Abstract
Background
Traumatic brain injuries (TBI) are associated with high risk of morbidity and mortality. Early outcome prediction in patients with TBI require reliable data input and stable prognostic models. The aim of this investigation was to analyze different CT classification systems and prognostic calculators in a representative population of TBI-patients, with known outcomes, in a neurointensive care unit (NICU), to identify the most suitable CT scoring system for continued research.
Materials and methods
We retrospectively included 158 consecutive patients with TBI admitted to the NICU at a level 1 trauma center in Sweden from 2012 to 2016. Baseline data on admission was recorded, CT scans were reviewed, and patient outcome one year after trauma was assessed according to Glasgow Outcome Scale (GOS). The Marshall classification, Rotterdam scoring system, Helsinki CT score and Stockholm CT score were tested, in addition to the IMPACT and CRASH prognostic calculators. The results were then compared with the actual outcomes.
Results
Glasgow Coma Scale score on admission was 3–8 in 38%, 9–13 in 27.2%, and 14–15 in 34.8% of the patients. GOS after one year showed good recovery in 15.8%, moderate disability in 27.2%, severe disability in 24.7%, vegetative state in 1.3% and death in 29.7%. When adding the variables from the IMPACT base model to the CT scoring systems, the Stockholm CT score yielded the strongest relationship to actual outcome. The results from the prognostic calculators IMPACT and CRASH were divided into two subgroups of mortality (percentages); ≤50% (favorable outcome) and > 50% (unfavorable outcome). This yielded favorable IMPACT and CRASH scores in 54.4 and 38.0% respectively.
Conclusion
The Stockholm CT score and the Helsinki score yielded the closest relationship between the models and the actual outcomes in this consecutive patient series, representative of a NICU TBI-population. Furthermore, the Stockholm CT score yielded the strongest overall relationship when adding variables from the IMPACT base model and would be our method of choice for continued research when using any of the current available CT score models.
Similar content being viewed by others
Background
Traumatic brain injury (TBI) is a major cause of death and disability worldwide [1,2,3]. Risk stratification and early outcome prediction is important for triage and clinical decision making as well as for communication with the patients’ relatives and for clinical audit purposes. The need for accurate outcome prediction has opened the field of prognostic modeling in TBI. Historically, prognostication relied merely on clinical information, and although this is still important, more complex models also include information about radiological features, laboratory results and biomarkers [4, 5]. The most commonly cited models, CRASH score [6] and IMPACT score [7], combine clinical and radiological information to make individual prognoses.
Studies of these models have identified four important prognostic variables following TBI: pupillary response, Glasgow Coma Scale (GCS) score, patient age, and radiological findings on computed tomography (CT) [7]. These prognostic features have been displayed graphically, enabling risk stratification in relation to outcome, and ultimately, provide a tool to aid in clinical decision making [8, 9]. The CRASH and IMPACT models are continuously validated on new patient series [10,11,12,13,14].
The CRASH trial was an international multicenter study which aimed to determine the effects on death and disability of a short-term corticosteroid infusion following significant head injury, and the first results were published in 2004. However, this trial eventually led to the presentation of a prognostic calculator in TBI [6]. The International Mission on Prognosis and Analysis of randomized Controlled Trials in TBI (IMPACT) studies were initiated in 2003 and continued over a 10-year period. A collaboration that commenced with the Medical Research Council of Great Britain (MRC), CRASH, led to the presentation of an enhanced prognostic calculator that included predictors of outcome in three prognostic models of increasing complexity; a Core model based on injury severity and demographics (including age, motor score and pupillary reactivity), an Extended model additionally including CT information (based on Marshall CT classification) and secondary insults (hypoxia and hypotension); and a Lab model additionally including glucose and hemoglobin values [15]. The results of CRASH and IMPACT prognostic calculators are presented as the predicted probability in percent of 6 month mortality and unfavourable outcome, where 0% responds to no risk of mortality or unfavourable outcome and 100% an extremely high risk of mortality and unfavourable outcome.
However, several other investigations have aimed to improve the classification of the CT variables, and for this study, four other CT classification scores were included in addition to the CRASH score and the IMPACT score. These scores were the Marshall CT classification [16], the Rotterdam scoring system [17], the Helsinki CT score [18] and the Stockholm CT score [19] (see Supplementary Table 1 for an overview). While the Marshall CT classification has been considered “gold standard”, albeit limitations, the Rotterdam scoring system was based on Marshall CT classification with the addition of other CT variables for a better estimated prognosis [17]. The Helsinki CT score and Stockholm CT score are similar to the previous mentioned scores, with their own individual variables [18, 19].
The aim of this investigation was to test and analyze different CT classification systems and prognostic calculators in a representative population of TBI-patients, with known outcomes, in a neurointensive care unit (NICU), to identify the most suitable CT scoring system for clinical use and continued research.
Methods
Patient cohort
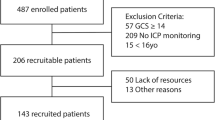
All consecutive adult patients with TBI, admitted to the neuro-intensive care unit (NICU) at the level 1 trauma center at Sahlgrenska University Hospital, in Gothenburg, Sweden from 2012 to 2016 were identified (n = 188), and 158 patients were included. Exclusion criteria were subacute injury, missing person data, patient transferred from another neurotrauma center and penetrating injury, see Fig. 1. Baseline data and clinical variables were retrieved from the medical records. These variables included: date of trauma, gender, age, trauma mechanism, GCS score, pupillary response, best motor response, signs of hypoxia or hypotension, blood glucose levels, hemoglobin levels (Hb), any signs of extracranial injuries, and whether or not the patient had any anticoagulatory medications.
The trauma mechanisms were classified according to the categories fall from height, assault, unclear reason of trauma (where the patient was found unconscious), and traffic accidents. Any extracranial injuries which by themselves required hospital care (in addition to the intracranial injuries) were also registered in the data material. These could include injuries to the upper and lower limbs, vertebral column, thoracic or abdominal injuries as well as facial injuries.
The traffic accidents included any accidents where a vehicle (car, bus, tram, motorcycle, bicycle, or trailer) either collided with another vehicle, with a person, or as a single vehicle accident.
Patients initially admitted to local hospitals in the area who were in need of neurointensive care were transferred to the level 1 trauma center at Sahlgrenska University Hospital and the NICU. After treatment, the patients were again transferred back to their local hospital for continued care and rehabilitation.
Radiological CT scores and prognostic models
CT examinations were reviewed, and data for the variables of the different CT scores was registered. The first CT scans upon arrival were retrieved rather than the “worst” CT scans. The Marshall CT classification, Rotterdam scoring system, Helsinki and Stockholm CT scores were tested, and the IMPACT and CRASH prognostic calculators were applied [7].
The results of the CRASH and IMPACT calculators are presented as continuous variables from 0 to 100%, where 0% represents no risk for mortality or unfavorable outcome at 6 months and 100% a guaranteed risk for mortality or unfavorable outcome at 6 months. For this study, we dichotomized the predicted outcomes, i.e. ≤50% was assessed as favorable outcome (GOS 4–5), and > 50% was assessed as unfavorable outcome GOS [1,2,3].
Outcome
Glasgow Outcome Scale (GOS) was used for global evaluation of outcome. GOS is a grading scale ranging from 1 (dead) to 5 (full recovery). An unfavorable outcome was defined as GOS 1–3 and favorable outcome as GOS 4–5. The assessment of GOS scores was based on interviews with the patients or their next of kin by a specially trained intensive care nurse (VH) one year post injury [20].
Statistics
The statistical analyses were made using statistical software SPSS, version 25. Statistical significance was set at p < 0.05. The categorical variables were analyzed by frequencies and cross tables which are presented in numbers and percentages. The continuous variables are presented as medians and interquartile ranges due to non-normal distributed data. To show the correlation between the CT scoring systems and GOS, mean plots were used. The plots were made by variance analyses (one-way ANOVA). For validation and comparison of the different CT scoring systems, we used two statistical methods; logistical regression analyses and area under the receiver operating characteristic curve (AUC).
Logistical regression analyses were used to measure the association and accuracy between the different CT scoring systems and GOS. This yielded Nagelkerke’s pseudo-R2 which in turn gave a value between 0 and 1, where 0 equals no explanatory variation between the outcome and the model, and 1 equals fully explanatory variation.
Furthermore, AUC was used to compare with the measures of Nagelkerke’s pseudo-R2 which also gives a value of the variance between the outcome and the model. AUC gave a value between 0.5–1, where 0.5 indicates nothing more than random chance and 1 indicates a perfect association. To be able to perform the aforementioned analyses, GOS score was dichotomized in two ways. First GOS 1–3 versus 4–5 (unfavorable versus favorable outcome) and GOS 1 versus GOS 2–5 (dead versus alive).
Finally, a multivariable analysis by logistic regression was performed to further indicate the individual CT scoring systems’ value when added to the IMPACT base model. These variables included pupillary response, glucose, Hb level, age, and GCS on admission.
Ethics
This study has been approved by the Swedish Ethical Review Authority (2019–04330). Formal consent from the patients was waived by the Review Authority.
Results
158 patients with TBI were included during the study period, see Fig. 1. The mean age was 59 years and 70% were male. Fall from heights and traffic accidents were the most common trauma mechanisms (55 and 22%, respectively). The demographics, CT variables and outcomes are presented in Table 1.
GCS scores were assessed upon admission, and the patients were divided into three groups depending on total score; severe TBI (GCS 3–8), moderate TBI (GCS 9–13) and mild TBI (GCS 14–15). In total, 60 patients presented with a severe TBI (38%), 43 patients with moderate TBI (27%) and 55 patients (35%) with mild TBI. Acute subdural hematomas were the most predominant finding present in 85% of the patients. The results from the prognostic calculators IMPACT and CRASH yielded a favorable IMPACT score in 54.4% of the cohort, and a favorable CRASH score in 38.0%. GOS at one year after discharge showed good recovery in 15.8%, moderate disability in 27.2%, severe disability in 24.7%, and death in 29.7%. See Table 1.
All CT scores, as well as the IMPACT and CRASH prognostic models, were tested in relation to the actual outcomes of the patients. The relationships between CT scores, prognostic models and outcome, according to GOS, are given in Fig. 2A-D. To illustrate the relationship between the different CT scoring systems, i.e., Stockholm (A), Helsinki (B), Rotterdam (C), Marshall (D), with outcome (GOS), means plots were used where the y-axis shows the CT scoring system, and the x-axis shows GOS. This illustrates that the higher the CT scoring value, the worse the outcome and vice versa. The relationships between GOS and IMPACT and CRASH, respectively, are illustrated in Fig. 3A-B.
The statistical association and accuracy between the different CT-scoring systems and GOS are given in Table 2, for favorable versus unfavorable outcome, and in Table 3 for dead versus alive. As seen in Table 2, the Stockholm CT score remained strongest when comparing with other scoring systems, followed by the Helsinki CT score. The Helsinki score was superior in predicting survival (Table 3). When adding variables from IMPACT base model to the CT scores, the Stockholm CT score yielded statistical significance in relationship to actual outcome (see Table 4).
Discussion
In this study, we aimed to investigate suitable CT variables and prognostic models to use in clinical practice and research in patients with known outcomes after TBI. There was a significant correlation between the predicted outcome from the scoring systems and the known outcome in the patient series. The Helsinki CT score was most accurate in predicting survival, and the Stockholm CT score was most accurate in predicting unfavorable versus favorable outcome. Furthermore, when the Stockholm CT score was added to the IMPACT base model, this yielded the strongest prognostic relationship compared to any of the other CT scoring systems.
The superior accuracy in the Stockholm and Helsinki CT scores, compared with the Marshall classification and Rotterdam scoring system, might be explained by the inclusion of additional variables, but there were also differences in how the variables were registered and how they affected prognosis. When comparing the Stockholm CT score to the other scores, there was a clear difference in the analyses of traumatic subarachnoid hemorrhage (tSAH). In the Stockholm CT score, absence or presence of intraventricular hemorrhage (IVH) analyzed, as well as the absence or presence of tSAH, both in the convexities and in the basal cisterns, in addition to the volume of the hematoma. The Marshall classification neither included tSAH nor IVH and the Rotterdam score only analyzed the absence or presence of IVH or tSAH, but did not discriminate between the two, and the Helsinki CT score analyzed only the absence or presence of IVH. Considering these factors, it is reasonable to believe that tSAH plays an important role in the prognosis of TBI patients, i.e. a worse outcome in the presence of tSAH, which has also been shown in previous studies [21]. One reason for this could be more complicated management as traumatic subarachnoid blood might case harmful complications such as vasospasm, ischemia, hydrocephalus, and electrolyte disturbances [22].
Another difference between the scoring systems concerned epidural hematomas (EDH), where the Helsinki CT score and the Stockholm CT score considered the presence of EDH a positive prognostic factor, and if present, subtracted points for EDH from the total sum; hence, yielding a more favorable outcome [19]. However, in the Rotterdam scoring system, the presence of EDH was considered a negative sign and increased the risk of poor outcome [17]. In the Marshall classification, hematomas or contusions greater than 25 cm3 were considered, but EDH was not specifically analyzed. Furthermore, the Stockholm CT score was the only modality which analyzed midline shift as a continuous variable and was also the only scoring system considering if signs of diffuse axonal injury (DAI) were present on CT. Even though DAI is primarily diagnosed on magnetic resonance imaging (MRI) scans, signs of DAI on CT scans could indicate severe consequences [23, 24]. Measuring midline shift as a continuous variable and including signs of DAI might explain why the Stockholm CT score was marginally more accurate than the Helsinki CT score in outcome prediction.
We found that adding relevant CT variables to the IMPACT base model yielded improved prognostic measurements. This motivates further investigations to optimize CT findings to increase prognostic accuracy which, in turn, can aid decision making in the clinical setting. From the results of this investigation, we would suggest the use of the Stockholm CT score or Helsinki CT score as the method of choice when predicting prognosis in patients with TBI. For future perspectives, we expect to see additional and more complex prognostic models in TBI, applying the results from large multi-center investigations such as the CENTER-TBI study that includes CT variables as well as biomarkers and clinical baseline data [25].
Future prospectives in TBI prognostication
Native CT scan is part of the routine trauma protocol used at patient admission. The prognostic scores investigated in this study use findings on a native CT scan for prognostication. However, data suggests that perfusion CT scans could be superior in prognostication after TBI [26]. As CT perfusion measures cerebral vascular autoregulation, it could be useful in the guidance of TBI treatment [27, 28]. Further studies on TBI prognostication should include CT perfusion and aim to address physiology as well as associated injuries to improve prognosis and overall care for these patients.
Strengths and limitations
The patients in this investigation were highly representative of TBI-patients in NICU. Only a small number of patients were lost to follow-up, and the standards of care adhere to the Brain trauma foundation guidelines [29] and are also routine practice in other Scandinavian and international level 1 trauma centers and NICU. Data collection was retrieved by a limited number of individuals in the team, and the radiological examinations were performed according to a routine trauma protocol and available for assessment.
One limitation was that the study was retrospective and relied on data from medical records. Moreover, when commencing this study, one aim was to compare the known patient outcome with the predicted outcome from the different prognostic models for each individual. The reason for this was to assess whether the prognostic tools yielded a poorer outcome compared to reality – or in fact a more positive outcome – and in turn, this would be another guide in the clinical setting. However, we could not find a statistical model to analyze this, due to the differences in the nature of the values of GOS and the prognostic tools. Furthermore, we assessed GOS one-year post-injury, and the prognostic models generally present outcomes at 6 months. This was a limitation in the view of comparison, and we suspect that the outcomes in our patients would be better, given the six additional months of recovery. However, the improvement beyond 6 months seems limited in reported literature [30, 31].
A possible limitation of the study was that different CT machines and protocols were used during the study period. However, for native CT scans, this has less effect compared to more advanced modalities. Additionally, a Swedish survey from 2016 showed that homogenous examination protocols for CT head scans were used in trauma and 98% used native CT scans without contrast [32]. All scanners at the different hospitals in the region underwent regular maintenance by the vendors and sequences were optimized by the hospitals for clinical evaluation of traumatic brain injuries.
Conclusion
There was a significant correlation between the predicted outcomes from the scoring systems and the known outcomes in this patient material, representative for a NICU TBI-population. The Stockholm CT score and the Helsinki score yielded the closest relationship between the models and the actual outcomes. Furthermore, the Stockholm CT score yielded the strongest overall relationship when adding variables from IMPACT base model and would be our method of choice for continued research when using any of the current available CT scoring models.
Availability of data and materials
The datasets used and analyzed during the current study are available from the corresponding author on reasonable request.
Abbreviations
- CT:
-
Computed tomography
- DAI:
-
Diffuse axonal injury
- EDH:
-
Epidural hematoma
- GCS:
-
Glasgow coma scale
- GOS:
-
Glasgow outcome scale
- IVH:
-
Intraventricular hemorrhage
- MRI:
-
Magnetic Resonance Imaging
- NICU:
-
Neurointensive Care Unit
- SAH:
-
Subarachnoid hemorrhage
- SDH:
-
Subdural hematoma
- TBI:
-
Traumatic brain injury
- tSAH:
-
Traumatic subarachnoid hemorrhage
References
Faul M, Coronado V. Epidemiology of traumatic brain injury. Handb Clin Neurol. 2015;127:3–13. https://doi.org/10.1016/B978-0-444-52892-6.00001-5.
Stalnacke BM, Saveman BI, Stenberg M. Long-term follow-up of disability, cognitive, and emotional impairments after severe traumatic brain injury. Behav Neurol. 2019;2019:9216931.
Tagliaferri F, Compagnone C, Korsic M, Servadei F, Kraus J. A systematic review of brain injury epidemiology in Europe. Acta Neurochir. 2006;148(3):255–68; discussion 68. https://doi.org/10.1007/s00701-005-0651-y.
Gao J, Zheng Z. Development of prognostic models for patients with traumatic brain injury: a systematic review. Int J Clin Exp Med. 2015;8(11):19881–5.
Czeiter E, Mondello S, Kovacs N, Sandor J, Gabrielli A, Schmid K, et al. Brain injury biomarkers may improve the predictive power of the IMPACT outcome calculator. J Neurotrauma. 2012;29(9):1770–8. https://doi.org/10.1089/neu.2011.2127.
Perel P, Arango M, Clayton T, Edwards P, Komolafe E, Poccock S, et al. Predicting outcome after traumatic brain injury: practical prognostic models based on large cohort of international patients. BMJ (Clinical research ed). 2008;336(7641):425–9. https://doi.org/10.1136/bmj.39461.643438.25.
Steyerberg EW, Mushkudiani N, Perel P, Butcher I, Lu J, McHugh GS, et al. Predicting outcome after traumatic brain injury: development and international validation of prognostic scores based on admission characteristics. PLoS Med. 2008;5(8):e165 discussion e.
Brennan PM, Murray GD, Teasdale GM. Simplifying the use of prognostic information in traumatic brain injury. Part 1: the GCS-pupils score: an extended index of clinical severity. J Neurosurg. 2018;128(6):1612–20. https://doi.org/10.3171/2017.12.JNS172780.
Murray GD, Brennan PM, Teasdale GM. Simplifying the use of prognostic information in traumatic brain injury. Part 2: graphical presentation of probabilities. J Neurosurg. 2018;128(6):1621–34. https://doi.org/10.3171/2017.12.JNS172782.
Olivecrona M, Koskinen LO. The IMPACT prognosis calculator used in patients with severe traumatic brain injury treated with an ICP-targeted therapy. Acta Neurochir. 2012;154(9):1567–73. https://doi.org/10.1007/s00701-012-1351-z.
Charry JD, Navarro-Parra S, Solano J, Moscote-Salazar L, Pinzón MA, Tejada JH. Outcomes of traumatic brain injury: the prognostic accuracy of various scores and models. Neurol Neurochir Pol. 2019;53(1):55–60. https://doi.org/10.5603/PJNNS.a2018.0003.
Maeda Y, Ichikawa R, Misawa J, Shibuya A, Hishiki T, Maeda T, et al. External validation of the TRISS, CRASH, and IMPACT prognostic models in severe traumatic brain injury in Japan. PLoS One. 2019;14(8):e0221791. https://doi.org/10.1371/journal.pone.0221791.
Bilgi K, Gopalakrishna KN, Chakrabarti D, Rao GSU. Outcome prediction of TBI: are there parameters that affect the IMPACT and CRASH models? World neurosurgery; 2020.
Wongchareon K, Thompson HJ, Mitchell PH, Barber J, Temkin N. IMPACT and CRASH prognostic models for traumatic brain injury: external validation in a south-American cohort. Inj Prev. 2020;26(6):546–54. https://doi.org/10.1136/injuryprev-2019-043466.
Maas AI, Murray GD, Roozenbeek B, Lingsma HF, Butcher I, McHugh GS, et al. Advancing care for traumatic brain injury: findings from the IMPACT studies and perspectives on future research. The Lancet Neurology. 2013;12(12):1200–10. https://doi.org/10.1016/S1474-4422(13)70234-5.
Marshall LF, Marshall SB, Klauber MR, Van Berkum CM, Eisenberg H, Jane JA, et al. The diagnosis of head injury requires a classification based on computed axial tomography. J Neurotrauma. 1992;9(Suppl 1):S287–92.
Maas AI, Hukkelhoven CW, Marshall LF, Steyerberg EW. Prediction of outcome in traumatic brain injury with computed tomographic characteristics: a comparison between the computed tomographic classification and combinations of computed tomographic predictors. Neurosurgery. 2005;57(6):1173–82 discussion −82.
Raj R, Siironen J, Skrifvars MB, Hernesniemi J, Kivisaari R. Predicting outcome in traumatic brain injury: development of a novel computerized tomography classification system (Helsinki computerized tomography score). Neurosurgery. 2014;75(6):632–46; discussion 46-7. https://doi.org/10.1227/NEU.0000000000000533.
Nelson DW, Nyström H, MacCallum RM, Thornquist B, Lilja A, Bellander BM, et al. Extended analysis of early computed tomography scans of traumatic brain injured patients and relations to outcome. J Neurotrauma. 2010;27(1):51–64. https://doi.org/10.1089/neu.2009.0986.
Wilson JT, Pettigrew LE, Teasdale GM. Structured interviews for the Glasgow outcome scale and the extended Glasgow outcome scale: guidelines for their use. J Neurotrauma. 1998;15(8):573–85. https://doi.org/10.1089/neu.1998.15.573.
Thelin EP, Nelson DW, Vehviläinen J, Nyström H, Kivisaari R, Siironen J, et al. Evaluation of novel computerized tomography scoring systems in human traumatic brain injury: an observational, multicenter study. PLoS Med. 2017;14(8):e1002368. https://doi.org/10.1371/journal.pmed.1002368.
Modi NJ, Agrawal M, Sinha VD. Post-traumatic subarachnoid hemorrhage: a review. Neurol India. 2016;64(Suppl):S8–s13.
Vieira RC, Paiva WS, de Oliveira DV, Teixeira MJ, de Andrade AF, de Sousa RM. Diffuse axonal injury: epidemiology, Outcome and Associated Risk Factors. Front Neurol. 2016;7:178.
Ljungqvist J, Nilsson D, Ljungberg M, Esbjörnsson E, Eriksson-Ritzén C, Skoglund T. Longitudinal changes in diffusion tensor imaging parameters of the corpus callosum between 6 and 12 months after diffuse axonal injury. Brain Inj. 2017;31(3):344–50. https://doi.org/10.1080/02699052.2016.1256500.
Dijkland SA, Retel Helmrich IRA, Nieboer D, van der Jagt M, Dippel DWJ, Menon D, et al. Outcome Prediction after Moderate and Severe Traumatic Brain Injury: External Validation of Two Established Prognostic Models in 1742 European patients. J Neurotrauma. 2020;38(10):1377-88.
Wintermark M, van Melle G, Schnyder P, Revelly JP, Porchet F, Regli L, et al. Admission perfusion CT: prognostic value in patients with severe head trauma. Radiology. 2004;232(1):211–20. https://doi.org/10.1148/radiol.2321030824.
Wintermark M, Chiolero R, Van Melle G, Revelly JP, Porchet F, Regli L, et al. Cerebral vascular autoregulation assessed by perfusion-CT in severe head trauma patients. J Neuroradiol. 2006;33(1):27–37. https://doi.org/10.1016/S0150-9861(06)77225-X.
Wintermark M, Chioléro R, van Melle G, Revelly JP, Porchet F, Regli L, et al. Relationship between brain perfusion computed tomography variables and cerebral perfusion pressure in severe head trauma patients. Crit Care Med. 2004;32(7):1579–87. https://doi.org/10.1097/01.CCM.0000130171.08842.72.
Carney N, Totten AM, O'Reilly C, Ullman JS, Hawryluk GW, Bell MJ, et al. Guidelines for the Management of Severe Traumatic Brain Injury, Fourth Edition. Neurosurgery. 2017;80(1):6–15. https://doi.org/10.1227/NEU.0000000000001432.
Pagulayan KF, Temkin NR, Machamer J, Dikmen SS. A longitudinal study of health-related quality of life after traumatic brain injury. Arch Phys Med Rehabil. 2006;87(5):611–8. https://doi.org/10.1016/j.apmr.2006.01.018.
Stocchetti N, Zanier ER. Chronic impact of traumatic brain injury on outcome and quality of life: a narrative review. Crit Care. 2016;20(1):148. https://doi.org/10.1186/s13054-016-1318-1.
Wiklund E, Koskinen SK, Linder F, Åslund PE, Eklöf H. Whole body computed tomography for trauma patients in the Nordic countries 2014: survey shows significant differences and a need for common guidelines. Acta Radiol. 2016;57(6):750–7. https://doi.org/10.1177/0284185115597718.
Acknowledgments
The authors would like to thank statistician Nashmil Ariai for aiding with the statististical analyses.
Funding
AC received financial support from The Gothenburg Society of Medicine. Funding was received from ALF, the Swedish national contract between the state and region in cooperation with the university and the hospital in purpose to fund education and clinical research to advance development in medicine and health care. Open Access funding provided by University of Gothenburg.
Author information
Authors and Affiliations
Contributions
JL, VH and DK were involved in the planning of the study and study design. DK, VH and JL performed the data collection. DK, JL and AC performed the manuscript writing and submission process. HOH and VH drafted the manuscript. All authors substantively revised the manuscript and has approved the submitted version.
Corresponding authors
Ethics declarations
Ethics approval and consent to participate
This study has been approved by the Swedish Ethical Review Authority (2019–04330). Formal consent from the patients was waived by the Review Authority.
Consent for publication
Not applicable.
Competing interests
All authors certify that they have no affiliations with or involvement in any organization or entity with any financial interest (such as honoraria; educational grants; participation in speakers’ bureaus; membership, employment, consultancies, stock ownership, or other equity interest; and expert testimony or patent-licensing arrangements), or non-financial interest (such as personal or professional relationships, affiliations, knowledge or beliefs) in the subject matter or materials discussed in this manuscript.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Additional file 1: Supplementary Table 1.
Overview of the different prognostic calculators and CT scores.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Khaki, D., Hietanen, V., Corell, A. et al. Selection of CT variables and prognostic models for outcome prediction in patients with traumatic brain injury. Scand J Trauma Resusc Emerg Med 29, 94 (2021). https://doi.org/10.1186/s13049-021-00901-6
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s13049-021-00901-6