Abstract
Background
Kawasaki disease (KD) is an acute febrile illness of unknown etiology and predictors for intravenous immunoglobulin (IVIG) resistance have been widely explored in recent decades. Neutrophil to lymphocyte platelet ratio (NLPR) was reported to be associated with the outcomes in many diseases. However, its relationship with IVIG resistance has not be explored.
Methods
The medical data of patients diagnosed with KD in Children’s Hospital of Soochow University between January 2019 and December 2020 were retrospectively reviewed and analyzed. Patients were trisected into three groups based on NLPR. Logistics regression was used to analyze the association between NLPR and IVIG resistance. Restricted cubic spine was used to exhibit the relationship. Sensitivity analysis and subgroup analysis were also carried out.
Results
A total of 803 patients were included in the present study (61.8% males; median age: 24 months). IVIG resistance occurred in 74 (9.2%) patients. Multivariable-adjusted analyses revealed higher NLPR (odds ratio [95% confidence interval]: 1.12 [1.00-1.24]) was an independent predictor of IVIG resistance, which was strengthened by sensitivity analyses. The association of NLPR and IVIG resistance was not modified by age, sex, CALs, or days of IVIG initiation ≤ 4.
Conclusion
NLPR may be a valuable prognostic marker in KD patients with IVIG resistance.
Similar content being viewed by others
Background
Kawasaki disease (KD) is an acute febrile illness of unknown etiology, which predominantly affects children under 5 years of age. With a predilection for coronary artery lesions (CALs), it has become the most common cause of acquired heart diseases in developed countries [1]. Given that intravenous immunoglobulin (IVIG) resistance is one of the most important predictors for CALs, predictors for IVIG resistance have been widely explored and prediction models have been extensively established in recent decades [2,3,4,5].
Among them were neutrophil to lymphocyte ratio (NLR) and platelet to lymphocyte ratio (PLR) [6, 7]. Recognized as potential biomarkers of baseline inflammatory process, both two ratios were now widely reported in cardiovascular diseases [8, 9], neurological diseases [10, 11], and rheumatic diseases [12, 13], et al. The low-cost and consequent wide and easy availability in daily clinical practice has made them popular in clinical practice, with a higher ratio often indicating a poor prognosis.
More recently, neutrophil to lymphocyte platelet ratio (NLPR), which was obtained by either NLR and platelet counts or neutrophil counts and PLR, was reported to have a predictive value in disease prognosis [14, 15]. Considering that both neutrophils and platelets were indicative of inflammation, we hypothesized that NLPR was also associated with IVIG resistance in KD. However, no study has been carried out to investigate its value in KD. In the present study, we aimed to explore the association between NLPR and IVIG resistance.
Methods
Study population
We retrospectively reviewed and collected the medical data of patients diagnosed with KD in Children’s Hospital of Soochow University between January 2019 and December 2020. All patients were diagnosed based on American Heart Association (AHA) guidelines [1], and the first day of fever onset was defined as the first day of the disease. Patients were excluded if they: (1) had incomplete CALs status and NLPR value; (2) didn’t have IVIG treatment; (3) initiated IVIG in other hospitals. Patients were treated with a total dose of 2 g/kg of IVIG on diagnosis together with aspirin of 30–50 mg/kg/day in the acute stage, followed by 3–5 mg/kg/d aspirin after defervescence for at least 72 h. For patients with IVIG resistance, a second dose of IVIG, together with 2-4 mg/kg/day intravenous pulse methylprednisolone was administered.
Data collection
Peripheral blood samples were routinely obtained within 24 h on admission and repeated 72 h after the administration of IVIG, or as appropriate. The laboratory data included white blood cell count (WBC) and its subpopulations, hematocrit, erythrocyte sedimentation rate (ESR), c-reactive protein (CRP), alanine aminotransferase (ALT), aspartate transaminase (AST), serum albumin, serum sodium, and serum potassium. If a laboratory indicator was performed two or more times before IVIG treatment, the results from the most recent IVIG were recorded. All patients received transthoracic echocardiography to record the status of the coronary arteries during the acute phase and were repeated before discharge and at 4 weeks, or as appropriate. The missing data are summarized in Additional file 1.
The study was reviewed and approved by the Ethics Committee of Children’s Hospital of Soochow University (No: 2020CS159). Informed consent was waived because of the retrospective nature of the study.
Definitions
KD was diagnosed based on the 2017 AHA guidelines [1]. CALs were defined when Z-scores were larger than 2.5 based on the Dallaire equations [16], and (or) clear irregularity of the coronary artery intima. IVIG resistance was termed as a persistent or recrudescent fever ≥ 38.0℃ for more than 72 h after the initiation of IVIG [1]. NLPR was calculated as follows:
Statistical analyses
All statistical analyses in this study were performed using R (version 4.0.4). The data were presented as the mean ± standard deviation (SD) or median and quartiles for continuous variables and the comparisons were performed using one-way ANOVA or Kruskal–Wallis H test. Categorical variables were presented as numbers with percentages and were compared using chi-square or Fisher’s exact test. In the multivariable analyses, adjustments were made for potential confounders from the IVIG-resistant Kobayoshi’s prediction model [2] and from background knowledge such as age, sex, CRP, AST, ALT, serum albumin, and sodium, days of IVIG initiation ≤ 4. Percentages of neutrophils and platelet counts were not adjusted for the collinearity with NLPR. We excluded variables with incomplete data for more than 20%. Otherwise, we determined the missing data by multiple imputation methods. NLPR was trisected in T1 (< 33rd percentile) T2 (33rd − 67th percentile), T3 (≥ 67th percentile), and were compared. Area under receiver operating characteristic (ROC) curve (AUC) was also calculated. A two-sided P < 0.05 was considered statistically significant.
We additionally carried out sensitivity analyses. First, we grouped NLPR into < 20th percentile (T1), 20th − 80th percentile (T2), and ≥ 80th percentile (T3) and did the multivariable logistic regression again. Second, we used variables in the present study and selected the confounders. Variables with P < 0.20 in the univariate analysis were included in the multivariable analysis.
Given that there are two crossovers of peripheral neutrophils and lymphocytes in childhood, which occur between 4 ~ 6 days after birth and 4 ~ 6 years old, additional interaction analyses were performed to determine possible effect modification by the subgroups. We grouped all participants into < 48 months and ≥ 48 months since that KD scarcely occurred in neonates. We didn’t group patients ≥ 48 months in more groups because there were only 38 patients ≥ 72 months. Besides, interaction analyses for sex, CALs, delayed IVIG treatment, and days of IVIG initiation ≤ 4 were also carried out.
Results
Patient characteristics
A total of 803 patients were included in the final analysis (Fig. 1), including 442 (61.8%) males and 273 (38.2%) females, with a male-to-female ratio of 1.62. The median age was 24 months. As shown in Table 1, patients in T3 were older, less likely to be IKD, and less likely to have delayed IVIG initiation. Besides, they had higher levels of CRP, ESR, hematocrit, AST, ALT, and lower levels of albumin, serum sodium, and potassium (P < 0.01). Moreover, patients in T3 tended to have longer days of hospitalization. 74 (9.2%) had IVIG resistance. Notably, patients in T3 had a higher rate of IVIG resistance than those in T1 and T2 (14.9% in T3, 6.7% in T2, and 6.0% in T1, respectively, see Table 2. However, no significant difference in CALs was found among the three groups (P = 0.159).
Relationship between NLPR and IVIG resistance
The results of the logistic analysis of IVIG resistance are shown in Table 3. In the univariate analysis, NLPR as a continuous variable was negatively associated with IVIG resistance (P = 0.001). Even after adjusting the confounders, the difference remained significant (P = 0.040). When NLPR was trisected, patients with middle NLPR (T2) and high NLPR (T3) were associated with a higher incidence of IVIG resistance. However, the difference in T2 was insignificant (odds ratio [95% confidence interval]: 1.21 [0.58, 2.53]). T3 had a higher incidence of IVIG resistance in both the crude and multivariable-adjusted models (odds ratio [95% confidence interval]: 2.76 [1.53, 5.20] and 2.59 [1.23, 5.58], respectively). P for trend in both two models were statistically significant (P < 0.05). NLPR was determined to be independently associated with IVIG resistance (Fig. 2). Subgroup analysis showed age, sex, CALs, IKD, delayed IVIG treatment, and days of IVIG initiation ≤ 4 did not modify the association between NLPR and IVIG resistance (Fig. 3).
The adjusted odds ratio (OR) for NLPR for IVIG resistance. The solid line indicates the odds ratio of NLPR. The shaded area represents the 95% confidence interval (CI). Dots indicate the OR ratio according to each group. Error bars represent the 95% CI. The vertical dotted lines trisected patients. NLPR: neutrophil to lymphocyte platelet ratio; IVIG: intravenous immunoglobulin
Sensitivity analyses
Our findings were preserved across the sensitivity analyses (Table 4). NLPR as a continuous variable and as a categorical variable were both independently associated with IVIG resistance (P < 0.05). The comparisons of variables in patients with and without IVIG resistance are shown in Additional file 2. The logistic analyses of IVIG resistance using data in the present study are shown in Additional file 3. Age, serum albumin, NLPR, and days of IVIG initiation ≤ 5 were selected as independent predictors of IVIG resistance.
ROC analysis
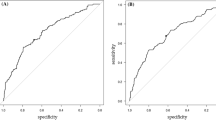
AUC of NLPR was 0.638 (95% confidence interval: [0.567–0.709]) with a sensitivity of 46% and a specificity of 81%. The cut-off value of NLPR was 1.8. The prediction model including NLPR, age, serum albumin, and days of IVIG initiation ≤ 4 had an AUC of 0.697 (95% confidence interval: [0.633–0.761]), with a sensitivity of 71% and a specificity of 64%.
Discussion
The retrospective study of 803 KD patients in the present study showed that a higher level of NLPR was associated with an increased incidence of IVIG resistance. Furthermore, the relationship was not modified by age, sex, CALs, IKD, or days of IVIG initiation.
The positive correlation between NLPR and IVIG resistance was in consistent with what we expected in clinical practice as elevated neutrophils and reduced platelet counts were both considered predictors of IVIG resistance in previous studies [2, 4, 5, 17]. On the other hand, a low level of lymphocyte count was also identified as an independent predictor in a recent study [17]. However, all these prediction models assessed neutrophils, lymphocytes, and platelet counts separately. Accordingly, percentages or absolute cell counts were used in each study with no unanimous standards.
NLPR, which was calculated by neutrophils, lymphocytes, and platelet counts, partly reflected the potential roles of all these variables simultaneously. Neutrophils are the most abundant type of white blood cells in peripheral blood and play crucial roles in inflammation and infection. A sudden increase in neutrophils led to increased oxygen intermediate, neutrophil elastase, and myeloperoxidase in the acute stage of KD [18,19,20], which on one hand caused self-destruction and on the other hand, reflected disease severity. Our results showed that the absolute count of lymphocytes and percentage of lymphocytes were both significantly lower in patients with IVIG resistance, which was in line with previous studies [17, 21]. The decrease in lymphocytes was a consequence of dynamically changed cell abundances in the acute stage of KD, with B cells decreasing and T cells varying among different subgroups [22].
Previous studies had pointed out that neutrophils infiltrated both the myocardium and the coronary arteries of the autopsied KD patients [23, 24], indicating a potential role of neutrophils in CALs and myocarditis. However, higher NLPR was not recognized as a predictor of CALs in the present study. Prediction of CALs, together with IVIG resistance was the issue of major interest in KD. However, although CALs were the consequence of disease severity and IVIG resistance was a form of disease severity, the risk factors for the two were not quite the same. The potential reasons still lay in the pathogenesis of KD and remained to be explored.
Of note, we found that patients with higher NLPR tended to have a more severe disease course, which was indicated by other laboratory variables, such as higher levels of CRP, ESR, AST, ALT, and lower levels of serum albumin, serum sodium, and potassium, almost all of which were referred to IVIG resistance in previous studies [1,2,3,4,5]. Besides, patients with higher NLPR were older and less likely to have delayed treatment, which could be explained by lymphocyte dominance in white blood cells in healthy children under 4–6 years old and a surge of platelets in the later stage of the disease course [25]. However, neither age nor days of IVIG initiation modified the relationship between NLPR and IVIG resistance. The result remained the same regarding sex, CALs, and IKD.
There were some limitations in the present study. First, selection biases existed due to the retrospective nature of the study. Second, numbers of prediction models had been established all over the world, with none of them having excellent performance in all populations [26,27,28]. The reasons lay in the methods to establish the models, the variables included in the analysis, the different genetic backgrounds of the study population, et al. Here we identified NLPR, age, serum albumin, and days of IVIG initiation ≤ 4 as predictors of IVIG resistance in the study population. Unfortunately, the performance of the prediction model was not excellent with regards to its AUC of 0.697. Further studies are warranted to explore a more accurate and applicable model in this area.
Conclusion
NLPR may be a valuable prognostic marker in KD patients with IVIG resistance.
Availability of data and materials
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Abbreviations
- NLPR :
-
Neutrophil to lymphocyte platelet ratio
- IVIG :
-
Intravenous immunoglobulin
- KD :
-
Kawasaki disease
- CALs :
-
Coronary artery lesions
- NLR :
-
Neutrophil to lymphocyte ratio
- PLR :
-
Platelet to lymphocyte ratio
- AHA :
-
American Heart Association
- WBC :
-
White blood cell
- ESR :
-
Erythrocyte sedimentation rate
- CRP :
-
C-reaction protein
- ALT :
-
Alanine aminotransferase
- AST :
-
Aspartate transaminase
- SD :
-
Standard deviation
- CI :
-
Confidence interval
- ROC :
-
Receiver operating characteristic
- AUC :
-
Area under the curve
References
McCrindle BW, Rowley AH, Newburger JW, Burns JC, Bolger AF, Gewitz M, et al. Diagnosis, treatment, and long-term management of Kawasaki Disease: A Scientific Statement for Health Professionals from the American Heart Association. Circulation. 2017;135(17):e927-e99.
Kobayashi T, Inoue Y, Takeuchi K, Okada Y, Tamura K, Tomomasa T, et al. Prediction of intravenous immunoglobulin unresponsiveness in patients with Kawasaki disease. Circulation. 2006;113(22):2606–12.
Sano T, Kurotobi S, Matsuzaki K, Yamamoto T, Maki I, Miki K, et al. Prediction of non-responsiveness to standard high-dose gamma-globulin therapy in patients with acute Kawasaki disease before starting initial treatment. Eur J Pediatr. 2007;166(2):131–7.
Egami K, Muta H, Ishii M, Suda K, Sugahara Y, Iemura M, et al. Prediction of resistance to intravenous immunoglobulin treatment in patients with Kawasaki disease. J Pediatr. 2006;149(2):237–40.
Tang Y, Yan W, Sun L, Huang J, Qian W, Ding Y, et al. Prediction of intravenous immunoglobulin resistance in Kawasaki disease in an East China population. Clin Rheumatol. 2016;35(11):2771–6.
Kawamura Y, Takeshita S, Kanai T, Yoshida Y, Nonoyama S. The combined usefulness of the neutrophil-to-lymphocyte and platelet-to-lymphocyte ratios in Predicting Intravenous Immunoglobulin Resistance with Kawasaki Disease. J Pediatr. 2016;178:281–4 e1.
Li C, Wu S, Shi Y, Liao Y, Sun Y, Yan H, et al. Establishment and validation of a Multivariate Predictive Scoring Model for Intravenous Immunoglobulin-Resistant Kawasaki Disease: a study of children from two Centers in China. Front Cardiovasc Med. 2022;9:883067.
Angkananard T, Inthanoo T, Sricholwattana S, Rattanajaruskul N, Wongsoasu A, Roongsangmanoon W. The predictive role of Neutrophil-to-lymphocyte ratio (NLR) and Mean platelet volume-to-lymphocyte ratio (MPVLR) for Cardiovascular events in adult patients with Acute Heart failure. Mediators Inflamm. 2021;2021:6889733.
Seropian IM, Romeo FJ, Pizarro R, Vulcano NO, Posatini RA, Marenchino RG, et al. Neutrophil-to-lymphocyte ratio and platelet-to-lymphocyte ratio as predictors of survival after heart transplantation. ESC Heart Fail. 2018;5(1):149–56.
Gong P, Liu Y, Gong Y, Chen G, Zhang X, Wang S, et al. The association of neutrophil to lymphocyte ratio, platelet to lymphocyte ratio, and lymphocyte to monocyte ratio with post-thrombolysis early neurological outcomes in patients with acute ischemic stroke. J Neuroinflammation. 2021;18(1):51.
Xu L, Gao TX, Chang SH, Jiang SM, Zhang LJ, Yang L. Role of lymphocyte-related immune-inflammatory biomarkers in detecting early progression of Guillain-Barre syndrome. J Clin Neurosci. 2022;105:31–6.
Erre GL, Paliogiannis P, Castagna F, Mangoni AA, Carru C, Passiu G, et al. Meta-analysis of neutrophil-to-lymphocyte and platelet-to-lymphocyte ratio in rheumatoid arthritis. Eur J Clin Invest. 2019;49(1):e13037.
Gasparyan AY, Ayvazyan L, Mukanova U, Yessirkepov M, Kitas GD. The platelet-to-lymphocyte ratio as an inflammatory marker in Rheumatic Diseases. Ann Lab Med. 2019;39(4):345–57.
Li S, Yu S, Qin J, Peng M, Qian J, Zhou P. Prognostic value of platelet count-related ratios on admission in patients with pyogenic liver abscess. BMC Infect Dis. 2022;22(1):636.
Nooh HA, Abdellateif MS, Refaat L, Kandeel EZ, Bayoumi A, Samra M, et al. The role of inflammatory indices in the outcome of COVID-19 cancer patients. Med Oncol. 2021;39(1):6.
Dallaire F, Dahdah N. New equations and a critical appraisal of coronary artery Z scores in healthy children. J Am Soc Echocardiogr. 2011;24(1):60–74.
Wu S, Liao Y, Sun Y, Zhang CY, Zhang QY, Yan H, et al. Prediction of intravenous immunoglobulin resistance in Kawasaki disease in children. World J Pediatr. 2020;16(6):7.
Niwa Y, Sohmiya K. Enhanced neutrophilic functions in mucocutaneous lymph node syndrome, with special reference to the possible role of increased oxygen intermediate generation in the pathogenesis of coronary thromboarteritis. J Pediatr. 1984;104(1):56–60.
Biezeveld MH, van Mierlo G, Lutter R, Kuipers IM, Dekker T, Hack CE, et al. Sustained activation of neutrophils in the course of Kawasaki disease: an association with matrix metalloproteinases. Clin Exp Immunol. 2005;141(1):183–8.
Yoshimura K, Tatsumi K, Iharada A, Tsuji S, Tateiwa A, Teraguchi M, et al. Increased nitric oxide production by neutrophils in early stage of Kawasaki disease. Eur J Pediatr. 2009;168(9):1037–41.
Tan XH, Zhang XW, Wang XY, He XQ, Fan C, Lyu TW, et al. A new model for predicting intravenous immunoglobin-resistant Kawasaki disease in Chongqing: a retrospective study on 5277 patients. Sci Rep. 2019;9(1):1722.
Xie Z, Huang Y, Li X, Lun Y, Li X, He Y, et al. Atlas of circulating immune cells in Kawasaki disease. Int Immunopharmacol. 2022;102:108396.
Takahashi K, Oharaseki T, Naoe S, Wakayama M, Yokouchi Y. Neutrophilic involvement in the damage to coronary arteries in acute stage of Kawasaki disease. Pediatr Int. 2005;47(3):305–10.
Harada M, Yokouchi Y, Oharaseki T, Matsui K, Tobayama H, Tanaka N, et al. Histopathological characteristics of myocarditis in acute-phase Kawasaki disease. Histopathology. 2012;61(6):1156–67.
Tremoulet AH, Jain S, Chandrasekar D, Sun X, Sato Y, Burns JC. Evolution of laboratory values in patients with Kawasaki disease. Pediatr Infect Dis J. 2011;30(12):1022–6.
Qian W, Tang Y, Yan W, Sun L, Lv H. A comparison of efficacy of six prediction models for intravenous immunoglobulin resistance in Kawasaki disease. Ital J Pediatr. 2018;44(1):33.
Song R, Yao W, Li X. Efficacy of four Scoring Systems in Predicting Intravenous Immunoglobulin Resistance in Children with Kawasaki Disease in a Children’s hospital in Beijing, North China. J Pediatr. 2017;184:120–4.
Davies S, Sutton N, Blackstock S, Gormley S, Hoggart CJ, Levin M, et al. Predicting IVIG resistance in UK Kawasaki disease. Arch Dis Child. 2015;100(4):366–8.
Acknowledgements
Not applicable.
Funding
This work was financially supported by the National Natural Science Foundation of China (grant number 81700439, 82000467, and 81900450), Gusu health personnel training project (grant number GSWS2019048, 2020054), Suzhou Science and Technology Development Plan Project (grant number SS202066), and Jiangsu province key medical young talents (grant number QNRC 2016765).
Author information
Authors and Affiliations
Contributions
YYL analyzed data and wrote the manuscript. YJT designed the study and revised the manuscript. BW, XL, QQX and HC collected and analyzed data. HTL designed the study. MHL and YMQ interpreted the data. All authors reviewed and approved the manuscript.
Corresponding authors
Ethics declarations
Ethics approval and consent to participate
The study was reviewed and approved by the Ethics Committee of Children’s Hospital of Soochow University (No: 2020CS159). Informed consent was waived because of the retrospective nature of the study. All methods were performed in accordance with the ethical standards as laid down in the Declaration of Helsinki and its later amendments or comparable ethical standards.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Additional file 1.
Missing values. Shows the missing data in laboratory variables.
Additional file 2.
Comparisons of variables in patients with and without IVIG resistance. Shows the comparisons of variables in patients with and without IVIGresistance.
Additional file 3.
Logistic analysis of IVIG resistance. Shows the univariate and multivariable logistic analyses of IVIGresistance using the present dataset
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Lu, Y., Tang, Y., Wang, B. et al. Predicting immunoglobulin resistance in Kawasaki disease: an assessment of neutrophil to lymphocyte platelet ratio. Ital J Pediatr 48, 208 (2022). https://doi.org/10.1186/s13052-022-01400-9
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s13052-022-01400-9