Abstract
Background
Heterogeneous respiratory system static compliance (CRS) values and levels of hypoxemia in patients with novel coronavirus disease (COVID-19) requiring mechanical ventilation have been reported in previous small-case series or studies conducted at a national level.
Methods
We designed a retrospective observational cohort study with rapid data gathering from the international COVID-19 Critical Care Consortium study to comprehensively describe CRS—calculated as: tidal volume/[airway plateau pressure-positive end-expiratory pressure (PEEP)]—and its association with ventilatory management and outcomes of COVID-19 patients on mechanical ventilation (MV), admitted to intensive care units (ICU) worldwide.
Results
We studied 745 patients from 22 countries, who required admission to the ICU and MV from January 14 to December 31, 2020, and presented at least one value of CRS within the first seven days of MV. Median (IQR) age was 62 (52–71), patients were predominantly males (68%) and from Europe/North and South America (88%). CRS, within 48 h from endotracheal intubation, was available in 649 patients and was neither associated with the duration from onset of symptoms to commencement of MV (p = 0.417) nor with PaO2/FiO2 (p = 0.100). Females presented lower CRS than males (95% CI of CRS difference between females-males: − 11.8 to − 7.4 mL/cmH2O p < 0.001), and although females presented higher body mass index (BMI), association of BMI with CRS was marginal (p = 0.139). Ventilatory management varied across CRS range, resulting in a significant association between CRS and driving pressure (estimated decrease − 0.31 cmH2O/L per mL/cmH20 of CRS, 95% CI − 0.48 to − 0.14, p < 0.001). Overall, 28-day ICU mortality, accounting for the competing risk of being discharged within the period, was 35.6% (SE 1.7). Cox proportional hazard analysis demonstrated that CRS (+ 10 mL/cm H2O) was only associated with being discharge from the ICU within 28 days (HR 1.14, 95% CI 1.02–1.28, p = 0.018).
Conclusions
This multicentre report provides a comprehensive account of CRS in COVID-19 patients on MV. CRS measured within 48 h from commencement of MV has marginal predictive value for 28-day mortality, but was associated with being discharged from ICU within the same period. Trial documentation: Available at https://www.covid-critical.com/study.
Trial registration: ACTRN12620000421932.
Similar content being viewed by others
Background
Millions of people have been infected by SARS-CoV-2 worldwide, and many of those have been hospitalized for respiratory complications associated with coronavirus disease-2019 (COVID-19). Many of those COVID-19 hospitalised patients have received mechanical ventilation (MV), due to the development of acute hypoxemic respiratory failure and acute respiratory distress syndrome (ARDS) [1,2,3,4]. To date, several landmark studies [5,6,7,8] have improved our understanding of COVID-19 pulmonary pathophysiology, but pulmonary derangement in COVID-19 and appropriate ventilatory management remains incompletely characterized.
Earlier reports on the pulmonary pathophysiology of COVID-19 patients reported conflicting results and extreme heterogeneity in levels of pulmonary shunting, static respiratory system compliance (CRS), [9,10,11,12] and substantial heterogeneity in lung recruitability [13, 14]. Adding further to the controversy over CRS in COVID-19 patients, Grasselli and collaborators [7] have compared findings from an Italian repository of COVID-19 ARDS with previous ARDS cases of different etiologies. They found statistically significant higher CRS in patients with COVID-19 ARDS. In addition, they found that patients who presented with lower CRS and higher D-dimer values had the greatest mortality risk. In line with these figures, in a small-case series, Chiumello and collaborators found that COVID-19 patients presented higher CRS levels in comparison with patients with ARDS from other etiologies and matched levels of hypoxemia [12]. Regrettably, those previous reports did not provide any information on how CRS progressed beyond a punctual assessment during the period of MV. In contrast, in another landmark study by Ferrando et al. [6], CRS figures from a Spanish database were very similar to previously published cohorts of ARDS patients. The authors also found that intensive care unit (ICU) discharge and mortality were not influenced by the initial levels of CRS.
In a pandemic caused by a novel virus, access to international data is vital, because it may help account for differences in populations, access to medical care, equipment and critical variations in clinical managements among countries. Thus, analysis of international repositories improves the overall understanding of a novel disease and helps establishing best practices to enhance outcome. One example of how single-center or single-country studies can influence medical care early in a pandemic, before being contradicted by subsequent international findings is the issue of CRS. Indeed, as this parameter can be markedly impacted by fine variations in ventilatory management, extrapolations from mono-center or single-country studies may be challenging. In early January 2020, the COVID-19 Critical Care Consortium incorporating the ExtraCorporeal Membrane Oxygenation for 2019 novel Coronavirus Acute Respiratory Disease (COVID-19–CCC/ECMOCARD) group was founded to investigate patients presenting to ICUs worldwide.
Here, we present a comprehensive appraisal of CRS in mechanically ventilated COVID-19 patients enrolled into the COVID-19–CCC/ECMOCARD international study, in order to understand the dynamics of CRS during the first week of mechanical ventilation and its potential impact on patient outcomes.
Materials and methods
Study design and oversight
The COVID-19-CCC/ECMOCARD is an international, multicentre, cohort observational study ongoing in 351 hospitals across 53 countries. The full study protocol is available elsewhere [15]. To summarize, participating hospitals obtained local ethics committee approval and a waiver of informed consent was granted in all cases. ISARIC/SPRINT-SARI data collection began at admission to hospital, while data collection for the COVID-19–CCC observational study commenced at admission to the ICU. De-identified patient data were collected retrospectively and stored via the REDCap electronic data capture tool, hosted at the University of Oxford, United Kingdom or Monash University, Melbourne, Australia.
Study population
We reviewed data of all patients admitted to the ICU at a COVID-19–CCC collaborating site, from January 14 through September 30, 2020, with a clinically suspected or laboratory confirmed diagnosis of SARS-CoV-2 infection, through naso-pharyngeal swab for real-time PCR SARS-CoV-2 detection. Of note, suspicion of SARS-CoV-2 infection was based on symptoms and onset of infection and was confirmed by the clinician when COVID-19 infection was the most likely cause of the symptoms experienced. Patients excluded were those under the age of 15 years or admitted to an ICU for other reasons. We focused our analysis on patients on controlled MV and with a computed CRS value within 48 h of MV commencement.
Definitions and pulmonary mechanics computations
CRS was calculated as: tidal volume (mL)/[(airway plateau pressure-PEEP (cmH2O))]. Of note, we provided to data collectors a detailed data dictionary, with instructions on how to collect airway plateau pressure values, via an inspiratory pause of approximately 3 s. We computed CRS using the first measured tidal volume, airway plateau pressure and PEEP values, within 48 h of MV commencement. In the sub-population of patients on controlled MV, without ECMO support, we analysed key pulmonary variables, such as tidal volume, positive end expiratory pressure (PEEP), static driving pressure, inspiratory fraction of oxygen (FiO2), and gas exchange, recorded during routine clinical practice and only. Tidal volume was reported in mL/kg of predicted body weight (PBW) [16].
Data collection
After enrolment, data on demographics, comorbidities, clinical symptoms and laboratory results were collected by clinical and research staff of the participating ICUs in an electronic case report form [15]. Details of respiratory and hemodynamic support, physiological variables, and laboratory results were collected daily. Of note, the worst daily values were preferentially recorded. The duration of MV and ICU stay, and hospital mortality were recorded. Analysis of daily data was restricted to the first seven days from commencement of MV.
Statistical analyses
Descriptive statistics summarised demographics, clinical signs on ICU admission, ICU management and clinical outcomes for the overall study cohort and subjects with baseline compliance measured within the first 48 h of controlled MV. Statistics were reported as medians (interquartile range) for continuous variables and numbers (percentage) for categorical variables. Linear regression was applied to summarise associations between baseline compliance with body mass index (BMI) (including interaction between BMI and sex), days from symptom onset to MV commencement and PaO2/FiO2, adjusted for BMI. Linear mixed modelling was used to investigate trends in compliance over time and associations with key respiratory parameters during the first 7 days of controlled MV. Models assumed a linear effect for days and a random intercept per subject to account for repeated measures. Consistent with exploratory analyses, BMI was included as a fixed effect to adjust for potential confounding in the clinical characteristics and management of patients with different BMI. Hypothesis testing was applied to all fixed effects, assuming a 5% level of statistical significance. Results were summarised graphically with uncertainty in estimated trends represented by 95% prediction intervals. Expected patient outcomes including length of ICU stay, duration of MV and risk of ICU mortality versus discharge were examined using multi-state modelling [17]. Compared with exploratory analyses of clinical outcomes, the multistate model accounted for ICU discharge and death as competing events and allowed data from all patients to be included, regardless of study follow-up time. The model comprised of four states, to describe patients prior to commencement of MV (non MV), on mechanical ventilation (MV), ICU discharged (Discharge) and mortality (Death). States were presented as percentage and standard error (SE) in the text. Patients extubated before death or discharge were assumed to transition between MV an non-MV states. State transitions were modelled by Cox proportional hazards, with patients censored at last known follow-up, up to 28 days from ICU admission. Follow-up analysis considered Cox proportional hazard regression to examine associations between baseline compliance and competing risks of ICU mortality and discharge, following commencement of MV. Baseline compliance was included as a linear effect, with age, sex, BMI and comorbidities (hypertension, chronic cardiac disease, chronic kidney disease) as additional covariates and adjusted for recruiting centre. A shared frailty term (Gamma distributed) was included to account for residual variation between study sites. Analyses were conducted using R version 3.6.2 or higher (The R Foundation).
Results
We studied 745 patients from 22 countries, who required admission to the ICU and MV from January 14 to December 31, 2020, and presented at least one value of CRS within the first seven days of MV. Among those, 597 (80%) had laboratory-confirmed diagnosis of SARS-CoV2 infection, while in 148 (20%), infection was clinically suspected. Enrolment rate, since January 2020, is reported in Fig. 1. CRS, within 48 h from endotracheal intubation, was available in 649 patients (Fig. 2). No association between CRS and days from onset of symptoms to commencement of MV was found (Fig. 3). Median CRS (IQR), within the first 48 h of mechanical ventilation, was 34.1 mL/cmH2O (26.4–44.0) and PaO2/FiO2 113.0 mmHg (84.0–161.3), without any linear association between these parameters. In particular, 16%, 46% and 38% of the patients presented with mild, moderate or severe hypoxemia, respectively (Fig. 4a). Female sex was associated with a significantly lower CRS than in males (95% CI of difference between genders: − 11.8 to − 7.4 mL/cmH2O p < 0.001) (Fig. 4b). Females also presented higher body mass index (BMI) (95% CI of difference between males and females: − 1.9 to − 5.5, p < 0.001), but as shown in Fig. 5, CRS and BMI were not linearly associated. Our model estimated that CRS was 37.57 cmH2O/mL (95% CI 36.5–38.6) upon commencement of MV (Fig. 6), with further worsening in the first seven days of MV (estimated decrease − 0.31 cmH2O/mL per day, 95% CI − 0.48 to − 0.14, p < 0.001). In addition, as detailed in Fig. 7, PaCO2, tidal volume, PEEP, driving pressure and FiO2 significantly varied across the range of CRS, and a significant association was found between inspiratory plateau pressure and CRS changes (Fig. 8).
Linear regression analysis of days from onset of symptoms to commencement of mechanical ventilation and static respiratory system compliance, based on the first measurement obtained within 48 h from commencement of mechanical ventilation, adjusted for body mass index. Dark black horizontal bar depicts median value, and upper and lower horizontal light black bars show 90th and 10th percentile. Days of onset of symptoms to commencement of mechanical ventilation was not associated with static respiratory system compliance (estimate 0.92 mL/cmH2O, 95% CI − 0.31–0.31 p = 0.417)
a Linear regression analysis of arterial partial pressure of oxygen (PaO2/FiO2) and respiratory system compliance (CRS), based on the first measurement obtained within 48 h from commencement of mechanical ventilation, with an interaction of gender and adjusted for body mass index (BMI). No statistically significant association was found between PaO2/FiO2 and CRS (estimate 0.49, 95% CI − 0.09–1.07 p = 0.100). Typical acute respiratory distress syndrome stratification groups [35] (severe, moderate and mild based on levels of hypoxemia) are highlighted in dark, medium and light grey, respectively. b Static respiratory system compliance (CRS) distribution by sex, based on the first measurement obtained within 48 h from commencement of mechanical ventilation. Dashed black lines depict median values for females and males
Linear regression analysis of static respiratory system compliance, based on the first measurement obtained within 48 h from commencement of mechanical ventilation, and body mass index with an interaction for sex. Per each graph, fitted line of the model is depicted and the upper and lower lines display the 95% predictive interval. Dark grey dots depict female patients, while light grey dots males. Static respiratory system compliance did not vary according to the body mass index (estimate − 0.12 cmH2O/mL, 95%CI − 0.29 to − 0.04, p = 0.139), but was associated with female sex (estimate − 10.73 cmH2O/mL, 95%CI − 18.54 to − 2.92, p = 0.007)
Static respiratory system compliance dynamics. Evolution of static respiratory system compliance over the first 7 days of mechanical ventilation, adjusted for body mass index. Under each day, the number of analysed patients is reported in parenthesis. Fitted line of the model is depicted, and the upper and lower lines display the 95% predictive interval. Respiratory system compliance varied during the first seven days of mechanical ventilation (estimate − 0.31 cmH2O/mL, 95%CI − 0.48 to 0.14, p < 0.001)
Linear Mixed model analysis of respiratory system compliance vs. crucial pulmonary variables during the first 7 days of mechanical ventilation (grey-scale coded bar for day 1 through 7 is reported on the right section of each graph and in parenthesis is reported the number of analysed patients). Per each graph, fitted line of the model is depicted and the upper and lower lines display the 95% predictive interval. All analyses are adjusted for body mass index. Static compliance of respiratory system was found to be associated with PaCO2 (estimated decrease − 0.11 mmHg, 95% CI − 0.15 to − 0.06, p < 0.001), tidal volume (estimated increase 0.04 mL/Kg of predicted body weight per day, 95% CI 0.03–0.04, p < 0.001), PEEP (estimated increase − 0.03 cmH2O, 95% CI 0.02–0.04, p < 0.001), driving pressure (estimated decrease − 0.31 cmH2O/L, 95% CI − 0.48 to 0.14, p < 0.001) and FiO2 (estimated decrease − 0.15%, 95% CI − 0.23 to − 0.06, p < 0.001). While PaO2/FiO2, was not significantly associated with static compliance of respiratory system (estimated increase 0.29 mmHg, 95% CI − 0.03 to 0.61, p = 072) PaO2/FiO2, ratio between arterial partial pressure of oxygen and inspiratory fraction of oxygen; PaCO2 arterial partial pressure of carbon dioxide; PEEP, positive end-expiratory pressure
Association of airway inspiratory plateau pressure with static respiratory system compliance. Linear Mixed model analysis of the association of respiratory system compliance with airway inspiratory plateau pressure during the first 7 days of mechanical ventilation (grey-scale coded bar for day 1 through 7 is reported on the right section of each graph and in parenthesis is reported the number of analysed patients). Fitted line of the model is depicted, and the upper and lower lines display the 95% predictive interval. Analysis is adjusted for body mass index. The model highlights significant association between respiratory system compliance and airway plateau pressure (estimated decrease − 0.22 cmH2O/L, 95% CI − 0.23 to − 0.21, p < 0.001), but based on the model prediction, airway plateau pressure remained predominantly below 30 cmH2O
Baseline characteristics upon ICU admission, applied interventions and outcomes, are summarized in Table 1. The most common interventions applied to the study population were use of antibiotics (96%), neuromuscular blocking agents (81%) and prone position (61%). The overall hospital mortality of the study population was 40%, and among those patients who died in the hospital or were discharged alive, the median (IQR) duration of MV was 11 days (6–18) and 14 days (8–23), respectively. Overall, 28-day ICU mortality, accounting for competing risks, was 35.6% (SE 1.7) and estimated 28-day mortality from commencement of MV was 37.1% (SE 1.7) (Fig. 9b). Cox proportional hazard analysis (Fig. 9c) demonstrated that age (hazard ratio 1.37, 95% CI 1.19–1.59, p < 0.001) and chronic cardiac diseases (HR 1.62, 95% CI 1.14–2.29, p < 0.001) were the only baseline factors associated with 28-day mortality risk. In addition, age (HR 0.77, 95% CI 0.66–0.83, p < 0.001), male sex (HR 0.59, 95% CI 0.44–0.79, p < 0.001), BMI (HR 0.86, 95% CI 0.79–0.95, p = 0.003) and CRS (+ 10 mL/cm H2O) (HR 1.14, 95% CI 1.02–1.28, p = 0.018) were associated with the chance of being discharge from the ICU within 28 days.
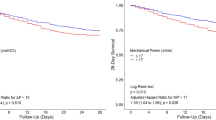
Multistate modelling and Cox regression analysis outcomes for patient with static compliance recorded within 48 h of commencing mechanical ventilation. a Multistate model structure for estimating expected outcomes up to 28 days from admission to intensive care unit (ICU). Modelled health states include not on invasive mechanical ventilation (non-MV), on mechanical ventilation (MV), ICU discharge and death. Patients start in the non-MV state if not mechanically ventilated upon or prior to ICU admission, or in the MV state otherwise. b Predicted probabilities of occupying health states up to 28 days from ICU admission. c Results of Cox proportional hazards modelling for risk of death and ICU discharge from commencement of mechanical ventilation. Covariates comprise age, body mass index (BMI), selected comorbidities (hypertension, chronic cardiac disease, chronic kidney disease) and baseline static compliance. Parameter estimates are presented as estimated hazard ratios with 95% confidence intervals (CI). Further details on factors significantly associated with assessed outcomes are available in the results section
Discussion
This large observational report from intensive care units throughout the world found that initial static respiratory system compliance was only associated with hazard of being discharged from the ICU within 28 days. The duration from onset of symptoms to commencement of MV did not influence CRS, and interestingly lower CRS was found in female patients. In the evaluated population, neuromuscular blocking agents and prone position were commonly applied and ventilatory management across CRS levels varied in terms of tidal volume, PEEP and FiO2, throughout the first 7 days of MV.
In comparison with previous reports on ARDS patients without COVID-19 [18], we similarly found that the majority of patients exhibited moderate hypoxemia, even when presented higher CRS. We also noted a larger range of CRS in line with previous studies [7, 8], but in contrast with values from a larger COVID-19 ARDS series from Spain [6]. Considering that we focused our analysis on static compliance of the respiratory system, without partitioning into the pulmonary and chest wall components [19, 20], it is interesting that CRS was not associated with BMI, suggesting that patients with higher BMI potentially presented also with higher lung compliance. Irrespective, we found lower CRS in female patients, who also presented higher BMIs. To the best of our knowledge, no studies have systematically investigated the effects of gender/BMI on COVID-19 severity; thus, whether obesity might be a crucial risk factor for ICU admission and mechanical ventilation, specifically in female patients, and its effects on lung compliance should be further explored. We also found that throughout the range of CRS values, plateau pressure was within what is typically presumed as lung protective ranges [21], but this resulted in potentially harmful driving pressures, specifically for patients with the lowest CRS values. As many of these patients were obese, this raises the question of whether these modest pressures might have increased the risk of pulmonary derecruitment, or in patients with normal BMI, the resulting driving pressure might have been related to pulmonary overdistention. These factors could have contributed to sustained hypoxemia and impaired lung function throughout the study period. In such circumstances, it is questionable whether MV guided by oesophageal pressure monitoring may have some benefits [22], but more research is needed to corroborate such reasoning.
Phenotypic subsets of COVID-19-associated ARDS have been proposed [9, 13, 23,24,25]. Recent study has also explored whether CRS—related phenotype patterns existed among patients with ARDS before the COVID-19 pandemic [26]. Various investigators [7, 27], who did not find significant CRS variability among COVID-19 patients requiring MV, questioned the overall clinical value of CRS in the COVID-19 population. In a very small case series, Gattinoni et al [9] found an initial CRS of 50 mL/cmH2O, but high levels of shunt fraction that could have explained the resulting severe hypoxemia. In subsequent study, Chiumello and collaborators found higher CRS in patient with COVID-19 ARDS and ARDS caused by other injuries, while matching for similar levels of PaO2/FiO2 [12]. Interestingly, these findings were in line with computed tomography studies results, corroborating higher proportion of normally aerated tissue in COVID-19 ARDS. In similar reports, heterogeneous pathophysiology among patients with different levels of pulmonary compliance has been implied [10, 25]. As corroborated by landmark post-mortem studies [28] and clinical studies [7, 29], SARS-CoV-2 heterogeneously affects pulmonary ventilation and perfusion. Hence, it could be argued that the use of CRS as key pathophysiological parameter to predict clinical evolution might be over simplistic and in-depth characterization of pulmonary pathophysiology should be recommended for COVID-19 patients, specifically when obese. Interestingly, our report is the first that specifically focused on the dynamics of CRS, rather than only baseline CRS. We found that CRS was not related to the duration from the onset of symptoms to commencement of MV, emphasising the need for inclusive data on mechanisms of lung injury in not ventilated COVID-19 patients [30]. The median CRS value found in our population was 34.1 mL/cmH2O, similar to findings by Ferrando et al. [6], not dissimilar to findings by Bellani et al. on patients with non-COVID-19 ARDS [31], but lower than figures recently reported by Grasselli [7] and Grieco [32] in COVID-19 patients. In addition, we found a further decrease in CRS during the first week of MV. This could have been related to the specific ventilatory management in our reported population, but such discrepancy further highlights the need of a comprehensive appraisal of pulmonary and chest wall mechanics in COVID-19 patients [20].
One of the most striking results was the continued use of high PEEP over the first seven days of MV, even in patients with high compliance. This seems counterintuitive, given that current recommendations in ARDS suggest decreasing PEEP, especially in the face of high compliance. As hypoxemia persisted even with high PEEP and high compliance, our results add to the hypothesis that maintaining high PEEP may worsen gas exchange from lung overdistension, resulting in increased dead space and intrapulmonary shunting. Other authors have speculated that using high levels of PEEP in COVID-19 patients with low recruitability may be detrimental, and that lowering PEEP may improve gas exchange and limit ventilator-induced lung injury [33]. Our results in this large cohort of patients from multiple global areas support this theory. Finally, we found that patients required two weeks of MV, and 28-day mortality in the overall population was 35.6%, with hospital mortality up to 40%. These figures are in line with mortality rates reported by Grasselli [7] in the subgroups characterized by low D-dimer, and mortality in severe-moderate COVID-19 ARDS, as corroborated by Ferrando [6]. Nevertheless, we found that CRS was only associated with the discharge from ICU within 28 days. Thus, the marginal clinical value of CRS as a predictor of mortality in COVID-19 patients calls for urgent identification of valuable markers that could inclusively describe pulmonary derangement and guide personalized treatment.
Strengths and limitations
Collaborations between international data collection efforts have the ability to answer many questions related to COVID 19 and to pave the way for future novel diseases to achieve rapid and global data access to help guide best practice. The international COVID-19 Critical Care Consortium study [15], in collaboration with the ISARIC/SPRINT-SARI networks [34], provides inferences not limited by ventilatory management specific to small patient cohort or single-country studies. In addition, in comparison with previous studies, we provided more granular data to inclusively appraise the dynamics of CRS in COVID-19 patients on MV and to study its association with laboratory, and clinical features. A few limitations of our observational study should also be emphasized. First, we centred our analysis on COVID-19 patients, without comparisons against previous repositories of patients with ARDS from different aetiologies. Yet, we provided a wide-ranging discussion of the characteristics of our population in the context of previous analyses in ARDS patients. Second, inferences on pulmonary perfusion disorders in our population can only be speculative, since D-dimer was only available in a small subset of patients (Table 1). Third, as reported by the enrolment rate (Fig. 1 Supplemental Digital Content), patients were mostly enrolled in the early phase of the pandemic, hence extrapolations from our findings should take into account potential biases related to overwhelmed critical care services. Fourthly, it is important to emphasise that we centred our analysis on CRS, but due to the complex respiratory pathophysiology in COVID-19 patients and the high percentage of patients with increased BMI, the use of oesophageal pressure monitoring to fully describe lung and chest wall compliances is advisable and should be prioritised in future investigations. Fifth, the majority of patients were admitted in centers located in North America, Europe and South America. Although these findings are in line with the global distribution of COVID-19 cases, extrapolations of our findings in other regions should be applied cautiously.
Conclusions
Our comprehensive appraisal of COVID-19 patients on MV from a large international observational study implies that expected CRS within 48 h from commencement of MV is not influenced by the duration from onset of symptoms to commencement of MV, but after intubation, a further decrease in CRS might be expected during the first week of ventilation. In addition, baseline CRS is associated with the chance of being discharged from the ICU within 28 days, but it is not a predictive marker of 28-day mortality. Based on potential inferences from our findings, future studies that could provide an in-depth characterization of lungs and chest wall compliance in COVID-19 patients will be critical to guide best practice in ventilatory management.
Availability of data materials
The datasets used and/or analysed during the current study are available from the corresponding author on reasonable request.
Abbreviations
- ARDS:
-
Acute respiratory distress syndrome
- COVID-19:
-
Coronavirus disease-2019
- COVID-19–CCC/ECMOCARD:
-
COVID-19 Critical Care Consortium incorporating the ExtraCorporeal Membrane Oxygenation for 2019 novel Coronavirus Acute Respiratory Disease
- FiO2 :
-
Inspiratory fraction of oxygen
- ICU:
-
Intensive care unit
- IQR:
-
Interquartile range
- MV:
-
Mechanical ventilation
- PBW:
-
Predicted body weight
- PEEP:
-
Positive end expiratory pressure
- C RS :
-
Static respiratory system compliance
References
Grasselli G, Zangrillo A, Zanella A, Antonelli M, Cabrini L, Castelli A, et al. Baseline characteristics and outcomes of 1591 patients infected with SARS-CoV-2 admitted to ICUs of the lombardy region, Italy. JAMA J Am Med Assoc. 2020;323:1574–81.
Guan W, Ni Z, Hu Y, Liang W, Ou C, He J, et al. Clinical characteristics of coronavirus disease 2019 in China. N Engl J Med. 2020;382:1708–20.
Richardson S, Hirsch JS, Narasimhan M, Crawford JM, McGinn T, Davidson KW, et al. Presenting characteristics, comorbidities, and outcomes among 5700 patients hospitalized with COVID-19 in the New York City area. JAMA J Am Med Assoc. 2020;323:E1-8.
Karagiannidis C, Mostert C, Hentschker C, Voshaar T, Malzahn J, Schillinger G, et al. Case characteristics, resource use, and outcomes of 10 021 patients with COVID-19 admitted to 920 German hospitals: an observational study. Lancet Respir Med [Internet]. Elsevier; 2020 [cited 2020 Aug 6];0. Available from: https://linkinghub.elsevier.com/retrieve/pii/S2213260020303167.
Gibson PG, Qin L, Puah SH. COVID-19 acute respiratory distress syndrome (ARDS): clinical features and differences from typical pre-COVID-19 ARDS. Med J Aust. 2020;2020:54-56.e1.
Ferrando C, Suarez-Sipmann F, Mellado-Artigas R, Hernández M, Gea A, Arruti E, et al. Clinical features, ventilatory management, and outcome of ARDS caused by COVID-19 are similar to other causes of ARDS. Intensive Care Med [Internet]. Springer; 2020 [cited 2020 Aug 13];1–12. Available from: https://doi.org/10.1007/s00134-020-06192-2.
Sacco F, Tonetti MT, Pizzilli G, Ranieri VM, di Radiologia Monteduro DF, Zompatori M, et al. Pathophysiology of COVID-19-associated acute respiratory distress syndrome: a multicentre prospective observational study. 2020; Available from: www.thelancet.com/respiratory.
Gattinoni L, Coppola S, Cressoni M, Busana M, Rossi S, Chiumello D. COVID-19 does not lead to a “Typical” acute respiratory distress syndrome. Am J Respir Crit Care Med. 2020;201:1299–300.
Gattinoni L, Coppola S, Cressoni M, Busana M, Chiumello D. Covid-19 does not lead to a “Typical” acute respiratory distress syndrome. Am J Respir Crit Care Med. 2020;2020:201.
Gattinoni L, Chiumello D, Rossi S. COVID-19 pneumonia: ARDS or not? [Internet]. Crit. Care. BioMed Central Ltd.; 2020 [cited 2020 Jun 6]. p. 154. Available from: https://doi.org/10.1186/s13054-020-02880-z
Schenck EJ, Hoffman K, Goyal P, Choi J, Torres L, Rajwani K, et al. Respiratory mechanics and gas exchange in COVID-19 associated respiratory failure. American Thoracic Society; 2020.
Chiumello D, Busana M, Coppola S, Romitti F, Formenti P, Bonifazi M, et al. Physiological and quantitative CT-scan characterization of COVID-19 and typical ARDS: a matched cohort study. Intensive Care Med [Internet]. Springer Science and Business Media Deutschland GmbH; 2020 [cited 2020 Nov 18];1–10. Available from: https://doi.org/https://doi.org/10.1007/s00134-020-06281-2
Beloncle FM, Pavlovsky B, Desprez C, Fage N, Olivier PY, Asfar P, et al. Recruitability and effect of PEEP in SARS-Cov-2-associated acute respiratory distress syndrome. Ann Intensive Care. Springer; 2020;10.
Pan C, Chen L, Lu C, Zhang W, Xia JA, Sklar MC, et al. Lung Recruitability in SARS-CoV-2 Associated Acute Respiratory Distress Syndrome: A Single-center, Observational Study. Am J Respir Crit Care Med. NLM (Medline); 2020;201.
ECMOCARD [Internet]. [cited 2020 Jun 2]. Available from: https://www.elso.org/COVID19/ECMOCARD.aspx.
Network ARDS, Brower RG, Matthay MA, Morris A, Schoenfeld D, Thompson BT, et al. Ventilation with lower tidal volumes as compared with traditional tidal volumes for acute lung injury and the acute respiratory distress syndrome. The Acute Respiratory Distress Syndrome Network. N Engl J Med [Internet]. 2000;342:1301–8. Available from: http://www.ncbi.nlm.nih.gov/pubmed/10793162.
Hazard D, Kaier K, Von Cube M, Grodd M, Bugiera L, Lambert J, et al. Joint analysis of duration of ventilation, length of intensive care, and mortality of COVID-19 patients: A multistate approach. BMC Med Res Methodol [Internet]. BioMed Central; 2020 [cited 2020 Oct 13];20:206. Available from: https://doi.org/10.1186/s12874-020-01082-z.
Bellani G, Laffey JG, Pham T, Fan E, Brochard L, Esteban A, et al. Epidemiology, patterns of care, and mortality for patients with acute respiratory distress syndrome in intensive care units in 50 countries. JAMA. 2016;315:788–800.
Pelosi P, Caironi P, Gattinoni L. Pulmonary and extrapulmonary forms of acute respiratory distress syndrome. Semin Respir Crit Care Med. 2001;22:259–68.
Chiumello D, Carlesso E, Cadringher P, Caironi P, Valenza F, Polli F, et al. Lung stress and strain during mechanical ventilation for acute respiratory distress syndrome. Am J Respir Crit Care Med. 2008;178:346–55.
Fan E, Del Sorbo L, Goligher EC, Hodgson CL, Munshi L, Walkey AJ, et al. An official American Thoracic Society/European Society of intensive care medicine/society of critical care medicine clinical practice guideline: Mechanical ventilation in adult patients with acute respiratory distress syndrome. Am J Respir Crit Care Med [Internet]. American Thoracic Society; 2017 [cited 2020 Oct 12];195:1253–63. Available from: https://pubmed.ncbi.nlm.nih.gov/28459336/.
Talmor D, Sarge T, Malhotra A, O’Donnell CR, Ritz R, Lisbon A, et al. Mechanical ventilation guided by esophageal pressure in acute lung injury. N Engl J Med. 2008;359:2095–104.
Marini JJ, Gattinoni L. Management of COVID-19 respiratory distress. JAMA J Am Med Assoc. 2020;323:2329–30.
Li X, Ma X. Acute respiratory failure in COVID-19: is it “typical” ARDS? Crit. Care. NLM (Medline); 2020. p. 198.
Gattinoni L, Chiumello D, Caironi P, Busana M, Romitti F, Brazzi L, et al. COVID-19 pneumonia: different respiratory treatments for different phenotypes? Intensive Care Med. Springer; 2020. p. 1.
Panwar R, Madotto F, Laffey JG, Van Haren FMP. Compliance Phenotypes in Early ARDS Before the COVID-19 Pandemic. Am J Respir Crit Care Med [Internet]. American Thoracic Society; 2020 [cited 2020 Oct 12]; Available from: https://pubmed.ncbi.nlm.nih.gov/32805143/.
Pan C, Chen L, Lu C, Zhang W, Xia JA, Sklar MC, et al. Lung recruitability in COVID-19–associated acute respiratory distress syndrome: A single-center observational study [Internet]. Am. J. Respir. Crit. Care Med. American Thoracic Society; 2020 [cited 2020 Aug 13]. p. 1294–7. Available from: https://doi.org/10.1164/rccm.202003-0527LE.
Ackermann M, Verleden SE, Kuehnel M, Haverich A, Welte T, Laenger F, et al. Pulmonary Vascular Endothelialitis, Thrombosis, and Angiogenesis in Covid-19. N Engl J Med [Internet]. Massachusetts Medical Society; 2020 [cited 2020 Jun 2];NEJMoa2015432. Available from: https://doi.org/10.1056/NEJMoa2015432.
Lang M, Som A, Mendoza DP, Flores EJ, Reid N, Carey D, et al. Hypoxaemia related to COVID-19: vascular and perfusion abnormalities on dual-energy CT. Lancet Infect. Dis. Lancet Publishing Group; 2020.
Cruces P, Retamal J, Hurtado DE, Erranz B, Iturrieta P, González C, et al. A physiological approach to understand the role of respiratory effort in the progression of lung injury in SARS-CoV-2 infection [Internet]. Crit. Care. BioMed Central Ltd; 2020 [cited 2021 Feb 7]. p. 494. Available from: https://doi.org/10.1186/s13054-020-03197-7.
Bellani G, Laffey JG, Pham T, Fan E, Brochard L, Esteban A, et al. Epidemiology, Patterns of Care, and Mortality for Patients With Acute Respiratory Distress Syndrome in Intensive Care Units in 50 Countries. JAMA [Internet]. 2016;315:788. Available from: internal-pdf://0.0.13.130/Epidemiology, Patterns of Care, and Mortality for.webarchive
Grieco DL, Bongiovanni F, Chen L, Menga LS, Cutuli SL, Pintaudi G, et al. Respiratory physiology of COVID-19-induced respiratory failure compared to ARDS of other etiologies. Crit Care [Internet]. NLM (Medline); 2020 [cited 2020 Oct 12];24:529. Available from: https://pubmed.ncbi.nlm.nih.gov/32859264/.
Roesthuis L, van den Berg M, van der Hoeven H. Advanced respiratory monitoring in COVID-19 patients: use less PEEP! Crit Care. Springer Science and Business Media LLC; 2020;24.
COVID-19 Clinical Research Resources · ISARIC [Internet]. [cited 2020 Oct 12]. Available from: https://isaric.tghn.org/covid-19-clinical-research-resources/.
Ranieri VM, Rubenfeld GD, Thompson BT, Ferguson ND, Caldwell E, Fan E, et al. Acute Respiratory Distress Syndrome. JAMA [Internet]. 2012;307:2526–33. Available from: https://doi.org/10.1001/jama.2012.5669.
Acknowledgements
We fully acknowledge statistical guidance by Adrian Barnett, Head Statistician of the COVID-19 Critical Care Consortium. We recognize the crucial importance of the ISARIC and SPRINT-SARI networks for the development and expansion of the COVID-19 Critical Care Consortium. We thank the generous support we received from ELSO and ECMOnet. We owe Li Wenliang, MD from the Wuhan Central Hospital an eternal debt of gratitude for reminding the world that doctors should never be censored during a pandemic. Finally, we acknowledge all members of the COVID-19 Critical Care Consortium and various collaborators.
Contributors
Prefix/First name/Last name | Site name |
|---|---|
Tala Al-Dabbous | Al Adan Hospital |
Dr Huda Alfoudri | |
Dr Mohammed Shamsah | |
Dr Subbarao Elapavaluru Ashley Berg Christina Horn | Allegheny General Hospital |
Dr Stephan Schroll | Barmherzige Bruder Regansburg |
Dr Jorge Velazco Wanda Fikes Ludmyla Ploskanych | Baylor Scott & White Health—Temple |
Dr Dan Meyer Maysoon Shalabi-McGuire Trent Witt Ashley Ehlers | Baylor University Medical Centre, Dallas |
Dr Lorenzo Grazioli | Bergamo Hospital |
Dr E. Wilson Grandin Jose Nunez Tiago Reyes | Beth Israel Deaconess Medical Centre |
Dr Mark Joseph Dr Brook Mitchell Martha Tenzer | Carilion Clinic |
Dr Ryuzo Abe Yosuke Hayashi | Chiba University Graduate School of Medicine |
Dr Hwa Jin Cho Dr In Seok Jeong | Chonnam National University Hospital |
Dr Nicolas Brozzi Dr Jaime Hernandez-Montfort | Cleveland Clinic—Florida |
Omar Mehkri Stuart Houltham | Cleveland Clinic—Ohio |
Dr Jerónimo Graf Rodrigo Perez | Clinica Alemana De Santiago |
Dr Roderigo Diaz Camila Delgado Joyce González Maria Soledad Sanchez | Clinica Las Condez |
Dr Diego Fernando Bautista Rincón Melissa Bustamante Duque Dr Angela Maria Marulanda Yanten | Clinica Valle de Lilli |
Dr Dan Brodie | Columbia University Medical Centre |
Dr Desy Rusmawatiningtyas | Dr Sardjito Hospital (Paediatrics) |
Gabrielle Ragazzo | Emory University Healthcare System |
Dr Azhari Taufik Dr Margaretha Gunawan Dr Vera Irawany Muhammad Rayhan Dr Elizabeth Yasmin Wardoyo | Fatmawati Hospital |
Dr Mauro Panigada Dr Chiara Martinet Dr Sebastiano Colombo Dr Giacomo Grasselli Dr Michela Leone Dr Alberto Zanella | Fondazione IRCCS Policlinico of Milan (Fondazione IRCCS Ca' Granda Ospedale Maggiore Policlinico) |
Prof Massimo Antonelli Dr Simone Carelli Domenico L. Grieco | Fondazione Policlinico Universitario Agostino Gemelli IRCCS |
Motohiro Asaki | Fujieda Municipal General Hospital |
Dr Kota Hoshino | Fukuoka University |
Dr Leonardo Salazar Laura Duarte | Fundación Cardiovascular de Colombia |
Dr Joseph McCaffrey Allison Bone | Geelong Hospital |
Dr David Thomson Dr Christel Arnold-Day Jerome Cupido Zainap Fanie Dr Malcom Miller Dr Lisa Seymore Dawid van Straaten | Groote Schuur Hospital |
Dr Ibrahim Hassan Dr Ali Ait Hssain Jeffrey Aliudin Al-Reem Alqahtani Khoulod Mohamed Ahmed Mohamed Darwin Tan Joy Villanueva Ahmed Zaqout | Hamad General Hospital—Weill Cornell Medical College in Qatar |
Dr Ethan Kurtzman Arben Ademi Ana Dobrita Khadija El Aoudi Juliet Segura | Hartford HealthCare |
Dr Gezy Giwangkancana | Hasan Sadikin Hospital (Adult) |
Dr Shinichiro Ohshimo | Hiroshima University |
Dr Koji Hoshino Saito Hitoshi Dr Yuka Uchinami | Hokkaido University Hospital |
Dr Javier Osatnik | Hospital Alemán |
Dr Anne Joosten | Hospital Civil Marie Curie |
Dr Antoni Torres Ana Motos Dr Minlan Yang | Hospital Clinic, Barcelona |
Carlos Luna | Hospital de Clínicas |
Francisco Arancibia | Hospital del Tórax |
Virginie Williams Alexandre Noel | Hospital du Sacre Coeur (Universite de Montreal) |
Dr Nestor Luque | Hospital Emergencia Ate Vitarte |
Dr Trieu Huynh Trung Sophie Yacoub | Hospital for Tropical Diseases |
Marina Fantini | Hospital Mater Dei |
Dr Ruth Noemi Jorge García Dr Enrique Chicote Alvarez | Hospital Nuestra Señora de Gracia |
Dr Anna Greti Oscar Lomeli | Hospital Puerta de Hierro |
Dr Adrian Ceccato | Hospital Universitari Sagrat Cor |
Dr Angel Sanchez | Hospital Universitario Sant Joan d’Alacant |
Dr Ana Loza Vazquez | Hospital Universitario Virgen de Valme |
Dr Ferran Roche-Campo | Hospital Verge de la Cinta de Tortosa |
Dr Divina Tuazon Dr Toni Duculan | Houston Methodist Hospital |
Hiroaki Shimizu | Kakogawa Acute Care Medical Center, Hyogo |
Marcelo Amato Luciana Cassimiro Flavio Pola Francis Ribeiro Guilherme Fonseca | INCOR (Universidade de São Paulo) |
Dr Heidi Dalton Dr Mehul Desai Dr Erik Osborn Hala Deeb | INOVA Fairfax Hospital |
Dr Antonio Arcadipane Claudia Bianco Raffaele Cuffaro Gennaro Martucci Giovanna Occhipinti Matteo Rossetti Chiara Vitiello | ISMETT |
Dr Sung-Min Cho Kate Calligy Dr Glenn Whitman | Johns Hopkins |
Dr Hiroaki Shimizu Dr Naoki Moriyama | Kakogawa Acute Care Medical Center |
Dr Jae-Burm Kim | Keimyung University Dong San Hospital |
Dr Nobuya Kitamura Takashi Shimazui | Kimitsu Chuo Hospital |
Dr Abdullah Al-Hudaib Dr Alyaa Elhazmi | King Faisal Specialist Hospital and Research Center |
Dr Johannes Gebauer | Klinikum Passau |
Dr Toshiki Yokoyama | Kouritu Tousei Hospital |
Dr Abdulrahman Al-Fares Esam Alamad Fatma Alawadhi Kalthoum Alawadi Dr Sarah Buabbas | Al-Amiri and Jaber Al-Ahmed Hospitals, Kuwait Extracorporeal Life Support Program |
Dr Hiro Tanaka | Kyoto Medical Centre |
Dr Satoru Hashimoto Masaki Yamazaki | Kyoto Prefectural University of Medicine |
Tak-Hyuck Oh | Kyung Pook National University Chilgok Hospital |
Dr Mark Epler Dr Cathleen Forney Jared Feister Katherine Grobengieser Louise Kruse Joelle Williamson | Lancaster General Health |
Dr Eric Gnall Dr Mara Caroline Sasha Golden Colleen Karaj Sherry McDermott Lynn Sher Dr Timothy Shapiro Lisa Thome Mark Vanderland Mary Welch | Lankenau Institute of Medical Research (Main Line Health) |
Prof Luca Brazzi | Le Molinette Hospital (Ospedale Molinette Torino) |
Dr Tawnya Ogston | Legacy Emanuel Medical Center |
Dr Dave Nagpal Karlee Fischer | London Health Sciences Centre |
Dr Roberto Lorusso Maria de Piero | Maastricht University Medical Centre |
Prof Mariano Esperatti | Mar del Plata Medical Foundation Private Community Hospital |
Dr Diarmuid O’Briain | Maroondah Hospital |
Dr Edmund G. Carton | Mater Misericordiae University Hospital |
Ayan Sen Amanda Palacios Deborah Rainey | Mayo Clinic College of Medicine |
Cassandra Seefeldt Dr Lucia Durham Dr Octavio Falcucci Amanda Emmrich Jennifer Guy Carling Johns Emily Neumann | Medical College of Wisconsin (Froedtert Hospital) |
Dr Nina Buchtele Dr Michael Schwameis | Medical University of Vienna |
Dr Stephanie-Susanne Stecher Delila Singh Dr Michaela Barnikel Lukas Arenz | Medical Department II, LMU Hospital Munich |
Dr Akram Zaaqoq Lan Anh Galloway Caitlin Merley | MedStar Washington Hospital Centre |
Dr Marc Csete Luisa Quesada Isabela Saba | Mount Sinai Medical Centre |
Dr Daisuke Kasugai Hiroaki Hiraiwa Taku Tanaka | Nagoya University Hospital |
Dr Eva Marwali Yoel Purnama Dr Santi Rahayu Dewayanti Dr Ardiyan Dr Debby Siagian | National Cardiovascular Center Harapan Kita |
Yih-Sharng Chen | National Taiwan University Hospital |
Prof John Laffey Dr Bairbre McNicholas Dr David Cosgrave | Galway University Hospitals |
Marlice VanDyk Sarah MacDonald | Netcare Unitas ECMO Centre |
Dr Ian Seppelt | Nepean Hospital |
Dr Indrek Ratsep Lauri Enneveer Kristo Erikson Dr Getter Oigus Andra-Maris Post Piret Sillaots | North Estonia Medical Centre |
Frank Manetta | Northwell Health |
Mamoru Komats | Obihiro-Kosei General Hospital |
Dr S. Veena Satyapriya Dr Amar Bhatt Marco Echeverria Juan Fiorda Alicia Gonzalez Dr Nahush A. Mokadam Johnny McKeown Joshua Pasek Haixia Shi Alberto Uribe | Ohio State University Medical Centre |
Dr Rita Moreno | Oklahoma Heart Institute |
Bishoy Zakhary Hannah Johnson Nolan Pow | Oregon Health and Science University Hospital (OHSU) |
Dr Marco Cavana Dr Alberto Cucino | Ospedale di Arco (Trento hospital) |
Prof Giuseppe Foti Dr Marco Giani Dr Vincenzo Russotto | Ospedale San Gerardo |
Prof Davide Chiumello Valentina Castagna Silvia Coppola | Ospedale San Paolo |
Dr Andrea Dell’Amore | Padua University Hospital (Policlinico of Padova) |
Dr Hoi-Ping Shum | Pamela Youde Nethersole Eastern Hospital |
Dr Alain Vuysteke | Papworth Hospitals NHS Foundation Trust |
Dr Asad Usman Andrew Acker Blake Mergler Nicolas Rizer Federico Sertic Benjamin Smood Alexandra Sperry Dr Madhu Subramanian | Penn Medicine (Hospital of the University of Pennsylvania) |
Dr Erlina Burhan Dr Navy Lolong Dr Ernita Akmal Prof Menaldi Rasmin Bhat Naivedh Dr Faya Sitompu | Persahabatan General Hospital |
Dr Peter Barrett Julia Daugherty Dr David Dean | Piedmont Atlanta Hospital |
Dr Antonio Loforte | Policlinico di S. Orsola, Università di Bologna |
Dr Irfan Khan Olivia DeSantis Dr Mohammed Abraar Quraishi | Presbyterian Hospital Services, Albuquerque |
Dr Gavin Salt | Prince of Wales |
Dr Dominic So Darshana Kandamby | Princess Margaret Hospital |
Dr Jose M. Mandei Hans Natanael | Prof Dr R. D. Kandou General Hospital—Paediatric |
Eka YudhaLantang Anastasia Lantang | Prof Dr R. D. Kandou General Hospital—Adult |
Anna Jung Dr Terese Hammond | Providence Saint John's Health Centre |
George Ng Dr Wing Yiu Ng | Queen Elizabeth Hospital, Hong Kong |
Dr Pauline Yeung | Queen Mary Hospital |
Dr Shingo Adachi | Rinku general medical center (and Senshu trauma and critical care center) |
Dr Pablo Blanco Ana Prieto Jesús Sánchez | Rio Hortega University Hospital |
Dr Meghan Nicholson | Rochester General Hospital |
Dr Michael Farquharson | Royal Adelaide Hospital |
Dr Warwick Butt Alyssa Serratore Carmel Delzoppo | Royal Children’s Hospital |
Dr Pierre Janin Elizabeth Yarad | Royal North Shore Hospital |
Dr Richard Totaro Jennifer Coles | Royal Prince Alfred Hospital |
Robert Balk Samuel Fox James Hays Esha Kapania Pavel Mishin Andy Vissing Garrett Yantosh | Rush University, Chicago |
Saptadi Yuliarto Dr Kohar Hari Santoso Dr Susanthy Djajalaksana | Saiful Anwar Malang Hospital (Brawijaya University) (Paediatrics) |
Dr Arie Zainul Fatoni | Saiful Anwar Malang Hospital (Brawijaya University) (Adult) |
Dr Masahiro Fukuda | Saiseikai Senri Hospital |
Prof Keibun Liu | Saiseikai Utsunomiya Hospital |
Prof Paolo Pelosi Dr Denise Battaglini | San Martino Hospital |
Dr Juan Fernando Masa Jiménez | San Pedro de Alcantara Hospital |
Dr Sérgio Gaião Dr Roberto Roncon-Albuquerque | São João Hospital Centre, Porto |
Jessica Buchner | Sentara Norfolk General Hospital |
Dr Young-Jae Cho Dr Sang Min Lee | Seoul National University Hospital |
Dr Su Hwan Lee | Severance Hospital |
Dr Tatsuya Kawasaki | Shizuoka Children's Hospital |
Dr Pranya Sakiyalak Prompak Nitayavardhana | Siriraj Hospital |
Dr Tamara Seitz | Sozialmedizinisches Zentrum Süd—Kaiser-Franz-Josef-Spital |
Rakesh Arora David Kent | St Boniface Hospital (University of Mannitoba) |
Dr Swapnil Parwar Andrew Cheng Jennene Miller | St George Hospital |
Daniel Marino Jillian E Deacon | St. Christopher's Hospital for Children |
Dr Shigeki Fujitani Dr Naoki Shimizu | St Marianna Medical University hospital |
Dr Jai Madhok Dr Clark Owyang | Stanford University Hospital |
Dr Hergen Buscher Claire Reynolds | St Vincent’s Hospital |
Dr Olavi Maasikas Dr Aleksandr Beljantsev Vladislav Mihnovits | Tartu University Hospital |
Dr Takako Akimoto Mariko Aizawa Dr Kanako Horibe Ryota Onodera | Teine Keijinkai Hospital |
Prof Carol Hodgson Meredith Young | The Alfred Hospital |
Timothy Smith Cheryl Bartone | The Christ Hospital |
Dr Timothy George | The Heart Hospital Baylor Plano, Plano |
Dr Kiran Shekar Niki McGuinness Lacey Irvine | The Prince Charles Hospital |
Brigid Flynn Abigail Houchin | The University of Kansas Medical Centre |
Dr Keiki Shimizu Jun Hamaguchi | Tokyo Metropolitan Medical Center |
Leslie Lussier Grace Kersker Dr John Adam Reich | Tufts Medical Centre (and Floating Hospital for Children) |
Dr Gösta Lotz | Universitätsklinikum Frankfurt (University Hospital Frankfurt)(Uniklinik) |
Dr Maximilian Malfertheiner Esther Dreier Dr Lars Maier | Universitätsklinikum Regensburg (Klinik für Innere Medizin II) |
Dr Neurinda Permata Kusumastuti | University Airlangga Hospital (Paediatric) |
Dr Colin McCloskey Dr Al-Awwab Dabaliz Dr Tarek B Elshazly Josiah Smith | University Hospital Cleveland Medical Centre (UH Cleveland hospital) |
Dr Konstanty S. Szuldrzynski Dr Piotr Bielański | University Hospital in Krakow |
Dr Yusuff Hakeem | University Hospitals of Leicester NHS Trust (Glenfield Hospital) |
Dr Keith Wille Rebecca Holt | University of Alabama at Birmingham Hospital (UAB) |
Dr Ken Kuljit S. Parhar Dr Kirsten M. Fiest Cassidy Codan Anmol Shahid | University of Calgary (Peter Lougheed Centre, Foothills Medical Centre, South Health Campus and Rockyview General Hospital) |
Dr Mohamed Fayed Dr Timothy Evans Rebekah Garcia Ashley Gutierrez Hiroaki Shimizu | University of California, San Francisco-Fresno Clinical Research Centre |
Dr Tae Song Rebecca Rose | University of Chicago |
Dr Suzanne Bennett Denise Richardson | University of Cincinnati Medical Centre |
Dr Giles Peek Dalia Lopez-Colon | University of Florida |
Dr Lovkesh Arora Kristina Rappapport Kristina Rudolph Zita Sibenaller Lori Stout Alicia Walter | University of Iowa |
Dr Daniel Herr Nazli Vedadi | University of Maryland—Baltimore |
Dr Lace Sindt Cale Ewald Julie Hoffman Sean Rajnic Shaun Thompson | University of Nebraska Medical Centre |
Dr Ryan Kennedy | University of Oklahoma Health Sciences Centre (OU) |
Dr Matthew Griffee Dr Anna Ciullo Yuri Kida | University of Utah Hospital |
Dr Ricard Ferrer Roca Cynthia Alegre Dr Sofia Contreras Dr JordI Riera | Vall d'Hebron University Hospital, Barcelona |
Dr Christy Kay Irene Fischer Elizabeth Renner | Washington University in St. Louis/Barnes Jewish Hospital |
Dr Hayato Taniguci | Yokohama City University Medical Center |
Gabriella Abbate Halah Hassan Dr Silver Heinsar Varun A Karnik Dr Katrina Ki Hollier F. O'Neill Dr Nchafatso Obonyo Dr Leticia Pretti Pimenta Janice D. Reid Dr Kei Sato Dr Kiran Shekar Aapeli Vuorinen Dr Karin S. Wildi Emily S. Wood Dr Stephanie Yerkovich | COVID-19 Critical Care Consortium |
Collaborators
Prefix/First name/Last name | Site name |
|---|---|
Dr Emma Hartley | Aberdeen Royal Infirmary (Foresterhill Health Campus) |
Bastian Lubis | Adam Malik Hospital |
Takanari Ikeyama | Aichi Childrens Health and Medical Center |
Balu Bhaskar | American Hospital |
Dr Jae-Seung Jung | Anam Korea University Hospital |
Sandra Rossi Marta Fabio Guarracino | Azienda Ospedaliero Universitaria Parma |
Prof Fabio Guarracino | Azienda Ospedaliero Universitaria Pisana |
Stacey Gerle | Banner University Medical Centre |
Emily Coxon | Baptist Health Louisville |
Dr Bruno Claro | Barts Hospital |
Dr. Gonzo Gonzalez-Stawinski | Baylor All Saints Medical Centre, Forth Worth |
Daniel Loverde | Billings Clinic |
Dr Vieri Parrini | Borgo San Lorenzo Hospital |
Dr Diarmuid O’Briain Stephanie Hunter | Box Hill Hospital |
Dr Angela McBride | Brighton and Sussex Medical School |
Kathryn Negaard Dr Phillip Mason | Brooke Army Medical Centre |
Dr Angela Ratsch | Bundaberg Hospital |
Dr Mahesh Ramanan Julia Affleck | Caboolture Hospital |
Ahmad Abdelaziz | Cairo University Hospital |
Dr Sumeet Rai Josie Russell-Brown Mary Nourse | Canberra Hospital |
Juan David Uribe | Cardio VID |
Dr Adriano Peris | Careggi Hospital |
Mark Sanders | Cedar Park Regional Medical Center |
Dominic Emerson | Cedars-Sinai Medical Centre |
Muhammad Kamal | Cengkareng Hospital |
Prof Pedro Povoa | Centro Hospitalar de Lisboa |
Dr Roland Francis | Charite-Univerrsitatsmedizi n Berlin |
Ali Cherif | Charles Nicolle University Hospital |
Dr Sunimol Joseph | Children’s Health Ireland (CHI) at Crumlin |
Dr Matteo Di Nardo | Children’s Hospital Bambino Gesù |
Micheal Heard | Children's Healthcare of Atlanta-Egleston Hospital |
Kimberly Kyle | Children's Hospital |
Ray A Blackwell | Christiana Care Health System's Centre for Heart and Vascular Health |
Dr Michael Piagnerelli Dr Patrick Biston | CHU de Charleroi |
Hye Won Jeong | Chungbuk National University Hospital |
Reanna Smith | Cincinnati Children's |
Yogi Prawira | Cipto Mangunkusumo Hospital |
Dr Giorgia Montrucchio Dr Gabriele Sales | Città della Salute e della Scienza Hospital—Turin, Italy |
Nadeem Rahman Vivek Kakar | Cleveland Clinic, Abu Dhabi |
Dr Michael Piagnerelli Dr Josefa Valenzuela Sarrazin | Clinica Las Condes |
Dr Arturo Huerta Garcia | Clínica Sagrada Família |
Dr Bart Meyns | Collaborative Centre Department Cardiac Surgery, UZ Leuven |
Marsha Moreno | Dignity Health Medical Group-Dominican |
Rajat Walia | Dignity Health St. Joseph's Hospital and Medical Center (SJHMC) |
Dr Annette Schweda | Donaustauf hospital |
Cenk Kirakli | Dr. Suat Seren Chest Diseases and Surgery Practice and Training Centre |
Estefania Giraldo | Fundación Clinica Shaio (Shaio Clinic) |
Dr Wojtek Karolak | Gdansk Medical University |
Dr Martin Balik | General University Hospital |
Elizabeth Pocock | George Washington University Hospital |
Evan Gajkowski | Giesinger Medical Centre |
Dr James Winearls Mandy Tallott | Gold Coast University Hospital |
Kanamoto Masafumi | Gunma University Graduate School of Medicine |
Dr Nicholas Barrett | Guy's and St Thomas NHS Foundation Trust Hospital |
Yoshihiro Takeyama | Hakodate City Hospital |
Sunghoon Park | Hallym University Sacred Heart Hospital |
Faizan Amin | Hamilton General Hospital |
Dr Erina Fina | Hasan Sadikin Hospital |
Dr Serhii Sudakevych | Heart Institute Ministry of Health of Ukraine |
Dr Angela Ratsch | Hervey Bay Hospital |
Patrícia Schwarz Ana Carolina Mardini | Hospital de Clínicas de Porto Alegre |
Ary Serpa Neto | Hospital Israelita Albert Einstein |
Dr Andrea Villoldo | Hospital Privado de Comunidad |
Alexandre Siciliano Colafranceschi | Hospital Pro Cardíaco |
Dr Alejandro Ubeda Iglesias | Hospital Punta de Europa |
Lívia Maria Garcia Melro Giovana Fioravante Romualdo | Hospital Samaritano Paulista |
Diego Gaia | Hospital Santa Catarina |
Helmgton Souza | Hospital Santa Marta |
Dr Diego Bastos | Hospital Cura D’ars Fortaleza |
Filomena Galas | Hospital Sirio Libanes |
Dr Rafael Máñez Mendiluce | Hospital Universitario de Bellvitge |
Alejandra Sosa | Hospital Universitario Esperanza (Universidad Francisco Marroquin) |
Dr Ignacio Martinez | Hospital Universitario Lucus Augusti |
Hiroshi Kurosawa | Hyogo Prefectural Kobe Children's Hospital |
Juan Salgado | Indiana University Health |
Dr Beate Hugi-Mayr | Inselspital University Hospital |
Eric Charbonneau | Institut Universitaire de Cardiologie et de Pneumologie de Quebec—Universite Laval |
Vitor Salvatore Barzilai | Instituto de Cardiologia do Distrito Federal—ICDF |
Veronica Monteiro | Instituto de Medicina Integral Prof. Fernando Figueira (IMIP) |
Rodrigo Ribeiro de Souza | Instituto Goiano de Diagnostico Cardiovascular (IGDC) |
Michael Harper | INTEGRIS Baptist Medical Center |
Hiroyuki Suzuki | Japan Red Cross Maebashi Hospital |
Celina Adams | John C Lincoln Medical Centre |
Dr Jorge Brieva | John Hunter Hospital |
George Nyale | Kenyatta National Hospital (KNH) |
Jihan Fatani Dr Faisal Saleem Eltatar | King Abdullah Medical City Specialist Hospital |
Dr. Husam Baeissa | King Abdullah Medical Complex |
Ayman AL Masri | King Salman Hospital NWAF |
Yee Hui Mok | KK Women's and Children's Hospital |
Masahiro Yamane | KKR Medical Center |
Hanna Jung | Kyung Pook National University Hospital |
Dr Matthew Brain Sarah Mineall | Launceston General Hospital |
Rhonda Bakken | M Health Fairview |
Dr Tim Felton | Manchester University NHS Foundation Trust—Wythenshawe |
Lorenzo Berra | Massachusetts General Hospital |
Gordan Samoukoviv Dr Josie Campisi | McGill University Health Centre |
Bobby Shah | Medanta Hospital |
Arpan Chakraborty | Medica Super speciality Hospital |
Monika Cardona | Medical University of South Carolina |
Harsh Jain | Mercy Hospital of Buffalo |
Dr Asami Ito | Mie University Hospital |
Brahim Housni | Mohammed VI University hospital |
Sennen Low | National Centre for Infectious Diseases |
Dr. Koji Iihara | National Cerebral and Cardiovascular Center |
Joselito Chavez | National Kidney and Transplant Institute |
Dr Kollengode Ramanathan | National University Hospital, Singapore |
Gustavo Zabert | National University of Comahue |
Krubin Naidoo | Nelson Mandela Children's Hospital |
Singo Ichiba | Nippon Medical School Hospital |
Randy McGregor | Northwestern Medicine |
Teka Siebenaler | Norton Children's Hospital |
Hannah Flynn | Novant Health (NH) Presbyterian Medical Centre |
Julia Garcia-Diaz Catherine Harmon | Ochsner Clinic Foundation |
Kristi Lofton | Ochsner LSA Health Shreveport |
Toshiyuki Aokage | Okayama University Hospital |
Kazuaki Shigemitsu | Osaka City General Hospital |
Dr Andrea Moscatelli | Ospedale Gaslini |
Dr Giuseppe Fiorentino | Ospedali dei Colli |
Dr Matthias Baumgaertel | Paracelsus Medical University Nuremberg |
Serge Eddy Mba | Parirenyatwa General Hospital |
Jana Assy | Pediatric and Neonatal Cardiac intensive care at the American University |
Holly Roush | Penn State Heath S. Hershey Medical Centre |
Kay A Sichting | Peyton Manning Children's Hospital |
Dr Francesco Alessandri | Policlinico Umberto, Sapienza University of Rome |
Debra Burns | Presbyterian Hospital, New York/Weill Cornell Medical Centre |
Ahmed Rabie | Prince Mohammed bin Abdulaziz Hospital |
Carl P. Garabedian | Providence Sacred Heart Children's Hospital |
Dr Jonathan Millar Dr Malcolm Sim | Queen Elizabeth II University Hospital |
Dr Adrian Mattke | Queensland Children’s Hospital |
Dr Danny McAuley | Queens University of Belfast |
Jawad Tadili | Rabat university hospital |
Dr Tim Frenzel | Radboud University Medical Centre |
Aaron Blandino Ortiz | Ramón y Cajal University Hospital |
Jackie Stone | Rapha Medical Centre |
Dr Alexis Tabah Megan Ratcliffe Maree Duroux | Redcliffe Hospital |
Dr Antony Attokaran | Rockhampton Hospital |
Dr Brij Patel | Royal Brompton &Harefield NHS Foundation Trust |
Derek Gunning | Royal Columbian Hospital |
Dr Kenneth Baillie | Royal Infirmary Edinburgh |
Dr Pia Watson | Sahlgrenska University Hospital |
Kenji Tamai | Saiseikai Yokohamashi Tobu Hospital |
Dr Gede Ketut Sajinadiyasa Dr Dyah Kanyawati | Sanglah General Hospital |
Marcello Salgado | Santa Casa de Misericordia de Juiz de Fora |
Assad Sassine | Santa Casa de Misericórdia de Vitoria |
Dr Bhirowo Yudo | Sardjito Hospital |
Scott McCaul | Scripps Memorial Hospital La Jolla |
Bongjin Lee | Seoul National University Children's Hospital |
Yoshiaki Iwashita | Shimane University Hospital |
Laveena munshi | Sinai Health Systems (Mount Sinai Hospital) |
Dr Neurinda Permata Kusumastuti | Soetomo General Hospital (FK UNAIR) |
Dr Nicole Van Belle | St. Antonius Hospital |
Ignacio Martin-Loeches | St James’s University Hospital |
Dr Hergen Buscher | St Vincent’s Hospital, Sydney |
Surya Oto Wijaya | Sulianti Saroso Hospital |
Dr Lenny Ivatt | Swansea Hospital |
Chia Yew Woon | Tan Tock Seng Hospital |
Hyun Mi Kang | The Catholic University of Seoul St Mary Hospital |
Erskine James | The Medical Centre Navicent Health |
Nawar Al-Rawas | Thomas Jefferson University Hospital |
Tomoyuki Endo | Tohoku Medical and Pharmaceutical University |
Dr Yudai Iwasaki | Tohoku University |
Dr Eddy Fan Kathleen Exconde | Toronto General Hospital |
Kenny Chan King-Chung | Tuen Mun Hospital |
Dr Vadim Gudzenko | UCLA Medical Centre (Ronald Regan) |
Dr Beate Hugi-Mayr | Universitätsspital Bern, Universitätsklinik für Herz- und Gefässchirurgie |
Dr Fabio Taccone | Universite Libre de Bruxelles |
Dr Fajar Perdhana | University Airlangga Hospital (Adult) |
Yoan Lamarche | University de Montreal (Montreal Heart Institute) |
Dr Joao Miguel Ribeiro | University Hospital CHLN |
Dr Nikola Bradic | University Hospital Dubrava |
Dr Klaartje Van den Bossche | University Hospital Leuven |
Gurmeet Singh | University of Aberta (Mazankowski Heart Institute) |
Dr Gerdy Debeuckelaere | University of Antwerp |
Dr Henry T. Stelfox | University of Calgary and Alberta Health Services |
Cassia Yi | University of California at San Diego |
Jennifer Elia | University of California, Irvine |
Shu Fang | University of Hong Kong |
Thomas Tribble | University of Kentucky Medical Center |
Shyam Shankar | University of Missouri |
Dr Paolo Navalesi | University of Padova |
Raj Padmanabhan | University of Pittsburgh Medical Centre |
Bill Hallinan | University of Rochester Medical Centre (UR Medicine) |
Luca Paoletti | University of South Carolina |
Yolanda Leyva | University of Texas Medical Branch |
Tatuma Fykuda | University of the Ryukus |
Jillian Koch | University of Wisconsin & American Family Children's Hospital |
Amy Hackman | UT Southwestern |
Lisa Janowaik | UTHealth (University of Texas) |
Jennifer Osofsky | Vassar Brothers Medical Center (VBMC) |
A/Prof Katia Donadello | Verona Integrated University Hospital |
Josh Fine | WellSpan Health—York Hospital |
Dr Benjamin Davidson | Westmead Hospital |
Andres Oswaldo Razo Vazquez | Yale New Haven Hospital |
Funding
University of Queensland; Wesley Medical Research; The Prince Charles Hospital Foundation; Fisher & Paykel; The Health Research Board of Ireland; Biomedicine international training research programme for excellent clinician-scientists; European Union’s research and innovation programme (Horizon 2020); la Caixa Foundation. Finally, Carol Hodgson is funded by a National Health and Medical Research Council Grant. Sally Schrapnel is funded by the Australian Research Council Centre of Excellence for Engineered Quantum Systems (Project number CE170100009).
Author information
Authors and Affiliations
Consortia
Contributions
GLB conceived the study, participated in its design and coordination and helped to draft the manuscript; JYS conceived the study, participated in its design and coordination and helped to draft the manuscript drafted the manuscript; HD participated in the design of the study and helped to draft the manuscript; NW performed the statistical analysis and helped to draft the manuscript; SS participated in the coordination of the study, performed the statistical analysis and helped to draft the manuscript; JPF participated in the design of the study and helped to draft the manuscript; BL performed the statistical analysis and helped to draft the manuscript; SH participated in the coordination of the study performed the statistical analysis and helped to draft the manuscript; AV performed the statistical analysis and helped to draft the manuscript; GB performed the statistical analysis and helped to draft the manuscript; JEM participated in the design of the study and helped to draft the manuscript; SF participated in the design and coordination of the study and helped to draft the manuscript; MP participated in the coordination of the study helped to draft the manuscript; JL participated in the coordination of the study helped to draft the manuscript; DB participated in the coordination of the study helped to draft the manuscript; EF participated in the coordination of the study helped to draft the manuscript; AT participated in the coordination of the study helped to draft the manuscript; DC participated in the coordination of the study helped to draft the manuscript; AC participated in the design of the study and helped to draft the manuscript; AE participated in collection of data and helped to draft the manuscript; CH participated in coordination and collection of data and helped to draft the manuscript; SI participated in collection of data and helped to draft the manuscript; CL participated in coordination and collection of data and helped to draft the manuscript; SM participated in coordination and collection of data and helped to draft the manuscript; AN participated in coordination and collection of data and helped to draft the manuscript; PY participated in coordination and collection of data and helped to draft the manuscript; MO participated in coordination and collection of data and helped to draft the manuscript; AP participated in coordination and collection of data and helped to draft the manuscript; HTT participated in collection of data and helped to draft the manuscript; JFF conceived the study, participated in its design and coordination and helped to draft the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Participating hospitals obtained local ethics committee approval, and a waiver of informed consent was granted in all cases.
Consent for publication
Not applicable.
Statistical analysis
Nicole White; Sally Shrapnel; Benoit Liquet; Samuel Hinton; Aapeli Vuorinem; Gareth Booth.
Competing interests
GLB and JF received research funds, through their affiliated institution from Fisher & Paykel. All remaining authors do not have any conflict of interest related to this report.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Li Bassi, G., Suen, J.Y., Dalton, H.J. et al. An appraisal of respiratory system compliance in mechanically ventilated covid-19 patients. Crit Care 25, 199 (2021). https://doi.org/10.1186/s13054-021-03518-4
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s13054-021-03518-4