Abstract
Importance
Emergency medical services (EMS) providers transiently ascend to high altitude for primary missions and secondary transports in mountainous areas in helicopters that are unpressurised and do not have facilities for oxygen supplementation. The decrease in cerebral oxygen saturation can lead to impairment in attention and reaction time as well as in quality of care during acute exposure to altitude.
Objective
The primary aim of the current study was to investigate the effect of oxygen supplementation on cognitive performance in Helicopter EMS (HEMS) providers during acute exposure to altitude.
Design, setting, and participants
This interventional, randomized, controlled, double-blind, cross-over clinical trial was conducted in October 2021. Each trial used a simulated altitude scenario equivalent to 4000 m, in which volunteers were exposed to hypobaric hypoxia with a constant rate of ascent of 4 m/s in an environmental chamber under controlled, replicable, and safe conditions. Trials could be voluntarily terminated at any time. Inclusion criteria were being members of emergency medical services and search and rescue services with an age between 18 and 60 years and an American Society of Anesthesiologists physical status class I.
Exposures
Each participant conducted 2 trials, one in which they were exposed to altitude with oxygen supplementation (intervention trial) and the other in which they were exposed to altitude with ambient air supplementation (control trial).
Main outcomes and measures
Measurements included peripheral oxygen saturation (SpO2), cerebral oxygenation (ScO2), breathing and heart rates, Psychomotor Vigilance Test (PVT), Digit-Symbol Substitution Test (DSST), n-Back test (2-BACK), the Grooved Pegboard test, and questionnaires on subjective performance, stress, workload, and positive and negative affect. Paired t-tests were used to compare conditions (intervention vs. control). Data were further analyzed using generalized estimating equations (GEE).
Results
A total of 36 volunteers (30 men; mean [SD] age, 36 [9] years; mean [SD] education, 17 [4] years) were exposed to the intervention and control trials. The intervention trials, compared with the control trials, had higher values of SpO2 (mean [SD], 97.9 [1.6] % vs. 86 [2.3] %, t-test, p = 0.004) and ScO2 (mean [SD], 69.9 [5.8] % vs. 62.1 [5.2] %, paired t-test, p = 0.004). The intervention trials compared with the control trials had a shorter reaction time (RT) on the PVT after 5 min (mean [SD], 277.8 [16.7] ms vs. 282.5 [15.3] ms, paired t-test, p = 0.006) and after 30 min (mean [SD], 276.9 [17.7] ms vs. 280.7 [15.0] ms, paired t-test, p = 0.054) at altitude. While controlling for other variables, there was a RT increase of 0.37 ms for each % of SpO2 decrease. The intervention trials showed significantly higher values for DSST number of correct responses (with a difference of mean [SD], 1.2 [3.2], paired t-test, p = 0.035). Variables in the intervention trials were otherwise similar to those in the control trials for DSST number of incorrect responses, 2-BACK, and the Grooved Pegboard test.
Conclusions and relevance
This randomized clinical trial found that oxygen supplementation improves cognitive performance among HEMS providers during acute exposure to 4000 m altitude. The use of oxygen supplementation may allow to maintain attention and timely reaction in HEMS providers. The impact of repeated altitude ascents on the same day, sleep-deprivation, and additional stressors should be investigated.
Trial registration NCT05073406, ClinicalTrials.gov trial registration.
Similar content being viewed by others
Introduction
Background
At high altitude (HA) there is lower barometric pressure and a lower partial pressure of oxygen in ambient air than at low altitude [1]. Emergency medical services (EMS) providers transiently ascend to HA for primary missions and secondary transports in mountainous areas in helicopters that are unpressurised and do not have facilities for oxygen supplementation [2]. They must provide medical treatment during potentially complex technical operations. In two randomized, controlled, single-blind, crossover trials, 48 providers active in Helicopter Emergency Medical Service (HEMS) showed impairments of attention and reaction time (RT), and of quality of care during simulated HA scenario [3, 4]. The impairments were correlated with the decreases in oxygen saturation. Other studies have shown cognitive impairment at altitudes above 3000 m with increases in procedural errors [5] and declines in working memory, and executive function or abstract reasoning [6, 7] in high-performance providers.
Importance
Crashes are one of the greatest hazards faced in both ambulance EMS [8] and HEMS missions [9, 10]. Reduced cognitive performance during HEMS missions at altitude could lead to accidents and to decreased quality of care. HEMS providers do not appear to be aware of the reduced performance during HA exposure [3, 4]. To our knowledge, no data have been reported on the efficacy of oxygen supplementation to prevent cognitive impairment in HEMS providers at altitude.
Goals of this investigation
The primary aim of the current study was to investigate the effect of oxygen supplementation on cognitive performance in HEMS providers during acute exposure to HA. We studied selected cognitive domains, including attention, working memory, psychomotor speed, fine motor skills, and visuomotor tracking. Secondary aims of the proposed study were to investigate subjective assessment of cognitive performance, mental stress, workload, and experienced positive and negative affect.
Methods
This interventional, randomized, controlled, double-blind, cross-over clinical trial was approved by the institutional review board of Bolzano (Protocol Number 0228969-BZ) and registered in ClinicalTrials.gov (Protocol Record NCT05073406). We conducted the study in adherence to the Declaration of Helsinki. All participants were informed about the possible risks of being exposed to altitude and gave written informed consent prior to enrollment. This study is reported following the Consolidated Standards Of Reporting Trials (CONSORT) reporting guideline.
Study population
The participants were healthy, unpaid, providers of EMS and search and rescue (SAR) services with occupational licenses. Participants of both sexes, with age between 18 and 60 years old and classified according to the American Society of Anesthesiologists (ASA) as class I were considered eligible [11]. Exclusion criteria were age below 18 years, age above 60 years, ASA class greater than I, a medical history of psychiatric disorders and neurological diseases, or severe altitude illness [12], and any acute disease. Participants were asked to avoid sleep deprivation and abstain from caffeine, alcohol consumption and smoking prior to the trials.
Randomization
A randomization list was created with the use of computer-generated pseudo-random numbers, balanced for sequence (intervention then control or control then intervention) and daytime (morning, afternoon). The sequence of cognition tests was randomized and balanced within each test session. Participants and researchers were blinded toward the oxygen supplementation.
Study protocol
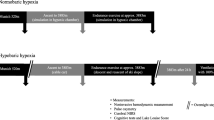
Each participant performed 2 trials (intervention and control) in a crossover design on the same day. Participants were divided into 9 groups of 4 participants each and the crossover design consisted of 2 study arms as shown in Fig. 1.
Experimental set-up. Panel a. Schematic representation of the study setting in the terraXcube in Bolzano, Italy, (on the left) for each study arm (4 participants) inside of the hypobaric chamber (upper panel) and of the control room (lower panel). Pictures (on the right) refer to the experimental setup inside the hypobaric chamber (upper panel) and in the control room (lower panel). Panel b. Study design. BR, breath rate; CT, cognitive tests; HR, heart rate; ScO2, cerebral oxygenation saturation; SpO2, peripheral oxygen saturation
In the intervention trial, each participant was exposed to altitude with continuous oxygen supplementation of 2 l/min via OxyMask (Southmedic Inc., Barrie, ON, Canada). In the placebo control trial, each participant was exposed to altitude with continuous ambient air supplementation of 2 l/min via OxyMask.
Prior to the study, participants completed a medical interview with a general medical visit (including self-assessments for sleep quality, depression/anxiety, perceived mental stress, and altitude exposure). Cognitive tests were administered three times during each trial: before the ascent (CT0), at 5 min (CT1) and 30 min (CT2) after the end of the ascent. After completing each cognitive test session, participants self-rated their cognitive performance and stress perception, as well as the positive and negative affect, and then performed the Grooved Pegboard test [13] twice, once with the dominant hand and once time with the non-dominant hand. At the end of each trial, participants rated their perceived workload.
Prior to the trial, participants rested for approximately 20–30 min to minimize anxiety and stress and performed a familiarization session of the cognitive tests and the Grooved Pegboard test. Trials could be voluntarily terminated at any time.
Setting and instrumentation
All trials were performed in the environmental chamber terraXcube, Eurac Research, Bolzano, Italy in October 2021 (Fig. 1). Experiments were conducted at a simulated altitude scenario equivalent to 4000 m, with volunteers exposed to hypobaric hypoxia at a constant rate of ascent of 4 m/s. terraXcube temperature, humidity, and carbon dioxide were continuously monitored and kept constant at normal indoor values.
Oxygen and air supplementation were delivered through OxyMask, designed to concentrate and redirect the flow of oxygen/air, preventing carbon dioxide re-breathing.
Belts for the monitoring system (Equivital EQ02, Hidalgo, UK) and sensors for oxygen saturation were fitted and participants received a technical introduction and safety briefing for terraXcube.
Cognitive tests were performed on dedicated laptops, which were placed on a separate desk for each participant. Participants were continuously monitored and guided (via radio commands) from the control room of terraXcube by research staff.
Measurements
Sustained attention was assessed with the Psychomotor Vigilance Test (PVT), visual attention and psychomotor speed with the Digit Symbol Substitution Test (DSST), and working memory with the 2-back Numerical (2-BACK) task. A brief 3-min version of the PVT was used, based on simple RT to visual stimuli, that occurred at random intervals varying from 2 to 5 s in steps of 200 ms, as previously described [3, 14,15,16,17]. In the PVT, RT (milliseconds [ms]) (excluding lapses and false starts), number of omission errors or “lapses” (defined as RTs ≥ 355 ms), false starts or errors of commission (defined as a response with no stimulus or RT < 100 ms), and performance score (defined as 1 minus the number of lapses and false starts divided by the number of valid stimuli including false starts ranging from 0 to 100%) were evaluated [15]. A computerised version of the DSST with 9 specific nonsense symbols was used, as previously described [3, 14, 18]. Test duration was fixed at 90 s, and the legend key was randomly reassigned at each administration. In the DSST, mean total number of correct and incorrect response pairs were evaluated. A 2-back Numerical (2-BACK) task of the n-back Numerical test was used [19]. Participants had to identify and indicate if the item currently presented was the same as the item presented 2 items earlier. In the 2-BACK, number of correct responses, number of missed responses, number of incorrect responses, mean RT of both correct and incorrect responses were evaluated. The software containing the three cognitive tests was installed on dedicated laptops as previously described [3, 14]. Six different versions of the DSST and the 2-BACK were administered across the multiple time points (test 1 and test 2, and CT0, CT1 and CT2) to avoid learning effects. The three tests were randomly assigned.
Fine motor skills, visuomotor tracking, and response speed (including motor inhibition and cognitive flexibility) were assessed with the Grooved Pegboard (Lafayette Instrument, Lafayette, IN, USA) [13]. Participants inserted 25 pegs into the grooved slots in a standardized order and as quickly as possible.
Perceived mental stress, anxiety and depression, and sleep quality were evaluated prior to the initiation of the study using the Perceived Stress Scale (PSS)-10 item [20], the Hospital Anxiety (HADS-A) and Depression (HADS-D) Scale [21], the Pittsburgh Sleep Quality Index (PSQI) [22], and the Insomnia Severity Index (ISI) [23]. Subjective performance and mental stress perception following each cognitive test session were evaluated using a visual analogue scale (VAS) [24]. Participants placed a mark on a 100-mm VAS horizontally positioned with the extremes labelled very good-very bad and low stress-high stress. Positive and negative affect were evaluated by the Positive and Negative Affect Schedule (PANAS) scale [25]. Participants rated their perceived workload using the National Aeronautics and Space Administration Task Load Index (NASA-TLX) questionnaire [26].
Physiological parameters measured continuously and noninvasively included heart rate (HR), derived from the 2-lead electrocardiogram, breathing rate (BR), SpO2 by a forehead sensor (EQ02, Hidalgo, UK), and ScO2 by near-infrared spectroscopy (NIRS), also by a forehead sensor (O3 Regional Oximetry, Masimo). The NIRS sensors were placed at a standardized frontotemporal location, high on the forehead to avoid any influences from the frontal or sagittal sinuses. The SpO2 sensor on the forehead was applied on the opposite side to the NIRS.
Statistical analysis
To achieve a power of 80% with p < 0.05, we estimated that we would need a sample size of 36 participants for an effect size of 0.67 between the oxygen supplementation tests and the control tests. For RT the clinically significant mean difference was 10 ± 15 ms [27].
We used SPSS for Windows software version 29.0 (SPSS, Chicago, IL, United States) to build the database and for statistical analysis. We used PRISM 10 (GraphPad Software) for visualizations. Continuous variables were expressed as mean and standard deviation (SD), whereas percentages were used for counted data. Paired t-tests were used to compare conditions (intervention vs. control). Data were further analyzed using generalized estimating equations (GEE) with condition and session (at 5 min and 30 min) as a within effect and using a first-order autoregressive (AR) [28] working correlation matrix. Predictors were condition (intervention, control), daytime (morning, afternoon), session, the interaction between daytime and session, gender, age above 33 years, education above 13 years, ISI score above 7, and the overall baseline (mean of morning and afternoon baseline measurements) as covariates. Instead of the condition effect, SpO2 at each timepoint was included in the model as a covariate. For graphical presentation, a heatmap was calculated showing the different means per session and variable for the conditions (intervention vs. control) [28]. For each variable z-scores were calculated and oriented so that higher values corresponded to better values. Colors were then assigned to all mean values according to percentiles.
We considered a two-sided p-value below 0.05 to be significant. We used a two-step rejection procedure to account for multiple hypothesis testing and to adjust p-values for a single predictor [29].
Results
Thirty-six volunteers were assessed for eligibility and enrolled to participate in the study (Fig. 2). All of them (30 men; mean [SD] age, 36 [8] years; mean [SD] education, 17 [4] years; 36 right-hand dominant) participated in both the intervention and control trials and were included in the data analysis. Characteristics of participants are reported in Table 1. Mean [SD] PSS score was 10.4 [5.4]. Mean [SD] ISI score was 3.7 [3.5]. Mean [SD] score of HADS-A was 2.9 [2.1] and HADS-D was 1.8 [1.6]. Mean [SD] PSQI score was 3.8 [1.8].
The heatmap shows the relatively different effects of oxygen supplementation in the intervention trials compared with the control trials on the parameters evaluated, with the green squares showing an improvement and the red squares showing a worsening (Fig. 3).
Heatmap representing the different means per session and variable for intervention (with O2) and control (without O2) conditions. For every variable z-scores were calculated and oriented such that higher values (green vs. red) correspond to better values. Colors were then assigned to all mean values according to percentiles. 2-BACK, 2-back Numerical task; BR, breath rate; DSST, Digit Symbol Substitution Test; HR, heart rate; mean, mean of values after 5 and 30 min; NASA-TLX, NASA Task Load Index; PANAS, Positive (P) and Negative (N) Affect Schedule; PEGBOARD, Grooved Pegboard test; PVT, Psychomotor Vigilance Test; RT, reaction time; ScO2, cerebral oxygenation saturation; SpO2, peripheral oxygen saturation; VAS, Visual Analogue Scale
The intervention trials compared with the control trials had higher values of mean SpO2 (mean [SD], 97.9 [1.6] % vs. 86.0 [2.3] %, paired t-test, p = 0.004) and mean ScO2 (mean [SD], 69.9 [5.8] % vs. 62.1 [5.2] %, paired t-test, p = 0.004) and comparable values for mean BR (mean [SD], 16.1 [2.9] bpm vs. 15.3 [3.0] bpm, paired t-test, p = 0.288). The intervention trials compared with the control trials had lower values of mean HR (mean [SD], 71.6 [10.7] bpm vs. 76.5 [10.1] bpm, paired t-test, p = 0.003) (Table 2 and Fig. 4 panel a,b,c).
Cognitive tests, physiological responses, performance perception and positive affect between intervention and control trials. Individual data is shown for intervention (blue) and control (red) for SpO2 (a), ScO2 (b), HR (c), PVT mean RT (d), DSST number of correct responses (g, VAS performance (h), and PANAS-P (i). Measurements of the same participant are connected by lines. Violin plots with 95% confidence intervals for the mean are shown in (e) for the within-person difference in reaction time in PVT separately for participants who had a baseline reaction time below or above 281 ms. A scatter plot with the respective regression line between SpO2 and the RT residual of the GEE model, when correcting for daytime, sequence, sex, age, ISI, HR, BR, and baseline RT, is shown in (f)
Primary outcomes
The intervention trials compared with the control trials had shorter RT on the PVT (mean [SD], 277.8 [16.7] ms vs. 282.5 [15.3] ms, paired t-test, p = 0.006) at 5 min and (mean [SD], 276.9 [17.7] ms vs. 280.7 [15.0] ms) paired t-test, p = 0.054) at 30 min as well as at the mean (mean of 5 and 30 min) (mean [SD], 277.3 [16.7] ms vs. 281.6 [14.5] ms, paired t-test, p = 0.005) (Table 2 and Fig. 4 panel d). The intervention trials compared with the control trials had similar performance scores and number of lapses on the PVT. The intervention trials compared with the control trials had a higher mean (mean of 5 and 30 min) number of correct responses (>mean [SD], 50.8 [6.5] vs. 49.6 [5.8], paired t- test, p = 0.035) and a similar number of incorrect responses on the DSST (Table 2 and Fig. 4 panel g). The intervention trials compared with the control trials had similar number of correct, missed, and incorrect responses, as well as the mean RT of both correct and incorrect responses on the 2-BACK (Table 2). The intervention trials compared with the control trials had shorter but not statistically significant different RTs in both the first part (mean [SD], 0.5 [0.1] ms vs. 0.6 [0.1] ms, paired t-test not corrected, p = 0.059) and the second part (mean [SD], 0.5 [0.1] ms vs. 0.6 [0.1] ms, paired t- test not corrected, p = 0.058) at 30 min. The intervention trials compared with the control trials had similar time to insert the pegs in the pegboard at the Grooved Pegboard test with the dominant hand (Table 2).
Secondary outcomes
The intervention trials compared with the control trials had lower but not statistically significant different mean VAS stress scores (mean [SD], 30.6 [21.1] mm vs. 36.7 [21.9] mm, paired t-test, p = 0.069), and mean VAS performance scores (mean [SD], (37.4 [17] mm vs. 45.3 [18.6] mm, paired t-test, p = 0.060) (Table 2 and Fig. 4 panel h). The intervention trials compared with the control trials had higher but not statistically significant different mean PANAS-P scores (mean [SD], 31.4 [7.3] vs. 29.1 [8.2]), paired t-test, p = 0.063 (Table 2 and Fig. 4 panel i). The intervention trials compared with the control trials had similar mean PANAS-N scores. The intervention trials compared with the control trials had similar mean NASA-TLX scores.
Other analyses for the primary outcomes
GEE analysis confirmed an independent effect of condition (intervention vs. control trials) on RT on the PVT (GEE, p = 0.017). GEE analysis also showed an independent influence of ISI on RT on the PVT (GEE, p = 0.026) (Table 3). Without condition effect, SpO2 showed a significant effect on RT on the PVT (GEE beta − 0.37, p < 0.001). Thus, for each % decrease in SpO2, there was an increase in RT of 0.37 ms (Fig. 4 panel f). Volunteers who were faster at baseline (RT ≤ 281 ms) showed a greater slowing of RT compared to those who were slower at baseline (RT > 281 ms) (mean difference in RT [SD] − 0.9 [8.9] ms, one sample t-test, p = 0.011 vs. − 2.6 [7.7] ms, one sample t-test, p = 0.171) (Fig. 4 panel e).
GEE analysis showed no independent influence of condition (intervention vs. control trials) on performance score (GEE, p = 0.904) and the number of lapses on the PVT (GEE, p = 0.694), and on the parameters analyzed on the DSST, 2-BACK and the Grooved Pegboard test (Table 3).
Discussion
This randomized clinical trial found that oxygen supplementation improves cognitive performance in HEMS providers during acute exposure to an altitude equivalent to 4000 m. The exposure in the control trial induced reduced sustained attention and timely reactions (i.e., slowing of RT). The increase in RT was inversely correlated to the decrease in oxygen saturation. Oxygen supplementation blunted the decrease in sustained attention and slowing of RT seen in the control trials. This suggests that the use of oxygen supplementation may be an effective countermeasure to improve occupational safety and health in providers of HEMS services operating at high altitude.
Cognitive impairment at HA is well known in aviation both in military and commercial contexts [30]. The rate and the length of hypoxia exposure in aviation compared to helicopter operations is substantially different. Previous studies demonstrated cognitive impairment at altitudes above 3000 m [5, 6, 31,32,33,34]. Nesthus et al. [5] reported more procedural errors during simulated flights in 20 private pilots at 3048 m and 3810 m. Bouak et al. [6] reported a decline in cognitive performance (i.e., short-term and working memory, executive function) at 4267 m and a lower positive mood (assessed with PANAS) in 16 military helicopter pilots. Pilmanis et al. [31] showed a minimal negative effect of simulated hypobaric hypoxia at 3658 m on cognitive performance (working memory and abstract reasoning) in 91 participants from military personnel. Peacock et al. [32] showed executive functioning impairment in 10 pilots at the simulated altitude of 3810 m with no effect on flight performance. Steinman et al. [33,34,35] showed that in helicopter crews exposed to a simulated altitude of 4572 m there was reduced alertness and awareness of the environment, decreased flight performance and increased RT [33,34,35] while there was no significant effect on flight performance at 3048 m [35]. We previously showed that acute exposure to an altitude of 5000 m of HEMS providers resulted in a slowing of RT that was not subjectively perceived, while psychomotor speed and decision making were not affected [3]. We found a decrease in sustained attention and lengthening of reaction times at 4000 m in EMS and SAR providers but no significant impairment of working memory, fine motor skills, visuomotor tracking and psychomotor speed. Increased reaction times were inversely correlated with the decrease in oxygen saturation, confirming previous findings that cognitive impairments at simulated altitude below 4000 m are insignificant [3, 7, 31].
There is an individual susceptibility and variability in responding to hypoxia (e.g., extent of the hypoxic ventilatory response, increased parasympathetic or sympathetic activity). Previous studies in aviation reported hypoxia symptoms in some individuals even at low altitudes [36, 37]. We found individual differences also in HEMS providers. There was a range of reaction times and the participants with faster reaction times at baseline (273.9 ms) had relatively greater lengthening of RTs in hypoxia without supplemental oxygen compared to the slower (289.4 ms) ones, but we did not find any influence of age or gender.
We found that oxygen supplementation improved the reduced sustained attention and timely reactions (i.e., slowing of RT) in HEMS providers during acute exposure to altitude. There was a positive correlation between cognitive performance and oxygenation level. Oxygen was administered at 2 l/min reaching a peripheral oxygen saturation of around 95–100% without administering an excess of oxygen and avoiding any carbon dioxide re-breathing through the use of an open design mask [38].
Our results are important for an evidence-based development of occupational safety regulation of providers operating in HEMS. Helicopters (e.g., H145, Airbus Helicopter SAS, Marignane, France; AW139, Leonardo, Cascina Costa di Samarate (VA), Italy; Bell 429, Bell, Fort Worth, TX, USA) operating in EMS transiently ascend to high altitude for primary missions and secondary transport in areas such as Europe, Colorado and Alaska in the USA [39], as well as in countries that have high mountain ranges in Asia and South America [40,41,42]. Helicopters do not need to fly at HA in the cruise phase unlike airplanes. Based on this assumption, the European Aviation Safety Agency stated that the need for oxygen is lower in HEMS mountain rescue operations because of the shorter time periods spent at altitude compared to general aviation [43]. Our results suggest a potential benefit in the use of oxygen supplementation for missions and transport at altitudes ≥ 4000 m also in HEMS operations. Reduced attention and increased reaction time can be observed during a single ascent of a HEMS mission [3, 44]. Aviation safety agencies and HEMS should consider oxygen supplementation based on the altitude, the time of exposure, the procedures [4, 45], as well as the impact of additional stressors. Multiple exposures to HA in daily practice can have an impact on cognition as reported by Robinson et al. [46] who reported flight performance deterioration during exposure to simulated 10,000 ft preceded by exposure to 25,000 ft. Some flight phases, such as take-off and landing, have been associated with increased number of HEMS accidents [10]. Sleep deprivation can be an important additional stressor. We found and independent effect of ISI on RT. A similar slowing of RT was reported in another population of health-care providers (i.e., nurses) due to sleep deprivation after night shift compared to day shift [47]. A lengthening of RT and an increase in self-reported tiredness was found for HEMS crew over a 5-week shift cycle [48].
Our results also showed that supplemental oxygen reduced the subjective mental stress level (as measured by the VAS) and increased the propensity for positive emotions (PANAS-P) thereby possibly affecting the management of other challenging situations.
Limitations
This was a simulation study. The results may not apply to real-world situations. The experimental protocol involved two consecutive ascents on a single day. In practice HEMS personnel may participate in multiple missions daily for multiple days, possibly causing larger decreases in cognitive performance. The clinical trial was run under controlled, replicable, and safe conditions that did not allow evaluation of the efficacy of oxygen supplementation in participants who experienced additional processive and systemic stressors. The computerized PVT lacks normative data taking into account different age and sex, as well as performance. The experimental protocol controlled the effect of sleep deprivation but it did not investigate the effect of multiple altitude ascents on the same day and other additional stressors.
Conclusions
This randomized clinical trial found that oxygen supplementation improves cognitive performance in HEMS providers during acute exposure to altitude. The use of oxygen supplementation may allow to maintain sustained attention and timely reactions in HEMS providers. The impact of repeated altitude ascents on the same day, weather conditions, time of the day, and additional stressors should be investigated.
Availability of data and materials
Data is available upon reasonable request.
References
Peacock AJ. ABC of oxygen: oxygen at high altitude. BMJ. 1998;317:1063–6. https://doi.org/10.1136/bmj.317.7165.1063.
Tomazin I, Ellerton J, Reisten O, Soteras I, Avbelj M; International Commission for Mountain Emergency Medicine. Medical standards for mountain rescue operations using helicopters: official consensus recommendations of the International Commission for Mountain Emergency Medicine (ICAR MEDCOM). High Alt Med Biol. 2011;12:335–41. https://doi.org/10.1089/ham.2010.1096.
Falla M, Hüfner K, Falk M, et al. Simulated acute hypobaric hypoxia effects on cognition in helicopter emergency medical service personnel – a randomized, controlled, single-blind, crossover trial. Hum Factors. 2024;66:404–23. https://doi.org/10.1177/00187208221086407.
Vögele A, van Veelen MJ, Dal Cappello T, et al. Effect of acute exposure to altitude on the quality of chest compression-only cardiopulmonary resuscitation in helicopter emergency medical services personnel: a randomized, controlled, single-blind crossover trial. J Am Heart Assoc. 2021;10:e021090. https://doi.org/10.1161/JAHA.121.021090.
Nesthus TE, Rush LI, Wreggit SS. Effects of Mild Hypoxia on Pilot Performances at General Aviation Altitudes. US Department of Transportation, Civil Aeromedical Institute, Federal Aviation Administration Report /FAA/ AM-97/9. 1997. National Technical Information Service, Springfield, Virginia, 22161, USA.
Bouak F, Vartanian O, Hofer K, Cheung B. Acute mild hypoxic hypoxia effects on cognitive and simulated aircraft pilot performance. Aerosp Med Hum Perform. 2018;89:526–35.
Legg SJ, Gilbey A, Hill S, et al. Effects of mild hypoxia in aviation on mood and complex cognition. Appl Ergon. 2016;53:357–63. https://doi.org/10.1016/j.apergo.2015.10.002.
From the Centers for Disease Control and Prevention. Ambulance crash-related injuries among Emergency Medical Services workers-United States, 1991–2002. JAMA. 2003;289:1628–9.
Milani M, Roveri G, Falla M, et al. Occupational accidents among search and rescue providers during mountain rescue operations and training events. Ann Emerg Med. 2023;81:699–705. https://doi.org/10.1016/j.annemergmed.2022.12.015.
Chesters A, Grieve PH, Hodgetts TJ. A 26-year comparative review of United Kingdom helicopter emergency medical services crashes and serious incidents. J Trauma Acute Care Surg. 2014;76:1055–60.
American Society of Anesthesiologists. ASA physical status classification system. Updated December 2020. Accessed November 7, 2022. https://www.asahq.org/standards-and-practice-parameters/statement-on-asa-physical-status-classification-system.
Roach RC, Hackett PH, Oelz O, et al. The 2018 lake louise acute mountain sickness score. High Alt Med Biol. 2018;19:4–6.
Tiffin J, Asher EJ. The Purdue pegboard; norms and studies of reliability and validity. J Appl Psychol. 1948;32:234–47.
Falla M, Papagno C, Dal Cappello T, et al. A prospective evaluation of the acute effects of high altitude on cognitive and physiological functions in lowlanders. Front Physiol. 2021;12: 670278. https://doi.org/10.3389/fphys.2021.670278.
Basner M, Mollicone D, Dinges DF. Validity and sensitivity of a brief psychomotor vigilance test (PVT-B) to total and partial sleep deprivation. Acta Astronaut. 2011;69:949–59. https://doi.org/10.1016/j.actaastro.2011.07.015.
Basner M, Savitt A, Moore TM, et al. Development and validation of the cognition test battery for spaceflight. Aerosp Med Hum Perform. 2015;86(11):942–52. https://doi.org/10.3357/AMHP.4343.2015.
Dinges DF, Powell JW. Microcomputer analyses of performance on a portable, simple visual RT task during sustained operations. Behav Res Methods Instrum Comput. 1985;17:652–5. https://doi.org/10.3758/BF03200977.
Wechsler D. Wechsler adult intelligence scale: WAIS-IV; Technical and interpretive manual. London: Pearson; 2008.
Kirchner WK. Age differences in short-term retention of rapidly changing information. J Exp Psychol. 1958;55:352–8.
Cohen S, Williamson G. Perceived stress in a probability sample of the United States. In: Spacapan S, Oskamp S, editors. The social psychology of health. Newbury Park: Sage; 1988.
Zigmond AS, Snaith RP. The hospital anxiety and depression scale. Acta Psychiatr Scand. 1983;67:361–70.
Buysse DJ, Reynolds CF 3rd, Monk TH, et al. The Pittsburgh Sleep Quality Index: a new instrument for psychiatric practice and research. Psychiatry Res. 1989;28:193–213.
Morin CM, Belleville G, Bélanger L, Ivers H. The Insomnia Severity Index: psychometric indicators to detect insomnia cases and evaluate treatment response. Sleep. 2011;34:601–8. https://doi.org/10.1093/sleep/34.5.601.
Hayes MHS, Paterson DG. Experimental development of the graphic rating method. Psychol Bull. 1921;18:98–9.
Watson D, Clark LA, Tellegen A. Development and validation of brief measures of positive and negative affect: the PANAS scales. J Pers Soc Psychol. 1988;54:1063–70.
Hart SG, Staveland LE. Development of NASA-TLX (task load index): results of empirical and theoretical research. In: Hancock PA, Meshkati N, editors. Human mental workload. Elsevier; 1988. p. 139–83. https://doi.org/10.1016/S0166-4115(08)62386-9.
Basner M, Moore TM, Nasrini J, Gur RC, Dinges DF. Response speed measurements on the psychomotor vigilance test: How precise is precise enough? Sleep. 2021;44:zsaa121. https://doi.org/10.1093/sleep/zsaa121.
Yang G, Cope L, He Z, Florea L. Jutils: a visualization toolkit for differential alternative splicing events. Bioinformatics. 2021;37:4272–4.
Li J. A two-step rejection procedure for testing multiple hypotheses. J Stat Plan Inference. 2008;138(6):1521–7.
Shaw DM, Cabre G, Gant N. Hypoxic hypoxia and brain function in military aviation: basic physiology and applied perspectives. Front Physiol. 2021;12: 665821. https://doi.org/10.3389/fphys.2021.665821.
Pilmanis AA, Balldin UI, Fischer JR. Cognition effects of low-grade hypoxia. Aerosp Med Hum Perform. 2016;87:596–603.
Peacock CA, Weber R, Sanders GJ, et al. Pilot physiology, cognition and flight performance during flight simulation exposed to a 3810-m hypoxic condition. Int J Occup Saf Ergon. 2017;23:44–9. https://doi.org/10.1080/10803548.2016.1234685.
Steinman Y, Groen E, Frings-Dresen MHW. Exposure to hypoxia impairs helicopter pilots’ awareness of environment. Ergonomics. 2021;64:1481–90.
Steinman Y, Groen E, Frings-Dresen MHW. Hypoxia impairs reaction time but not response accuracy in a visual choice reaction task. Appl Ergon. 2023;113:104079.
Steinman Y, van den Oord M, Frings-Dresen MHW, Sluiter JK. Flight performance during exposure to acute hypobaric hypoxia. Aerosp Med Hum Perform. 2017;88:760–7.
Cable GG. In-flight hypoxia incidents in military aircraft: causes and implications for training. Aviat Space Environ Med. 2003;74:169–72.
Smith AM. Hypoxia symptoms in military aircrew: long-term recall vs. acute experience in training. Aviat Space Environ Med. 2008;79:54–7. https://doi.org/10.3357/ASEM.2013.2008.
Lamb K, Piper D. Southmedic OxyMask(TM) compared with the Hudson RCI(®) Non-Rebreather Mask(TM): safety and performance comparison. Can J Respir Ther. 2016;52:13–5.
Heggie TW. Search and rescue in alaska’s national parks. Travel Med Infect Dis. 2008;6:355–61.
Giri S, Sharma U, Choden J, et al. Bhutan’s first emergency air medical retrieval service: the first year of operations. Air Med J. 2020;39:116–9.
Pietsch U, Knapp J, Mann M, et al. Incidence and challenges of helicopter emergency medical service (HEMS) rescue missions with helicopter hoist operations: analysis of 11,228 daytime and nighttime missions in Switzerland. Scand J Trauma Resusc Emerg Med. 2021;29:92.
Brugger H, Elsensohn F, Syme D, et al. A survey of emergency medical services in mountain areas of Europe and North America: official recommendations of the International Commission for Mountain Emergency Medicine (ICAR Medcom). High Alt Med Biol. 2005;6:226–37.
European Union Aviation Safety Agency. Helicopter emergency medical service performance and public interest sites. Opinion No 08/2022. In: Document library. 2022. www.easa.europa.eu/en/document-library/opinions/opinion-no-082022. Accessed 10 Jun 2024.
Nowacki J, Heekeren HR, Deuter CE, et al. Decision making in response to physiological and combined physiological and psychosocial stress. Behav Neurosci. 2019;133:59–67.
Pietsch U, Knapp J, Kreuzer O, et al. Advanced airway management in hoist and longline operations in mountain HEMS - considerations in austere environments: a narrative review This review is endorsed by the International Commission for Mountain Emergency Medicine (ICAR MEDCOM). Scand J Trauma Resusc Emerg Med. 2018;26:23.
Robinson FE, Horning D, Phillips JB. Preliminary study of the effects of sequential hypoxic exposures in a simulated flight task. Aerosp Med Hum Perform. 2018;89:1050–9. https://doi.org/10.3357/AMHP.5052.2018.
Behrens T, Burek K, Pallapies D, et al. Decreased psychomotor vigilance of female shift workers after working night shifts. PLoS ONE. 2019;14:e0219087.
Rose C, Ter Avest E, Lyon RM. Fatigue risk assessment of a Helicopter Emergency Medical Service crew working a 24/7 shift pattern: results of a prospective service evaluation. Scand J Trauma Resusc Emerg Med. 2023;31:72. https://doi.org/10.1186/s13049-023-01143-4.
Acknowledgements
We thank the colleagues from the terraXcube facility (Eurac Research, Italy) for support in experimental setting preparation. We thank the colleagues of the International Commission for Alpine Rescue (ICAR, Kloten, Switzerland) for invaluable scientific discussion. The authors thank the Department of Innovation, Research, University and Museums of the Autonomous Province of Bozen/Bolzano, Italy for covering the Open Access publication costs.
Funding
This research received no specific grant from any funding agency in the public, commercial, or not- for-profit sectors.
Author information
Authors and Affiliations
Contributions
MFa, MJvV, MFk, EMW, HB, KH and GS conceptualization. MFa, MJvV, HB and GS project administration and supervision. MFa, MJvV, MFk, EMW, BW, HB, KH and GS methodology. MFa, MJvV, GR, MM, AR, HB, KH and GS investigation. MFa, MJvV, MFk, EMW, HB, KH and GS data analysis and interpretation. MFa, MFk and GS original draft. MFa, MJvV, MFk, EMW, GR, MM, BW, AR, HB, KH, and GS review & editing.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
This randomized, controlled, crossover pilot study was approved by the Ethics Committee review board of Bolzano and the Ministry of Health of Italy (Protocol Number 0228969-BZ) and registered in ClinicalTrials.gov (Protocol Record NCT05073406). We conducted the study according to the Declaration of Helsinki. All participants were informed about the possible risks and gave written informed consent prior to enrolment. This study is reported following the Consolidated Standards Of Reporting Trials (CONSORT) reporting guideline.
Consent for publication
Not applicable.
Competing interests
No author has any conflict of interest with regard to items described in this article.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Falla, M., van Veelen, M.J., Falk, M. et al. Effect of oxygen supplementation on cognitive performance among HEMS providers after acute exposure to altitude: the HEMS II randomized clinical trial. Scand J Trauma Resusc Emerg Med 32, 65 (2024). https://doi.org/10.1186/s13049-024-01238-6
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s13049-024-01238-6