Abstract
Background
Sepsis is one of the main reasons for non-elective admission to pediatric intensive care units (PICUs), but little is known about determinants influencing outcome. We characterized children admitted with community-acquired sepsis to European PICUs and studied risk factors for mortality and disability.
Methods
Data were collected within the collaborative Seventh Framework Programme (FP7)-funded EUCLIDS study, which is a prospective multicenter cohort study aiming to evaluate genetic determinants of susceptibility and/or severity in sepsis. This report includes 795 children admitted with community-acquired sepsis to 52 PICUs from seven European countries between July 2012 and January 2016. The primary outcome measure was in-hospital death. Secondary outcome measures were PICU-free days censured at day 28, hospital length of stay, and disability. Independent predictors were identified by multivariate regression analysis.
Results
Patients most commonly presented clinically with sepsis without a source (n = 278, 35%), meningitis/encephalitis (n = 182, 23%), or pneumonia (n = 149, 19%). Of 428 (54%) patients with confirmed bacterial infection, Neisseria meningitidis (n = 131, 31%) and Streptococcus pneumoniae (n = 78, 18%) were the main pathogens. Mortality was 6% (51/795), increasing to 10% in the presence of septic shock (45/466). Of the survivors, 31% were discharged with disability, including 24% of previously healthy children who survived with disability. Mortality and disability were independently associated with S. pneumoniae infections (mortality OR 4.1, 95% CI 1.1–16.0, P = 0.04; disability OR 5.4, 95% CI 1.8–15.8, P < 0.01) and illness severity as measured by Pediatric Index of Mortality (PIM2) score (mortality OR 2.8, 95% CI 1.3–6.1, P < 0.01; disability OR 3.4, 95% CI 1.8–6.4, P < 0.001).
Conclusions
Despite widespread immunization campaigns, invasive bacterial disease remains responsible for substantial morbidity and mortality in critically ill children in high-income countries. Almost one third of sepsis survivors admitted to the PICU were discharged with some disability. More research is required to delineate the long-term outcome of pediatric sepsis and to identify interventional targets. Our findings emphasize the importance of improved early sepsis-recognition programs to address the high burden of disease.
Similar content being viewed by others
Background
Pediatric sepsis represents one of the most common reasons for pediatric intensive care unit (PICU) admission, and the prevalence and mortality in high-income countries has become comparable to that in adults [1,2,3,4,5]. In 2013, 10% of childhood deaths under the age of 5 years in high-income countries were attributable to infections, with the majority of acute infection-related deaths occurring in PICUs [6]. Recent reports have demonstrated the major impact of comorbidities with increasing rates of healthcare-associated infections [1, 2, 4, 5, 7,8,9].
In contrast, recent data on community-acquired sepsis are limited. Community-acquired sepsis represents specific patterns, affecting different hosts, and involving different pathogens, which may translate into different outcomes compared to healthcare-associated infections [10, 11]. In view of the need to develop improved strategies for early recognition and treatment of sepsis, as demanded by the recent resolution of the World Health Organization [12], it is imperative to assess contemporary characteristics of epidemiology and severity predictors for community-acquired sepsis [13]. Previous larger epidemiological sepsis studies have been predominantly based on hospital coding or PICU databases with mortality as the main outcome [1, 5]. A recent roadmap for future sepsis research highlighted the inherent limitations of such approaches, identifying the need to define the longer-term impact on survivors [14]. While increasing evidence in neonatal and adult patients demonstrates that new cognitive impairment, functional disability, and impaired quality of life are common amongst sepsis survivors [15,16,17,18], little is known about disability in pediatric sepsis survivors [19, 20].
The aim of this study was to characterize the clinical presentation, pathogens, mortality, and disability in children admitted to European PICUs with community-acquired sepsis, based on patients recruited through the multinational prospective European Childhood Life-threatening Infectious Disease Study (EUCLIDS).
Methods
Consortium and study sites
The EUCLIDS is a Seventh Framework Programme (FP7) project in the context of the European Union’s Research and Innovation funding program for 2007–2013. This large-scale prospective, multicenter, cohort study aimed to identify genes, and biological pathways that determine susceptibility and severity in life-threatening bacterial infections of childhood. The EUCLIDS clinical network includes predominantly academic pediatric hospitals that host a total of 52 PICUs from 7 European countries; Austria (9), Germany (7), Lithuania (1), The Netherlands (5), Spain (9), Switzerland (8), and the UK (13).
Study patients
From July 2012 to January 2016, patients aged 29 days to 18 years admitted with community-acquired sepsis to PICUs in participating centers were prospectively enrolled in the study. The 2005 pediatric consensus criteria for sepsis were used, dividing patients into those with sepsis, severe sepsis, or septic shock [21]. Healthcare-associated infections [22], patients undergoing bone marrow transplant, and patients already recruited who were readmitted within the same illness episode were excluded. Children with a central venous catheter at admission were not excluded. Although the consortium was specifically interested in patients with invasive meningococcal, pneumococcal, staphylococcal, salmonella, and group A streptococcal infections, representing the most common causes of community-acquired sepsis in children, patients with illness due to other organisms were included as well. Patients were recruited as early as possible in the illness within a time window from presentation to the time when culture results became available.
Ethical aspects
This study was conducted in accordance with the Declaration of Helsinki and Good Clinical Practice guidelines. The study protocol was approved by at least one ethical review board in every country (Coordinating Center Research Ethics Committee reference: 11/LO/1982) [23]. Written informed consent was obtained from parents or legal guardians. In the Swiss study [24, 25], consent was obtained for collection of blood for research, but waiver of consent for collection of anonymized epidemiological data was approved.
Clinical data collection
Data on clinical presentation, underlying disease, illness severity, management, microbiological results, and outcome were collected prospectively. Children were split into four age categories; infants (29 days to < 1 year), toddlers (≥ 1 year to < 5 years), school-aged children (≥ 5 years to < 12 years), and adolescents (≥ 12 years to < 18 years). Underlying conditions at admission to the PICU were classified following the pediatric complex chronic conditions classification system [26]. Illness severity was measured by the Pediatric risk of mortality score (PRISM) [27] and Pediatric Index of Mortality (PIM2) [28]. We studied lactate values obtained on admission, concomitant with PIM2 data collection. Invasive bacterial infections were defined as isolation by culture or PCR of a bacterial organism from a normally sterile site. We considered blood, cerebrospinal fluid, urine, bronchoalveolar lavage, joint aspirate, abscess aspirate, intraoperative swabs, and pleural aspirate as sterile sites. Urine positive for pneumococcal antigen was also considered as an invasive bacterial infection if patients met sepsis criteria. Positive cultures from sites such as endotracheal tube aspirate, nasopharyngeal aspirate, throat/nasal swabs, and wounds were not considered as sterile sites. We defined potentially vaccine-preventable infections as infections caused by pathogens that are included in currently available national immunization programs, with a focus on Haemophilus influenzae type B (HiB), meningococcus serogroups ACWY (MenACWY), meningococcus serogroup B (MenB), meningococcus serogroup C (MenC), pneumococcal conjugate vaccine 7 (PCV7, Prevnar, serotypes 4, 6B, 9V, 14, 18C, 19F and 23F), pneumococcal conjugate vaccine 10 (PCV10, Synflorix, additional serotypes 1, 5, 7F), pneumococcal conjugate vaccine 13 (PCV13, Prevnar 13, additional serotypes 3, 6A, 19A) and pneumococcal polysaccharide vaccine 23 (PPSV23, additional serotypes 2, 8, 9N, 10A, 11A, 12F, 15B, 17F, 20, 22F, 33F). Data on routine immunization schedules and uptake in the countries involved are presented in Additional file 1: Table S1. We classified patients as primary bloodstream infection and sepsis without a known source (grouped as no focus) versus patients with a clinical focus of infection. Patients admitted with systemic inflammatory response syndrome (SIRS) in the presence of suspected infection (i.e. sepsis), in whom a bacterial, viral, or fungal infection eventually could not be confirmed, were categorized as clinical presentation other.
Outcomes
The primary outcome measure was death in hospital, recorded as alive or death status at the time of hospital discharge. Secondary outcomes were assessed at time of hospital discharge and included disability, PICU-free days censored at day 28 (days alive and free from the need for intensive care), and hospital length of stay. Disability was defined as a Pediatric Overall Performance Category (POPC) scale > 1 [29], need for skin graft, amputation, or hearing loss. The POPC scale was determined either by direct observation or by chart review and ranges from 1 to 6: (1) good overall performance, (2) mild overall disability, (3) moderate overall disability, (4) severe overall disability, (5) coma or vegetative state, and (6) brain death. A description of these categories is presented in Additional file 1: Table S2 [29]. PICU-free days in patients who died were considered zero. All data were collected in web-based case report forms. Monthly telephone conferences, biannual meetings, clinical protocols including case definitions, data audits, and monitoring, ensured uniform procedures among study sites.
Statistical analysis
Categorical variables are presented as counts (percentages). We used the chi-Square test or Fisher’s exact test to compare frequency distributions between two categorical variables. Post-hoc Bonferroni correction for multiple testing was applied when we compared age groups with features on clinical presentation or pathogens. Continuous variables are presented either as mean (± standard deviation (SD)) for data with a parametric distribution or as median (interquartile range (IQR)) for non-parametric data. We tested differences between groups with analysis of variance (ANOVA) or Kruskal-Wallis and Student’s t test, or Mann-Whitney U test, as appropriate. Logistic regression (of binary outcome measures) and linear regression (of continuous outcome measures) were used to identify independent predictors. Variables with a P value <0.20 in the univariable analysis were included in the multivariable analysis. In the multivariable analysis, we only included one parameter of illness severity (PIM2), because of multicollinearity of the illness severity parameters. Area under the receiver operating characteristic (AUROC) curve analysis was applied to determine the Youden index. Sensitivity, specificity, positive predictive value (PPV), negative predictive value (NPV), positive likelihood ratio (PLR), and negative likelihood ratio (NLR) were calculated for the optimal cutoff value of lactate. Statistical analyses were performed with SPSS version 21 (Armonk, USA). Graphs were created in GraphPad Prism 5.00. A P value <0.05 was considered statistically significant.
Results
From July 2012 to January 2016, 795 children (54% male, median age 2.2 years (IQR 8 months to 6 years)) admitted with community-acquired sepsis to 52 PICUs in 7 European countries were enrolled (Fig. 1). Baseline characteristics by age category are presented in Table 1. An underlying condition was present in 288 patients (36%), of which prematurity and neonatal conditions (n = 87, 11%) and neurologic and neuromuscular conditions were most common (n = 70, 9%). A total of 466 patients (59%) presented with septic shock.
Clinical presentations and pathogens
Primary bloodstream infection and sepsis without a known source among patients with community-acquired sepsis accounted for 278 (35%) admissions to the PICU. The other most common clinical illnesses were meningitis/encephalitis (n = 182, 23%) and pneumonia (n = 149, 19%) (Additional file 1: Figure S1). Clinical presentation were similar across age groups, apart from osteomyelitis/septic arthritis, which was diagnosed more frequently in school-aged children than in infants (7.3% versus 0.4%, P value = 0.002).
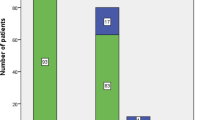
Bacterial etiology was confirmed in 428 patients (54%), including 334 patients (42%) with a positive blood culture, and pathogen distribution was associated with age (Fig. 2). Neisseria meningitidis was the most commonly identified pathogen (n = 131, 31%), of which serogroup B was most prevalent (n = 89, 68%), followed by Streptococcus pneumoniae (n = 78, 18%, of which serotypes 3 (n = 7, 14%) and 10A (n = 6, 12%) were most commonly identified in those with serotyping information available (n = 51)) (Additional file 1: Table S3).
Invasive pathogens in patients with community-acquired sepsis admitted to the pediatric intensive care unit. Invasive pathogens (n = 428) by age category. Numbers are higher as three patients had mixed bacterial infections (one patient with Staphylococcus aureus/gram-negative bacteria, two patients with gram-negative/gram-positive bacteria). The y-axis represents the percentage of respective pathogen within the age category, i.e. percentages of all pathogens within an age category add up to 100%. d, days; m, months
Of the 466 patients with septic shock, an invasive bacterial infection was confirmed in 255 patients (55%). N. meningitidis (n = 91, 36%) and group A streptococcus (n = 49, 19%) were the most commonly identified pathogens, followed by Streptococcus pneumoniae (n = 33, 13%).
Therapy
Invasive ventilation was used in 519 patients (69%) (median length of invasive respiratory support 5 days, IQR 3–8, n = 43 with missing data) and vasoactive agents for 418 patients (57%) (median 3 days, IQR 2–5 days, n = 56 with missing data). Infants needed invasive ventilation more frequently than adolescents (73% versus 58%, P value = 0.03).
Mortality and PICU-free survival
Of the 795 children admitted to PICU with community-acquired sepsis, 51 patients (6%) died. Mortality increased to 10% (n = 45) in patients with septic shock. Univariable analysis showed that the presence of bacteremia (odds ratio (OR) 4.4, 95% confidence interval (CI) 2.3–8.4, P < 0.001) and infections caused by S. pneumoniae (OR 2.5, 95% CI 1.2–5.1, P = 0.01) were associated with mortality in patients with sepsis (Table 2). In addition, illness severity, as measured by PRISM score, PIM2 score, invasive ventilation, the need for inotropes, and higher lactate at PICU admission, were also associated with sepsis mortality. The AUROC for lactate as a predictor of mortality was 0.723 (95% CI 0.624–0.822), with an optimal cutoff value of 2.2 mmol/L (sensitivity 0.78, specificity 0.60) (Additional file 1: Figure S2).
Infection caused by S. pneumoniae (OR 4.1, 95% CI 1.1–16.0, P = 0.04) and illness severity (PIM2 score OR 2.8, 95% CI 1.3–6.1, P < 0.01) remained independently significantly associated with mortality in multivariable analysis. A trend towards higher mortality was observed for bacteremia (OR 7.4, 95% CI 1.0–56.6, P = 0.06). PICU mortality did not differ significantly across age categories or countries. Also, the presence of an underlying condition at admission to the PICU was not associated with mortality.
The median PICU-free days to day 28 were 23 days (IQR 18–25) and the median hospital length of stay was 12 days (IQR 8–21). PIM2 score (B = − 0.202, P < 0.001), invasive S. pneumoniae infections (B = − 0.161, P = 0.02), and invasive Staphylococcus aureus infections (B = − 0.163, P = 0.01) were independent predictors of PICU-free days. PIM2 score (B = 0.270, P < 0.001), pneumonia (B = 0.145, P = 0.04), and invasive Staphylococcus aureus infections (B = 0.234, P = 0.001) were independent predictors of hospital length of stay (Additional file 1: Table S4).
Disability
Data on disability at discharge were available on 558/744 survivors (75%). Of these patients, 173/558 (31%) were discharged with disability including 71 patients (13%) with mild overall disability, 39 (7%) with moderate overall disability, 50 (9%) with severe overall disability, 4 (0.7%) who had undergone amputation, 2 (0.4%) with hearing loss, and 7 (1.3%) who had undergone skin graft. Toddlers (34%) and school-aged children (42%) were more often discharged with disability than infants (21%, P value <0.05).
Among survivors who did not have an underlying condition at admission to PICU, i.e. previously healthy children, 24% (83/349 patients whose data were available) were discharged with some disability. Disability data were available on 339/421 (81%) survivors of septic shock. In these, 120/339 (35%) patients had disability at PICU discharge, including 45 (13%) with mild overall disability, 29 (9%) with moderate overall disability, 35 (10%) with severe overall disability, 4 (1.2%) who had undergone amputation, and 7 (2.0%) who had undergone skin graft. Outcome as measured by mortality and POPC score was worst in patients admitted with pneumonia (Additional file 1: Figure S3) and in patients with invasive bacterial infections caused by Streptococcus pneumoniae (Additional file 1: Figure S4). When comparing patients discharged with and without disability, by univariable and multivariable analysis, the PIM2 score (OR 3.4, 95% CI 1.8–6.4, P < 0.001) and infections caused by Streptococcus pneumoniae (OR 5.4, 95% CI 1.8–15.8, P < 0.01) were independent predictors of disability (Table 3).
Economic impact of vaccine-preventable infections
Assuming an average cost per PICU day of 4000 € and 1000 € per day on a general ward, we estimate an average cost of 42,000 € per vaccine-preventable episode of severe community-acquired infection requiring admission to a PICU. This calculation was based on the mean hospital length of stay (18 days), including mean PICU length of stay (8 days), of the subgroup of patients with vaccine-preventable infections (n = 149). Within our consortium, a total of 149 vaccine-preventable cases reflect 43 cases per year. The impact on cost and resource utilization for the hospitals included was estimated at almost 2 million € for potentially vaccine-preventable infections annually.
Discussion
This prospective multicenter study of 795 children admitted with community-acquired sepsis to European PICUs demonstrates the substantial burden of severe invasive bacterial disease, despite widespread immunization programs, predominantly affecting previously healthy children [30]. Almost one third of survivors (31%) were discharged with disability ranging from mild to severe.
We observed a crude mortality rate of 6% in children admitted with sepsis. Other studies have reported higher mortality of up to 29% in high-income countries, which may relate to the large number of hospital-acquired infections with a disproportionate impact of high-risk patients such as those with oncologic conditions or those undergoing transplant in other cohorts [1, 2, 4, 5, 8, 31,32,33]. The enrolment criteria in our study were based on the 2005 consensus pediatric sepsis definition, and we included patients with sepsis in addition to patients with severe sepsis and septic shock, which may account for the lower mortality observed. However, most study patients were admitted to the PICU because of single or multiple organ dysfunction, and hence would be expected to meet the Sepsis-3-based sepsis definitions too [21, 34]. The limitations of current pediatric sepsis definitions including the low predictive accuracy of SIRS [35], and the need to adapt Sepsis-3 for pediatric age groups, have been highlighted recently [36].
We observed that 1 out of 3 sepsis survivors were discharged with a disability, including 1 in 10 with severe disability and/or amputation. Notably, 24% of previously healthy children left the hospital with some form of disability. While there is a lack of large studies on pediatric sepsis long-term outcomes, similar incidence of disability has been reported in two other studies, with a decline in functional status observed in 28 to 34% of pediatric sepsis survivors [4, 20]. Others have observed impaired neuropsychological performance and impaired educational functioning [19]. Our findings highlight the need to include disability as an outcome measure in pediatric sepsis trials in the future. More research is required to delineate the nature of the disabilities and to study the add-on effect of sepsis when underlying conditions are already present. Disability in children with underlying conditions could be evaluated more accurately in the future by reporting changes in performance scales between admission and discharge.
Independent risk factors for death, disability, and PICU-free days were illness severity - reflected by severity scores - and invasive pneumococcal infections. Our findings indicate that while current PICU severity scores were calibrated against mortality, PIM performs very well to predict disability as well, which indicates that some patients predicted to die survive, yet with a major impact on functional status. Larger studies are urgently needed to assess long-term impact, as this patient group is at high risk of prolonged dependency on health support, reduced school and work life performance, and reduced quality of life, resulting in an under recognized disproportionate impact of sepsis on our society [14]. Lactate was associated with mortality and the optimal cutoff value of 2.2 mmol/L in serum supports using lactate as a trigger threshold in National Institute for Health and Care Excellence (NICE) UK guidelines [37]. Previous studies have demonstrated the strong association between lactate and mortality, and indicated that both arterial and venous serum lactate level can be used for risk stratification [38,39,40,41]. Importantly, our study demonstrated that increased lactate levels at PICU admission were associated with disability too.
An invasive bacterial infection was confirmed in half of the children, which is comparable to pathogen detection rates from 30 to 65% in other studies [1, 4, 5, 33]. The most common community-acquired invasive pathogens in our study were meningococci - especially serogroup B (menB) - and pneumococci - especially serotypes 3 and 10A. Recently, a menB vaccine (Bexsero®) has been licensed for active immunization against menB and this vaccine has been implemented in the Czech Republic and UK routine immunization schedules [42, 43]. It had been anticipated that this vaccine would cover approximately 70 to 80% of MenB strains, depending on geographical region and age [30, 42]. Preliminary data report 93% vaccine uptake of two doses by 12 months of age [44]. Future studies should determine the impact on disease and herd protection. Meningococcal serogroup C immunization resulted in a significant drop in incidence of over 80% in the UK and over 90% in The Netherlands [45,46,47]. In contrast, invasive MenW disease is increasing and careful monitoring in the coming years is necessary [48]. Immunization against pneumococcal disease is recommended in almost all European countries, and has been proven effective in the decline of invasive pneumococcal infections [49]. In the post-immunization era, the incidence of non-vaccine serotypes has however increased, suggesting serotype replacement [49]. Additionally, vaccine failure does occur. Primary immunodeficiency is present in up to 26% of children > 2 years of age with invasive pneumococcal infections after introduction of vaccination, indicating that infected patients should undergo immunological investigations [50]. Pneumococcal serotype 3 is included in Prevnar 13 (PCV13), but not in Synflorix (PCV10). Limited vaccine effectiveness for serotype 3 has been reported previously [51, 52]. Pathogen detection is complicated by early administration of antibiotics and by low circulating microbial loads [53]. Therefore, new diagnostics to improve pathogen detection and optimal antimicrobial therapy are urgently needed [54].
Despite widespread vaccination campaigns in Europe effectively targeting invasive pneumococcal disease [43, 55,56,57], the burden due to these potentially vaccine-preventable infections has remained considerable: 17 % of patients with pneumococcal infections have died, while 35% of the patients have been discharged with some form of disability. The pneumococcal mortality rate in our study is slightly higher than mortality rates from other PICU studies [2, 8, 58]. However, those studies relied on ICD-9 codes for organisms and did not evaluate specific culture results. Therefore, a number of infections might have been classified based on non-sterile site cultures (e.g. nasopharyngeal aspirate), and thus could have identified a colonized location, whereas we confirmed each invasive pneumococcal infection by sterile site positive detection. On the other hand, our definition of invasive pneumococcal infection might have skewed the results away from respiratory infections, towards central nervous system infections, as we considered blood, pleural aspirate, and bronchoalveolar lavage as invasive detection sites for pneumonia. These sites are not routinely screened in patients with pneumonia. Nevertheless, our findings emphasize the importance and the need to continuously improve current immunization programs. Additionally, reducing potentially vaccine-preventable infections will have a beneficial effect on economic resources. We estimated an average cost of 42,000 € per vaccine preventable episode of severe community-acquired infection requiring PICU. In comparison, a recent Australian and New Zealand study [5] estimated the mean cost per ICU and ward admission for sepsis and septic shock of AUS$62062 (equal to 39,000 €). Beside direct costs in severe cases requiring PICU admission, the total economic impact of vaccine-preventable diseases encompasses direct costs in cases not requiring PICU admission, and indirect costs related to loss of revenue of caregivers and long-term costs related to permanent disability.
This study has several limitations, most of them relating to the design of this large, international consortium. First, the primary aim of our consortium was to identify genes associated with susceptibility and severity of invasive meningococcal, pneumococcal, staphylococcal, and group A streptococcal infections, which might have caused an enrollment bias in favor of infections caused by these organisms. Therefore, data on the prevalence and distribution of pathogens need to be interpreted with caution. Second, due to the genetic basis of this study, a bias towards enrollment of previously healthy children might have occurred. In our cohort, only 36% of patients had an underlying condition at admission, whereas in other studies percentages varying from 49 to 77% are reported, yet these studies included hospital-acquired infections too [1, 2, 4, 5, 7, 8]. Third, we assessed disability by the easily applicable and well-validated pediatric overall performance scale at discharge [29, 59]. However, there is a fair amount of disability data missing, possibly because of loss to follow up after patients had been transferred back to the local hospital prior to discharge. For patients with disability data available, we did not take further recovery after discharge into account. Fourth, the EUCLIDS consortium represents a network of institutions active in infectious diseases research and was not designed to provide population-based coverage. Hence we are unable to compare findings between countries and we are unable to estimate the impact of vaccine-preventable disease on mortality, morbidity, and costs at population level. Finally, our consortium includes multiple centers from multiple countries, representing a different epidemiological context, healthcare structures, and case-mix. Nevertheless, this study includes the largest prospectively enrolled contemporary cohort of children with community-acquired sepsis in high-income countries. Because previous pediatric sepsis studies included healthcare-associated infections, results from this study especially have implications for policy makers in public health, e.g. to develop immunization strategies. Last, this report differs from previous reports because we included disability as an outcome measure, thereby meeting the need to improve our understanding of the short-term physical effects of sepsis and understanding the implications for sepsis survivors [14].
Conclusions
This report from high-income countries describes a large cohort of children admitted with community-acquired sepsis to European PICUs, providing contemporary assessment of the epidemiology and characteristics of one of the most common reasons for PICU admission. Our study demonstrates the substantial burden caused by community-acquired sepsis, predominantly affecting previously healthy children. One out of three survivors was discharged with disability, indicating an urgent need for improved recognition, treatment, and follow up of children with sepsis.
Abbreviations
- AUROC:
-
Area under the receiver operating characteristic
- CI:
-
Confidence interval
- EUCLIDS:
-
European Childhood Life-threatening Infectious Disease Study
- FP7:
-
7th Framework Program
- HiB:
-
Haemophilus influenzae type B
- IQR:
-
Interquartile range
- MenACWY:
-
Meningococcus serogroups ACWY
- MenB:
-
Meningococcus serogroup B
- MenC:
-
Meningococcus serogroup C
- MenW:
-
Meningococcus serogroup W
- NLR:
-
Negative likelihood ratio
- NPV:
-
Negative predictive value
- OR:
-
Odds ratio
- PCV10:
-
Pneumococcal conjugate vaccine 10, Synflorix
- PCV13:
-
Pneumococcal conjugate vaccine 13, Prevnar 13
- PCV7:
-
Pneumococcal conjugate vaccine 7, Prevnar
- PICU:
-
Pediatric Intensive Care Unit
- PIM2:
-
Pediatric Index of Mortality
- PLR:
-
Positive likelihood ratio
- POPC:
-
Pediatric Overall Performance Category
- PPSV23:
-
Pneumococcal polysaccharide vaccine 23
- PPV:
-
Positive predictive value
- PRISM:
-
Pediatric risk of mortality score
- SD:
-
Standard deviation
- SE:
-
Standard error
- SIRS:
-
Systemic inflammatory response syndrome
References
Hartman ME, Linde-Zwirble WT, Angus DC, Watson RS. Trends in the epidemiology of pediatric severe sepsis. Pediatr Crit Care Med. 2013;14(7):686–93.
Ruth A, McCracken CE, Fortenberry JD, Hall M, Simon HK, Hebbar KB. Pediatric severe sepsis: current trends and outcomes from the Pediatric Health Information Systems database. Pediatr Crit Care Med. 2014;15(9):828–38.
Kaukonen KM, Bailey M, Suzuki S, Pilcher D, Bellomo R. Mortality related to severe sepsis and septic shock among critically ill patients in Australia and New Zealand, 2000-2012. JAMA. 2014;311(13):1308–16.
Weiss SL, Fitzgerald JC, Pappachan J, Wheeler D, Jaramillo-Bustamante JC, Salloo A, Singhi SC, Erickson S, Roy JA, Bush JL, et al. Global epidemiology of pediatric severe sepsis: the sepsis prevalence, outcomes, and therapies study. Am J Respir Crit Care Med. 2015;191(10):1147–57.
Schlapbach LJ, Straney L, Alexander J, MacLaren G, Festa M, Schibler A, Slater A, Group APS. Mortality related to invasive infections, sepsis, and septic shock in critically ill children in Australia and New Zealand, 2002-13: a multicentre retrospective cohort study. Lancet Infect Dis. 2015;15(1):46–54.
Liu L, Oza S, Hogan D, Perin J, Rudan I, Lawn JE, Cousens S, Mathers C, Black RE. Global, regional, and national causes of child mortality in 2000-13, with projections to inform post-2015 priorities: an updated systematic analysis. Lancet. 2015;385(9966):430–40.
Balamuth F, Weiss SL, Neuman MI, Scott H, Brady PW, Paul R, Farris RW, McClead R, Hayes K, Gaieski D, et al. Pediatric severe sepsis in U.S. children's hospitals. Pediatr Crit Care Med. 2014;15(9):798–805.
Watson RS, Carcillo JA, Linde-Zwirble WT, Clermont G, Lidicker J, Angus DC. The epidemiology of severe sepsis in children in the United States. Am J Respir Crit Care Med. 2003;167(5):695–701.
Zingg W, Hopkins S, Gayet-Ageron A, Holmes A, Sharland M, Suetens C, group EPs. Health-care-associated infections in neonates, children, and adolescents: an analysis of paediatric data from the European Centre for Disease Prevention and Control point-prevalence survey. Lancet Infect Dis. 2017;17(4):381–9.
Diekema DJ, Beekmann SE, Chapin KC, Morel KA, Munson E, Doern GV. Epidemiology and outcome of nosocomial and community-onset bloodstream infection. J Clin Microbiol. 2003;41(8):3655–60.
Groeneveld AB. Risk factors for increased mortality from hospital-acquired versus community-acquired infections in febrile medical patients. Am J Infect Control. 2009;37(1):35–42.
Reinhart K, Daniels R, Kissoon N, Machado FR, Schachter RD, Finfer S. Recognizing sepsis as a global health priority - a WHO resolution. N Engl J Med. 2017;377(5):414–7.
Schnitzler E, Iolster T. Burden of sepsis in children: perspectives from pediatric intensive care. Pediatr Crit Care Med. 2012;13(5):596–7.
Cohen J, Vincent JL, Adhikari NK, Machado FR, Angus DC, Calandra T, Jaton K, Giulieri S, Delaloye J, Opal S, et al. Sepsis: a roadmap for future research. Lancet Infect Dis. 2015;15(5):581–614.
Iwashyna TJ, Ely EW, Smith DM, Langa KM. Long-term cognitive impairment and functional disability among survivors of severe sepsis. JAMA. 2010;304(16):1787–94.
Winters BD, Eberlein M, Leung J, Needham DM, Pronovost PJ, Sevransky JE. Long-term mortality and quality of life in sepsis: a systematic review. Crit Care Med. 2010;38(5):1276–83.
Yende S, Austin S, Rhodes A, Finfer S, Opal S, Thompson T, Bozza FA, LaRosa SP, Ranieri VM, Angus DC. Long-term quality of life among survivors of severe sepsis: analyses of two international trials. Crit Care Med. 2016;44(8):1461–7.
Schlapbach LJ, Aebischer M, Adams M, Natalucci G, Bonhoeffer J, Latzin P, Nelle M, Bucher HU, Latal B, Swiss Neonatal N, et al. Impact of sepsis on neurodevelopmental outcome in a Swiss national cohort of extremely premature infants. Pediatrics. 2011;128(2):e348–57.
Als LC, Nadel S, Cooper M, Pierce CM, Sahakian BJ, Garralda ME. Neuropsychologic function three to six months following admission to the PICU with meningoencephalitis, sepsis, and other disorders: a prospective study of school-aged children. Crit Care Med. 2013;41(4):1094–103.
Farris RW, Weiss NS, Zimmerman JJ. Functional outcomes in pediatric severe sepsis: further analysis of the researching severe sepsis and organ dysfunction in children: a global perspective trial. Pediatr Crit Care Med. 2013;14(9):835–42.
Goldstein B, Giroir B, Randolph A, International Consensus Conference on Pediatric S. International pediatric sepsis consensus conference: definitions for sepsis and organ dysfunction in pediatrics. Pediatr Crit Care Med. 2005;6(1):2–8.
Horan TC, Andrus M, Dudeck MA. CDC/NHSN surveillance definition of health care-associated infection and criteria for specific types of infections in the acute care setting. Am J Infect Control. 2008;36(5):309–32.
Klobassa DS, Binder A, Glennie L, Van Leeuwen E, Martinon-Torres F, Villanueva-Gonzalez I, Cebey-Lopez M, Carrol E, Bojang K, Anderson S, et al. Federalism massively impairs paediatric research - lessons learned from a FP7 funded multicentre project. In: 34th Annual Meeting of the European Society for Paediatric Infectious Diseases (ESPID). Brighton; 2016.
Giannoni E, Berger C, Stocker M, Agyeman P, Posfay-Barbe KM, Heininger U, Konetzny G, Niederer-Loher A, Kahlert C, Donas A, et al. Incidence and outcome of group B streptococcal sepsis in infants in Switzerland. Pediatr Infect Dis J. 2016;35(2):222–4.
Agyeman PKA, Schlapbach LJ, Giannoni E, Stocker M, Posfay-Barbe KM, Heininger U, Schindler M, Korten I, Konetzny G, Niederer-Loher A, et al. Epidemiology of blood culture-proven bacterial sepsis in children in Switzerland: a population-based cohort study. Lancet Child Adolesc Health. 2017;1(2):124–33.
Feudtner C, Feinstein JA, Zhong W, Hall M, Dai D. Pediatric complex chronic conditions classification system version 2: updated for ICD-10 and complex medical technology dependence and transplantation. BMC Pediatr. 2014;14:199.
Pollack MM, Ruttimann UE, Getson PR. Pediatric risk of mortality (PRISM) score. Crit Care Med. 1988;16(11):1110–6.
Slater A, Shann F, Pearson G, Paediatric Index of Mortality study G. PIM2: a revised version of the Paediatric Index of Mortality. Intensive Care Med. 2003;29(2):278–85.
Fiser DH. Assessing the outcome of pediatric intensive care. J Pediatr. 1992;121(1):68–74.
Parikh SR, Newbold L, Slater S, Stella M, Moschioni M, Lucidarme J, De Paola R, Giuliani M, Serino L, Gray SJ, et al. Meningococcal serogroup B strain coverage of the multicomponent 4CMenB vaccine with corresponding regional distribution and clinical characteristics in England, Wales, and Northern Ireland, 2007-08 and 2014-15: a qualitative and quantitative assessment. Lancet Infect Dis. 2017;17(7):754–62.
Shime N, Kawasaki T, Saito O, Akamine Y, Toda Y, Takeuchi M, Sugimura H, Sakurai Y, Iijima M, Ueta I, et al. Incidence and risk factors for mortality in paediatric severe sepsis: results from the national paediatric intensive care registry in Japan. Intensive Care Med. 2012;38(7):1191–7.
van Paridon BM, Sheppard C, G GG, Joffe AR, Alberta Sepsis N. Timing of antibiotics, volume, and vasoactive infusions in children with sepsis admitted to intensive care. Crit Care. 2015;19:293.
Wolfler A, Silvani P, Musicco M, Antonelli M, Salvo I, Italian Pediatric Sepsis Study Group. Incidence of and mortality due to sepsis, severe sepsis and septic shock in Italian pediatric intensive care units: a prospective national survey. Intensive Care Med. 2008;34(9):1690–7.
Schlapbach LJ. Time for Sepsis-3 in children? Pediatr Crit Care Med. 2017;18(8):805–6.
Schlapbach LJ, Straney L, Bellomo R, MacLaren G, Pilcher D. Prognostic accuracy of age-adapted SOFA, SIRS, PELOD-2, and qSOFA for in-hospital mortality among children with suspected infection admitted to the intensive care unit. Intensive Care Med. 2018;44(2):179–88. https://doi.org/10.1007/s00134-017-5021-8. Epub 2017 Dec 19.
Schlapbach LJ, Kisson N: Pediatric sepsis definitions - an urgent need for change. JAMA Pediatr. 20181;172(4):312–14. https://doi.org/10.1001/jamapediatrics.2017.5208.
Tavare A, O'Flynn N. Recognition, diagnosis, and early management of sepsis: NICE guideline. Br J Gen Pract. 2017;67(657):185–6.
Schlapbach LJ, MacLaren G, Festa M, Alexander J, Erickson S, Beca J, Slater A, Schibler A, Pilcher D, Millar J, et al. Prediction of pediatric sepsis mortality within 1 h of intensive care admission. Intensive Care Med. 2017;43(8):1085–96. https://doi.org/10.1007/s00134-017-4701-8. Epub 2017 Feb 20.
Scott HF, Brou L, Deakyne SJ, Kempe A, Fairclough DL, Bajaj L. Association between early lactate levels and 30-day mortality in clinically suspected sepsis in children. JAMA Pediatr. 2017;171(3):249–55.
Schlapbach LJ, MacLaren G, Straney L. Venous vs arterial lactate and 30-day mortality in pediatric sepsis. JAMA Pediatr. 2017;171(8):813.
Morin L, Ray S, Wilson C, Remy S, Benissa MR, Jansen NJ, Javouhey E, Peters MJ, Kneyber M, De Luca D, et al. Refractory septic shock in children: a European Society of Paediatric and Neonatal Intensive Care definition. Intensive Care Med. 2016;42(12):1948–57.
Watson PS, Turner DP. Clinical experience with the meningococcal B vaccine, Bexsero((R)): prospects for reducing the burden of meningococcal serogroup B disease. Vaccine. 2016;34(7):875–80.
European Centre for Disease Prevention and Control (ECDC) - Vaccine Schedule [https://vaccine-schedule.ecdc.europa.eu/]. Accessed April 2018.
Public health England. Preliminary vaccine coverage estimates for the meningococcal B (MenB) immunisation programme for England, update from August to December 2017. Health Protection Report, Public Health England 2018, Volume 12(Number 3). https://assets.publishing.service.gov.uk/government/uploads/system/uploads/attachment_data/file/677275/hpr0318_menb.pdf.
Bijlsma MW, Brouwer MC, Spanjaard L, van de Beek D, van der Ende A. A decade of herd protection after introduction of meningococcal serogroup C conjugate vaccination. Clin Infect Dis. 2014;59(9):1216–21.
Trotter CL, Ramsay ME. Vaccination against meningococcal disease in Europe: review and recommendations for the use of conjugate vaccines. FEMS Microbiol Rev. 2007;31(1):101–7.
Trotter CL, Andrews NJ, Kaczmarski EB, Miller E, Ramsay ME. Effectiveness of meningococcal serogroup C conjugate vaccine 4 years after introduction. Lancet. 2004;364(9431):365–7.
Ladhani SN, Beebeejaun K, Lucidarme J, Campbell H, Gray S, Kaczmarski E, Ramsay ME, Borrow R. Increase in endemic Neisseria meningitidis capsular group W sequence type 11 complex associated with severe invasive disease in England and Wales. Clin Infect Dis. 2015;60(4):578–85.
Savulescu C, Krizova P, Lepoutre A, Mereckiene J, Vestrheim DF, Ciruela P, Ordobas M, Guevara M, McDonald E, Morfeldt E, et al. Effect of high-valency pneumococcal conjugate vaccines on invasive pneumococcal disease in children in SpIDnet countries: an observational multicentre study. Lancet Respir Med. 2017;5(8):648–56.
Gaschignard J, Levy C, Chrabieh M, Boisson B, Bost-Bru C, Dauger S, Dubos F, Durand P, Gaudelus J, Gendrel D, et al. Invasive pneumococcal disease in children can reveal a primary immunodeficiency. Clin Infect Dis. 2014;59(2):244–51.
Slotved HC, Dalby T, Harboe ZB, Valentiner-Branth P, Casadevante VF, Espenhain L, Fuursted K, Konradsen HB. The incidence of invasive pneumococcal serotype 3 disease in the Danish population is not reduced by PCV-13 vaccination. Heliyon. 2016;2(11):e00198.
Andrews NJ, Waight PA, Burbidge P, Pearce E, Roalfe L, Zancolli M, Slack M, Ladhani SN, Miller E, Goldblatt D. Serotype-specific effectiveness and correlates of protection for the 13-valent pneumococcal conjugate vaccine: a postlicensure indirect cohort study. Lancet Infect Dis. 2014;14(9):839–46.
Kellogg JA, Manzella JP, Bankert DA. Frequency of low-level bacteremia in children from birth to fifteen years of age. J Clin Microbiol. 2000;38(6):2181–5.
Herberg JA, Kaforou M, Wright VJ, Shailes H, Eleftherohorinou H, Hoggart CJ, Cebey-Lopez M, Carter MJ, Janes VA, Gormley S, et al. Diagnostic test accuracy of a 2-transcript host RNA signature for discriminating bacterial vs viral infection in febrile children. JAMA. 2016;316(8):835–45.
Whitney CG, Farley MM, Hadler J, Harrison LH, Bennett NM, Lynfield R, Reingold A, Cieslak PR, Pilishvili T, Jackson D, et al. Decline in invasive pneumococcal disease after the introduction of protein-polysaccharide conjugate vaccine. N Engl J Med. 2003;348(18):1737–46.
Weil-Olivier C, van der Linden M, de Schutter I, Dagan R, Mantovani L. Prevention of pneumococcal diseases in the post-seven valent vaccine era: a European perspective. BMC Infect Dis. 2012;12:207.
Esposito S, Principi N. Impacts of the 13-valent pneumococcal conjugate vaccine in children. J Immunol Res. 2015;2015:591580.
Klobassa DS, Zoehrer B, Paulke-Korinek M, Gruber-Sedlmayr U, Pfurtscheller K, Strenger V, Sonnleitner A, Kerbl R, Ausserer B, Arocker W, et al. The burden of pneumococcal meningitis in Austrian children between 2001 and 2008. Eur J Pediatr. 2014;173(7):871–8.
Pollack MM, Holubkov R, Funai T, Clark A, Moler F, Shanley T, Meert K, Newth CJ, Carcillo J, Berger JT, et al. Relationship between the functional status scale and the pediatric overall performance category and pediatric cerebral performance category scales. JAMA Pediatr. 2014;168(7):671–6.
Acknowledgements
We would like to acknowledge the EUCLIDS consortium (http://www.euclids-project.eu). See the additional file for a full list of consortium members.
Funding
This work was supported by the European Seventh Framework Programme for Research and Technological Development (FP7) under EUCLIDS Grant Agreement number 279185. The Swiss Pediatric Sepsis Study was funded by grants from the Swiss National Science Foundation (342730_153158/1), the Swiss Society of Intensive Care, the Bangerter Foundation, the Vinetum and Borer Foundation, and the Foundation for the Health of Children and Adolescents. This research was also supported by the National Institute for Health Research Newcastle Biomedical Research Centre based at Newcastle Hospitals NHS Foundation Trust and Newcastle University. These funders were not involved in the design of the study, collection, analysis, interpretation of data, or in writing the manuscript.
Availability of data and materials
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Author information
Authors and Affiliations
Consortia
Contributions
Study conception or design: NPB, LJS, GJD, JAHe, RG, STA, CGF, EDC, WZ, ML, MF, FMT, JAHa, and ME. Acquisition, analysis, or interpretation of data: NPB, LJS, GJD, JAHa, and ME. Drafting or revising the manuscript: all authors. Final approval of the submitted manuscript and agreed to be accountable for all aspects of the work: all authors.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
This study was conducted in accordance with the Declaration of Helsinki and Good Clinical Practice guidelines. The study protocol was approved by at least one ethical review board in every country (Coordinating center Research Ethics Committee reference: 11/LO/1982).
Consent for publication
Written informed consent was obtained from parents or legal guardians. In the Swiss study, consent was obtained if feasible, but collection of anonymized epidemiological data was approved for all patients. All authors have provided consent for publication of the manuscript.
Competing interests
The authors declare that they have no competing interests
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Additional file
Additional file 1:
Figures S1-S4, Tables S1-S4, and the EUCLIDS consortium author list. (DOCX 145 kb)
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated.
About this article
Cite this article
Boeddha, N.P., Schlapbach, L.J., Driessen, G.J. et al. Mortality and morbidity in community-acquired sepsis in European pediatric intensive care units: a prospective cohort study from the European Childhood Life-threatening Infectious Disease Study (EUCLIDS). Crit Care 22, 143 (2018). https://doi.org/10.1186/s13054-018-2052-7
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s13054-018-2052-7