we supplement zinc in COVID-19 patients? Evidence from meta-analysis. Polish Arch Intern Med. 2021.
Chen X, Zhao B, Qu Y, Chen Y, Xiong J, Feng Y, et al. Detectable serum SARS-CoV-2 viral load (RNAaemia) is closely correlated with drastically elevated interleukin 6 (IL-6) level in critically ill COVID-19 patients. Clin Infect Dis. 2020;71:1937–42.
Al-Saleh IA, Al-Jaloud A, Al-Doush I, El-Din G. The distribution of selenium levels in Saudi dairy farms: a preliminary report from Al-Kharj. J Environ Pathol Toxicol Oncol. 1999;18:37–46.
Fromonot J, Gette M, Ben Lassoued A, Guéant JL, Guéant-Rodriguez RM, Guieu R. Hypozincemia in the early stage of COVID-19 is associated with an increased risk of severe COVID-19. Clin Nutr. 2021. https://doi.org/10.1016/j.clnu.2021.04.042.
Short SAP, Gupta S, Brenner SK, Hayek SS, Srivastava A, Shaefi S, et al. D-dimer and Death in critically ill patients with coronavirus disease 2019. Crit Care Med. 2021;49:e500.
Perera M, el Khoury J, Chinni V, Bolton D, Qu L, Johnson P, et al. Randomised controlled trial for high-dose intravenous zinc as adjunctive therapy in SARS-CoV-2 (COVID-19) positive critically ill patients: trial protocol. BMJ Open. 2020;10:e040580.
Huang I, Pranata R, Lim MA, Oehadian A, Alisjahbana B. C-reactive protein, procalcitonin, D-dimer, and ferritin in severe coronavirus disease-2019: a meta-analysis. Ther Adv Respir Dis. 2020;14:1753466620937175. https://doi.org/10.1177/1753466620937175.
Acknowledgements
We would like to acknowledge all of the investigators in the Saudi critical care pharmacy research (SCAPE) platform who participated in this project. Additionally, we thank Dr. Hakeam A. Hakeam for his support during the study execution.
Funding
None.
Author information
Authors and Affiliations
Contributions
KS and OA equally contributed to the conception and design of the research; AS, GK, AK, SA, RK, RV and AA contributed to the design of the research; KS, OA, AS, GK, AK, SA, RK, RV, AAB, RG and AA contributed to the acquisition and analysis of the data; KS, OA, AS, GK contributed to the interpretation of the data; KS, OA, AS, SA, AK, RK, AA, AAB, RG and RV drafted the manuscript. All authors critically revised the manuscript, agree to be fully accountable for ensuring the integrity and accuracy of the work, and read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
The study was approved in November 19th, 2020 by King Abdullah International Medical Research Center Institutional Review Board, Riyadh, Saudi Arabia (Reference No: RC20/589/R). Participants' confidentiality was strictly observed throughout the study using the anonymous unique serial number for each subject and restricting data only to the investigators. Informed consent was not required due to the research's method as per the policy of the governmental and local research center.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Additional file 1: Table e1
Patients Baseline characteristics before and after propensity-score matching.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Al Sulaiman, K., Aljuhani, O., Al Shaya, A.I. et al. Evaluation of zinc sulfate as an adjunctive therapy in COVID-19 critically ill patients: a two center propensity-score matched study. Crit Care 25, 363 (2021). https://doi.org/10.1186/s13054-021-03785-1
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s13054-021-03785-1