Abstract
Objectives
To develop emergency medical dispatch (EMD) centre prediction models with high sensitivity and satisfying specificity to identify high-priority patients and patients suitable for non-emergency care respectively, when assessing patients with chest pain.
Methods
Observational cohort study of 2917 unselected patients with chest pain who contacted an EMD centre in Sweden due to chest pain during 2018. Multivariate logistic regression was applied to develop models predicting low-risk or high-risk condition, that is, occurrence of time-sensitive diagnosis on hospital discharge.
Results
Prediction models were developed for the identification of patients suitable for high- and low-priority dispatch, using 11 and 10 variables respectively. The area under the receiver-operating characteristic curve (AUROC) for the high-risk prediction model was 0.79 and for the low-risk model it was 0.74. When applying the high-risk prediction model, 56% of the EMS missions were given highest priority, compared with 65% with the current standard. When applying the low-risk model, 7% were given the lowest priority compared to 1% for the current standard. The new prediction models outperformed today’s dispatch priority accuracy in terms of sensitivity as well as positive and negative predictive value in both high- and low-risk prediction. The low-risk model predicted almost six times as many patients as having low-risk conditions compared with today’s standard. This was done without increasing the number of high-risk patients wrongly assessed as low-risk.
Conclusions
By introducing prediction models, based on logistic regression analyses, using variables obtained by standard EMD-questions on age, sex, medical history and symptomology, EMD prioritisation can be improved compared with using current criteria index-based ones. This will allow a more efficient emergency medical services resource allocation.
Similar content being viewed by others
Background
Chest pain is one of the most common reasons for calling the emergency medical dispatch (EMD) centre, accounting for more than ten percent of all calls [1]. Chest pain can be caused by several high-risk conditions where time is crucial, such as myocardial infarction, aortic aneurysm and pulmonary embolism. For this reason, high sensitivity is warranted for EMD assessment of patients with chest pain. However, in most cases chest pain is benign [2]. The desire to attain high sensitivity and the relative rareness of high-risk conditions give rise to substantial over-triage, giving patients with low-risk conditions the highest dispatch priority [2, 3]. In addition, the proportion of emergency medical services (EMS) patients in whom chest pain is caused by acute myocardial infarction (AMI) has decreased during the last decades [2, 4, 5]. During the same time period, the overall number of EMS requests has increased [1, 6, 7] altering EMD call probabilities. The increased utilisation of the EMS may partly be explained by numerous information campaigns to the public encouraging them to call the EMD when experiencing chest pain [8,9,10,11]. However, both EMS utilisation and EMD prioritisation are complex and affected by several factors, such as patients’ socio-economic background. For example, high socio-economic status shortens EMS response times for patients with chest pain [12,13,14]. Furthermore, EMS utilisation is higher in low-income areas [15, 16] while a smaller proportion are given highest priority by the EMD [17] or receive advanced prehospital care [12].
A displacement effect results from the combination of four factors: a general increase in EMS utilisation [1, 6, 7]; chest pain being a common cause for EMD contact [1]; a decreasing proportion of patients with chest pain from AMI [2, 4, 5]; and substantial over-triage of patients with chest pain [2, 5]. This means that patients with non-urgent healthcare needs are given high priority and occupy limited EMS resources at the cost of delaying EMS care for those with more urgent needs. This displacement effect can be compared with the well-known phenomenon of emergency department crowding, when an increase in patient volume negatively affects the quality and promptness of care provided, leading to an increase in adverse events [18,19,20].
For cardiac arrest, one of the most urgent conditions in EMS care, EMS response times in Sweden have doubled during the last decades [21]. This may probably be explained at least partly by the displacement effect described above. Estimations claim that lowering the cardiac arrest response times to the 1990 level would double the survival rates [21].
The increasing number of EMD contacts due to chest pain, the advice to the public to contact the EMD when experiencing chest pain, and the decreasing proportion of high-risk conditions all emphasise the need for more accurate assessment and prioritisation at the EMD. Improving the accuracy of EMD prioritisation of patients with chest pain would lead to more patients with high-risk conditions having an ambulance dispatched with high priority and fewer patients with low-risk conditions being given high priority, thereby reducing over-triage. This would result in more effective EMS resource coordination and influence the displacement effect positively. Identifying patients with chest pain suitable for other modes of transportation than ambulance would also influence the displacement effect positively.
The aim of this study has been to develop an EMD prediction model with high sensitivity and satisfying specificity to identify high-priority patients and patients suitable for non-emergency care when assessing patients with chest pain.
Methods
This study is part of the BRIAN research programme (BRöstsmärta I AmbulaNs (Swedish), EMS Chest Pain (English)). The cohort, methods and previous results have been described elsewhere [22, 23] and are therefore summarised briefly.
Setting
In Sweden, 112 is the emergency number used by all EMD centres. In the county studied, the EMD centre is run by the company SOS Alarm AB, owned by the Swedish Government and the Swedish Association of Local Authorities and Regions. At the EMD centre, the emergency medical call is assessed by an emergency medical dispatch operator. The EMD operator does not need any formal medical education but must have undergone six months of training and certification before starting to handle emergency medical calls single-handed. The EMD operator uses the “Swedish Index for Medical Priority Dispatch” (SIMPD) when setting the priority of the EMS mission. The SIMPD is a criteria-based index adapted from a Norwegian version that was developed from the Medical Priority Dispatch System (MPDS) used worldwide [24,25,26]. The SIMPD has just over 30 chapters of which chest pain is one. The EMD operator uses the SIMPD to assign a priority level from 1 (the most urgent) to 3 (least urgent) [27]. The patient may also be referred to waiting at home, other modes of transportation than ambulance or contacting a non-emergency care agency for medical consultation such as a primary healthcare centre [24]. If the operator is in doubt when assessing an emergency medical call, a registered nurse may be consulted [24, 27].
The county studied covers an area of 5500 km2 and had 329,000 inhabitants in 2018. These are served by two emergency hospitals. The EMS consist of eight ambulance stations with 19 ambulances during daytime. This ambulance fleet is coordinated by an EMD operator who determines which ambulance should be allocated to which EMS mission. In 2018, approximately 30,000 patient-related EMS missions were carried out (inter-hospital site transports excluded).
Study population
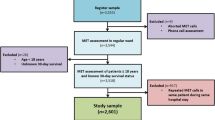
A total of 3121 EMS missions were carried out in the county catchment area including patients ≥ 18 years old and with a chief complaint of chest pain (assigned Rapid Emergency Triage and Treatment System (RETTS) code 5, i.e. chest pain, according to EMS personnel on scene [28]). All these missions were eligible for inclusion. After excluding patients declining to participate and patients who were lost to follow-up, 2917 EMS missions remained and were consecutively included in the study.
Data collection
Each patient was tracked throughout the emergency healthcare chain, from EMD prioritisation to hospital discharge. Data on symptoms was retrieved using a 15-item questionnaire [22] embedded in the digital EMS record available bedside. Previous medical history and diagnosis on hospital discharge were collected from the hospital medical records. Thus data on symptoms and previous medical history was not collected during the emergency medical call, but later in the healthcare chain.
Endpoint
The primary endpoint was a risk classification group, in terms of a low- or high-risk condition. All patients were classified as having either a low-, intermediate- or high-risk condition as the cause of their chest pain. The assessment was based on the final diagnosis on discharge from hospital according to the physician in charge. A high-risk condition was defined as a time-sensitive condition with high risk of death and in need of immediate hospital care. An intermediate-risk condition was defined as a condition probably in need of hospital care, but for which time was not a critical factor. A low-risk condition was defined as a final diagnosis with no medical need for prompt hospital care. An overview of how the different diagnoses were classified along with a more detailed description of how this risk classification was carried out can be found in a previous publication [22]. In the EMD setting this could be translated to:
-
High-risk condition—in need of highest dispatch priority.
-
Intermediate-risk condition—ambulance dispatch with a lower priority is appropriate.
-
Low-risk condition—no need for ambulance dispatch, referral to alternative modes of transportation or contacting a non-emergency care agency are appropriate.
Data imputation
The general idea is iteratively to train a machine learning model on complete variables to predict unknown instances of any missing variable. The list of complete variables is updated at every iteration by including the newly imputed variable, thereby using better and better data for every iteration. We leveraged the MissForest algorithm that iteratively builds Random Forest models to impute missing instances in the variables of interest [29], to improve the prerequisites for multivariate analysis. Random Forest combines the predictions from several regression trees to produce more accurate predictions and bootstrapping to reduce the risk of overfitting [30].
Statistical analysis
After imputation, the complete data set of 2917 EMS missions was randomly divided into two sets [31]: one prediction model training set including 2049 (70%) EMS missions and one validation set including 868 (30%) EMS missions. Two prediction models were generated from the training set, one for low- and one for high-risk prediction.
Stepwise forward logistic regression was used when generating the prediction models. To limit the number of variables in the models, thereby easing clinical usage and strengthening generalisability, strict thresholds for variable entry and exit were applied (p ≤ 0.001 for both).
For the high-risk model, a low (10%) endpoint probability cut-off was set to ensure high sensitivity, thereby reducing the risk for wrongly down-prioritising patients with high-risk conditions. For the low-risk model, a high (90%) endpoint probability cut-off was set to ensure high specificity, thereby reducing the risk of assessing patients with high-risk conditions as suitable for non-ambulance dispatch.
McNemar’s test [32, 33] was used to analyse possible differences between SIMPD/EMD prioritisation and prioritisation based on the prediction models developed.
All statistical analyses were carried out using IBM SPSS Statistics 27. Imputation was implemented using Python (Scikit-learn package).
Results
Of the 868 EMS missions included in the validation set, 17% concerned patients with high-risk conditions. The corresponding figures for intermediate-risk and low-risk conditions were 15% and 68% respectively. The median age was 72 years old (Q25–Q75, 58–82), and 50% were men (Table 1). This is equivalent to both the complete set described in detail in the previous report [22] and the training set used for prediction model development in this study, which indicates successful randomisation.
The stepwise forward logistic regression generated a high-risk condition prediction model with 11 different variables. The variables most indicative of a high-risk condition were pain during activity, intense pain and pain in right arm (Table 2).
The stepwise forward logistic regression generated a low-risk condition prediction model with 10 different variables. Most variables had an odds ratio of less than one, thereby signifying reduced chance of a low-risk condition. The variables with increased odds ratios for a low-risk condition were previous history of angina pectoris and sudden pain debut (within seconds) (Table 3). The models for high- and low-risk prediction respectively shared six variables. In total, if applying both models at the same time, 15 different variables are included.
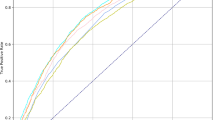
The area under the receiver-operating characteristic curve (AUROC) for the high-risk model was 0.79 when applied to the validation set. For the low-risk prediction model, the AUROC was 0.74 (Table 4).
In the validation set, 65% of the EMS missions were given highest priority by the EMD operator when using the SIMPD. The corresponding figure for lowest priority was 1%. When the high-risk prediction model was applied instead, 56% of the EMS missions were given highest priority. When the low-risk model was applied, 7% were given the lowest priority. The new prediction models outperformed the SIMPD regarding sensitivity as well as positive and negative predictive value for both high- and low-risk prediction. The SIMPD was slightly better than the new prediction models concerning specificity when identifying patients with low-risk conditions. When comparing the models developed here with the SIMPD, using McNemar’s test, both the high- and low-risk differed significantly from the SIMPD (p-value < 0.001).
Three patients with high-risk conditions were wrongly assessed by both the SIMPD and the low-risk prediction model as having low-risk conditions. However, the low-risk model predicted almost six times as many patients as having low-risk conditions, and thereby displayed a lower misclassification rate. The three high-risk patients wrongly classified by the low-risk prediction model suffered from non-ST-elevation myocardial infarction (NSTEMI), pulmonary embolism, and sepsis respectively. Two of the high-risk patients wrongly assigned lowest priority by the SIMPD suffered from NSTEMI and one from unstable angina.
Discussion
In this study, we show that prediction models based on ten and eleven variables respectively outperform the decision support tool (SIMPD) currently used. They outperform both in the identification of patients needing an ambulance with highest priority and patients with less urgent medical needs who could be referred to transport/care other than EMS transport to the emergency department.
The prediction model variables included can be obtained by the EMD operator by asking questions which must be considered standard when evaluating patients with chest pain: “Is your breathing affected?” “Can you describe the character of your pain?” “What part of your chest is hurting?” etc. along with standard EMD information regarding age, sex and medical history. Such questions could be integrated into a computer-aided dispatch system and automatically supplied to the EMD operator when chest pain is selected as the chief complaint. Rawshani et al. [34] have previously shown that such an approach is feasible when EMD operators are instructed to ask ten questions regarding symptoms and previous medical history when handling calls concerning patients with chest pain. If such questions are asked and the answers obtained are entered onto a computer to allow the prediction model to estimate the likelihood of a high- or low-risk condition, the results of that estimation can support the EMD operator when assessing the appropriate priority.
This study shows that the accuracy of the SIMPD currently used is quite modest in terms of identifying patients with high-risk or low-risk conditions. By applying the newly developed prediction models, accuracy can be improved and still decrease the number of high priority dispatches. Thus more patients with urgent care needs are given high priority and at the same time sparse EMS resources are used more effectively. Even if the accuracy of the newly developed prediction models is not perfect, they outperform the current standard model.
The misclassification rate was 5% for patients predicted as having low-risk conditions but actually having high-risk conditions. Thus it does not seem feasible for the EMD operator to refer patients with chest pain to primary care instead of the emergency department, and still provide acceptable patient safety [35, 36]. This is also in line with previously developed prediction models [37]. If an ambulance with lowest priority or a single-manned unit is instead dispatched to patients predicted as having low-risk conditions, patient safety can be maintained while still allowing more effective EMS resource utilisation compared with the SIMPD.
Gellerstedt et al. [5] developed a prediction model to be used at the EMD centre to identify patients with life-threatening conditions among patients with chest pain. This model had a sensitivity of 88% which is equivalent to the high-risk model developed in this study. The corresponding figure for the SIMPD is 77%, the same as in our study. The results of the Gellerstedt et al. [5] study support our finding that computer-based prediction models based on logistic regression analyses outperform a criteria-based index such as the SIMPD regarding the identification of high-risk patients, without increasing the dispatch of EMS resources with highest priority. It therefore seems reasonable to advocate the introduction of more advanced and dynamic decision support systems at the EMD centre than the current criteria-based ones.
Strengths and limitations
A key strength is the unselected inclusion of EMS missions comprising an almost complete population of EMS chest pain patients for one year, which improves generalisability. Gellerstedt et al. [5] report comparable data on demographics and priority distribution for their cohort which further strengthens the external validity of our findings.
Another strength is the use of two different sets for model training and validation. This increases the generalisability of the results, and also makes it more likely that the newly developed prediction models will also perform well in other cohorts. However, external validation using an out-of-sample data set is needed before considering clinical implementation.
One weakness of this study is that it is based on data collected by EMS personnel on scene but not during emergency medical calls. It is possible that data collected by the EMD might differ in terms of pain intensity for example, or assessment of paleness. This also makes it difficult to assess the practicability of assigning the EMD operator to obtain the data required for the prediction models. Since this data can be obtained by asking conventional questions on demographics and symptomology, we judge that in most cases this would not entail any problems, which is also supported by Rawshani et al. [34].
Furthermore, the use of non-EMD data did not allow us to investigate whether obtaining the data would extend the length of the emergency medical call. EMD centre calls concerning chest pain patients with high-risk conditions, when using the SIMPD, last for a median time of 4 min [38], and median time from dispatch to EMS arrival is 10 min in the county studied [22]. A possible extension of call length by a few percent is thus unlikely to affect patient outcome, especially since most EMS chest pain dispatches concern patients with low-risk conditions. One should also be aware that it is common practice in some cases to dispatch an ambulance on information retrieved early in the emergency medical call and then reclassify the priority, or even cancel the EMS mission, as the call progress continues and further questions are asked. This limits the delay of EMS response times due to long-lasting emergency medical calls. On the other hand, this approach may complicate the use of the prediction models developed as they require complete data to provide a prediction. However, it is reasonable to assume that this potential delay would be compensated for by the more optimised resource allocation enabled by more accurate assessments and prioritisations.
The results regarding the accuracy of the SIMPD are probably not directly transferable to other EMD support systems such as the MPDS, from which the SIMPD was developed some decades ago [24]. The SIMPD and the MPDS have evolved independently of each other for several years. However, the design and structure of the SIMPD and the MPDS remain the same and the potential benefits of applying prediction models such as the one developed in this study probably also apply to the MPDS.
Conclusions
We conclude that by introducing prediction models based on logistic regression analyses, using variables obtained by standard EMD questions on age, sex, medical history and symptomology, EMD prioritisation can be improved compared with using current criteria index-based ones. This would allow more efficient EMS resource allocation. We advocate external validation of available computer-based prediction models to allow implementation at the EMD centre, to support the operators in their decision-making.
Availability of data and materials
The datasets generated and analysed during the current study are not publicly available due the integrity of patient privacy but are available from the corresponding author on reasonable request and if approved by the Regional Ethical Review Board in Lund.
Abbreviations
- AMI:
-
Acute Myocardial Infarction
- AUROC:
-
Area Under the Receiver-Operating Characteristic Curve
- EMD:
-
Emergency Medical Dispatch
- EMS:
-
Emergency Medical Services
- MPDS:
-
Medical Priority Dispatch System
- NSTEMI:
-
Non-ST-elevation myocardial infarction
- SIMPD:
-
Swedish Index for Medical Priority Dispatch
References
Pittet V, Burnand B, Yersin B, Carron PN. Trends of pre-hospital emergency medical services activity over 10 years: a population-based registry analysis. BMC Health Serv Res. 2014;14:380.
Rawshani A, Larsson A, Gelang C, Lindqvist J, Gellerstedt M, Bang A, et al. Characteristics and outcome among patients who dial for the EMS due to chest pain. Int J Cardiol. 2014;176(3):859–65.
Feldman MJ, Verbeek PR, Lyons DG, Chad SJ, Craig AM, Schwartz B. Comparison of the medical priority dispatch system to an out-of-hospital patient acuity score. Acad Emerg Med. 2006;13(9):954–60.
Thang ND, Karlson BW, Bergman B, Santos M, Karlsson T, Bengtson A, et al. Characteristics of and outcome for patients with chest pain in relation to transport by the emergency medical services in a 20-year perspective. Am J Emerg Med. 2012;30(9):1788–95.
Gellerstedt M, Rawshani N, Herlitz J, Bång A, Gelang C, Andersson JO, et al. Could prioritisation by emergency medicine dispatchers be improved by using computer-based decision support? A cohort of patients with chest pain. Int J Cardiol. 2016;220:734–8.
Schehadat MS, Scherer G, Groneberg DA, Kaps M, Bendels MHK. Outpatient care in acute and prehospital emergency medicine by emergency medical and patient transport service over a 10-year period: a retrospective study based on dispatch data from a German emergency medical dispatch centre (OFF-RESCUE). BMC Emerg Med. 2021;21(1):29.
Lowthian JA, Cameron PA, Stoelwinder JU, Curtis A, Currell A, Cooke MW, et al. Increasing utilisation of emergency ambulances. Aust Health Rev. 2011;35(1):63–9.
Blohm M, Hartford M, Karlson BW, Karlsson T, Herlitz J. A media campaign aiming at reducing delay times and increasing the use of ambulance in AMI. Am J Emerg Med. 1994;12(3):315–8.
Nehme Z, Cameron PA, Akram M, Patsamanis H, Bray JE, Meredith IT, et al. Effect of a mass media campaign on ambulance use for chest pain. Med J Aust. 2017;206(1):30–5.
Thuresson M, Haglund P, Ryttberg B, Herlitz J, Nilsson U. Impact of an information campaign on delays and ambulance use in acute coronary syndrome. Am J Emerg Med. 2015;33(2):297–8.
Cartledge S, Finn J, Straney L, Ngu P, Stub D, Patsamanis H, et al. The barriers associated with emergency medical service use for acute coronary syndrome: the awareness and influence of an Australian public mass media campaign. Emerg Med J. 2017;34(7):466–71.
Govindarajan A, Schull M. Effect of socioeconomic status on out-of-hospital transport delays of patients with chest pain. Ann Emerg Med. 2003;41(4):481–90.
Hsia RY, Huang D, Mann NC, Colwell C, Mercer MP, Dai M, et al. A US National Study of the association between income and ambulance response time in cardiac arrest. JAMA Netw Open. 2018;1(7): e185202.
Sepehrvand N, Alemayehu W, Kaul P, Pelletier R, Bello AK, Welsh RC, et al. Ambulance use, distance and outcomes in patients with suspected cardiovascular disease: a registry-based geographic information system study. Eur Heart J Acute Cardiovasc Care. 2020;9(1_suppl):45–58.
Aitavaara-Anttila M, Liisanantti J, Ehrola A, Spalding M, Ala-Kokko T, Raatiniemi L. Use of prehospital emergency medical services according to income of residential area. Emerg Med J. 2020;37(7):429–33.
Søvsø MB, Bech BH, Christensen HC, Huibers L, Christensen EF, Christensen MB. Sociodemographic characteristics associated with contacts to emergency medical services and out-of-hours primary care: an observational study of 2.3 million citizens. Clin Epidemiol. 2020;12:393–401.
Magnusson C, Zelano J. High-resolution mapping of epilepsy prevalence, ambulance use, and socioeconomic deprivation in an urban area of Sweden. Epilepsia. 2019;60(10):2060–7.
Pines JM, Pollack CV, Diercks DB, Chang AM, Shofer FS, Hollander JE. The association between emergency department crowding and adverse cardiovascular outcomes in patients with chest pain. Acad Emerg Med. 2009;16(7):617–25.
Carter EJ, Pouch SM, Larson EL. The relationship between emergency department crowding and patient outcomes: a systematic review. J Nurs Scholarsh. 2014;46(2):106–15.
Diercks DB, Roe MT, Chen AY, Peacock WF, Kirk JD, Pollack CV, et al. Prolonged emergency department stays of non-ST-segment-elevation myocardial infarction patients are associated with worse adherence to the American College of Cardiology/American Heart Association guidelines for management and increased adverse events. Ann Emerg Med. 2007;50(5):489–96.
Holmén J, Herlitz J, Ricksten SE, Strömsöe A, Hagberg E, Axelsson C, et al. Shortening ambulance response time increases survival in out-of-hospital cardiac arrest. J Am Heart Assoc. 2020;9(21): e017048.
Wibring K, Lingman M, Herlitz J, Amin S, Bång A. Prehospital stratification in acute chest pain patient into high risk and low risk by emergency medical service: a prospective cohort study. BMJ Open. 2021;11(4): e044938.
Wibring K, Lingman M, Herlitz J, Ashfaq A, Bång A. Development of a prehospital prediction model for risk stratification of patients with chest pain. Am J Emerg Med. 2021;51:26–31.
Ek B, Svedlund M. Registered nurses’ experiences of their decision-making at an Emergency Medical Dispatch Centre. J Clin Nurs. 2015;24(7–8):1122–31.
Sporer KA, Johnson NJ. Detailed analysis of prehospital interventions in medical priority dispatch system determinants. West J Emerg Med. 2011;12(1):19–29.
Clawson J, Olola C, Heward A, Patterson B, Scott G. The Medical Priority Dispatch System’s ability to predict cardiac arrest outcomes and high acuity pre-hospital alerts in chest pain patients presenting to 9-9-9. Resuscitation. 2008;78(3):298–306.
Torlén K, Kurland L, Castrén M, Olanders K, Bohm K. A comparison of two emergency medical dispatch protocols with respect to accuracy. Scand J Trauma Resusc Emerg Med. 2017;25(1):122.
Magnusson C, Herlitz J, Axelsson C. Patient characteristics, triage utilisation, level of care, and outcomes in an unselected adult patient population seen by the emergency medical services: a prospective observational study. BMC Emerg Med. 2020;20(1):7.
Stekhoven DJ, Buhlmann P. MissForest-non-parametric missing value imputation for mixed-type data. Bioinformatics. 2012;28(1):112–8.
Shah AD, Bartlett JW, Carpenter J, Nicholas O, Hemingway H. Comparison of random forest and parametric imputation models for imputing missing data using MICE: a CALIBER study. Am J Epidemiol. 2014;179(6):764–74.
Altman DG, Vergouwe Y, Royston P, Moons KG. Prognosis and prognostic research: validating a prognostic model. BMJ. 2009;338: b605.
Shapiro DE. The interpretation of diagnostic tests. Stat Methods Med Res. 1999;8(2):113–34.
Gellerstedt M, Bang A, Herlitz J. Could a computer-based system including a prevalence function support emergency medical systems and improve the allocation of life support level? Eur J Emerg Med. 2006;13(5):290–4.
Rawshani N, Rawshani A, Gelang C, Herlitz J, Bang A, Andersson JO, et al. Could ten questions asked by the dispatch center predict the outcome for patients with chest discomfort? Int J Cardiol. 2016;209:223–5.
Pope JH, Aufderheide TP, Ruthazer R, Woolard RH, Feldman JA, Beshansky JR, et al. Missed diagnoses of acute cardiac ischemia in the emergency department. N Engl J Med. 2000;342(16):1163–70.
Than M, Herbert M, Flaws D, Cullen L, Hess E, Hollander JE, et al. What is an acceptable risk of major adverse cardiac event in chest pain patients soon after discharge from the Emergency Department? A clinical survey. Int J Cardiol. 2013;166(3):752–4.
Alotaibi A, Alghamdi A, Reynard C, Body R. Accuracy of emergency medical services (EMS) telephone triage in identifying acute coronary syndrome (ACS) for patients with chest pain: a systematic literature review. BMJ Open. 2021;11(8): e045815.
Wibring K, Herlitz J, Lingman M, Bång A. Symptom description in patients with chest pain: a qualitative analysis of emergency medical calls involving high-risk conditions. J Clin Nurs. 2019.
Acknowledgements
We want to thank the following persons: Margaret Myers for language editing. Ulf Strömberg for statistical support.
Funding
Open access funding provided by University of Gothenburg. This study has been funded by the Department of Ambulance and Prehospital Care, Region Halland, and the Scientific Council of Region Halland (HALLAND-209901). The funding bodies had no role in the design, conduct, interpretation or writing of the report on this research.
Author information
Authors and Affiliations
Contributions
KW, ML, JH, and AB planned the data collection. KW, ML, JH, and AB designed the study. KW carried out data analysis. KW, ML, JH and AB contributed to writing the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
In this study, all patients eligible for inclusion were subject to an opt-out procedure. The study was approved by the Regional Ethical Review Board in Lund (Dno 2017/212).
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Wibring, K., Lingman, M., Herlitz, J. et al. The potential of new prediction models for emergency medical dispatch prioritisation of patients with chest pain: a cohort study. Scand J Trauma Resusc Emerg Med 30, 34 (2022). https://doi.org/10.1186/s13049-022-01021-5
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s13049-022-01021-5