Abstract
Background
Infants with rule-out infections are responsible for the majority of empirical antibiotics treatment (EAT) in neonatal intensive care units (NICUs), particularly very preterm infants (VPIs). Antibiotic overuse has been linked to adverse outcomes. There is a paucity of data on the association between EAT and clinical outcomes (containing the nutritional outcomes) of VPIs without infection-related morbidities.
Methods
Clinical data of VPIs admitted in 28 hospitals in 20 provinces of China from September 2019 to December 2020 were collected. EAT of VPIs was calculated as the number of days with initial usage in the first week after birth, and then categorized into 3 groups (antibiotic exposure: none, 1-4 days, and > 4 days). Clinical characteristics, nutritional status , and the short-term clinical outcomes among 3 groups were compared and analyzed.
Results
In total, 1834 VPIs without infection-related morbidities in the first postnatal week were enrolled, including 152 cases (8.3%) without antibiotics, 374 cases (20.4%) with EAT ≤4 days and 1308 cases (71.3%) with EAT > 4 days. After adjusting for the confounding variables, longer duration of EAT was associated with decreased weight growth velocity and increased duration of reach of full enteral feeding in EAT > 4 days group (aβ: -4.83, 95% CI: − 6.12 ~ − 3.53; aβ: 2.77, 95% CI: 0.25 ~ 5.87, respectively) than those receiving no antibiotics. In addition, the risk of feeding intolerance (FI) in EAT > 4 days group was 4 times higher than that in non-antibiotic group (aOR: 4.14, 95%CI: 1.49 ~ 13.56) and 1.8 times higher than that in EAT ≤4 days group (aOR: 1.82, 95%CI: 1.08 ~ 3.17). EAT > 4 days was also a risk factor for greater than or equal to stage 2 necrotizing enterocolitis (NEC) than those who did not receive antibiotics (aOR: 7.68, 95%CI: 1.14 ~ 54.75) and those who received EAT ≤4 days antibiotics (aOR: 5.42, 95%CI: 1.94 ~ 14.80).
Conclusions
The EAT rate among uninfected VPIs was high in Chinese NICUs. Prolonged antibiotic exposure was associated with decreased weight growth velocity, longer duration of reach of full enteral feeding, increased risk of feeding intolerance and NEC ≥ stage 2. Future stewardship interventions to reduce EAT use should be designed and implemented.
Similar content being viewed by others
Background
Given the immaturity of immune system, premature newborns are at a higher risk of infectious diseases such as sepsis, with atypical clinical manifestations, rapid disease progress, and high mortality [1, 2]. Therefore, prescription of empirical antibiotics treatment (EAT) is common among premature infants in neonatal intensive care unit (NICU). Over 75% of very low birth weight infants (VLBWI) and over 90% of extremely preterm infants (< 28 weeks of gestation) receive empirical antibiotics within the first postnatal week due to risk of early-onset sepsis (EOS) [3, 4]. Encouragingly, a large reduction in antibiotic use among preterm infants is observed and believing this is the result of increasing national focus on antibiotic overuse and misuse over the last decade. There was a marked reduction in the proportion of early antibiotics exposure declining from 82% in 2009 to 66% in 2018 across Norway including 4932 infants with a gestational age < 32 weeks, owing to the implementation of national antimicrobial stewardship [5]. Nevertheless, inappropriate antibiotic use and prolonged antibiotic durations still existed and became a big concern in premature newborns with rule-out infections [6]. Cohort and retrospective studies demonstrated that early EAT in very premature infants (VPIs) can increase the risk of poor prognosis, such as bronchopulmonary dysplasia (BPD), late-onset sepsis (LOS), neonatal necrotizing enterocolitis (NEC) and even death [6, 7]. It can also increase the risk of childhood asthma and obesity [8, 9]. Additionally, there was a dose-effect relationship (positive correlation) between duration of EAT and severity of those diseases [6, 7]. However, the most concerning finding was that 92% of EAT courses for rule-out sepsis were longer than 3 days with a median duration of 8 days [10]. Currently, clinicians do not pay enough attention to the harmfulness of EAT in preterm infants with rule-out infections, and the effect of in-hospital EAT on the clinical outcomes of VPIs without infection-related morbidities has not been sufficiently investigated. Thus, the aims of this retrospective cohort study were to compare the short-term outcomes of VPIs without infection-related morbidities who received different durations of empirical antibiotic exposure with initial usage in the first postnatal week, particularly on the nutritional outcomes.
Methods
Study population and design
The data of this study came from a prospective multicenter study that involved the influencing factors of extrauterine growth retardation (EUGR) in VPIs from different regions of China (The clinical trial registration: chictr.org.cn; Number: ChiCTR1900023418; Date of first registration: 26/05/2019). The study was approved by the Ethics Committee of the Women and Children’s Hospital, School of Medicine, Xiamen University (No. KY-2019-016). In the study, clinical data of VPIs in NICU were collected from 28 tertiary first-class hospitals in 20 provinces of China from September 2019 to December 2020.
Inclusion criteria
Infants born with gestational age (GA) < 32 week and admitted to the participating NICUs within 24 hours after birth.
Exclusion criteria
(I) Infants with congenital malformations and metabolic diseases; (II) infants who died, whose treatment was interrupted and led to an automatic discharge due to parental wishes based on financial constraints; (III) infants with incomplete medical record information. Based on this, infants who developed culture-proven sepsis, high-risk category of early-onset sepsis (EOS) with RCL score 3 (Table 1) [11], clinically diagnosed LOS, NEC, or other confirmed infectious diseases (including pneumonia, urinary tract infection, intracranial infection, or skin infection) during the first postnatal week were excluded.
Study data set
This was a retrospective multicenter cohort study. VPIs who met the inclusion criteria were divided into three groups according to the duration of empirical use of antibiotics: non-antibiotic group, EAT ≤4 days group, EAT > 4 days group. The demographic and clinical data were collected including perinatal information (e.g. GA, BW, the use of antenatal steroids, birth mode, Apgar score at 5 minutes, maternal complications during pregnancy), and primary clinical outcome occurring after the first postnatal week, such as greater than or equal to stage 2 NEC (NEC ≥ stage 2), hospital-acquired infections (HAI), hemodynamically significant patent ductus arteriosus (hsPDA), severe intraventricular hemorrhage (IVH) (grade 3 or 4), periventricular leukomalacia (PVL), moderate and severe BPD, parenteral nutrition associated cholestasis (PNAC), and stage 3 through 5 retinopathy of prematurity (ROP) in either eye. We also collected nutrition related data, including breast feeding, use of breast milk fortifier, cumulative fasting time, and duration of parenteral nutrition.
Study definitions
Early empirical antibiotics treatment (EAT)
With initial usage in the first week after birth, suspected bacterial infection among the VPIs was managed with empirical antibiotics depending on the suspected infection site, perinatal condition, treatment response, and based on local bacterial drug resistance surveillance data but did not have culture-proven infection.
Definitions related to EOS and LOS
(I) Risk classification of EOS (Suspected EOS): The probability of EOS was assessed using an easy-to-use scoring system based on risk factors (R), clinical symptoms (C), and laboratory findings (L) (Table 1) [11]. One point was given if one or more of the three areas of RCL were positive. The minimum score was 0, and the maximum score was 3 points. The neonates were stratified into three risk categories of EOS according to RCL score. Low-risk category defined as neonates with one or no abnormal finding of the three areas of RCL, total score 0 or 1; Medium-risk category defined as neonates with abnormal findings in two of the three areas of RCL, total score 2; High-risk category defined as neonates with risk factors, clinical signs, and abnormal routine laboratory values, total score 3.
(II) Culture-proven EOS: neonates 1-3 days with positive blood/CSF culture, and total RCL score ≥ 1;
(III) Clinically diagnosed late-onset sepsis (LOS): neonates aged > 3 days with clinical symptoms, and conform to any of the following conditions (1) two or more of abnormal routine laboratory values (white blood cells, immature/total neutrophil, C-reactive protein, platelets, and procalcitonin); (2) CSF examination indicates purulent meningitis; (3) bacterial DNA is detected in blood samples [12].
(IV) Culture-proven LOS: neonates aged > 3 days with positive blood/CSF culture and clinical symptoms.
Definitions related to nutrition management
(I) Days of reach of full enteral feeding were defined as the duration of oral feeding reaching 150 ml/ (kg.d); (II) Age of oral calorie attainment was defined as age of oral total calorie reaching 110 kcal/ (kg.d); (III) Weight growth velocity (GV) (after regaining birth weight) was calculated using an exponential model [13]; (IV) Breast-feeding was defined as the amount of breastfeeding accounting for more than 50% of the total enteral feeding during hospitalization; (V) EUGR was defined as postnatal weight below the 10th percentile of the expected growth for the postmenstrual age (PMA) at the time of discharge or 36 weeks PMA, and was evaluated with the 2013 Fenton Preterm Growth Chart [14]; (VI) The diagnostic criteria of feeding intolerance (FI) were in line with the Clinical guidelines for the diagnosis and treatment of FI in preterm infants (2020) [15].
Definitions related to primary clinical outcome
(I) Moderate to severe BPD was defined as requirement of oxygen therapy, positive pressure ventilation, or mechanical ventilation at the corrected GA of 36 weeks or at discharge (whichever comes first) according to National Institutes of Health (NIH) 2001 definition [16]; (II) There is no consensus on the definition of a hsPDA, in our study, hsPDA was defined as patent ductus arteriosus (PDA) catheter diameter > 1.5 mm, left atrium-to-aortic root (LA/Ao) ratio ≥ 1.4 accompanied by one of the following clinical manifestations: heart murmur, tachycardia (sustained ≥160 beats/min), increased respiration, increased pulse pressure (> 25 mmHg), hypotension, flushing, or cardiac dilation [17]; (III) Greater than or equal to stage 2 NEC based on the Bell criteria [18]; (IV) Hospital-acquired infections (HAI) was defined as confirmed infectious diseases including LOS, meningitis, pneumonia, or urinary tract infection with/without positive culture results after the first postnatal week (all occurring after EAT use); (V) Severe IVH (grade 3 or 4) based on the Papile criteria [19]; (VI) ROP staging was performed in agreement with international classification [20], and ROP requiring intervention was defined as ROP requiring intravitreal drug injection, laser therapy, or surgery; (VII) The diagnoses of arenteral nutrition associated cholestasis (PNAC), and PVL established by referring to Practical Neonatology (5th edition) [21].
Statistics analysis
The counting data rate (%) indicated that comparison between groups was performed using χ2 test or Fisher exact probability method. Kolmogorov-Smirnov test was used to evaluate whether the measurement data conformed to the normal distribution. The measurement data of the normal distribution were expressed by \(\overline{x}\pm s\), and the two independent samples t-test was for comparison between groups, while the measurement data of non-normal distribution was expressed by M (Q1, Q3). The ranks sum test was used for group comparisons. Multivariate analysis was performed using binary logistic regression analysis and linear regression analysis. All statistical analyses were conducted using a software program (SPSS, version 26.0; IBM, Armonk, NY, USA), with statistical significance evaluated using 2-sided P values at the 5% testing level.
Results
General information
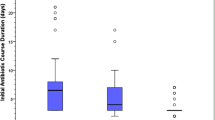
A total of 2600 cases who met the inclusion criteria were enrolled through a prospective multicenter study of VPIs-EUGR from September 2019 to December 2020. Of these, 766 infants were excluded from analysis, including 86 cases with incomplete medical record data, 369 cases with EOS [317 cases with high-risk category of EOS (RCL score 3), 52 cases with culture-proven EOS], 64 cases with LOS during the first postnatal week (23 cases with culture-proven LOS, 41 cases with clinically diagnosed LOS,), 5 cases of early NEC (within 7 days after birth) and 242 cases of other infectious diseases within 7 days after birth such as pneumonia, urinary tract infection, intracranial infection, or skin infection. Therefore, the remaining 1834 uninfected infants during the first postnatal week were enrolled in the analysis. Among them, there were 152 cases (8.3%) who not treated with antibiotics, 374 cases (20.4%) with EAT≤4 days and 1308 cases (71.3%) with EAT > 4 days (Fig. 1). The total rate of EAT was 91.7% (1682/1834). Among 1682 infants who received EAT, 1501(89.2%) had treatment initiated at Day 0, and a total of 2622 courses were prescribed. There were 1095(65.1%), 294 (17.5%), and 293 (17.4%) infants who received 1, 2, and more than 2 courses of antibiotics, respectively. The rate of EAT in VPIs without infection-related morbidities among the hospitals involved in this study fluctuated between 41.3 and 97.6%. The median time of EAT usage during hospitalization was 9 days; 61.4% of them were 1-10 days (Fig. 2). There were 946 (56.2%), 687 (40.8%), 436 (25.9%), and 149 (8.9%) infants who received a ampicillin, third-generation cephalosporin, piperacillin tazobactam, and carbapenem, respectively.
Comparison of perinatal and clinical data
Results showed that infants with lower GA and BW, more postnatal corticosteroid use, longer duration of mechanical ventilation and hospital stay, had higher level of empirical antibiotics usage (all P < 0.001). More empirical antibiotics usage was found in male infants and infants with Apgar score ≤ 7 at 5 min (P = 0.016 and 0.012, respectively). There was no significant difference in the use of empirical antibiotics for multiple births, cesarean section, small for gestational age (SGA), premature rupture of membranes (PROM) > 18 hours, completed antenatal steroids, gestational diabetes mellitus (GDM), and gestational hypertension among the three groups (all P > 0.05) (Table 2).
Comparison of nutritional outcomes
After adjusting for confounding variables, linear regression analysis indicated that Weight growth velocity of EAT ≤4 days group (aβ: -3.68, 95%CI:-5.12 ~ − 2.24) and EAT > 4 days group (aβ:-4.83, 95%CI: − 6.12 ~ − 3.53) was lower compared to that of the non-antibiotic group. Weight GV of EAT > 4 days group was lower than that in EAT ≤4 days group (aβ:-1.15, 95%CI:-1.96 ~ − 0.28). Days of reach of full enteral feeding in EAT > 4 days group was longer than that in non-antibiotic group (aβ: 2.77, 95% CI: 0.25 ~ 5.87). The risk of feeding intolerance (FI) in EAT > 4 days group was 4 times higher than that in non-antibiotic group (aOR: 4.14, 95%CI: 1.49 ~ 13.56) and 1.8 times higher than that in EAT ≤4 days group (aOR: 1.82, 95%CI:1.08 ~ 3.17). There was no significant difference in the duration of parenteral nutrition, the age of oral calorie attainment and the incidence of EUGR among the three groups (all P > 0.05) (Table 3).
Effect of empirical antibiotics treatment on clinical outcomes
Univariate analysis revealed significant differences in the incidence of NEC ≥ stage 2, HAI, hsPDA, moderate and severe BPD and parenteral nutrition associated cholestasis (PNAC) (all P < 0.05) among the three groups (Table 4). After adjusting for factors that may affect the clinical outcomes, multivariate analysis showed that the risk of NEC ≥ stage 2 in EAT > 4 days group was 7.7 times higher than that of the non-antibiotic group (aOR: 7.68, 95%CI: 1.14 ~ 54.75) and 5.4 times higher than that in the EAT ≤4 days group (aOR: 5.42, 95%CI: 1.94 ~ 14.80). The risk of hsPDA incidence in EAT > 4 days group was 3.3 times higher than that of the non-antibiotic group (aOR: 3.28, 95%CI: 1.48 ~ 9.03) and 2.8 times higher than that in EAT ≤4 days group (aOR: 2.75, 95%CI: 1.54 ~ 4.88) (Table 5).
Discussion
Antibiotics are among the most commonly prescribed drugs in the NICU, despite absence of infection in most cases [22]. In the early stage of the disease or when the culture results are not available, up to 94% of antibiotics usage in the NICU is via EAT [22] and long-term use of antibiotics often appears in clinical practice. However, in VPIs, those clinical manifestations such as respiratory and circulatory instability, increased heart rate, feeding intolerance (FI), or body temperature fluctuation are not reliable indicators of infection [23]. Additionally, application of non-specific inflammatory indexes, such as C-reactive protein (CRP), procalcitonin, white blood cell count, or platelet count to determine duration of antibiotics use is questionable [24]. It is more recognized that continuous normal values of biomarkers like CRP and procalcitonin over the first 48 hours of age can help to eliminate infection and shorten the course of antibiotics [25].
The rate of antibiotics use in hospitalized premature infants remains high. An investigation from 24 tertiary medical institutions indicated that the average ratio of antibiotic use to hospitalization was 53.0%, with a maximum of 91.4% among VLBWI and ELBWI during hospitalization in China [26]. In a retrospective study from 297 academic and community hospitals across the United States [3], the majority of premature infants had early antibiotic initiation (31,715 VLBW infants [78.6%] and 11,264 ELBW infants [87.0%]). Collectively, a total of 11,669 cases (84.9%) of VLBWI from grade III NICU in Canada received EAT during hospitalization between 2010 and 2014 [6]. In our study, high frequency use of antibiotics (91.7% of EAT in VPIs without infection-related morbidities, even reaching 100% in some hospitals) and long course antibiotic use were also been found; 71.3% of EAT was used for more than 4 days, and the median period was 9 days, which exceed that of USA by more than 5 days [27] and that of Norway by more than 4 days [5]. Although there remains controversy on antibiotics duration when the cultures are negative, a 48-hour course with negative culture is sufficient for rule-out sepsis and will result in dramatic reduction of antibiotic use [25]. Moreover, a lower BW and GA, longer mechanical ventilation time, Apgar score ≤ 7 at 5 minutes, or postnatal corticosteroid use were found to be associated with increased EAT use and prolonged EAT. The high rate of EAT in newborns may be due to the following reasons: (I) Physicians believe that premature infants or invasive procedures may be associated with bacterial infection; (II) Postnatal corticosteroid immunosuppression may cause infection according to the belief of Chinese doctors, but there is no evidence-based basis for this. It is worth recommending that several recent studies used delivery characteristics to identify premature infants at lower risk of EOS. Puopolo and Mukhopadhyay conducted a study of 15,433 infants born at 22 to 28 weeks’ gestation in Neonatal Research Network centers from 2006 to 2014, those born by cesarean delivery with membrane rupture at delivery and absence of clinical chorioamnionitis were significantly less likely to have confirmed EOS [2]. Additionally, the neonatal EOS calculator is a clinical risk stratification tool increasingly used to guide the use of empirical antibiotics for newborns [28].
In recent years, numerous studies have demonstrated that unnecessary or long-term use of antibiotics can increase the risk of adverse clinical outcomes in premature infants, and prolonged use of broad-spectrum antibiotics can cause strong selective pressure on microorganisms, which will in turn induce drug-resistance [29]. In this study, a significant positive correlation was found between the risk of NEC ≥ stage 2 and EAT usage or prolonged EAT. Antibiotic-induced gut microbiota dysbiosis in preterm infants have been linked to the pathogenesis of NEC, which is consistent with our findings [30]. A large retrospective cohort study of 4039 extremely low birth weight infants by Cotten et al. [31], found that prolonged EAT (≥5 days) tended to be associated with NEC or death and NEC alone, with a ~ 4% increase in the odds of NEC or dying and a ~ 7% increase in the odds of NEC alone for each additional day of initial EAT. A meta-analysis of 13 studies involving 7901 premature infants, reported that the initial EAT ≥5 days correlated with the risk of NEC [32]. Similar associations were observed for hsPDA in our study. It has been reported that gentamicin, tobramycin, and other aminoglycoside antibiotics can relax arterial smooth muscle and delay the closure of PDA, with an increased risk of hsPDA [33]. However, no aminoglycoside antibiotics were used in our case. There is a paucity of biological data explaining the mechanism between antibiotic exposure and hsPDA, and whether other antibiotics have this adverse effect needs further study.
Moreover, clinicians tended to suspect infections when VPIs showed increased heart rate, blood pressure fluctuation and shortness of breath before the diagnosis of hsPDA, which resulted an increase in EAT.
Our study suggests that early empiric antibiotic exposure may result in decreased weight growth velocity, increased duration of reach of full enteral feeding, and higher risk of feeding intolerance among VPIs. There is a growing literature on the deleterious impact of antibiotics on neonatal nutritional outcomes. Martinez et al.found that early empiric antibiotic use was associated with delayed feeding tolerance in premature infants [34]. The gut microbiota of preterm infants influence nutrient absorption and this may be disrupted following antibiotics use [35]. An immature ontogenesis of the enteric microbiota may affect the pathophysiology of feeding intolerance [36]. Although there is no difference in the incidence of EUGR at 36 weeks of GA, our study points out that prolonged EAT has adverse effects on nutrition management of VPIs in a short-term process, which is not conducive to the nutritional development of VPIs during this period and may may contribute to NEC. On the basis of these results, we recommend that EAT should be avoided where possible, and the duration of antibiotic use should be shortened to reduce the incidence of adverse nutritional outcomes in premature infants, which can potentially reduce time of hospital stay and may save health-care resources.
This study had several limitations. Firstly, some indicators such as chorioamnionitis during pregnancy, antibiotics during pregnancy, cesarean section with no labor onset, neonatal umbilical vascular catheterization, central venous catheterization, and pulmonary surfactant use were not considered because of too many missing values. These confounding factors may have influenced the clinical outcomes. However, after adjusting for known confounding factors, the conclusion that prolonged use of EAT during hospitalization was associated with adverse effects on the clinical outcomes and nutrition management of VPIs did not change. Secondly, the data presented here come from a prospective multicenter study on factors influencing VPIs-EUGR in China, which excluded patients who died during hospitalization. Therefore, the effect of EAT on death as a clinical outcome was not studied. Thirdly, there is no equipoise at neonatal pediatrics treatment level and the policies of care in hospitals in China, which may also affect the short-term clinical outcome of newborns.
Conclusions
In this study, we found that the utilization rate of EAT starting within 1 week after birth among VPIs was high despite absence of infection, particularly in younger gestational age and lower birth weight groups. Prolonged EAT within the first postnatal week was associated with decreased weight growth velocity, longer duration of reach of full enteral feeding, increased risk of feeding intolerance and NEC ≥ stage 2 after the first postnatal week. Therefore, stronger policies regarding the initiation and continuation of EAT among VPIs should be created to reduce the frequency and duration of EAT in Chinese NICUs.
Availability of data and materials
The datasets generated and/or analysed during the current study are not publicly available but are available from the corresponding author on reasonable request. Due to the data were used under license for the current study, and so are not publicly available.
Abbreviations
- EAT:
-
Empirical antibiotics treatment
- VPIs:
-
Very preterm infants
- FI:
-
Feeding intolerance
- NEC:
-
Necrotizing enterocolitis
- HAI:
-
Hospital-acquired infections
- hsPDA:
-
hemodynamically significant patent ductus arteriosus
- NICU:
-
Neonatal intensive care unit
- VLBWI:
-
Very low birth weight infants
- ELBWI:
-
Extremely low birth weight infants
- BPD:
-
Bronchopulmonary dysplasia
- LOS:
-
Late-onset sepsis
- EUGR:
-
Extrauterine growth retardation
- GA:
-
Gestational age
- BW:
-
Birth weight
- EOS:
-
Early-onset sepsis
- CSF:
-
Cerebrospinal fluid
- RDS:
-
Respiratory distress syndrome
- FI:
-
Feeding intolerance
- IVH:
-
Intraventricular hemorrhage
- PVL:
-
Periventricular leukomalacia
- PNAC:
-
Parenteral nutrition associated cholestasis
- ROP:
-
Retinopathy of prematurity
- SGA:
-
Gestational age
- GDM:
-
Gestational diabetes mellitus
- CDC:
-
Centers for Disease Control and Prevention
References
Perin J, Mulick A, Yeung D, Villavicencio F, Lopez G, Strong KL, et al. Global, regional, and national causes of under-5 mortality in 2000-19: an updated systematic analysis with implications for the sustainable development goals. Lancet Child Adolesc Health. 2022;6(2):106–15. https://doi.org/10.1016/S2352-4642(21)00311-4.
Puopolo KM, Mukhopadhyay S, Hansen Pupp I, Cotten CM, Stoll BJ, Sanchez PJ, et al. Identification of extremely premature infants at low risk for early-onset Sepsis. Pediatrics. 2017;140(5):e20170925. https://doi.org/10.1542/peds.2017-0925.
Flannery DD, Ross RK, Mukhopadhyay S, Tribble AC, Puopolo KM, Gerber JS. Temporal trends and center variation in early antibiotic use among premature infants. JAMA Netw Open. 2018;1(1):e180164. https://doi.org/10.1001/jamanetworkopen.2018.0164.
Mukhopadhyay S, Sengupta S, Puopolo KM. Challenges and opportunities for antibiotic stewardship among preterm infants. Arch Dis Child Fetal Neonatal Ed. 2019;104(3):F327–32. https://doi.org/10.1136/archdischild-2018-315412.
Vatne A, Hapnes N, Stensvold HJ, Dalen I, Guthe HJ, Støen R, et al. Early empirical antibiotics and adverse clinical outcomes in infants born very preterm: a population-based cohort. J Pediatr. 2022;S0022-3476(22):00851–4. https://doi.org/10.1016/j.jpeds.2022.09.029.
Ting JY, Synnes A, Roberts A, Deshpandey A, Dow K, Yoon EW, et al. Association between antibiotic use and neonatal mortality and morbidities in very low-birth-weight infants without culture-proven Sepsis or necrotizing enterocolitis. JAMA Pediatr. 2016;170(12):1181–7. https://doi.org/10.1001/jamapediatrics.2016.2132.
Ting JY, Roberts A, Sherlock R, Ojah C, Cieslak Z, Dunn M, et al. Duration of initial empirical antibiotic therapy and outcomes in very low birth weight infants. Pediatrics. 2019;143(3):e20182286. https://doi.org/10.1542/peds.2018-2286.
Slob EMA, Brew BK, Vijverberg SJH, Kats CJAR, Longo C, Pijnenburg MW, et al. Early-life antibiotic use and risk of asthma and eczema: results of a discordant twin study. Eur Respir J. 2020;55(4):1902021. https://doi.org/10.1183/13993003.02021-2019.
Saari A, Virta LJ, Sankilampi U, Dunkel L, Saxen H. Antibiotic exposure in infancy and risk of being overweight in the first 24 months of life. Pediatrics. 2015;135(4):617–26. https://doi.org/10.1542/peds.2014-3407.
Jiang S, Zhang L, Yan W, Li S, Han J, Zhou Q, et al. Antibiotic use in neonatal intensive care units in China: a multicenter cohort study. J Pediatr. 2021;239:136–142.e4. https://doi.org/10.1016/j.jpeds.2021.08.067.
Stocker M, van Herk W, EI Helou S, Dutta S, Fontana MS, FABA S, et al. Procalcitonin-guided decision making for duration of antibiotic therapy in neonates with suspected early-onset sepsis: a multicentre, randomised controlled trial (NeoPIns). Lancet. 2017;390(10097):871–81. https://doi.org/10.1016/S0140-6736(17)31444-7.
Subspecialty group of neonatology, the society of pediatric, Chinese medical association; Professional committee of infectious diseases, Neonatology Society, Chinese Medical Doctor Association. Expert consensus on the diagnosis and management of neonatal sepsis (version 2019). Chin J Pediatr. 2019;57(4):252–7. https://doi.org/10.3760/cma.j.issn.0578-1310.2019.04.005.
Patel AL, Engstrom JL, Meier PP, Kimura RE. Accuracy of methods for calculating postnatal growth velocity for extremely low birth weight infants. Pediatrics. 2005;116(6):1466–73. https://doi.org/10.1542/peds.2004-1699.
Fenton TR, Kim JH. A systematic review and meta-analysis to revise the Fenton growth chart for preterm infants. BMC Pediatr. 2013;13:59. https://doi.org/10.1186/1471-2431-13-59.
Evidence-Based Medicine Group, Neonatologist society, Chinese Medical Doctor Association. Clinical guidelines for the diagnosis and treatment of feeding intolerance in preterm infants (2020). Chin J Contemp Pediatr. 2020;22(10):1047–55. https://doi.org/10.7499/j.issn.1008-8830.2008132.
Gilfillan M, Bhandari A, Bhandari V. Diagnosis and management of bronchopulmonary dysplasia. BMJ. 2021;375:n1974. https://doi.org/10.1136/bmj.n1974.
Jain A, Shah PS. Diagnosis, evaluation, and management of patent ductus arteriosus in preterm neonates. JAMA Pediatr. 2015;169(9):863–72. https://doi.org/10.1001/jamapediatrics.2015.0987.
Bell MJ, Ternberg JL, Feigin RD, Keating JP, Marshall R, Barton L. Neonatal necrotizing enterocolitis. Therapeutic decisions based upon clinical staging. Ann Surg. 1978;187(1):1–7. https://doi.org/10.1097/00000658-197801000-00001.
Papile LA, Burstein J, Burstein R, Koffler H. Incidence and evolution of subependymal and intraventricular hemorrhage: a study of infants with birth weights less than 1,500 gm. J Pediatr. 1978;92(4):529–34. https://doi.org/10.1016/s0022-3476(78)80282-0.
Chiang MF, Quinn GE, Fielder AR, Ostmo SR, Paul Chan RV, Berrocal A, et al. International classification of retinopathy of prematurity, Third Edition. Ophthalmology. 2021;128(10):e51–68. https://doi.org/10.1016/j.ophtha.2021.05.031.
Shao XM, Ye HM, Qiu XS. Practice of neonatology. 5th ed. Beijing: People’s Medical Publishing House; 2019.
Tzialla C, Borghesi A, Serra G, Stronati M, Corsello G. Antimicrobial therapy in neonatal intensive care unit. Ital. J Pediatr. 2015;41:27. https://doi.org/10.1186/s13052-015-0117-7.
Rudd KE, Johnson SC, Agesa KM, Shackelford KA, Tsoi D, Kievlan DR, et al. Global, regional, and national sepsis incidence and mortality, 1990-2017: analysis for the global burden of disease study. Lancet. 2020;395(10219):200–11. https://doi.org/10.1016/S0140-6736(19)32989-7.
Cantey JB, Lee JH. Biomarkers for the diagnosis of neonatal Sepsis. Clin Perinatol. 2021;48(2):215–27. https://doi.org/10.1016/j.clp.2021.03.012.
Cantey JB, Wozniak PS, Pruszynski JE, Sánchez PJ. Reducing unnecessary antibiotic use in the neonatal intensive care unit (SCOUT): a prospective interrupted time-series study. Lancet Infect Dis. 2016;16(10):1178–84. https://doi.org/10.1016/S1473-3099(16)30205-5.
Wang M, Yue S, Lin J, Gao XR, Peng XM, Chen MY, et al. A multicenter survey of antibiotic use in very and extremely low birth weight infants in Hunan Province. Chin J Contemp Pediatr. 2020;22(6):561–6. https://doi.org/10.7499/j.issn.1008-8830.1912085.
van Herk W, el Helou S, Janota J, Hagmann C, Klingenberg C, Staub E, et al. Variation in current Management of Term and Late-preterm Neonates at risk for early-onset Sepsis: an international survey and review of guidelines. Pediatr Infect Dis J. 2016;35(5):494–500. https://doi.org/10.1097/INF.0000000000001063.
Laccetta G, Ciantelli M, Tuoni C, Sigali E, Miccoli M, Cuttano A. Early-onset sepsis risk calculator: a review of its effectiveness and comparative study with our evidence-based local guidelines. Ital J Pediatr. 2021;47(1):73. https://doi.org/10.1186/s13052-021-01028-1.
Bell BG, Schellevis F, Stobberingh E, Goossens H, Pringle M. A systematic review and meta-analysis of the effects of antibiotic consumption on antibiotic resistance. BMC Infect Dis. 2014;14:13. https://doi.org/10.1186/1471-2334-14-13.
Baranowski JR, Claud EC. Necrotizing enterocolitis and the preterm infant microbiome. Adv Exp Med Biol. 2019;1125:25–36. https://doi.org/10.1007/5584_2018_313.
Cotten CM, Taylor S, Stoll B, Goldberg RN, Hansen NI, Sánchez PJ, et al. Prolonged duration of initial empirical antibiotic treatment is associated with increased rates of necrotizing enterocolitis and death for extremely low birth weight infants. Pediatrics. 2009;123(1):58–66. https://doi.org/10.1542/peds.2007-3423.
Rina P, Zeng Y, Ying J, Qu Y, Mu D. Association of initial empirical antibiotic therapy with increased risk of necrotizing enterocolitis. Eur J Pediatr. 2020;179(7):1047–56. https://doi.org/10.1007/s00431-020-03679-4.
Vucovich MM, Cotton RB, Shelton EL, Goettel JA, Ehinger NJ, Poole SD, et al. Aminoglycoside-mediated relaxation of the ductus arteriosus in sepsis-associated PDA. Am J Physiol Heart Circ Physiol. 2014;307(5):H732–40. https://doi.org/10.1152/ajpheart.00838.2013.
Martinez FE, Ferri WAG, Leone CR, de Almeida MFB, Guinsburg R, Meneses JDA, et al. Early empiric antibiotic use is associated with delayed feeding tolerance in preterm infants: a retrospective analysis. J Pediatr Gastroenterol Nutr. 2017;65(1):107–10. https://doi.org/10.1097/MPG.0000000000001490.
Yee AL, Miller E, Dishaw LJ, Gordon JM, Ji M, Dutra S, et al. Longitudinal microbiome composition and stability correlate with increased weight and length of very-low-birth-weight infants. mSystems. 2019;4(1):e00229–18. https://doi.org/10.1128/mSystems.00229-18.
Indrio F, Riezzo G, Tafuri S, Ficarella M, Carlucci B, Bisceglia M, et al. Probiotic supplementation in preterm: feeding intolerance and hospital cost. Nutrients. 2017;9(9):965. https://doi.org/10.3390/nu9090965.
Acknowledgments
We would like to thank all participants of the Chinese Multicenter EUGR Collaborative Group, which consisted of: Department of Neonatology, Women and Children’s Hospital, School of Medicine, Xiamen University, Xiamen, Fujian, China (YZ, QY, WS, LT, ZZ, and XL); Department of Neonatology, The Third Affiliated Hospital of Guangzhou Medical University, Guangzhou, Guangdong, China (FW, Qianxin Tian, and Qiliang Cui); Department of Pediatrics, Shengjing Hospital of China Medical University, Shenyang, Liaoning, China (JM, Yuan Yuan, and Ling Ren); Department of Neonatology, Guiyang Maternity and Child Health Hospital/Guiyang Children’s Hospital, Guiyang, Guizhou, China (LL, Bizhen Shi and Yumei Wang); Department of Pediatrics, Peking University Third Hospital, Beijing, China (YC, Jinghui Zhang, and XT); Department of Neonatology, Children’s Hospital of Fudan University, Shanghai, China (RZ, Yan Zhu); Department of Neonatology, Maternal and Child Hospital of Guangdong Province, Guangzhou, Guangdong, China (XY, Jingjing Zou); Department of Neonatology, General Hospital of Ningxia Medical University, Yinchuan, Ningxia, China (YQ, Yuhuai Li, and Baoyin Zhao); Department of Neonatology, Children’s Hospital of Hebei Province, Shijiazhuang, Hebei, China (Shuhua Liu and LM); Department of Neonatology, Children’ Hospital of Nanjing Medical University, Nanjing, Jiangsu, China (Ying Xu and RC); Department of Neonatology, The First Hospital of Jilin University, Changchun, Jilin, China (Wenli Zhou and HW); Department of Neonatology, Quanzhou Maternity and Children’s Hospital, Quanzhou, Fujian, China (Zhiyong Liu and DC); Department of Pediatrics, Tongji Hospital, Tongji Medical College, Huazhong University of Science and Technology, Wuhan, Hubei, China (Jinzhi Gao, Jing Liu, and Ling Chen); Department of Neonatology, Liaocheng People’s Hospital, Liaocheng, Shandong, China (Cong Li, Chunyan Yang, and Ping Xu); Department of Neonatology, the Affiliate Hospital of Inner Mongolia Medical University, Hohhot, Inner Mongolia, China (Yayu Zhang, Sile Hu, and Hua Mei); Department of Neonatology, Suzhou Municipal Hospital, Suzhou, Jiangsu, China (Zuming Yang, Zongtai Feng, and Sannan Wang); Department of Neonatology, The Third Affiliated Hospital of Zhengzhou University, Zhengzhou, Henan, China (Eryan Meng, Lihong Shang, and Falin Xu); Department of Neonatology, Chengdu Women’ and Children’s Central Hospital, School of Medicine, University of Electronic Science and Technology of China, Chengdu, Sichuan, China (Shaoping Ou and Rong Ju); Department of Neonatology, Hunan Children’s Hospital, Changsha, Hunan, China (Guinan Li and Juan Yi); Department of Neonatology, People’s Hospital of Xinjiang Uygur Autonomous Region, Urumqi, Xinjiang, China (Long Li and Yongqiao Liu); Department of Neonatology, Guangzhou Women and Children’s Medical Center, Guangzhou, Guangdong, China (Zhe Zhang and Meigui Wu); Department of Neonatology, Shanghai Children’s Medical Center, Shanghai, China (Fei Bei and Ye Liu); Department of Neonatology, Children’s Hospital of Chongqing Medical University, Chongqing, China (Chun Deng and Huijie Yang); Department of Neonatology, The First People’s Hospital of Yulin, Yulin,Guangxi, China (Ping Su and Shifeng Chen); Department of Neonatology, the People’s Hospital of Baoji, Baoji, Shanxi, China (Lingying Luo and Linlin Wang); Department of Pediatrics, Affiliated Hospital of Qingdao University, Qingdao, Shandong, China (Xiaohong Liu and Lihua Yan); Departments of Neonatology, Shandong Provincial Hospital Affiliated to Shandong First Medical University, Jinan, Shandong, China (Lijun Wang and Xiaokang Wang); and Departments of Neonatology, Xi’an Children’s Hospital, Xi’an, Shanxi, China (Shuqun Yu and Qiaomian Zhu).
Funding
This study was supported by Guidance Project of Xiamen Medical and Health in 2019 (3502Z20199077) and Guidance Project of Xiamen Medical and Health in 2021 (3502Z20214ZD1225).
Author information
Authors and Affiliations
Consortia
Contributions
XL, XT, and YZ conceptualized and designed the study. YZ, QY, FW, JM, LL, RZ, WS, LT, YC, XY,YQ, LM, RC, HW, DC, ZZ, and the other consortium members (the Chinese Multicenter EUGR Collaborative Group) carried out the clinical data collection. YZ, QY, FW, JM, LL, and RZ analyzed and interpreted the clinical data. YZ and QY wrote the first draft of this manuscript. XL, XT, and YZ reviewed and revised the manuscript. All authors read and approved the final manuscript.
Corresponding authors
Ethics declarations
Ethics approval and consent to participate
The study was approved by the Ethics Committee of the Women and Children’s Hospital, School of Medicine, Xiamen University (No. KY-2019-016), recognized by all participating hospitals. All methods were carried out in accordance with relevant guidelines and regulations. Written informed consent was obtained for the collection of data from all legal representatives of participants.
Consent for publication
Not applicable.
Competing interests
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Zhu, Y., Yang, Q., Wu, F. et al. The impact of early empirical antibiotics treatment on clinical outcome of very preterm infants: a nationwide multicentre study in China. Ital J Pediatr 49, 14 (2023). https://doi.org/10.1186/s13052-023-01414-x
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s13052-023-01414-x