Abstract
Introduction
The understanding of coagulopathies in trauma has increased interest in thromboelastography (TEG®) and thromboelastometry (ROTEM®), which promptly evaluate the entire clotting process and may guide blood product therapy. Our objective was to review the evidence for their role in diagnosing early coagulopathies, guiding blood transfusion, and reducing mortality in injured patients.
Methods
We considered observational studies and randomized controlled trials (MEDLINE, EMBASE, and Cochrane databases) to February 2014 that examined TEG®/ROTEM® in adult trauma patients. We extracted data on demographics, diagnosis of early coagulopathies, blood transfusion, and mortality. We assessed methodologic quality by using the Newcastle-Ottawa scale (NOS) for observational studies and QUADAS-2 tool for diagnostic accuracy studies.
Results
Fifty-five studies (12,489 patients) met inclusion criteria, including 38 prospective cohort studies, 15 retrospective cohort studies, two before-after studies, and no randomized trials. Methodologic quality was moderate (mean NOS score, 6.07; standard deviation, 0.49). With QUADAS-2, only three of 47 studies (6.4%) had a low risk of bias in all domains (patient selection, index test, reference standard and flow and timing); 37 of 47 studies (78.8%) had low concerns regarding applicability. Studies investigated TEG®/ROTEM® for diagnosis of early coagulopathies (n = 40) or for associations with blood-product transfusion (n = 25) or mortality (n = 24). Most (n = 52) were single-center studies. Techniques examined included rapid TEG® (n =12), ROTEM® (n = 18), TEG® (n = 23), or both TEG® and rapid TEG® (n = 2). Many TEG®/ROTEM® measurements were associated with early coagulopathies, including some (hypercoagulability, hyperfibrinolysis, platelet dysfunction) not assessed by routine screening coagulation tests. Standard measures of diagnostic accuracy were inconsistently reported. Many abnormalities predicted the need for massive transfusion and death, but predictive performance was not consistently superior to routine tests. One observational study suggested that a ROTEM® -based transfusion algorithm reduced blood-product transfusion, but TEG®/ROTEM®-based resuscitation was not associated with lower mortality in most studies.
Conclusions
Limited evidence from observational data suggest that TEG®/ROTEM® tests diagnose early trauma coagulopathy and may predict blood-product transfusion and mortality in trauma. Effects on blood-product transfusion, mortality, and other patient-important outcomes remain unproven in randomized trials.
Similar content being viewed by others
Introduction
The emerging understanding of early coagulopathies and their clinical consequences after severe trauma have created a search for better coagulation assays. Current routine screening coagulation tests (RSCTs), such as activated partial thromboplastin time (aPTT) and prothrombin time (PT), have limited utility to diagnose early trauma coagulopathies and direct their treatment. Neither test predicts the extent of bleeding in critically ill or trauma patients [1], and a recent systematic review concluded that they are inappropriate for trauma [2]. The cell-based understanding of hemostasis [3], emphasizing tissue factor (TF) as the initiator of coagulation and the role of platelets, has challenged the clotting cascade concept that underlies RSCTs. The cell-based model and the need for shorter turnaround time (TAT) for tests to guide transfusion in bleeding trauma patients have propelled interest in thromboelastography (TEG® ; Hemoscope Corporation, Niles, IL, USA) and thromboelastometry (ROTEM® ; Tem International GmbH).
TEG® and ROTEM® are based on the principle that the result of the hemostatic process is a clot whose physical properties determine patients’ hemostatic status. These tests provide global information on the dynamics of clot development, stabilization, and dissolution, reflecting in vivo hemostasis, and assess both thrombosis and fibrinolysis [4]. The additional information from TEG®/ROTEM® is based on their performance in whole blood [4], whereas RSCTs are performed in plasma, without the cellular components of platelets and tissue-bearing cells.
By systematically searching for relevant studies, we sought to evaluate the evidence that the use of TEG® and ROTEM® in adult traumatically injured patients (a) diagnoses trauma coagulopathies on admission to hospital, (b) guides transfusion, and (c) reduces mortality.
Materials and methods
This descriptive systematic review was reported in accordance with Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines [5].
Information sources and search technique
With the assistance of an experienced librarian, we searched MEDLINE (1946 to February 2014), EMBASE (1947 to February 2014), and Cochrane Controlled Trials Register (from inception to February 2014) to identify studies of thromboelastography and thromboelastometry in trauma. We used a sensitive search strategy combining MeSH headings and the key words “thromboelastography” AND “trauma,” “thromboelastometry” AND “trauma,” “thromboelastography” AND “injury,” “thromboelastometry” AND “injury,” TEG® AND “trauma,” TEG® AND “injury,” ROTEM® AND “trauma,” and ROTEM® AND “injury.” Search terms were defined a priori and by reviewing the MeSH terms of articles identified in preliminary literature searches. Two authors (LTL, AKS) independently reviewed the abstracts of all articles identified by the literature search and selected articles for detailed review if either reviewer considered them potentially relevant. We also searched the bibliographies of all articles selected for detailed review and all relevant published reviews to find any other studies potentially eligible for inclusion. We did not search conference proceedings. No language restrictions were imposed; we translated two studies in Spanish and Italian and engaged a medical student to translate one Chinese study that was ultimately excluded. Details of the search strategies are in Additional file 1.
Eligibility criteria and study selection
Studies were eligible for inclusion if they were observational studies or randomized controlled trials (RCTs) that evaluated TEG®/ROTEM® in adult trauma patients and reported outcomes related to diagnosis of coagulopathies (hypocoagulation, hypercoagulation, platelet dysfunction, hyperfibrinolysis (HF), TAT), transfusion management (prediction of massive transfusion (MT), and transfusion guidance), or mortality (prediction and reduction). Studies were excluded if they enrolled only burn patients or enrolled patients in other surgical specialties, or were case reports or case series. Two independent reviewers (LTL, AKS) reviewed all full-text versions of all potentially eligible studies. Agreement between reviewers was assessed by using the Cohen κ [6]. In case of disagreement, consensus was reached by discussion with a third author (BN, NKJA).
Data abstraction and analysis
We abstracted data from included studies on study objective, setting and study design, patient selection, clinical and demographic characteristics, TEG®/ROTEM® technique, RSCT technique, presence of comparison group, blood-product transfusion, and mortality. Two authors (LTL, AKS) independently assessed study methodology based on the Newcastle-Ottawa Scale for cohort studies [7] and QUADAS-2 [8] for quality assessment of diagnostic accuracy studies. For studies that did not report diagnostic accuracy, we supplemented the Newcastle-Ottawa scale by assessing the description of TEG®/ROTEM® performance. In applying the Newcastle-Ottawa scale, we considered management by TEG®/ROTEM® to be the relevant exposure and a nonexposed cohort to be one that was managed without TEG®/ROTEM®. We considered the following outcomes: (a) diagnostic performance of TEG®/ROTEM® parameters compared with RSCT (PT, aPTT, INR, platelet count, fibrinogen) for early coagulopathies, (b) utilization of blood products red blood cells (RBCs), fresh frozen plasma (FFP), platelets concentrate (PLT), fibrinogen concentrate (FC), cryoprecipitate, prothrombin complex concentrate (PCC), and (c) mortality. Because of clinical and methodologic heterogeneity among studies, we anticipated reporting results qualitatively instead of conducting meta-analyses.
Results
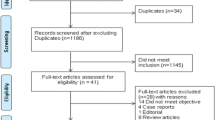
The electronic search identified 1,352 potentially relevant studies. After evaluating 82 full-text manuscripts, 55 met inclusion criteria (Figure 1). An excellent agreement was reached between the reviewers for study inclusion (κ = 0.82). References for the excluded studies are in Additional file 1.
Study characteristics
All 55 studies were observational (Table 1). Forty studies [9]-[48] addressed the use of TEG®/ROTEM® in diagnosing early coagulopathies; 25 studies [9],[15],[16],[22],[23],[25],[26],[29],[31],[32],[35]-[37],[41],[45],[47]-[56] examined associations with transfusion; and 24 studies [14],[16],[21],[23],[29],[32],[33],[36]-[38],[40],[45],[47],[48],[51],[54],[56]-[63] examined associations with mortality. Only three [40],[41],[61] were conducted in multiple centers. Thirty-eight studies were prospective cohorts [9]-[14],[16],[17],[19],[20],[24]-[28],[30],[32],[33],[36],[38],[40]-[48],[50],[52],[53],[55]-[58],[61],[62], two were before-after [37],[63], and 15 were retrospective cohorts [15],[18],[21]-[23],[29],[31],[34],[35],[39],[49],[51],[54],[59],[60]. The techniques used for TEG® and ROTEM® varied: in 28 studies [9],[11]-[14],[17],[20],[22],[26],[28],[30],[33],[38]-[41],[44],[45],[47],[49],[50],[52]-[55],[58],[59],[61],[63], the tests were done at 37°C; in nine [10],[15],[16],[18],[19],[21],[37],[43],[57], at the patient’s temperature; in 16, there was no description of the temperature [23]-[25],[29],[31],[32],[34]-[36],[42],[46],[48],[51],[56],[60],[62]; and one study performed the test at different temperatures [27]. Rapid TEG® (r-TEG® ), a technique with fresh whole blood using a solution containing TF as the coagulation trigger, was used in 12 studies [17],[18],[21],[25],[33]-[35],[37],[43],[49],[52],[54]; ROTEM® , in 18 studies [12],[14],[20],[22],[23],[26],[28],[31],[32],[38],[40],[42],[50],[51],[55],[58]-[60]; TEG® , in 23 studies [9]-[11],[13],[15],[16],[19],[24],[27],[29],[30],[36],[39],[41],[44],[45],[53],[56]-[58],[61]-[63]; and two studies performed TEG® and r-TEG® [17],[46] in the same cohort of patients.
Demographic data
The 55 studies included 12,489 patients. The mean or median age of the patients ranged from 24 to 74 years (mean of mean or median age across all studies, 40 years). The majority were male (9,858 patients, 78.9%), and the mean injury severity score (ISS) [64] ranged from 9 to 55 (mean of means in all studies, 25.3). Studies included trauma with or without traumatic brain injury (TBI) (n = 49), isolated TBI (n = 4), and burns with all types of trauma (n = 2). The median (interquartile range (IQR)) sample size of the included studies was 87 (52 to 235).
Methodologic quality
The overall methodologic quality of the studies was moderate (Table 2). Most studies (n = 36) included consecutive patients [10],[12]-[14],[16],[19],[21]-[27],[30]-[35],[37]-[41],[45],[46],[50]-[52],[54]-[56],[58]-[60],[63]. Fifty-two (94.5%) studies were not controlled [9]-[11],[13]-[36],[38]-[50],[52]-[62]. The three (5.5%) controlled studies [37],[51],[63] had comparable control groups managed without TEG®/ROTEM®. Eleven (20%) studies used healthy [12],[13],[19],[27],[41],[42],[44],[61] or other hospitalized trauma [24],[32],[60] controls to examine associations between TEG®/ROTEM® abnormalities and outcomes. Nearly all (n = 53, 96.4%) studies had adequate follow-up. The mean Newcastle-Ottawa score (n = 55 studies) was 6.07 (SD, 0.49; possible range, 1 to 9).
We assessed 47 studies of diagnostic accuracy by using QUADAS-2 (Table 3). Considering the domains of patient selection, index test, reference standard, and flow and timing, only three studies (6.4%) [32],[37],[39] had low risk of bias in all; 24 (51.1%) [10],[16],[21]-[26],[30],[31],[33]-[35],[38],[40],[44],[45],[47],[50],[52],[54],[55],[58],[62] had low and unclear risks; and 20 (42.5%) [9],[11]-[15],[17]-[20],[29],[36],[42],[48],[49],[53],[57],[59]-[61] had high risk of bias in at least one domain. In the applicability section, 37 studies (78.8%) [9]-[11],[16]-[18],[21]-[26],[29]-[40],[44],[45],[47],[48],[50],[52]-[55],[57]-[59],[62] had low concerns, and 10 studies (21.2%) [12]-[15],[19],[20],[42],[49],[60],[61] had at least one domain with high concern. The remaining eight studies that that did not evaluate diagnostic performance against a reference standard are represented in Table 4.
Outcomes
The included studies collectively reported on hypocoagulability, hypercoagulability, platelet dysfunction, and hyperfibrinolysis. We summarize the parameters of TEG®/ROTEM® used for diagnosis, turnaround times (TATs), and results concerning prediction, reduction, or guidance of transfusion and prediction or reduction of mortality. Details of all studies without a control group (for clinical outcomes) or a reference standard (for diagnostic performance) are given in Table 5.
Diagnosis of early trauma coagulopathies
Hypercoagulability
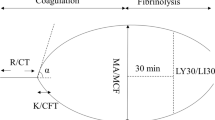
Six studies demonstrated hypercoagulability in trauma patients not detected by RSCT. Hypercoagulability was defined mostly by the manufacturer of both TEG® and ROTEM® devices, and the reference standards (where reported) were Doppler ultrasound, CT angiography, or a surgical procedure demonstrating thrombus. One study [11] demonstrated that 62% of trauma patients were hypercoagulable according to TEG® (R <3.7) on the first day after injury with normal RSCT values. Another study [19] showed higher TEG® α-angle (which reflects the degree of fibrin cross-linking) and MA (maximal amplitude) in trauma compared with RSCT (PT and aPTT) (P <0.05), indicating a hypercoagulable state. A study [24] detected increased G (shear elastic modulus strength (5,000 – MA)/(100 – MA), which reflects clot strength) in a cohort of trauma patients after splenectomy, who had more thromboembolic events, compared with patients treated nonoperatively. Another study of rapid TEG® (r-TEG® ) [18] showed that increased G was associated with thromboembolic complications (OR, 1.25; 95% CI, 1.12 to 1.39), after controlling for thromboprophylaxis and using the reference standards discussed earlier. When TEG® [27] was performed 24 hours after injury, trauma patients were more hypercoagulable compared with healthy volunteers across a broad range of temperatures (32°C to 38°C).
Finally, a cohort study [34] suggested an association between admission MA and pulmonary embolism (OR, 3.5 for MA >65; 95% CI, 1.69 to 7.23; and OR, 5.8 for MA >72; 95% CI, 2.85 to 11.77), after controlling for gender, race, age, and ISS.
Hyperfibrinolysis (HF)
Only one study [14] compared HF detected with ROTEM® with a laboratory gold standard and showed that ROTEM® had satisfactory diagnostic properties for HF, defined by laboratory measurement of euglobin lysis time (ELT). However, the sample size was very small (n = 23, of which five had HF), limiting the strength of inferences.
Platelet dysfunction
With TEG® platelet-mapping (PM) test, a study [13] showed that patients with TBI had more platelet dysfunction on admission, measured by lower platelet response to arachidonic acid (AA) but not to adenosine diphosphate (ADP), compared with non-TBI trauma patients, alcohol abusers, and healthy volunteers (P <0.001). Another study [41], using the same technique, found lower platelet response to both AA and ADP in trauma patients versus healthy volunteers (P <0.0001). With ROTEM® , one study [42], comparing healthy volunteers with trauma patients, speculated that an observed difference in clot strength arose from platelet dysfunction. A related study [65] of ROTEM® at emergency department (ED) admission demonstrated significantly lower values of platelet component of clot elasticity (MCE EXTEM, ROTEM® extrinsically activated test with TF and MCE FIBTEM, ROTEM® fibrin-based extrinsically activated test with TF and the platelet inhibitor cytochalasin D) in trauma nonsurvivors vs. survivors (P = 0.0012).
Hypocoagulability
Six studies directly compared TEG® or ROTEM® with RSCT, with variable results for diagnostic performance. One study [9] demonstrated that TEG® detected hypocoagulability in 45 (85.5%) of 52 patients, whereas only one (1.9%) of 52 was hypocoagulable by elevated PT/aPTT and two (3.8%) of 52 were hypercoagulable by elevated platelet count. In a cohort [12] of trauma patients and healthy volunteers, EXTEM A15 ≥ 32 mm (amplitude at 15 minutes) and FIBTEM A10 ≥ 5 mm were sensitive (87% and 91%) and specific (100% and 85%) for detection of PT >1.5 of control value and fibrinogen <1 g/L, respectively. Doran [20] found that 16 (64%) of 25 patients were hypocoagulable by ROTEM® trace, and only 10% had abnormal RSCT (P = 0.0005). In contrast, another study [39] (n = 219) found that TEG® -R (R, reaction time, defined as the time until a clot firmness of 2 mm is achieved, corresponding to CT, clotting time, in ROTEM® ) performed worse than INR for the diagnosis of vitamin K deficiency in trauma patients (clotting factor activity used as gold standard). TEG® -R (compared with INR >1.5) had a sensitivity of 33% (67% for INR), specificity of 95% (98% for INR), positive predictive value (PPV) of 47% (84% for INR), and negative predictive value (NPV) of 92% (96% for INR). In another cohort [26] (n = 300), ROTEM® parameters of CFT (clot-formation time), α-angle, A5 (clot amplitude 5 minutes after CT) and MCF (maximum clot firmness) were significantly different in the group with coagulopathy, defined by INR >1.2. A5 ≥ 35 mm had a sensitivity of 77% and specificity of 87% for the detection of coagulopathy. Finally, a recent large study [40] (n = 517) found that ROTEM® EXTEM, and FIBTEM measures of A5 and MCF were significantly correlated with fibrinogen levels (EXTEM A5, r2 = 0.35, and MCF, r2 = 0.26; FIBTEM A5, r2 = 0.44, and MCF, r2 = 0.27; all P <0.001). The sensitivity/specificity of EXTEM A5 < 36 mm (FIBTEM A5 < 9.5 mm) for discriminating patients with admission fibrinogen <1.5 g/L were 53%/87% (78%/70%; ROC AUC 0.8, 95% CI 0.7 to 0.9 for both).
One study [46] comparing the same parameters measured by r-TEG® and conventional kaolin-activated TEG® found strong correlation for MA (marker of platelet function; r = 0.80); moderate correlation for G (overall clot strength; r = 0.70), k (speed of clot formation; r = 0.66), and α-angle (degree of fibrin cross-linking; r = 0.38); and poor correlation for LY30 (degree of fibrinolysis; r = 0.19).
Although TEG® and r-TEG® may be moderately sensitive in detecting abnormal clot strength, they have not differentiated between fibrinogen and platelet contributions to clot integrity. Recent studies of the TEG® -based functional fibrinogen assay (FF) have examined the relative contribution each. By using Kaolin TEG® MA to define coagulopathy, Johansson [45] showed that TEG® -FF MA and G were lower in hypocoagulable patients and significantly higher in hypercoagulable patients compared with patients with normal Kaolin TEG® MA (P <0.001). In another study [47], coagulopathic patients (INR ≥1.3) had lower admission fibrinogen contribution to MA than did noncoagulopathic patients (24.7% versus 31.2%; P <0.05). Platelet contribution to MA was higher than fibrinogen at all time points, decreased over time, and stabilized at 72 hours (69.4% at 0 hours, 56.2% at 72 hours). In contrast, fibrinogen contribution to MA increased over time and stabilized at 72 hours (30.6% at 0 hours, 43.8% at 72 hours).
Turnaround times
Four studies reported on the use of TEG® and ROTEM® as POC devices. Carroll [16] demonstrated no statistical difference in the diagnosis of acute trauma coagulopathies when collecting blood on site or 1 hour after admission in the emergency department (ED), except for a small but statistically significant change in MA (60.6 (SD 11.1) mm on site and 63.4 (SD 12.1) mm in ED; P = 0.014). A cohort study [17] demonstrated that r-TEG® had a shorter TAT (time to MA) by a median (IQR) of 10.8 (1.1 to 18.5) minutes compared with TEG® . In another cohort [26], laboratory PT had a median TAT of 78 minutes (IQR, 62 to 103 minutes), whereas ROTEM® A5 was available by 5 minutes.
Finally, in another study [25], early r-TEG® values (ACT [activated clotting time], k-time, and r-value) were available within 5 minutes, late r-TEG® values (MA and α-angle) within 15 minutes, and RSCTs within 48 minutes (P <0.001 for all comparisons with r-TEG® ).
Blood transfusion
Prediction of massive transfusion (MT) and any transfusion
Several studies compared ROTEM® and TEG® parameters with RSCTs for prediction of MT (defined by most studies as transfusion of ≥10 RBC units within 24 hours of trauma). Davenport [26] found better sensitivity (71% versus 42%) for ROTEM® A5 ≥ 35 mm versus INR >1.2, but worse specificity (85% versus 94%). Another study [31] found that FIBTEM (0 to 3 mm) had the highest AUC (0.84; 95% CI, 0.79 to 0.88) among ROTEM® tests for prediction of MT, but hemoglobin (AUC 0.87; 95% CI, 0.83 to 0.91) and PT (AUC, 0.87; 95% CI, 0.83 to 0.90) were better predictors.
A third study [22] showed multiple ROTEM® tests to be associated with MT; in a multivariable analysis limited by few events, hemoglobin ≤100 g/L (OR, 18.18; 95% CI, 2.73 to 125.00) was a stronger predictor of MT than abnormal MCF (OR, 8.47; 95% CI, 1.19 to 62.50). Similarly, RCSTs and TEG® had similar abilities to predict MT (G AUC 0.89; 95% CI, 0.89 to 0.96; INR AUC, 0.92; 95% CI, 0.86 to 0.98; PTT AUC 0.90; 95% CI, 0.83 to 0.97) [54].
ROTEM® parameters significantly associated with MT include increased CFT [22],[31], decreased MCF [22],[31], prolonged EXTEM and INTEM CT (intrinsically activated test using ellagic acid, clotting time) [31] and FIBTEM A10 (ROC AUC, 0.83; 95% CI, 0.78 to 0.87) [31]. For TEG® , statistically significant differences in α angle, MA, K, G (at 1 hour), and estimated lysis according to transfusion need (minimal, moderate, or massive) were reported [21]. Another study [36] found that patients with HF, defined by estimated lysis >15%, had a greater need for MT (76.9% versus 8.7%; adjusted OR, 19.1; 95% CI, 3.6 to 101.3).
Finally, a recent study demonstrated that a TEG® LY30 (percentage decrease in clot amplitude at 30 minutes after MA) of 3% or greater had poor sensitivity (31%) but high specificity (91%) for predicting MT [56].
Investigators have also compared TEG®/ROTEM® with RCST for prediction of any blood-product transfusion, generally guided by RSCT or a massive transfusion protocol (MTP). One study [52] found that r-TEG® α-angle <74.7 degrees had higher sensitivity (84%) but lower specificity (57%) to predict transfusion of any blood product versus INR >1.2 (38%, 88%), INR >1.5 (19%, 96%), or aPTT >60 (5%, 98%); fibrinogen <3 g/L had the highest sensitivity (90%) and similar specificity (48%). Another study [26] showed a similar pattern for ROTEM® A5 ≥ 35 mm in prediction of any RBC (sensitivity 33%, specificity 88%) or FFP (36%, 87%) compared with INR >1.2 (17%, 96% for RBC; 21%, 96% for FFP).
Considered in isolation, many TEG®/ROTEM® abnormalities have been associated with transfusion of specific blood products. Reduced TEG® MA was associated with transfusion of RBC [15],[29], FFP [29], and PLT [29]; TEG® ACT predicted RBC, FFP, and PLT transfusions within the first 2 hours [25]; α-angle <56 predicted MT of RBC, FFP, PLT, and cryoprecipitate [35]; and patients with r-TEG® -defined maximum rate of thrombin generation (MRTG) ≥9.2 mm/min at 3 hours received significantly less RBC, FFP, and cryoprecipitate in the first 6 hours [37]. A study combining several markers [53] showed that patients considered coagulopathic based on TEG® (R, K, α-angle, and MA) received more RBC (10 versus 0), FFP (7 versus 0), and PLT (3 versus 0) in the first 24 hours (P <0.05). More recently, the inhibition of ADP function was correlated with transfusion in the first 6 hours (59.6% inhibition (0 RBC) versus 96.1% inhibition (>1 RBC), P = .025) [41], and TEG® -defined hypocoagulable patients required more uncross-matched blood (adjusted P = 0.004) and FFP (adjusted P <0.001) at 24 hours compared with normocoagulable and hypercoagulable patients [48].
Finally, patients requiring FFP had a significantly lower admission fibrinogen contribution to MA by the FF assay (26.6% versus 30.6%; P <0.05). In contrast, one study [16] showed no significant differences in TEG® parameters (R, K, α-angle, MA, LY60 (percentage decrease in clot amplitude at 60 minutes after MA) in patients transfused with RBC, FFP, or PLT versus not; only MA-ADP, a measure of ADP-platelet activation, correlated with transfusion of any blood product (P = 0.004).
Similarly, nonsurviving patients with isolated TBI had more ROTEM® -defined coagulation abnormalities and received more RBCs (P = 0.016), FC (P = 0.01) and PCC (P <0.001) than survivors [23], and higher FIBTEM MCF was associated with decreased RBC transfusion (adjusted OR, 0.92 per 1-unit increase, 95% CI, 0.87 to 0.98) [32]. Patients with HF [55], based on plasmin-antiplasmin complex levels and ROTEM® maximum lysis (ML) >15%, required more transfusions of RBC, FFP, PLT, and cryoprecipitate (P <0.05 for all comparisons).
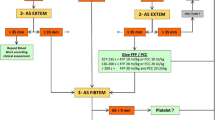
Few data exist on TEG®/ROTEM® to monitor coagulopathy in response to transfusion. Rourke et al.[40] showed that administration of high doses of FC (6 to 12 g) or cryoprecipitate (30 U) restored EXTEM and FIBTEM A5 and MCF to the level of patients with minor injuries; fibrinogen levels were maintained but not augmented after FC administration. Changes in ROTEM® -determined CT, A5, and MCF after the transfusion of 4 units of RBC, dependent on the RBC/FFP ratio (≥1:1, 1:2 to 3:4, <1:2) [50].
TEG®/ROTEM®-guided transfusions versus conventional guidance
A modeling study [49] (n = 44) suggested that transfusion guided by r-TEG® versus RSCT would reduce the proportion of patients needing blood-product transfusion from 73.1% to 53.9% (P = 0.03), driven by reductions in FFP administration (61.5% with INR trigger versus 26.9% with r-TEG® -ACT trigger, P = 0.003). No difference was predicted for transfusion of PLT (P = 0.32) or cryoprecipitate (P = 0.18). A cohort study reported lower exposure to blood products in 80 patients with ROTEM® -guided FC and PCC compared with a historical group (n = 601) for whom FFP and PLT transfusions were guided by clinical decision (generally RSCT) [51]. Transfusion of RBC (PLT) was avoided in 29% (91%) of patients in the ROTEM® -guided FC and PCC group compared with 3% (56%) in the RSCT-guided FFP and PLT groups (P <0.001).
Mortality
Prediction
TEG® and ROTEM® parameters have been compared with RCSTs for prediction of mortality. For prediction of coagulopathy-related death, G had a similar adjusted AUC (0.93, 95% CI, 0.87 to 0.98) compared with INR (AUC, 0.88; 95% CI, 0.80 to 0.97; P = 0.11) and aPTT (AUC, 0.89; 95% CI, 0.81 to 0.97; P = 0.19) [54]. In another analysis, r-TEG® G, MRTG, and total thrombin generation (TG) (P = 0.03 for each) discriminated between survivors and nonsurvivors, in contrast to INR at 6 hours (P = 0.10) [37]. Another study found that TEG® -defined hypercoagulable patients had lower 24-hour mortality (0 versus 5.5% (normocoagulable) versus 27.8% (hypocoagulable), 10 deaths total, adjusted P <0.001) and hospital mortality (11.1% versus 5.5% versus 38.9%, 20 deaths total, adjusted P <0.001) [48]. With ROTEM® , a study [23] showed similar discrimination for mortality in TBI by using EXTEM with cytochalasin D (FIBTEM) MCF (AUC, 0.77; 95% CI, 0.66 to 0.85) and aPTT (AUC, 0.79; 95% CI, 0.69 to 0.87). A second study (n = 334; 26 early deaths) [32] using RSCTs, ROTEM® , and clinical judgment to guide transfusion, demonstrated significant correlations among PT, aPTT, fibrinogen, platelet count, and ROTEM® measurements (all |Spearman r| >0.5). In separate logistic regression analyses, each adjusted for hemoglobin and base excess, PT, aPTT, CT, CFT, MCF, LI (all EXTEM) and FIBTEM MCF were associated with 24-hour mortality. However, the predictive abilities of ROTEM® measurements and RSCTs were not directly compared.
Considering TEG®/ROTEM® measurements alone, weak clot strength [16],[21],[29],[32],[37],[45],[47],[54],[57],[58],[61],[62] and HF [14],[16],[21],[23],[32],[33],[35],[36],[38],[40],[45],[55],[56],[60] have been associated with morbidity and mortality. For example, ROTEM® -defined HF was associated with multiorgan dysfunction syndrome (MODS) (n = 115; 63.2% in patients with HF versus 24.6% in patients without HF, P = 0.004) [38] and hospital mortality (n = 115; 52.2% versus 12.9%, P <0.001) [38]; five of five versus nine of 82, P <0.05 [14]). Similarly in another study [36], TEG® -defined HF (estimated percentage lysis >15%) was associated with increased 24-hour mortality (69.2% versus 1.9% without HF; adjusted OR, 55.8; 95% CI, 7.2 to 432.3), but the number of deaths (n = 12) was extremely small. Holcomb [35] showed that r-TEG® LY-30, along with most other r-TEG® parameters, was associated with mortality; the logistic regression models were not described. More recently, a study [47] examined associations of the FF assay with mortality and demonstrated that a higher admission contribution of FF to MA predicted lower mortality (unadjusted hazard ratio, 0.815; P <0.001; 95% CI and adjusted model not reported).
Effect of TEG®/ROTEM®-guided transfusion
Several observational studies have examined whether TEG®/ROTEM®-guided transfusion reduced mortality after trauma and found no consistent effect. A small before/after study (n = 68) found lower crude mortality in patients in whom resuscitation was guided by r-TEG® (29% versus 65%) but did not report statistical testing or an adjusted analysis [37]. In a retrospective cohort study of massively bleeding patients (n = 131) transfused FC, PLT, and PCC using ROTEM® guidance, the observed mortality was 24.4%, which was lower than the expected mortality by TRISS (33.7%; P = 0.032) but similar to expected mortality by RISC (28.7%, P >0.05) [59]. Similarly, another cohort study of trauma patients (n = 681) found no difference in mortality between those treated in one center with ROTEM® -guided administration of FC and PCC (7.5%) and a multicenter control group treated with plasma transfusion (10%, P = 0.69) guided by the usual RSCT-guided clinical practice [51]. A before/after study [63] (n = 289) compared outcomes in trauma patients transfused with at least 6 U RBC in the first 24 hours according to TEG® -driven practice to a later period when a MTP not guided by TEG® was used. Overall, unadjusted mortality was unchanged, and MTP versus TEG® -directed care was not associated with mortality in multivariable analysis.
Discussion
Main findings
Our systematic review found 55 studies of TEG®/ROTEM® examining the diagnosis of trauma coagulopathies, including hypocoagulation, hypercoagulation, platelet dysfunction and fibrinolysis; guidance of blood-product administration; and associations with mortality. To our knowledge, this review is the first to summarize the literature on the use of TEG® and ROTEM® in trauma. The overall methodologic quality of included studies was moderate. No RCTs were reported; most cohort studies lacked clinically similar control groups managed without TEG®/ROTEM®, and standard measures of diagnostic accuracy were inconsistently reported. Observational data suggest that TEG® and ROTEM® may have adequate diagnostic properties for abnormalities identified by RSCTs and may identify additional coagulation disorders. However, the effect of these tests on the need for blood-product transfusion and mortality is unclear.
Studies in different but related clinical settings, not included in this systematic review, have also investigated TEG® and ROTEM® . Two studies of ROTEM® in a mixed population with shock [66] and noncardiac surgery [67] demonstrated rapid and useful results to guide decisions in hemostatic resuscitation. Other studies with mixed trauma and nontrauma populations [68]-[71] demonstrated good ability of ROTEM® to predict the need for MT, a clinically useful outcome regardless of management approach (laboratory abnormality-directed versus blood-product ratio based). Another possible advantage of these tests is that timely results may avoid FFP transfusion and subsequent FFP-related adverse events. However, relevant RCTs to test these hypotheses are lacking.
Other randomized [72]-[74] and observational [75]-[77] studies in cardiac surgery, burns, and mixed perioperative settings found reduced blood product transfusion and improved clinical outcomes after implementation of POC coagulation-management algorithms guided by ROTEM® . In contrast, existing observational studies in trauma patients do not suggest an effect of TEG®/ROTEM®-based transfusion protocols on clinically important outcomes, including mortality.
Strengths and weaknesses of this study
Major limitations of this review are related to the quality of the included studies. Reproducible technical standards for the performance of TEG®/ROTEM® were lacking, and inconsistent reporting of 2 × 2 tables precluded calculation of summary diagnostic test-performance measures and exploration of threshold effects. A major problem faced by diagnostic studies of trauma coagulopathy is the ambiguous nature of the gold standard, given that RSCTs may not provide an adequate description of all associated abnormalities. No RCTs exist in trauma patients, aside from one that enrolled burns patients exclusively [73], and the quality of the observational studies is modest. Studies differed in the use of TEG® or ROTEM® , and the few studies [78],[79] that compared TEG® and ROTEM® concluded that these methods are not interchangeable.
Studies also examined different patient populations, transfusion triggers, and transfusion protocols, limiting direct comparisons and generalizability. Clinical differences between many included studies and contemporary practice include substitution of FFP for clotting factors concentrate such as PCC, FC, and cryoprecipitate. Other methods for analysis of platelet dysfunction have been developed, such as platelet function analyzer (PFA-100) and multiple platelet function analyzer (Multiplate). These analyzers monitor different aspects of platelet function and appear to be technically reliable and practical POC devices, despite limitations [65],[80]-[82].
Two systematic reviews of TEG®/ROTEM® exist for nontrauma populations. A Cochrane review [83] included nine RCTs, mostly in cardiac surgery, that compared transfusion guided by TEG®/ROTEM® with transfusion guided by clinical judgment, RSCTs, or both in severely bleeding patients. The review found that TEG®/ROTEM® reduced blood loss by a mean of 85 ml (95% CI, 29 to 141 ml) but had no effect on mortality.
Another systematic review [84] included 16 observational studies and two RCTs in patients with sepsis and concluded that TEG®/ROTEM® (compared with RSCTs) detect impaired fibrinolysis, which may help to discriminate between sepsis and systemic inflammatory response syndrome (SIRS). However, limitations of data prevented conclusions regarding the value of TEG®/ROTEM® to identify patients with sepsis who could benefit from anticoagulants.
Conclusions
In summary, our systematic review demonstrated limited but rapidly growing observational evidence on the use of TEG® and ROTEM® in trauma. Both methods may be useful for diagnosis of early trauma coagulopathies, specifically hypocoagulability, hypercoagulability, hyperfibrinolysis, and platelet dysfunction. They may also be used to direct blood and blood-product transfusion; effects on patient-important outcomes are uncertain. The existing literature helps clinicians to appreciate the potential impact of these novel methods on transfusion guidance and outcomes in trauma. However, adequately powered and methodologically sound RCTs will be required to prove positive effects on blood-product transfusion and patient-important outcomes.
Key messages
The literature on TEG® and ROTEM® in trauma is limited by the lack of randomized controlled trials and the moderate quality of observational studies.
TEG® and ROTEM® may be superior to routine screening coagulation tests to promptly diagnose early trauma coagulopathy, including hypocoagulability, hyperfibrinolysis, hypercoagulability, and platelet dysfunction.
Many TEG® and ROTEM® abnormalities predict the need for massive transfusion and predict death, but predictive performance is not consistently superior to routine screening coagulation tests.
Limited evidence from one observational study suggests that a ROTEM® -based transfusion algorithm reduces the amount of blood and blood products transfused.
TEG® and ROTEM® -based resuscitation for bleeding trauma patients is not associated with lower mortality in most observational studies, but the question requires evaluation in randomized trials.
Authors’ contributions
LTL participated in the study design, data collection, data analysis, drafting of the manuscript, and revision of the manuscript. BN participated in the study design and manuscript revision. AKS participated in data collection and data analysis. SR participated in study design and manuscript revision. NKJA participated in the study design, data collection, data analysis, drafting of the manuscript, and revision of the manuscript. All authors approved the final manuscript.
Additional file
Abbreviations
- A10:
-
Clot amplitude at 10 minutes
- A15:
-
clot amplitude at 15 minutes
- A5:
-
clot amplitude at 5 minutes after CT (in ROTEM® )
- AA:
-
arachidonic acid
- ACT:
-
activated clotting time
- ADP:
-
adenosine diphosphate
- aPTT:
-
activated partial thromboplastin time
- AT:
-
antithrombin
- ATC:
-
acute trauma coagulopathy
- AUC:
-
area under the curve
- BD:
-
base deficit
- BE:
-
base excess
- BP:
-
blood pressure
- CFT:
-
clot-formation time
- CI:
-
confidence interval
- CLI:
-
clot lysis index (residual clot firmness in percentage of MCF at a certain time after CT)
- CT:
-
clotting time
- ED:
-
emergency department
- ELT:
-
euglobulin lysis time
- EXTEM:
-
extrinsically activated test with tissue factor
- F 1 + 2:
-
prothrombin fragment 1 + 2
- FF:
-
functional fibrinogen test
- FFP:
-
fresh frozen plasma
- FIBTEM:
-
fibrin-based extrinsically activated test with tissue factor and the platelet inhibitor cytochalasin D
- G:
-
shear elastic modulus strength ([5,000 – MA]/[100 – MA])
- HCR:
-
hemostatic control resuscitation
- HF:
-
hyperfibrinolysis
- INR:
-
international normalized ratio
- INTEM:
-
intrinsically activated test using ellagic acid
- IQR:
-
interquartile range
- ISS:
-
injury severity score
- K:
-
kinetic time (time between 2 and 20 mm amplitude achieved in TEG® )
- LI30:
-
lysis index (residual clot firmness in percentage of MCF) 30 minutes after CT in ROTEM® )
- LI60:
-
lysis index (residual clot firmness in percentage of MCF) 60 minutes after CT in ROTEM® )
- LR:
-
logistic regression
- LY30:
-
percentage decrease in clot amplitude at 30 minutes after MA in TEG®
- LY60:
-
percentage decrease in clot amplitude at 60 minutes after MA in TEG®
- MA:
-
maximal amplitude
- MCF:
-
maximal clot firmness
- ML:
-
maximal lysis
- MODS:
-
multiple organ-dysfunction syndrome
- MRTG:
-
maximal rate of thrombin formation
- MT:
-
massive transfusion
- NOS:
-
Newcastle-Ottawa scale
- NPV:
-
negative predictive value
- OR:
-
odds ratio
- PCC:
-
prothrombin complex concentrate
- PE:
-
pulmonary embolism
- PF:
-
primary fibrinolysis
- PFA-100:
-
platelet-function analyzer
- PLT:
-
platelet concentrate
- PM:
-
platelet mapping
- POC:
-
point-of-care
- PPV:
-
positive predictive value
- PRISMA:
-
preferred reporting items for systematic reviews and meta-analyses
- PT:
-
prothrombin time
- QUADAS:
-
quality assessment of diagnostic accuracy studies
- R:
-
reaction time (time from starting the test until 2-mm amplitude can be detected in TEG® )
- RBC:
-
red blood cell
- RCT:
-
randomized controlled trial
- RISC:
-
revised injury severity classification
- ROC:
-
receiver operating curve
- ROTEM®:
-
rotational thromboelastometry
- RSCT:
-
routine screening coagulation test
- r-TEG®:
-
rapid thromboelastography
- RTS:
-
revised trauma score
- SBP:
-
systolic blood pressure
- SD:
-
standard deviation
- SIRS:
-
systemic inflammatory response syndrome
- TAT:
-
thrombin-antithrombin complex
- TBI:
-
traumatic brain injury
- TE:
-
thromboembolic event
- TEG®:
-
thromboelastography
- TEG® -PM:
-
TEG® platelet mapping
- TF:
-
tissue factor
- TG:
-
thrombin generation
- TNF-α:
-
tumor necrosis factor alpha
- TRISS:
-
trauma injury severity score
- α angle:
-
rate of clot formation
References
Chowdhury P, Saayman AG, Paulus U, Findlay GP, Collins PW: Efficacy of standard dose and 30 ml/kg fresh frozen plasma in correcting laboratory parameters of haemostasis in critically ill patients. Br J Haematol. 2004, 125: 69-73. 10.1111/j.1365-2141.2004.04868.x.
Lier H, Bottiger BW, Hinkelbein J, Krep H, Bernhard M: Coagulation management in multiple trauma: a systematic review. Intensive Care Med. 2011, 37: 572-582. 10.1007/s00134-011-2139-y.
Hoffman M, Monroe DM: A cell-based model of hemostasis. Thromb Haemost. 2001, 85: 958-965.
Da Luz LT, Nascimento B, Rizoli S: Thrombelastography (TEG® ): practical considerations on its clinical use in trauma resuscitation.Scand J Trauma Resusc Emerg Med 2013, 21:29.,
Moher D, Liberati A, Tetzlaff J, Altman DG: Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. Int J Surg. 2010, 8: 336-341. 10.1016/j.ijsu.2010.02.007.
Cohen J: Weighted kappa: nominal scale agreement with provision for scaled disagreement or partial credit. Psychol Bull. 1968, 70: 213-220. 10.1037/h0026256.
The Newcastle-Ottawa Scale (NOS) for assessing the quality of nonrandomised studies in meta-analyses. []. Accessed March 2013., [http://www.ohri.ca/programs/clinical_epidemiology/oxford.htm]
Whiting PF, Rutjes AW, Westwood ME, Mallett S, Deeks JJ, Reitsma JB, Leeflang MM, Sterne JA, Bossuyt PM: QUADAS-2: a revised tool for the quality assessment of diagnostic accuracy studies. Ann Intern Med. 2011, 155: 529-536. 10.7326/0003-4819-155-8-201110180-00009.
Kaufmann CR, Dwyer KM, Crews JD, Dols SJ, Trask AL: Usefulness of thrombelastography in assessment of trauma patient coagulation. J Trauma. 1997, 42: 716-722. 10.1097/00005373-199704000-00023.
Watts DD, Trask A, Soeken K, Perdue P, Dols SJ, Kaufmann C: Hypothermic coagulopathy in trauma: effect of varying levels of hypothermia on enzyme speed, platelet function, and fibrinolytic activity. J Trauma. 1998, 44: 846-854. 10.1097/00005373-199805000-00017.
Schreiber MA, Differding J, Thorborg P, Mayberry JC, Mullins RJ: Hypercoagulability is most prevalent early after injury and in female patients. J Trauma. 2005, 58: 475-481. 10.1097/01.TA.0000153938.77777.26.
Rugeri L, Levrat A, David JS, Delecroix E, Floccard B, Gros A, Allaouchiche A, Negrier C: Diagnosis of early coagulation abnormalities in trauma patients by rotation thrombelastography. J Thromb Haemost. 2007, 5: 289-295. 10.1111/j.1538-7836.2007.02319.x.
Nekdulov M, Bellander B, Blomback M, Wallen HN: Platelet dysfunction in patients with severe traumatic brain injury. J Neurotrauma. 2007, 24: 1699-1706. 10.1089/neu.2007.0322.
Levrat A, Gros A, Rugeri L, Inaba K, Floccard B, Negrier C, David JS: Evaluation of rotation thrombelastography for the diagnosis of hyperfibrinolysis in trauma patients. Br J Anaesth. 2008, 100: 792-797. 10.1093/bja/aen083.
Plotkin AJ, Wade CE, Jenkins DH, Smith KA, Noe JC, Park MS, Perkins JG, Holcomb JB: A reduction in clot formation rate and strength assessed by thrombelastography is indicative of transfusion requirements in patients with penetrating injuries. J Trauma. 2008, 64: S64-S68. 10.1097/TA.0b013e318160772d.
Carroll RC, Craft RM, Langdon RJ, Clanton CR, Snider CC, Wellons DD, Dakin PA, Lawson CM, Enderson BL, Kurek SJ: Early evaluation of acute traumatic coagulopathy by thrombelastography. Transl Res. 2009, 154: 34-39. 10.1016/j.trsl.2009.04.001.
Jeger V, Zimmermann H, Exadaktylos AK: Can rapidTEG® accelerate the search for coagulopathies in the patient with multiple injuries?. J Trauma. 2009, 66: 1253-1257. 10.1097/TA.0b013e31819d3caf.
Kashuk JL, Moore EE, Sabel A, Barnett C, Haenel J, Le T, Pezold M, Lawrence J, Biffl WL, Cothren CC, Johnson JL: Rapid thrombelastography (r-TEG® ) identifies hypercoagulability and predicts thromboembolic events in surgical patients. Surgery. 2009, 146: 764-774. 10.1016/j.surg.2009.06.054.
Park MS, Martini WZ, Dubick MA, Salinas J, Butenas S, Kheirabadi BS, Pusateri AE, Vos JA, Guymon CH, Wolf SE, Mann KG, Holcomb JB: Thromboelastography as a better indicator of hypercoagulable state after injury than prothrombin time or activated partial thromboplastin time. J Trauma. 2009, 67: 266-276. 10.1097/TA.0b013e3181ae6f1c.
Doran CM, Woolley T, Midwinter MJ: Feasibility of using rotational thromboelastometry to assess coagulation status of combat casualties in a deployed setting. J Trauma. 2010, 69: S40-S48. 10.1097/TA.0b013e3181e4257b.
Kashuk JL, Moore EE, Sawyer M, Wohlauer M, Pezold M, Barnett C, Biffl WL, Burlew CC, Johnson JL, Sauaia A: Primary fibrinolysis is integral in the pathogenesis of the acute coagulopathy of trauma. Ann Surg. 2010, 252: 434-444.
Leemann H, Lustenberger T, Talving P, Kobayashi L, Bukur M, Brenni M, Brűesch M, Spahn DR, Keel MJB: The role of rotation thromboelastometry in early prediction of massive transfusion. J Trauma. 2010, 69: 1403-1409. 10.1097/TA.0b013e3181faaa25.
Schochl H, Solomon C, Traintinger S, Nienaber U, Tacacs-Tolnai A, Windhofer C, Bahrami S, Voelckel W: Thromboelastometric (ROTEM® ) findings in patients suffering from isolated severe traumatic brain injury. J Neurotrauma. 2011, 28: 2033-2041. 10.1089/neu.2010.1744.
Watters JM, Sambasivan CN, Zink K, Kremenevskiy I, Englehart MS, Underwood SJ, Schreiber MA: Splenectomy leads to a persistent hypercoagulable state after trauma. Am J Surg. 2010, 199: 646-651. 10.1016/j.amjsurg.2010.01.015.
Cotton BA, Faz G, Hatch QM, Radwan ZA, Podbielski J, Wade C, Kozar RA, Holcomb JB: Rapid thrombelastography delivers real-time results that predict transfusion within 1 hour of admission. J Trauma. 2011, 71: 407-417. 10.1097/TA.0b013e31821e1bf0.
Davenport R, Manson J, De’Ath H, Platton S, Coates A, Allard S, Hart D, Pearse R, Pasi J, MacCallum P, Stanworth S, Brohi K: Functional definition and characterization of acute traumatic coagulopathy. Crit Care Med. 2011, 39: 1-7. 10.1097/CCM.0b013e3182281af5.
Differding JA, Underwood SJ, Van PY, Khaki RA, Spoerke NJ, Schreiber MA: Trauma induces a hypercoagulable state that is resistant to hypothermia as measured by thrombelastogram. Am J Surg. 2011, 201: 587-591. 10.1016/j.amjsurg.2011.01.012.
Jansen JO, Luke D, Davies E, Spencer P, Kirkman E, Midwinter MJ: Temporal changes in ROTEM® -measured coagulability of citrated blood samples from coagulopathic trauma patients. Injury. 2013, 44: 36-39. 10.1016/j.injury.2011.12.003.
Nystrup KB, Windeløv NA, Thomsen AB, Johansson PI: Reduced clot strength upon admission, evaluated by thrombelastography (TEG® ), in trauma patients is independently associated with increased 30-day mortality.Scand J Trauma Resusc Emerg Med 2011, 19:52.,
Ostrowski SR, Sørensen AM, Larsen CF, Johansson PI: Thrombelastography and biomarker profiles in acute coagulopathy of trauma: a prospective study.Scand J Trauma Resusc Emerg Med 2011, 19:64.,
Schöchl H, Cotton B, Inaba K, Nienaber U, Fischer H, Voelckel W, Solomon C: FIBTEM provides early prediction of massive transfusion in trauma.Crit Care 2011, 15:R265.,
Tauber H, Innerhofer P, Breitkopf R, Westermann I, Beer R, El Attal R, Strasak A, Mittermayr M: Prevalence and impact of abnormal ROTEM® assays in severe blunt trauma: results of the `Diagnosis and Treatment of Trauma-Induced Coagulopathy (DIA-TRE-TIC) study’. Br J Anaesth. 2011, 107: 378-387. 10.1093/bja/aer158.
Cotton BA, Harvin JA, Kostousouv V, Minei KM, Radwan ZA, Schöchl H, Wade CE, Holcomb JB, Matijevic N: Hyperfibrinolysis at admission is an uncommon but highly lethal event associated with shock and prehospital fluid administration. J Trauma Acute Care Surg. 2012, 73: 365-370. 10.1097/TA.0b013e31825c1234.
Cotton BA, Minei KM, Radwan ZA, Matijevic N, Pivalizza E, Podbielski J, Wade CE, Kozar RA, Holcomb JB: Admission rapid thrombelastography predicts development of pulmonary embolism in trauma patients. J Trauma Acute Care Surg. 2012, 72: 1470-1477. 10.1097/TA.0b013e31824d56ad.
Holcomb JB, Minei KM, Scerbo ML, Radwan ZA, Wade CE, Kozar RA, Gill BS, Albarado R, McNutt MK, Khan S, Adams PR, McCarthy JJ, Cotton BA: Admission rapid thrombelastography can replace conventional coagulation tests in the emergency department experience with 1974 consecutive trauma patients. Ann Surg. 2012, 256: 476-486. 10.1097/SLA.0b013e3182658180.
Ives C, Inaba K, Branco BC, Okoye O, Schöchl H, Talving P, Lam L, Shulman I, Nelson J, Demetriades D: Hyperfibrinolysis elicited via thromboelastography predicts mortality in trauma. J Am Coll Surg. 2012, 215: 496-502. 10.1016/j.jamcollsurg.2012.06.005.
Kashuk JL, Moore EE, Wohlauer M, Johnson JL, Pezold M, Lawrence T, Biffl WL, Burlew CCC, Barnett C, Sawyer M, Sauaia A: Initial experiences with point-of-care rapid thrombelastography for management of life-threatening postinjury coagulopathy. Transfusion. 2012, 52: 23-33. 10.1111/j.1537-2995.2011.03264.x.
Kutcher ME, Cripps MW, McCreery RC, Crane IM, Greenberg MD, Cachola LM, Redick BJ, Nelson MF, Cohen MJ: Criteria for empiric treatment of hyperfibrinolysis after trauma. J Trauma Acute Care Surg. 2012, 73: 87-93. 10.1097/TA.0b013e3182598c70.
Nascimento B, Mahoos MA, Callum J, Capone A, Pacher J, Tien H, Rizoli S: Vitamin K-dependent coagulation factor deficiency in trauma: a comparative analysis between international normalized ratio and thromboelastography. Transfusion. 2012, 2: 7-13. 10.1111/j.1537-2995.2011.03237.x.
Rourke C, Curry N, Khan S, Taylor R, Raza I, Davenport R, Stanworth S, Brohi K: Fibrinogen levels during trauma hemorrhage, response to replacement therapy, and association with patient outcomes. J Thromb Haemost. 2012, 10: 1342-1351. 10.1111/j.1538-7836.2012.04752.x.
Wohlauer MV, Moore EE, Thomas S, Sauaia A, Evans E, Harr J, Silliman CC, Ploplis V, Castellino FJ, Walsh M: Early platelet dysfunction: an unrecognized role in the acute coagulopathy of trauma. J Am Coll Surg. 2012, 214: 739-746. 10.1016/j.jamcollsurg.2012.01.050.
Woolley T, Midwinter M, Spencer P, Watts S, Doran C, Kirkman E: Utility of interim ROTEM® values of clot strength, A5 and A10, in predicting final assessment of coagulation status in severely injured battle patients. Injury. 2013, 44: 593-599. 10.1016/j.injury.2012.03.018.
Chapman BC, Moore EE, Barnett C, Stovall RT, Biffl WL, Burlew CC, Bensard DD, Jurkovich GJ, Pieracci FM: Hypercoagulability following blunt solid abdominal organ injury: when to initiate anticoagulation. Am J Surg. 2013, 206: 917-923. 10.1016/j.amjsurg.2013.07.024.
Harr JN, Moore EE, Ghasabyan A, Chin TL, Sauaia A, Banerjee A, Silliman CC: Functional fibrinogen assay indicates that fibrinogen is critical in correcting abnormal clot strength following trauma. Shock. 2013, 39: 45-49.
Johansson PI, Sørensen AM, Larsen CF, Windeløv NA, Stensballe J, Perner A, Rasmussen LS, Ostrowski SR: Low hemorrhage-related mortality in trauma patients in a Level I trauma center employing transfusion packages and early thromboelastography-directed hemostatic resuscitation with plasma and platelets. Transfusion. 2013, 53: 3088-3099. 10.1111/trf.12214.
Lee TH, McCully BH, Underwood SJ, Cotton BA, Cohen MJ, Schreiber MA: Correlation of conventional thrombelastography and rapid thrombelastography in trauma. Am J Surg. 2013, 205: 521-527. 10.1016/j.amjsurg.2013.01.016.
Kornblith LZ, Kutcher ME, Redick BJ, Calfee CS, Vilardi RF, Cohen MJ: Fibrinogen and platelet contributions to clot formation: implications for trauma resuscitation and thromboprophylaxis. J Trauma Acute Care Surg. 2014, 76: 255-263. 10.1097/TA.0000000000000108.
Branco BC, Inaba K, Ives C, Okoye O, Shulman I, David JS, Schöchl H, Rhee P, Demetriades D: Thromboelastogram evaluation of the impact of hypercoagulability in trauma patients. Shock. 2014, 41: 200-207. 10.1097/SHK.0000000000000109.
Kashuk JL, Moore EE, Le T, Lawrence J, Pezold M, Johnson JL, Cothren CC, Biffl WL, Barnett C, Sabel A: Noncitrated whole blood is optimal for evaluation of postinjury coagulopathy with point-of-care rapid thromboelastography. J Surg Res. 2009, 156: 133-138. 10.1016/j.jss.2009.03.046.
Davenport R, Curry N, Manson J, De’Ath H, Coates A, Rourke C, Pearse R, Stanworth S, Brohi K: Hemostatic effects of fresh frozen plasma may be maximal at red cell ratios of 1:2. J Trauma. 2011, 70: 90-96. 10.1097/TA.0b013e318202e486.
Schöchl H, Nienaber U, Maegele M, Hochleitner G, Primavesi F, Steitz B, Arndt C, Hanke A, Voelckel W, Solomon C: Transfusion in trauma: thromboelastometry-guided coagulation factor concentrate-based therapy versus standard fresh frozen plasma-based therapy.Crit Care 2011, 15:R83.,
Jeger V, Willi S, Liu T, Yeh DD, DeMoya M, Zimmermann H, Exadaktylos AK: The rapid TEG® α-angle may be a sensitive predictor of transfusion in moderately injured blunt trauma patients.Sci World J 2012, [], [http://www.hindawi.com/journals/tswj/2012/821794/]
Ostrowski SR, Johansson PI: Endothelial, glycocalyx degradation induces endogenous heparinization in patients with severe injury and early traumatic coagulopathy. J Trauma Acute Care Surg. 2012, 73: 60-66. 10.1097/TA.0b013e31825b5c10.
Pezold M, Moore EE, Wohlauer M, Sauaia A, Gonzalez E, Banerjee A, Silliman CC: Viscoelastic clot strength predicts coagulation-related mortality within 15 minutes. Surgery. 2012, 151: 48-54. 10.1016/j.surg.2011.06.023.
Raza I, Davenport R, Rourke C, Platton S, Manson J, Spoors C, Khan S, De'Ath HD, Allard S, Hart DP, Pasi KJ, Hunt BJ, Stanworth S, MacCallum PK, Brohi K: The incidence and magnitude of fibrinolytic activation in trauma patients. J Thromb Haemost. 2013, 11: 307-314. 10.1111/jth.12078.
Chapman MP, Moore EE, Ramos CR, Ghasabyan A, Harr JN, Chin TL, Stringham JR, Sauaia A, Silliman CC, Banerjee A: Fibrinolysis greater than 3% is the critical value for initiation of antifibrinolytic therapy. J Trauma Acute Care Surg. 2013, 75: 961-967. 10.1097/TA.0b013e3182aa9c9f.
Park MS, Salinas J, Wade CE, Wang J, Martini W, Pusateri AE, Merrill GA, Chung K, Wolf SE, Holcomb JB: Combining early coagulation and inflammatory status improves prediction of mortality in burned and nonburned trauma patients. J Trauma. 2008, 64: S188-S194. 10.1097/TA.0b013e318160a5a3.
Schöchl H, Frietsch T, Pavelka M, Jambor C: Hyperfibrinolysis after major trauma: differential diagnosis of lysis patterns and prognostic value of thromboelastometry. J Trauma. 2009, 67: 125-131. 10.1097/TA.0b013e31818b2483.
Schöchl H, Nienaber U, Hofer G, Voelckel W, Jambor C, Scharbert G, Kozek-Langenecker S, Solomon C: Goal-directed coagulation management of major trauma patients using thromboelastometry (ROTEM® )-guided administration of fibrinogen concentrate and prothrombin complex concentrate.Crit Care 2010, 14:R55.,
Theusinger OM, Wanner GA, Emmert MY, Billeter A, Eismon J, Seifert B, Simmen HP, Spahn DR, Baulig W: Hyperfibrinolysis diagnosed by rotational thromboelastometry (ROTEM® ) is associated with higher mortality in patients with severe trauma. Anesth Analg. 2011, 113: 1003-1012. 10.1213/ANE.0b013e31822e183f.
Davis PK, Musunuru H, Walsh M, Cassady R, Yount R, Losiniecki A, Moore EE, Wohlauer MV, Howard J, Ploplis VA, Castellino FJ, Thomas SG: Platelet dysfunction is an early marker for traumatic brain injury-induced coagulopathy. Neurocrit Care. 2013, 18: 201-208. 10.1007/s12028-012-9745-6.
Kunio NR, Differding JA, Watson KM, Stucke RS, Schreiber MA: Thrombelastography-identified coagulopathy is associated with increased morbidity and mortality after traumatic brain injury. Am J Surg. 2012, 203: 584-588. 10.1016/j.amjsurg.2011.12.011.
Tapia NM, Chang A, Norman M, Welsh F, Scott B, Wall MJ, Mattox KL, Suliburk J: TEG® -guided resuscitation is superior to standardized MTP resuscitation in massively transfused penetrating trauma patients. J Trauma Acute Care Surg. 2013, 74: 378-386. 10.1097/TA.0b013e31827e20e0.
Baker SP, O’Neill B, Haddon W, Long WB: The injury severity score: a method for describing patients with multiple injuries and evaluating emergency care. J Trauma. 1974, 14: 187-196. 10.1097/00005373-197403000-00001.
Solomon C, Traintinger S, Ziegler B, Hanke A, Rahe-Meyer N, Voelckel W, Schöchl H: Platelet function following trauma: a multiple electrode aggregometry study. Thromb Haemost. 2011, 106: 322-330. 10.1160/TH11-03-0175.
Reed MJ, Nimmo AF, McGee D, Manson L, Neffendorf AE, Moir L, Donaldson LS: Rotational thrombolelastometry produces potentially clinical useful results within 10 min in bleeding emergency department patients: the DEUCE study. Eur J Emerg Med. 2013, 20: 160-166. 10.1097/MEJ.0b013e3283561261.
Görlinger K, Dirkmann D, Solomon C, Hanke AA: Fast interpretation of thromboelastometry in non-cardiac surgery: reliability in patients with hypo-, normo-, and hypercoagulability. Br J Anaesth. 2013, 110: 222-230. 10.1093/bja/aes374.
Inaba K, Branco BC, Rhee P, Blackbourne LH, Holcomb JB, Teixeira PG, Shulman I, Nelson J, Demetriades D: Impact of plasma transfusion in trauma patients who do not require massive transfusion. J Am Coll Surg. 2010, 210: 957-965. 10.1016/j.jamcollsurg.2010.01.031.
Borgman MA, Spinella PC, Holcomb JB, Blackbourne LH, Wade CE, Lefering R, Bouillon B, Maegele M: The effect of FFP:RBC ratio on morbidity and mortality in trauma patients based on transfusion prediction score. Vox Sang. 2011, 101: 44-54. 10.1111/j.1423-0410.2011.01466.x.
Shanwell A, Andersson TM, Rostgaard K, Edgren G, Hjalgrim H, Norda R, Melbye M, Nyrén O, Reilly M: Post-transfusion mortality among recipients of ABO-compatible but non-identical plasma. Vox Sang. 2009, 96: 316-323. 10.1111/j.1423-0410.2009.01167.x.
Inaba K, Branco BC, Rhee P, Holcomb JB, Blackbourne LH, Shulman I, Nelson J, Demetriades D: Impact of ABO-identical vs ABO-compatible non-identical plasma transfusion in trauma patients. Arch Surg. 2010, 145: 899-906. 10.1001/archsurg.2010.175.
Weber CF, Görlinger K, Meininger D, Herrmann E, Bingold T, Moritz A, Cohn LH, Zacharowski K: Point-of-care testing: a prospective, randomized clinical trial of efficacy in coagulopathic cardiac surgery patients. Anesthesiology. 2012, 117: 531-547. 10.1097/ALN.0b013e318264c644.
Schaden E, Kimberger O, Kraincuk P, Baron DM, Metnitz PG, Kozek-Langenecker S: Perioperative treatment algorithm for bleeding burn patients reduces allogeneic blood product requirements. Br J Anaesth. 2012, 109: 376-381. 10.1093/bja/aes186.
Girdauskas E, Kempfert J, Kuntze T, Borger MA, Enders J, Fassl J, Falk V, Mohr FW: Thromboelastometrically guided transfusion protocol during aortic surgery with circulatory arrest: a prospective, randomized trial. J Thorac Cardiovasc Surg. 2010, 140: 1117-1124. 10.1016/j.jtcvs.2010.04.043.
Görlinger K, Fries D, Dirkmann D, Weber CF, Hanke AA, Schöchl H: Reduction of fresh frozen plasma requirements by perioperative point-of-care coagulation management with early calculated goal-directed therapy. Transfus Med Hemother. 2012, 39: 104-113. 10.1159/000337186.
Görlinger K, Dirkmann D, Hanke AA, Kamler M, Kottenberg E, Thielmann M, Jakob H, Peters J: First-line therapy with coagulation factor concentrates combined with point-of-care coagulation testing is associated with decreased allogeneic blood transfusion in cardiovascular surgery: a retrospective, single-center cohort study. Anesthesiology. 2011, 115: 1179-1191.
Fassl J, Matt P, Eckstein F, Filipovic M, Gregor M, Zenklusen U, Seeberger MD, Bolliger D: Transfusion of allogeneic blood products in proximal aortic surgery with hypothermic circulatory arrest: effect of thromboelastometry-guided transfusion management. J Cardiothorac Vasc Anesth. 2013, 27: 1181-1188. 10.1053/j.jvca.2013.02.009.
Sankarankutty A, Nascimento B, da Luz LT, Rizoli S: TEG® and ROTEM® in trauma: similar test but different results?World J Emerg Surg 2012, 7:S3.,
Venema LF, Post WJ, Hendriks HG, Huet RC, de Wolf JT, de Vries AJ: An assessment of clinical interchangeability of TEG® and ROTEM® thromboelastographic variables in cardiac surgical patients. Anesth Analg. 2010, 111: 339-344. 10.1213/ANE.0b013e3181e368bc.
Kutcher ME, Redick BJ, McCreery RC, Crane IM, Greenberg MD, Cachola LM, Nelson MF, Cohen MJ: Characterization of platelet dysfunction after trauma. J Trauma Acute Care Surg. 2012, 73: 13-19. 10.1097/TA.0b013e318256deab.
Görlinger K, Jambor C, Hanke AA, Dirkmann D, Adamzik M, Hartmann M, Rahe-Meyer N: Perioperative coagulation management and control of platelet transfusion by point-of-care platelet function analysis. Transfus Med Hemother. 2007, 34: 396-411. 10.1159/000109642.
Adamzik M, Görlinger K, Peters J, Hartmann M: Whole blood impedance aggregometry as a biomarker for the diagnosis and prognosis of severe sepsis.Crit Care 2012, 16:R204.,
Afshari A, Wikkelso A, Brok J, Møller AM, Wetterslev J: Thrombelastography (TEG® ) or thromboelastometry (ROTEM® ) to monitor haemotherapy versus usual care in patients with massive transfusion.Cochrane Database Syst Rev 2011, 3, CD007871.,
Møller MC, Meijers JCM, Vroom MB, Juffermans NP: Utility of thromboelastography and/or thromboelastometry in adults with sepsis: a systematic review.Critical Care 2014, 18:R30.,
Acknowledgements
We thank Mr. Henry Lam for assistance with the search strategy, Michelle Hayes for retrieval of the full text of some studies, and Maria Chiu for assistance with translation. This study received no funding. Bartolomeu Nascimento was the 2010 to 2011 National Blood Foundation Grant recipient for the conduct of research related to coagulopathy in trauma.
Author information
Authors and Affiliations
Corresponding author
Additional information
Competing interests
Dr. Rizoli is a member of a Scientific Advisory Board to CSL Behring, manufacturer of fibrinogen concentrate. He is also the recipient of a Canadian Institute of Health Research (CIHR) New Investigator award in partnership with NovoNordisk Canada, manufacturer of NovoSeven (recombinant factor VII).
Electronic supplementary material
Authors’ original submitted files for images
Below are the links to the authors’ original submitted files for images.
Rights and permissions
This article is published under an open access license. Please check the 'Copyright Information' section either on this page or in the PDF for details of this license and what re-use is permitted. If your intended use exceeds what is permitted by the license or if you are unable to locate the licence and re-use information, please contact the Rights and Permissions team.
About this article
Cite this article
Da Luz, L.T., Nascimento, B., Shankarakutty, A.K. et al. Effect of thromboelastography (TEG®) and rotational thromboelastometry (ROTEM®) on diagnosis of coagulopathy, transfusion guidance and mortality in trauma: descriptive systematic review. Crit Care 18, 518 (2014). https://doi.org/10.1186/s13054-014-0518-9
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s13054-014-0518-9