Abstract
Background
Nutrition exerts a fundamental role in the prevention of obesity (OB). The aim of this study was to assess the extent to which well recognized risk factors for early OB can be associated to overweight (OW) or OB under a standardized nutritional approach and surveillance in toddlers.
Methods
The eligible population was represented by 676 toddlers aged 24–36 months, assigned to 18 primary care pediatricians trained on nutritional issues who shared a standardized nutritional approach. Six-hundred-twenty-nine children (333 boys), mean age 27.8 ± 4.2 months were effectively included in this observational study. Parents received nutritional advice with particular emphasis to proteins and sugar composition supported by leaflets and reinforced at each visit. Body mass index was assessed at the age of 24–36 months. The following individual and family risk factors were considered: gestational age, birth weight, eutocic/caesarean delivery, milk feeding history, household smoking or antibiotics exposure, parents’ weight, height and educational level. Prevalence of OW/OB was compared to a group of 742 toddlers (373 boys) under usual care.
Results
Under a standardized nutritional counselling, 28.1% toddlers were classified as OW/OB compared to 36.9% toddlers under usual care (p = 0.005). In unadjusted models, parental OW/OB was significantly associated to OW/OB in toddlers (p < 0.01), while high birth weight did not reach statistical significance (p = 0.07). In adjusted models, including all the explanatory variables studied, only paternal OW/OB vs. normal weight was significantly associated to OW/OB in toddlers (OR 2.035, 95% confidence interval 1.206–3.436). No protective effect of exclusive breast feeding during the first 6 months of age was demonstrated.
Conclusions
Toddlers under a standardized nutrition counselling focused to limit protein and simple sugars, showed lower prevalence of OW/OB compared to usual care. Healthy promotion activities should take into account the influence of paternal BMI on the offspring adiposity.
Similar content being viewed by others
Background
Overweight (OW) and obesity (OB) in children represent public health concerns worldwide, where 41 milions of children under 5 years of age and 340 milions of children and adolescents between 5 and 19 years of age have been reported to be OW or OB, respectively [1].
The last data provided by “Okkio alla salute”, the Italian surveillance system in children aged 8–9 years [2]) showed that the prevalence of OW was 20.4% and OB 9.4%, with higher rates in Southern Italy in 2019. Specifically, the highest prevalence of OW (25.4%) and OB (18.8%) was reported in the Campania region [2].
Several early-life factors significantly contribute to the development of obesity. During the “first 1000 days”, a window of life that includes the pre-conceptional period, maternal influences and nutritional factors play a peculiar role in weight gain compared to other environmental factors, like physical activity and sedentary behaviours. Besides genetic predisposition, high pre-pregnancy body mass index (BMI), increased gestational weight gain and diabetes mellitus are among the main risk factors for early development of obesity [3]. Instead, less explored is the paternal contribution during this early age. With regard to nutritional factors, an important role has been attributed to formula feeding, early age at complementary feeding and increased intake of energy [4]. Specifically, protein intake excess, namely lactoproteins [5, 6] in infants, has been associated to an early adiposity rebound in toddlers, which is predictive of OB persistance and presence of comorbidities later in life [7,8,9]. Other environmental risk factors for early adiposity, such as type of delivery, birth weight, parental education level, household smoking or early antibiotic therapy have been also explored [3, 10,11,12].
Prevention strategies of childhood obesity are more feasible and successful in the first years of life. For instance, a systematic review of systematic reviews on the effects of nutritional exposure or interventions in children under 3 years, underscored the promising effect of lowering the protein intake of infant formula or complementary food on the risk of later OW/OB [13]. Indeed, a previous study conducted by three primary care pediatricians from our research group demonstrated that simple but specific dietary advices, focused on the choice and amount of food with low protein content and sugar, were associated to a reduction of the frequency of OW and OB from 26.3 to 13.9% (defined according to the BMI thresholds proposed by the Centers for Disease Control and Prevention) in toddlers living in Campania, an Italian region with high prevalence of OW/OB [14]. Subsequently, this nutritional approach was standardized and implemented among specifically trained Pediatricians working in the Campania region.
As far as we know, there are no studies that compared the association between risk factors for early development of excess weight gain and the prevalence of OW/OB in children under a preventive standardized nutritional counselling.
Therefore, the aim of this prospective study was to assess the extent to which well recognized risk factors for early OB can be associated to overweight (OW) or OB under a standardized nutritional approach and surveillance in toddlers directed at OB prevention.
Methods
The Regional Health Service provides cost-free health care to all children from birth to 14 years. In particular, each child has a scheduled number of visits from 1 to 144 months, 4 of which from 6 to 36 months of life. Health care is delivered by primary care pediatricians, who are provided with a medical software to store the demographic and clinical data of their child population. This particular organization allowed us to design the following prospective study in the primary care setting. The study was approved by the Ethical Committee “Campania Sud” of the ASL Napoli 3 Sud (protocol number 16, 3/2/2016) and was performed in accordance with the 1975 Declaration of Helsinki revised in 1983. To ensure data protection and confidentiality, data extracted from the medical records were anonimyzed before being uploaded in a database for analyses. Informed consent was obtained by parents of the chidren enrolled in the study.
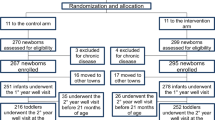
Briefly, the study involved the entire population of children aged 24–36 months old born from 1 October 2015 to 31 march 2016, under the care of 18 primary care pediatricians working in the Campania region, who participated to an update training about nutrition in the first 36 months of life in order to prevent obesity. After the training, pediatricians shared a protocol of weaning and feeding to be used in all children up to 36 months under their care. Nutrition counselling provided to parents was specifically focused on animal and vegetal protein content from different food sources, avoid abundant portions of food rich in animal proteins, and limit sugar and sugar-sweetened beverages. Leaflets containing practical examples and pictures of portions were delivered according to the different nutrition phases (weaning, 6 to 12 months, 12 to 36 months). The recommended daily protein amount corresponded approximatively to 1.3 gr/kg between 6 and 12 months and 2 gr/kg after 12 months of age; in any case, it was lower than 3.5 gr/kg considered at risk for overweight [15, 16]. The eligible population was represented by 676 children; 47 were excluded for the following reasons: change of residence, change of the registered pediatrician, missing visit at 24–36 months. Therefore 629 children (333 boys, 296 girls), mean age 27.8 ± 4.2 months were effectively included in the study. Children underwent well-child visits according to the following time sheet: every three months until 12 months of age; every 4 months from 12 to 24 months; every 6 months from 24 to 36 months of age. The well-child visits were increased from 4 (as scheduled according to the Italian Health Service organization) to 7 and they were devoted to assess parents’dietary behaviour and reinforce nutrition advice.
The following individual and family data were available: gestational age, birth weight, type of delivery (eutocic/cesarean), milk feeding history, exposure to household smoking or antibiotics exposure in the first 24 months of life, parents’ weight, height and educational level. Antibiotic exposure was assessed using outpatient prescriptions and patient-reported medications recorded at all primary care visits between birth and 23 months of age. The limit of at least 4 doses during this period was considered a risk factor [17].
Body weight, lenght (under 24 months of age) and height (≥ 24 months) were measured between 24 and 36 months in each office by the same pediatrician, who was specifically trained in anthropometric standard procedures [18]. Weight was measured on mechanical beam scales, lenght/height were measured to the nearest 0.1 cm using a paidometer or a stadiometer, respectively with the child wearing only underwear and no shoes.
BMI (kg/m2) was calculated as body weight divided by the square of height. Categories of normal weight (NW), overweight (OW) and obesity (OB) for children were defined according to the BMI thresholds proposed by the WHO for the children [19], or to the adult cut off of 18.5, 25 and 30 kg/m2 for the parents [20]. Parental educational level was categorized as low (elementary and middle school) or high (high school or degree).
Nine primary care pediatricians working in the same region, who did not participate to the nutritional training and did not share the standardized approach, provided anthropometric data of 738 toddlers (373 boys, 365 girls) born in the same period (mean age 27.6 ± 4.2 months), under their usual care and scheduled number of well-child visits.
Statistical analysis
Data are expressed as means and standard deviations, medians and interquartile ranges, frequencies (%) and 95% confidence interval (CI). All variables had skewed distribution. Between-groups differences were evaluated by using the Mann Whitney test. Chi-square or Fisher’s exact test, as appropriate, were used to compare proportions. Spearman correlation test was used for correlation analyses. Unadjusted and adjusted binary logistic regression analyses were used and OR with corresponding 95% Confidence Intervals were calculated to evaluate the association of OW/OB status and exposure variables (prenatal/postnatal or family potential risk factors) while controlling for confounding.
A P value < 0.05 was considered statistically significant. The statistical analysis was performed using the IBM SPSS Statistics, Version 28.0. Armonk, NY: IBM Corp.
Results
The overall prevalence of OW/OB in toddlers under standardized care and surveillance was 28.1% (22.1% OW, 6% OB) compared to 36.9% (OW 28.3, OB 8.7%) in children under usual care (p = 0.005). Age, BMI-SDS and % of BMI categories did not differ by sex (Table 1).
Demographic, anthropometric, prenatal/neonatal or family data were compared between NW and OW/OB toddlers under standardized care and surveillance (Table 2). No differences were found with regard to most variables, except for higher parental BMI values and higher percentage of OW/OB parents in children with OW/OB (Table 2).
Correlation analyses showed a slight relationship between BMI z score and birth weight (rho 0.134, p < 0.001; father’s BMI (rho 0.148, p < 0.001) and mother’s BMI (rho 0.110, p < 0.007).
Mother’s BMI was significantly correlated with birth weight (rho 0.124, p = 0.002); father s’ BMI (rho 0.237, p < 0.001), mother’s education level (rho − 0.134, p < 001) and fathers’education level (rho − 0.195, p < 0.001). Moreover, father’s BMI was significantly correlated with fathers’education level (rho − 0.141, p < 0.001).
The associations between toddlers’ OW/OB status and the different risk factors for OW/OB were explored by logistic regression analyses. The unadjusted and adjusted Odds ratios and 95% CI are synthetized in Table 3. The presence of OW/OB in fathers or mothers (P < 0.01) and, at a lesser but not significant extent, high birth weight (P = 0.07) were associated with OW/OB in toddlers in the unadjusted model (Model 1). In the adjusted models, caesarean delivery was significantly associated with OW/OB in toddlers compared to vaginal delivery (ORs between 1.696 and 1.788, p < 0.01) after controlling for prenatal/neonatal, environmental variables and maternal OW/OB (Models 2–4); the statistical significance disappeared after including the paternal OW/OB variable (Model 5). The presence of OW/OB in fathers was associated with a nearly twofold increase in the association with OW/OB in toddlers, independently of all the prenatal/neonatal, environmental factors, the presence of maternal OW/OB and paternal and maternal education status (Models 5 and 6).
Discussion
Our results underline that under a standardized nutritional counselling and surveillance, 28.1% toddlers were classified as OW/OB compared to 36.9% toddlers under usual care. The presence of OW/OB in one or both parents was a significant risk factor in toddlers with OW/OB. After adjustment for all the risk variables, only the presence of OW/OB in fathers was the explanatory variable for OW/OB in toddlers under a standardized nutritional approach.
Pediatric primary care is regarded as an important setting for pediatric obesity prevention and treatment [21, 22]. Pediatricians have an important role in educating parents about specific health behaviors devoted to control energy balance since the very first months of life. In particular, nutrition interventions in the first two years of life can have a positive impact on a child’s BMI, provided that intervention are maintained later in life [23]. Specifically, prospective cohort studies support an association between higher intake of proteins, mainly of animal origin, and increased BMI or BMI z-scores in healthy, well-nourished children from Western countries [24]. Early-life determinants, beginning in the intrauterine life and continuing through the first years of life, may influence the progression of weight gain throughout the life course. However, the efficacy of a nutritional preventive intervention may be weakened by the presence of non modifiable (genetic, epigenetic) or enviromental influences. Our prospective study was designed to assess the extent to which well recognized risk factors for early obesity characterize toddlers with OW/OB under a standardized nutritional counselling and surveillance.
Our results underline that the presence of OW/OB in one or both parents was a significant risk factor in toddlers with OW/OB in unadjusted analyses. In particular, the OR for OW/OB was a 1.6 times higher whether either fathers or mothers were affected by OW/OB compared to their normal weight conterpart, confirming previous results. The strict association berween parental obesity and early childhood obesity can be interpreted as a consequence of the complex interplay among genetic, epigenetic and environmental factors. By comparing the strenght of the association of BMI between parents and their offspring, several Authors reported that this association was stronger in mothers, when the birth weight was considered; in this case the reason may be ascribed to the intrauterine environment related to maternal BMI [25, 26]. On the contrary, associations between parents and offsprings adiposity did not differ between fathers and mothers when children were measured at later ages [27, 28]. In particular, a previous cohort study performed in the Netherlands found a similar association of both paternal and maternal BMI with preschool overweight, after controlling for 34 putative parental, fetal, and infant factors with overweight risk [29]. At variance with this latter study, we found that the association of OB status between the parent-child dyad disappeared when the mothers were considered, after controlling for several risk factors that are associated with maternal obesity, while it persisted in the fathers. Indeed, maternal OB is linked to greater risk of preterm birth, large-for-gestational-age babies or lower breastfeeding rates [30] thus probably explaining why the association was not confirmed in the adjusted models. Our findings underline the independent role of paternal BMI on children’s adiposity in the very firs years of life, that can be supported by genetic and epigenetic influences [31] and/or family environment about parenting practice. Despite food parenting is most often guided by mothers in the first 2–3 years of age, we cannot exclude that nowadays fathers may directly influence feeding practice in different ways and levels during the first few years of life [32].
We did not find any influence of low birth weight on the prevalence of OW/OB in our cohort; indeed, this association has been usually reported after the age of 8 years [16]. Other determinants, such as large birth weight or preterm delivery did not appear to be associated to the risk of OW/OB in our cohort.
Increasing evidences points to cesarean delivery as a possible risk factor for obesity, but the underlying mechanism of this association remains unclear [33, 34]. Of note, we found that the OR of OW/OB in toddlers born by caesarean compared to vaginal delivery was significant as long as parental OW/OB status was not included to the model, thus explaining that the impact of caesarean delivery is reduced by adding stronger risk factors. The effect of cesarean delivery on children’s obesity has been reported to be higher in places where the cesarean delivery rate was > 10% (RR = 1.30, 95% CI: 1.10–1.50) [35]. Interestingly, the rate of cesarean births in 2021 in the Campania region was the highest in Italy (50.2% vs. 31.2%) as well as the prevalence of OW/OB [2, 36], therefore this possible risk factor deserves further research. Indeed, other studies have shown that the association between caesarean delivery and OW/OB may be invalidated by further subgroup analyses. For instance, Li et al. [37] found that the overall OR for OW/OB after cesarean delivery was 1.32 (95% CI 1.15–1.51) in children berween 3–8 years of age, but it remained significant only for OB (OR 1.40, 95% CI 1.17, 1.67) and not for OW (OR 1.22, 95% CI 0.99, 1.50). Furthermore, a prospective study performed in United States reported an unadjusted OR for OB of 2.40 (1.60 to 3.62) in children at 3 years of age born from caesarean delivery, that decreased to 2.10 (1.36, 3.23) when adjusted for several child (age, sex and birth weight) and maternal predictors (age, education, race/ethnicity, prepregnancy BMI). Of note, this association was significant only in mothers with normal pre-pregnancy weight [38].
Our study does not support that excludive breast-feeding up to six months protects from OW/OB, nor that antibiotic exposure or household smoking are significant risk factors for it. Concerning breastfeeding, we cannot exclude that more prolonged periods of breastfeeding while children received nutritionally adequate and safe complementary foods might have exerted a protective role in our setting.
Our study has some weaknesses: parents’BMI was based on self-reported height and weight, and individual and family data of the control group were lacking. The strenght of our study is the large number of trained pediatricians, who provided periodically health visits and nutritional education to a large sample of toddlers and families in the first 24–36 months of life. In addition, the inclusion of a variety of prenatal, postnatal and family risk factors for early obesity, which are frequently interrelated each other, allowed us to perform statistical analyses adjusted for several confounders.
Conclusions
Our study performed in a region with the highest prevalence of OW/OB in children highlights that nutritional counseling focused on protein and sugar intakes during the first years of life should be implemented among Pediatricians working in a primary care setting. Healthy promotion activities should take into account the influence of paternal BMI on the offspring adiposity.
Data availability
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Abbreviations
- BMI:
-
Body Mass Index
- CI:
-
Confidence Interval
- OB:
-
Obesity
- OW:
-
Overweight
- SDS:
-
Standard Deviation Score
References
World Health Organization Obesity and overweight. https://www.who.int/news-room/fact-sheets/detail/obesity-and-overweight, accessed on 21/02/2024.
EpiCentro. Istituto Superiore Sanità (ISS). OKKio alla salute. Dati nazionali e regionali 2019. https://www.epicentro.iss.it/okkioallasalute/indagine-2019-dati. accessed on 21/02/2024.
Woo Baidal JA, Locks LM, Cheng ER, Blake-Lamb TL, Perkins ME, Taveras EM. Risk factors for childhood obesity in the first 1,000 days: a systematic review. Am J Prev Med. 2016;50:761–79. https://doi.org/10.1016/j.amepre.2015.11.012
Mameli C, Mazzantini S, Zuccotti GV. Nutrition in the First 1000 days: the origin of childhood obesity. Int J Environ Res Public Health. 2016;13:838. https://doi.org/10.3390/ijerph13090838
Appleton J, Russell CG, Laws R, Fowler C, Campbell K, Denney-Wilson E. Infant formula feeding practices associated with rapid weight gain: a systematic review. Matern Child Nutr. 2018;14:e12602. https://doi.org/10.1111/mcn.12602
Luque V, Closa-Monasterolo R, Escribano J, Ferré N. Early Programming by Protein Intake: The Effect of Protein on Adiposity Development and the Growth and Functionality of Vital Organs. Nutr Metab Insights. 2015; 8:49–56. doi: 10.4137/NMI.S29525. eCollection 2015.
Pimpin L, Jebb S, Johnson L, Wardle J, Ambrosini GL. Dietary protein intake is associated with body mass index and weight up to 5 y of age in a prospective cohort of twins. Am J Clin Nutr. 2016;103:389–97. https://doi.org/10.3945/ajcn.115.118612
Rolland-Cachera MF, Akrout M, Péneau S. Nutrient intakes in early life and risk of obesity. Int J Environ Res Public Health. 2016;13:564. https://doi.org/10.3390/ijerph13060564
Weber M, Grote V, Closa-Monasterolo R, Escribano J, Langhendries JP, Dain E, et al. Lower protein content in infant formula reduces BMI and obesity risk at school age: follow-up of a randomized trial. Am J Clin Nutr. 2014;99:1041–51. https://doi.org/10.3945/ajcn.113.064071
Bider-Canfield Z, Martinez MP, Wang X, Yu W, Bautista MP, Brookey J, et al. Maternal obesity, gestational diabetes, breastfeeding and childhood overweight at age 2 years. Pediatr Obes. 2017;12:171–8. https://doi.org/10.1111/ijpo.12125
Parrino C, Vinciguerra F, La Spina N, Romeo L, Tumminia A, Baratta R, et al. Influence of early-life and parental factors on childhood overweight and obesity. J Endocrinol Invest. 2016;39:1315–21. https://doi.org/10.1007/s40618-016-0501-1
Scott FI, Horton DB, Mamtani R, Haynes K, Goldberg DS, Lee DY, et al. Administration of antibiotics to children before age 2 years increases risk for childhood obesity. Gastroenterology. 2016;151:120–e95. https://doi.org/10.1053/j.gastro.2016.03.006
Patro-Gołąb B, Zalewski BM, Kołodziej M, Kouwenhoven S, Poston L, Godfrey KM et al. Nutritional interventions or exposures in infants and children aged up to 3 years and their effects on subsequent risk of overweight, obesity and body fat: a systematic review of systematic reviews. Obes Rev. 2016;17:1245-57. https://doi.org/10.1111/obr.12476. Erratum in: Obes Rev. 2018;19:1620.
Limauro R, Gallo P, Cioffi L, Antignani A, Cioffi V, Calella P, et al. Clinical audit in the pediatric primary care office and overweight prevention in toddlers. BMC Pediatr. 2020;20:163. https://doi.org/10.1186/s12887-020-02076-y
SINU Società Italiana di Nutrizione Umana. LARN Livelli Di Assunzione Di Riferimento Di Nutrienti ed energia per la popolazione italiana. IV Revisione. Milano SICS; 2014.
Hörnell A, Lagström H, Lande B, Thorsdottir I. Protein intake from 0 to 18 years of age and its relation to health: a systematic literature review for the 5th Nordic Nutrition recommendations. Food Nutr Res. 2013;57. https://doi.org/10.3402/fnr.v57i0.21083
Bailey LC, Forrest CB, Zhang P, Richards TM, Livshits A, DeRusso PA. Association of antibiotics in infancy with early childhood obesity. JAMA Pediatr. 2014;168:1063–9. https://doi.org/10.1001/jamapediatrics.2014.1539
Gallo P, Cioffi L, Limauro R, Farris E, Bianco V, Sassi R, et al. SGA Children in Pediatric Primary Care: what is the best choice, large or small? A 10-Year prospective longitudinal study. Glob Pediatr Health. 2016;3:2333794X16659993. https://doi.org/10.1177/2333794X16659993
WHO Multicentre Growth Reference Study Group WHO child. Growth standards based on length/height, weight and age. Acta Paediatr Suppl. 2006;450:76–85. https://doi.org/10.1111/j.1651-2227.2006.tb02378.x
Jensen MD, Ryan DH, Apovian CM, Ard JD, Comuzzie AG, Donato KA, American College of Cardiology/American Heart Association Task Force on Practice Guidelines and The Obesity Society. 2013 AHA/ACC/TOS guideline for the management of overweight and obesity in adults: a report of the. J Am Coll Cardiol. 2014; 63(25 Pt B):2985–3023. doi: 10.1016/j.jacc.2013.11.004. Erratum in: J Am Coll Cardiol. 2014 1;63(25 Pt B):3029–3030.
Seburg EM, Olson-Bullis BA, Bredeson DM, Hayes MG, Sherwood NE. A review of primary care-based childhood obesity Prevention and Treatment interventions. Curr Obes Rep. 2015;4:157–73. https://doi.org/10.1007/s13679-015-0160-0
Daniels SR, Hassink SG, COMMITTEE ON NUTRITION. The role of the Pediatrician in Primary Prevention of obesity. Pediatrics. 2015;136:e275–92. https://doi.org/10.1542/peds.2015-1558
Koplin JJ, Kerr JA, Lodge C, Garner C, Dharmage SC, Wake M, et al. Infant and young child feeding interventions targeting overweight and obesity: a narrative review. Obes Rev. 2019;20(Suppl 1):31–44. https://doi.org/10.1111/obr.12798
Arnesen EK, Thorisdottir B, Lamberg-Allardt C, Bärebring L, Nwaru B, Dierkes J, et al. Protein intake in children and growth and risk of overweight or obesity: a systematic review and meta-analysis. Food Nutr Res. 2022;66. https://doi.org/10.29219/fnr.v66.8242
Xue F, Willett WC, Rosner BA, Forman MR, Michels KB. Parental characteristics as predictors of birthweight. Hum Reprod. 2008;23:168–77. https://doi.org/10.1093/humrep/dem316
Lie RT, Wilcox AJ, Skjaerven R. Maternal and paternal influences on length of pregnancy. Obstet Gynecol. 2006;107:880–5. https://doi.org/10.1097/01.AOG.0000206797.52832.36
Patel R, Martin RM, Kramer MS, et al. Familial associations of adiposity: findings from a cross-sectional study of 12,181 parental-offspring trios from Belarus. PLoS ONE. 2011;6:pe14607. https://doi.org/10.1371/journal.pone.0014607
Fleten C, Nystad W, Stigum H, Skjaerven R, Lawlor DA, Davey Smith G, Naess O. Parent-offspring body mass index associations in the Norwegian mother and child cohort study: a family-based approach to studying the role of the intrauterine environment in childhood adiposity. Am J Epidemiol. 2012;176:83–92. https://doi.org/10.1093/aje/kws134
Heppe DH, Kiefte-de Jong J, Durmuş B, et al. Parental, fetal, and infant risk factors for preschool overweight: the Generation R Study. Pediatr Res. 2013;73:120–7. https://doi.org/10.1038/pr.2012.145
Marchi J, Berg M, Dencker A, Olander EK, Begley C. Risks associated with obesity in pregnancy, for the mother and baby: a systematic review of reviews. Obes Rev. 2015;16:621–38. https://doi.org/10.1111/obr.12288
Noor N, Cardenas A, Rifas-Shiman SL, Pan H, Dreyfuss JM, Oken E, Hivert MF, James-Todd T, Patti ME, Isganaitis E. Association of Periconception Paternal Body Mass Index with persistent changes in DNA methylation of offspring in Childhood. JAMA Netw Open. 2019;2:e1916777. https://doi.org/10.1001/jamanetworkopen.2019.16777
Thullen M, Majee W, Davis AN. Co-parenting and feeding in early childhood: reflections of parent dyads on how they manage the developmental stages of feeding over the first three years. Appetite. 2016;105:334–43. https://doi.org/10.1016/j.appet.2016.05.039
Mueller NT, Shin H, Pizoni A, Werlang IC, Matte U, Goldani MZ, et al. Delivery mode and the transition of pioneering gut-microbiota structure, composition and predicted metabolic function. Genes. 2017;8:364. https://doi.org/10.3390/genes8120364
Shokry E, Marchioro L, Uhl O, Bermúdez MG, García-Santos JA, Segura MT, et al. Investigation of the impact of birth by cesarean section on fetal and maternal metabolism. Arch Gynecol Obstet. 2019;300:589–600. https://doi.org/10.1007/s00404-019-05213-w
Zhang S, Qin X, Li P, Huang K. Effect of Elective Cesarean Section on children’s obesity from birth to adolescence: a systematic review and Meta-analysis. Front Pediatr. 2022;9:793400. https://doi.org/10.3389/fped.2021.793400
Li HT, Zhou YB, Liu JM. The impact of cesarean section on offspring overweight and obesity: a systematic review and meta-analysis. Int J Obes. 2013;37:893–9. https://doi.org/10.1038/ijo.2012.195
Huh SY, Rifas-Shiman SL, Zera CA, Edwards JW, Oken E, Weiss ST, Gillman MW. Delivery by caesarean section and risk of obesity in preschool age children: a prospective cohort study. Arch Dis Child. 2012;97:610–6. https://doi.org/10.1136/archdischild-2011-301141
Certificato di assistenza al parto (CeDAP). Analisi dell’evento nascita - Anno 2021. Available at www. https://www.salute.gov.it/portale/documentazione/p6_2_2_1.jsp?lingua=italiano&id=3264, accessed on 21/02/2024.
Acknowledgements
The Authors are grateful to the following primary care Pediatricians for providing anthropometric measurements of toddlers aged 24–36 months of life: P. Canale; P. Chianese; V. Di Crosta; N. Gasparini; F. Iaccarino; S. Manetti; A. Martucci; R. Palumbo; G. Sannoner.
Funding
This manuscript was supported by the Italian Federation of Pediatricians (F.I.M.P.) Study Center, Naples, Italy.
Author information
Authors and Affiliations
Contributions
RL, LC and GV had full access to all of the data in the study and were responsible for the study conceptualization. RL and GV drafted the manuscript. RL, LC, VB, VcC, AC, DDG, AE, EF, PG, MG, AI, AmI, MTLV, LR, PS, RS, CS, NS had a role in collecting the data. LC and GV did the statistical analysis. VtC, AA supported the creation of nutritional advice and leaflets. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
The Ethical Committee “Campania Sud” of the ASL Napoli 3 Sud approved the study protocol (approval n. 16, 3/2/2016). Informed consent was obtained by parents or guardians of the children enrolled in the study. All methods were carried out following the Declaration of Helsinki.
Consent for publication
Not applicable.
Conflict of interest
The authors have no conflict of interest to declare.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Limauro, R., Cioffi, L., Bianco, V. et al. Nutritional counselling and risk factors for obesity: an observational study in toddlers. Ital J Pediatr 50, 115 (2024). https://doi.org/10.1186/s13052-024-01668-z
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s13052-024-01668-z