Abstract
Background
Methylation of the p16 promoter resulting in epigenetic gene silencing—known as p16 epimutation—is frequently found in human colorectal cancer and is also common in normal-appearing colonic mucosa of aging individuals. Thus, to improve clinical care of colorectal cancer (CRC) patients, we explored the role of age-related p16 epimutation in intestinal tumorigenesis.
Methods
We established a mouse model that replicates two common genetic and epigenetic events observed in human CRCs: Apc mutation and p16 epimutation. We conducted long-term survival and histological analysis of tumor development and progression. Colonic epithelial cells and tumors were collected from mice and analyzed by RNA sequencing (RNA-seq), quantitative PCR, and flow cytometry. We performed single-cell RNA sequencing (scRNA-seq) to characterize tumor-infiltrating immune cells throughout tumor progression. We tested whether anti-PD-L1 immunotherapy affects overall survival of tumor-bearing mice and whether inhibition of both epigenetic regulation and immune checkpoint is more efficacious.
Results
Mice carrying combined Apc mutation and p16 epimutation had significantly shortened survival and increased tumor growth compared to those with Apc mutation only. Intriguingly, colon tumors with p16 epimutation exhibited an activated interferon pathway, increased expression of programmed death-ligand 1 (Pdl1), and enhanced infiltration of immune cells. scRNA-seq further revealed the presence of Foxp3+ Tregs and γδT17 cells, which contribute to an immunosuppressive tumor microenvironment (TME). Furthermore, we showed that a combined therapy using an inhibitor of DNA methylation and a PD-L1 immune checkpoint inhibitor is more effective for improving survival in tumor-bearing mice than blockade of either pathway alone.
Conclusions
Our study demonstrated that age-dependent p16 epimutation creates a permissive microenvironment for malignant transformation of polyps to colon cancer. Our findings provide a mechanistic rationale for future targeted therapy in patients with p16 epimutation.
Similar content being viewed by others
Introduction
Although an extensive catalog of DNA methylation alterations has been detected in patients with colorectal cancer (CRC), elucidating the functional contributions of these modifications during tumor initiation and maintenance remains an important unmet need. Mitotically stable gene silencing resulting from epigenetic alteration of promoter DNA methylation, known as epimutation, was proposed by Robin Holliday [1] as one possible mechanism for loss of tumor suppressor function in Knudson’s classic two-hit gene inactivation model [2]. Importantly, unlike genetic mutations, epigenetic mechanisms are intrinsically malleable and thus represent attractive therapeutic targets for improving clinical care of CRC patients [3, 4]. To this end, we seek to better understand the mechanisms by which epimutations frequently observed in cancer cells contribute to carcinogenesis.
Epimutation of the cyclin-dependent kinase inhibitor 2A (CDKN2A) gene, also known as p16, is among the most common epigenetic events in human CRCs [5, 6], and this modification is frequently detected in preneoplastic lesions [7, 8]. Indeed, p16 epimutation originates in normal colon tissues, where it occurs as a function of aging, a phenomenon that is conserved in both humans and mice [9, 10]. Notably, such age-associated epimutations are preferentially located on CpG islands marked with polycomb complex [10] and p16, which controls cell cycle progression, promotes cellular senescence, and is one of the best documented polycomb-bound genes [11, 12]. Prompted by these observations, we created a mouse model of engineered p16 promoter hypermethylation, which leads to accelerated p16 epimutation in somatic tissues during aging and predisposes mice to spontaneous tumor development [13]. This established mouse model of p16 epimutation enables us to answer clinically relevant questions that cannot easily be addressed using in vitro systems. Of particular interest, we can determine whether p16 epimutation drives malignant progression of intestinal tumors and if reversal of these epigenetic defects in p16 suppresses tumor growth.
Many cancers arise through successive accumulation of genetic and epigenetic alterations that collectively drive disease progression and metastasis [14]. In this regard, genetically engineered mouse models have provided direct evidence that combined mutations can accelerate the growth of intestinal tumors and promote the development of a malignant phenotype. Published studies have established such a connection between p16 inactivation and driver gene mutations (e.g., in Apc and Braf) in intestinal tumorigenesis models [15,16,17]. However, these studies have only focused on investigating the functional effect of p16 gene mutations, which rarely occur in human CRCs. In addition, most mouse studies used young animals of 6 − 8 weeks of age, which is equivalent to approximately 15 − 20-year-old humans. As a result, many of the biological processes that underlie the age-dependency of cancer were not considered.
Here, to determine whether age-dependent p16 epimutation promotes intestinal carcinogenesis in combination with Apc mutation, we generated a mouse model that replicates these two events, which are commonly observed in human CRC [18]. Using this model, we found that p16 epimutation promotes the malignant transformation of intestinal tumors initiated by Apc mutation. In addition, colon tumors with combined Apc mutation and p16 epimutation exhibit a remarkable immune phenotype with high levels of interferon signaling and programmed death-ligand 1 (Pdl1) expression. Single-cell RNA sequencing (scRNA-seq) analysis further revealed dynamic changes in the tumor microenvironment (TME) in Apc-mutant mice with p16 epimutation, identifying distinct immune cell subpopulations that contribute to T cell dysfunction and tumor immune evasion. Lastly, we found that a combined therapy involving inhibition of both DNA methylation and the PD-L1 immune checkpoint improves survival in our mouse model of CRC, suggesting that such combined therapies may hold clinical promises for CRC patients with p16 epimutation.
Materials and methods
Experimental animals
The mouse lines used in this study have been described previously; in brief, p16cis/cis mice [13] were created by targeted knock-in of a 140-bp DNA sequence (cis-element) that facilitates spread of DNA methylation at the p16 promoter in cis. ApcMin/+ mice [19] carrying a heterozygous germline mutation at codon 850 of the Apc gene were crossed with p16cis/cis mice to generate ApcMin/+; p16cis/cis mice. All mice were on a C57BL/6 background. Details for PCR genotyping assays, including primer sequences and PCR conditions, are summarized in Supplementary Table 1. All animal research was performed in accordance with the NIH Guide for Care and Use of Laboratory Animals and approved by the Baylor College of Medicine Animal Care and Use Committee. Mice were monitored bi-weekly by determining body weight measurements and assessing rectal prolapse size. We used the following criteria for early euthanasia: > 20% weight loss, progression of rectal prolapse to greater than 3 mm of tissue protrusion, and/or severe signs of a moribund condition.
Cell culture
Primary MEFs (cis-MEFs) were isolated from p16cis/cis mice at embryonic day 12.5 (E12.5) using the Pierce™ Mouse Embryonic Fibroblast Isolation Kit (Thermo Fisher Scientific, Waltham, MA, USA). As a control, we isolated MEFs (ctr-MEFs) from mice carrying a control element knocked into the same targeted site, as previously described [13]. MEFs were cultured in Dulbecco’s Modified Eagle Medium (DMEM; Gibco, Thermo Fisher Scientific), containing 10% fetal bovine serum (FBS; R & D Systems, Minneapolis, MN, USA), 2-mM L-glutamine (Gibco, Thermo Fisher Scientific), and 1% penicillin/streptomycin (Invitrogen, Thermo Fisher Scientific). To remove the cis-element, we infected cis-MEFs at passage 19 with adenovirus Ad5CMVCre (Gene Vector Core, Baylor College of Medicine, Houston, TX, USA). After PCR validation to ensure complete excision of the cis-element, MEFs were exposed to DAC (Sigma-Aldrich, St. Louis, MO, USA) at varying concentrations (0.2, 0.5, or 1 μM) or phosphate-buffered saline (PBS) for 72 h, as previously described [20].
Whole-genome bisulfite sequencing (WGBS) analysis
WGBS was performed and analyzed as previously described [21, 22]. Briefly, we used 500 ng genomic DNA from primary MEFs, i.e., ctr-MEF at passage 7 and cis-MEF at passage 12. Sonicated, adaptor-ligated DNA was treated with sodium bisulfite by the EZ DNA Methylation-Direct kit (Zymo Research). The bisulfite modified DNA was amplified (18 cycles) using adaptor-specific primers and fragments of 200–500 bp were isolated. The quantity and size distribution of libraries were determined using the Pico Green fluorescence assay and the Agilent 2100 Bioanalyzer, respectively. Each library was sequenced as 150 bp paired end reads with planned 30 × coverage per sample. The reads were mapped to mouse genome (mm10) using BSMAP with default parameters [23]. Using the “CAMDA” function in “CAMDA.py” toolkit47, the average methylation ratio of each CpG was calculated as the number of unconverted CpGs divided by the total number of read covering that CpG. All WGBS data been uploaded to GEO and are available at the accession number GSE214032.
CRISPR-mediated targeted demethylation
We used the dCas9-SunTagTET1 system [24] to perform targeted p16 promoter demethylation. Plasmids containing p16-specific gRNA or non-targeting control gRNA were constructed using an all-in-one vector from Addgene (#82,559; Watertown, MA, USA) and Gibson Assembly (NEB, Ipswich, MA, USA). gRNA sequences are listed in Supplementary Table 2. Transfection of cis-MEFs was performed with equimolar amounts of plasmid, using Lipofectamine 20,000 Reagent (Life Technologies, Thermo Fisher Scientific), according to the manufacturer’s instructions. The dCas9-SunTag system contains the scFv-GFP-TET1CD fusion protein which enables FACS sorting to isolate vector-expressing cells. At 48 h post-transfection, MEFs were sorted using a FACS Aria Fusion flow cytometer (BD Biosciences, Franklin Lakes, NJ, USA) to isolate GFP-positive cells for p16 methylation and expression analyses.
DNA methylation and gene expression analysis
Quantitative bisulfite-pyrosequencing analyses to measure DNA methylation was performed as previously described [13, 25]. Bisulfite sequencing of cloned PCR products was used to confirm methylation of CpG sites. Primer sequences and sequencing assays are listed in Supplementary Table 3. Quantitative qRT-PCR was performed to measure mouse p16 and ERV expression levels as previously described [13, 26]. Assay designs are summarized in Supplementary Table 4. All experiments were carried out in triplicate and relative gene expression was normalized to β-actin expression on an ABI Step OnePlus Detection System.
Histology
For histological analyses, mouse intestines and tumors were fixed in 10% neutral buffered formalin. Fixed tissues were paraffin-embedded, sectioned, and stained with H&E, according to standard laboratory protocols at the Cellular and Molecular Morphology Core at the Texas Medical Center Digestive Diseases Center.
RNA-seq analysis
RNA-seq was performed using 1-μg RNA extracted from colonic crypts, as previously described [22, 27]. Prior to sequencing, RNA was subjected to quality control analysis using an Agilent 2100 Bioanalyzer with the Agilent RNA 6000 Nano Kit (Agilent Technologies, Santa Clara, CA, USA). RNA-seq libraries were prepared using standard BGI protocols (mRNA enrichment by rRNA depletion and oligo dT selection) and sequenced on the BGISEQ-500 platform (BGI Group, Shenzhen, China), with a planned sequencing depth of 25 million reads per sample. BOWTIE2 software [28] was used for efficient realignment of RNA sequences, and gene expression levels for each sample were calculated with RSEM [29]. For DEG analysis, we used DEseq2 [30] and ranked genes by fold change (> 2) and adjusted P-value based on multiple testing correction (Bonferroni). All RNA-seq data have been uploaded to GEO and are available at the accession number GSE213568.
Microsatellite instability (MSI) analysis by fluorescent PCR
Five microsatellite loci were analyzed in colon tumors from ApcMin/+ and ApcMin/+; p16cis/cis mice, including three mononucleotide (BAT-24, BAT-59, and BAT-67) and two dinucleotide markers (DiMit79 and TG27) based on the published recommendations [16, 31, 32]. PCR primers and fluorescent labeling are summarized in Supplementary Table 5. For PCR amplifications, we used DNA isolated from colon tumors as well as healthy tissues (liver). PCR fragments were separated on a 3730 DNA analyzer (Applied Biosystems) following the manufacture’s protocol and the raw data were analyzed with GeneMapper 5 software. If the profiles of all markers are identical to those seen in normal tissues, the tumor is classified as microsatellite stable (MSS).
Tumor preparation and flow cytometry
Colon tumors were dissected, cut into small pieces, and further dissociated into a single-cell suspension using the Mouse Tumor Dissociation Kit (Miltenyi Biotec, Bergisch Gladback, North Rhine-Westphalia, Germany) and a gentleMACS™ Octo Dissociator (Miltenyi Biotech), according to the manufacturer’s instructions. Digested tumors were filtered through 70 μM filters, washed with PBS, and then subjected to flow cytometry analysis. For each sample, red blood cells were removed using Red Blood Cell Lysis Solution (Miltenyi Biotech), and the remaining cells were treated with TruStain FcX™ PLUS blocking solution (BioLegend, San Diego, CA, USA). Cells were then analyzed with the Zombie Yellow Fixable Viability Kit (BioLegend) for live/dead staining and labelled using the following antibodies: PE/Dazzle™ 594 anti-CD45 (clone 30-F11), APC anti-CD3 (clone 17A2), APC/Cy7 anti-CD4 (clone RM4-5), Alexa Fluor® 700 anti-CD8a (clone 53–6.7), Brilliat Violet 421™ anti-CD11b (clone M1/70), PE anti-Ly6G/Ly6C (Gr-1) (clone RB6-8C5), all of which were purchased from BioLegend. Samples were acquired on a BD FACSymphony A5 High-Parameter Cell Analyzer (BD Biosciences).
scRNA-seq
Colon tumors and adjacent normal tissues were collected and processed on the same day for scRNA-seq. Single-cell suspensions were prepared from tumor samples and normal colonic mucosa using the Tumor Dissociation Kit (Miltenyi Biotec) or with gentle TrypLE (Thermo Fisher Scientific) dissociation, respectively. Single live cells were isolated by flow cytometry, and 3’ gene expression libraries were generated using the Chromium Next GEM Single Cell 3’ Kit v3.1 (10 × Genomics, Pleasanton, CA, USA). These were then subjected to scRNA-seq as described previously [33]. In brief, single cells, reverse transcription (RT) reagents, gel beads containing barcoded oligonucleotides, and oil were loaded on a Chromium controller (10 × Genomics) to generate single-cell Gel Bead-in-Emulsions (GEMS) on which full length cDNA was synthesized and barcoded for each single cell. GEMS were then broken, and cDNAs from each single cell were pooled, cleaned up using Dynabeads MyOne Silane Beads (Invitrogen, Thermo Fisher Scientific), and amplified by PCR. The amplified products were then fragmented to an optimal size prior to end-repair, A-tailing, and adaptor ligation. The final library was sequenced on the Illumina Novaseq 6000 platform. All scRNA-seq data have been uploaded to GEO and are available at the accession number GSE213568.
scRNA-seq data analysis
We used Seurat v4.0 [34] for scRNA-seq data processing and analysis. Cells were filtered to include only those with < 5% mitochondrial DNA, RNA counts between 250 and 30,000, and 250 to 5000 features. Doublets were removed using DoubletFinder [35], according to a recent benchmarking paper [36]. The homotypic proportion was estimated through unsupervised clustering using the Leiden algorithm [37]. To eliminate batch effects, MNNCorrect [38] and Seurat integration [34] were applied separately to the dataset with default parameters. MNNCorrect successfully grouped cell types together but left significant batch effects within clusters. Seurat integration grouped cell types together and successfully mixed batches. The remaining analysis was performed using the results from Seurat integration.
Optimal Uniform Manifold Approximation and Projection (UMAP) [39] parameters were determined using scDEED (manuscript under submission). Briefly, integrated data were clustered using the Leiden algorithm with resolution 2.5. Cells were annotated manually using markers found through Seurat’s FindAllMarkers. Note that besides the Leiden algorithm, the data were also clustered and annotated using the Louvain algorithm [40] at the same resolution. Based on marker analysis, some Louvain clusters contained multiple cell types, whereas the Leiden clusters displayed a more homogenous composition. Leiden clustering results were therefore used for final annotations and further analysis. Clusters with multiple unlikely cell type signatures and high ranking pANN scores from DoubletFinder (ranked in their original batch) were also removed.
DEGs were determined using the Wilcoxon Rank–Sum Test, MAST [41], and Clipper [42]. Analysis was restricted to genes with a log-fold change of at least 0.25 across two conditions. Correction for multiple testing in the Wilcoxon Rank–Sum Test and MAST was performed with the Benjamini–Hochberg method. Only genes found to be significant by all three methods were reported as DEGs. GO and Kyoto Encyclopedia of Genes and Genomes (KEGG) analysis was performed with ClusterProfiler [43].
Drug treatments
We began drug treatments in ApcMin/+; p16cis/cis mice at 12–15 weeks of age, when intestinal tumors had been established. For single-agent therapy, mice were randomly assigned to receive either 250 μg anti-PD-L1 (clone 10F.9G2, BioXCell) or the same amount of IgG2b isotype control (clone LTF-2, BioXCell). Treatments were administered by i.p. injection every 3 days, for a total of six treatments. For combination therapy, mice were treated with DAC (1.0 mg/kg, i.p.) on Monday and anti-PD-L1 or control IgG2b on Thursday for 6 or 10 consecutive weeks in females and males, respectively.
3D tumor organoid culture
Colonic tumor organoids were cultured as described previously with some modifications [44]. Briefly, colon tumor tissues were minced and enzymatically digested with 1-mg/ml collagenase type IV (Gibco, Thermo Fisher Scientific) for 30 min at 37 °C, with intermittent shaking. Digested cell fragments were washed with cold washing buffer, containing Ham's F-12 Nutrient Mix, 5% FBS, 15-Mm HEPES, 2.5-μM Rock-inhibitor Y-27632, 2-mM L-glutamine, 1% penicillin/streptomycin, 0.25-µg/mL amphotericin B, 50-µg/mL gentamicin, and 100-μg/ml Primocin™, and then filtered through a 70-μm cell strainer. Dissociated cells were suspended in Matrigel® Matrix (Corning Inc, Corning, NY, USA) at equal amounts and plated in pre-warmed 24-well plates with WRNE culture medium, containing Wnt-3A, R-spondin 3, and Noggin. Media was changed every 3 days, and cells were passaged after 5–7 days. To enrich for tumorigenic organoids, after two passages, organoids were grown and maintained in NE medium, without Wnt-3A and R-spondin. For DAC treatment, organoids were exposed to various concentrations of DAC, ranging from 0.2 to 2 μM. Cell growth was monitored using the CellTiter-Glo 3D Cell Viability Assay (Promega, Madison, WI, USA) based on the amount of ATP present, according to the manufacturer’s instructions. Luminescence was detected with a GloMax Discover Microplate Reader (Promega).
Statistical analysis
All statistical analyses were performed using GraphPad Prism v6. Quantitative DNA methylation and expression results are expressed as the mean ± standard error of the mean (SEM). Significance was determined using the two-tailed Student’s t-test or one way ANOVA of more than two groups, and P < 0.05 was considered statistically significant.
Results
Engineered p16 epimutation recapitulates key features of age-associated epigenetic silencing
We previously generated a mouse model of p16 epimutation through targeted knock-in of a 140-bp Alu-related DNA sequence (cis-element) that facilitates the spread of DNA methylation at promoter CpG islands (CGIs) in cis [13, 20]. Here, to validate our approach for modeling age-associated epigenetic events, we generated primary mouse embryonic fibroblasts (MEFs) from cis- or control (ctr)-element knock-in mice. We mapped DNA methylation patterns in an approximately 1 kb region at p16 promoter and determined whether engineered p16 epimutation leads to spontaneous immortalization of primary MEFs resulting from loss of p16 function. Indeed, as measured during cell passaging, we observed initial methylation seeding within the cis-element, followed by increased methylation gradually spreading toward the endogenous p16 promoter at later passages (Fig. 1A). Consistent with these observations, this increase in p16 promoter methylation results in transcriptional suppression of p16 in cis-element knock-in MEFs (cis-MEF), and these cells can be expanded well beyond the senescence checkpoint (i.e., at eight passages when ctr-MEFs are senescent; Fig. 1B). Furthermore, using whole genome bisulfite sequencing (WGBS) for a comprehensive DNA methylation profiling, we showed that cis-element-mediated approach specifically induces p16 promoter hypermethylation; for example, the neighboring p19Arf (p14Arf in humans) and p15Ink4b promoters, located > 11 kb and > 27 kb away, respectively, were not affected (Fig. 1C).
Engineered p16 epimutation recapitulates key features of age-associated epigenetic silencing. A p16 DNA methylation profiles in cis-MEF following serial passaging (p). A schematic of the p16 promoter of the cis-element knock-in allele with the CpG maps is shown. B Kinetics of p16 promoter methylation and mRNA expression in ctr-MEF and cis-MEF. Methylation levels were averaged from CpGs from − 814 bp to − 589 bp relative to the TSS. Note that cis-MEF cells grew for more than 25 passages; in contrast, the controls (ctr-MEF) entered growth arrest at passage 8 (p8) via p16 up-regulation. The p16 gene expression is relative to β–actin. C UCSC Genome Browser tracks showing the DNA methylation status of p16, p19, and p15 in the INK4/ARF locus on chromosome 4. The chromosomal coordinates are annotated on the top. The WGBS tracks show DNA methylation profiling in ctr-MEF (p7) and cis-MEF (p10). The height of each bar represents the methylation level of an individual CpG between 0 and 1 (100%). Promoters are indicated by angled arrows, and the knock-in location is indicated by an asterisk. D Comparisons of p16 promoter methylation in cis-MEF before and after Ad-Cre mediated cis-element excision with increasing doses of DAC. The culture passage number of cis-MEF is at p23. E Side-by-side comparisons of p16 expression in cis-MEF under the same conditions as (D). For (D) and (E), data are shown as mean ± SEM with individual values from three independent experiments. P values were determined by a one-way ANOVA test. F dCas9-SunTagTET1-mediated targeted demethylation of p16 in cis-MEF. p16-gRNA indicates GFP-positive cells treated with a p16-specific gRNA that binds the promoter region from − 792 bp to − 769 bp relative to the TSS. Ctr indicates GFP-negative cells without targeted demethylation. Data are shown from three independent experiments. G Targeted promoter demethylation resulted in p16 gene reactivation. The culture passage number of cis-MEF is at p23. P values were determined by a two-tailed Student’s t-test
Next, to rule out the possibility that p16 methylation is secondary to gene silencing (e.g., the cis-element recruits other silencers), we used adenovirus-Cre (Ad5CMVCre, referred to hereafter as Ad-Cre) to remove the loxP-flanked cis-element in cis-MEFs. We found that both p16 promoter methylation (Fig. 1D) and gene silencing (Fig. 1E) are stably maintained, even two months after cis-element removal. In addition, treatment with the hypomethylating agent 5-aza-2’-deoxycytidine (DAC) restores p16 expression in a dose-dependent manner. We then applied the CRISPR method [24] and used the dCas9-SunTagTET1 system to selectively demethylate DNA at the p16 promoter. Remarkably, we found that one p16-specific sgRNA (p16-gRNA) induces greater demethylation at neighboring CpGs (i.e., within 200 bp of the p16-gRNA target site) than treatment with DAC at the standard dose of 0.5 µM (Fig. 1F). Interestingly, methylation levels at CpGs within the p16-gRNA-binding site are unchanged in p16-gRNA-transfected MEFs, perhaps because they are inaccessible to the TET1 demethylase. Nevertheless, selective CRISPR-mediated promoter demethylation also induces p16 expression (Fig. 1G). These results demonstrate that our approach successfully models p16 epimutation in which the p16 gene activity is directly controlled by the engineered promoter methylation.
p16 epimutation cooperates with Apc mutation to drive adenoma–carcinoma progression
To further characterize the effects of engineered p16 epimutation in vivo, we focused on the intestine which represents the most rapidly renewing tissue. To assess changes in DNA methylation during normal aging, we generated p16cis/+ mice carrying the cis-element knock-in at one allele and used forward primers specific for knock-in or WT sequences to measure p16 promoter methylation at each allele separately. As shown in Fig. 2A, we found that age-dependent p16 promoter methylation is markedly accelerated by the cis-element within intestinal tissues. Moreover, this p16 promoter hypermethylation exhibits a clonal expansion pattern (Fig. 2B). Because methylation was measured at the whole-tissue level, this result indicates that de novo methylation accumulates in long-lived intestinal stem cells. We hypothesized that the clonal expansion of p16 methylation with age may provide selective advantages for subsequent accumulation of molecular changes to promote colon cancer development.
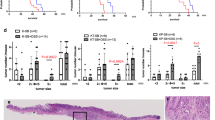
Age-dependent p16 epimutation cooperates with Apc mutation to promote colon cancer. A Accelerated methylation of p16cis alleles compared with WT alleles in intestinal tissues during aging as determined by bisulfite pyrosequencing. B Bisulfite PCR, cloning, and sequencing show a clonal expansion pattern associated with p16 promoter hypermethylation in aged intestines. Each row represents a single sequenced molecule. Black and white circles represent methylated and unmethylated CpGs, respectively. C The addition of p16 epimutation in Apc-mutant mice shortened survival. Survival was compared using the Kaplan–Meier method in ApcMin/+ mice (10 males and 16 females) and ApcMin/+; p16cis/cis mice (15 males and 14 females). D At 15 wk of age, ApcMin/+; p16cis/cis mice developed more tumors in the distal small intestines compared to ApcMin/+ and ApcMin/+; p16cis/+ mice. E Tumor number vs. size in the distal small intestines. F More colon tumors were found in the ApcMin/+; p16cis/cis mice at 15 wk of age compared to the other two groups. For figures (D–F), data are mean ± SEM with individual values. P values were determined by a two-tailed Student’s t-test. G H&E staining of the colon tumor sections revealed histologic features of malignant transformation in ApcMin/+; p16cis/cis mice. Yellow lines indicate tubular adenomas with extensive high-grade dysplasia, and arrows indicate focally invasive adenocarcinoma. Scale bars: 100 μm
To then test the functional requirement for p16 epimutation in intestinal tumorigenesis, we bred the p16cis allele into ApcMin/+ mice to generate the following groups of animals: (1) ApcMin/+ (control), (2) ApcMin/+; p16cis/+ (p16 epimutation at one allele), and (3) ApcMin/+; p16cis/cis (p16 epimutation at both alleles). As shown in Fig. 2C, we found that ApcMin/+; p16cis/cis mice display significantly shortened overall survival compared to ApcMin/+ mice (median 18 vs. 25 wk; P = 0.001). To further determine the cause of this early mortality in ApcMin/+; p16cis/cis mice, we assessed the whole bowel tumor formation under a dissecting microscope (Fig. S1A). Notably, we detected a greater number of tumors within the distal regions of the small intestine in ApcMin/+; p16cis/cis mice than in both ApcMin/+ and ApcMin/+; p16cis/+ mice at 15 wk of age (Fig. 2D). Moreover, tumors in ApcMin/+; p16cis/cis mice were found to be significantly larger than those in ApcMin/+ and ApcMin/+; p16cis/+ mice (Fig. 2E). Interestingly, we also observed a twofold increase in tumor number within the colons of ApcMin/+; p16cis/cis mice relative to the other two groups (Fig. 2F). Histological analysis further revealed a substantially increased incidence of high-grade dysplasia and intramucosal carcinoma in colons from ApcMin/+; p16cis/cis mice relative to ApcMin/+ mice (71% vs. 33%). In addition, ApcMin/+; p16cis/cis mice display aggressive pathological features, such as focal invasion of muscularis mucosa, a hallmark of malignancy that is not present in ApcMin/+ mice (Fig. 2G, Fig. S1B-C). Collectively, these observations from our in vivo model system demonstrate a direct pathogenic role for age-dependent p16 epimutation in CRC development.
p16 epimutation in Apc-mutant colon tumors induces proinflammatory immune responses
To identify genes regulated by p16 epimutation that contribute to intestinal tumorigenesis, we performed RNA sequencing analysis (RNA-seq) and compared transcriptome profiles of colonic mucosa from ApcMin/+; p16cis/cis mice with those from ApcMin/+ mice at 15 wk of age. From this analysis, we identified a total of 103 differentially expressed genes (DEGs), including 77 downregulated and 26 up-regulated genes (Fig. 3A). Gene Ontology (GO) analysis of downregulated DEGs revealed enrichment of terms associated with cellular metabolic processes, particularly those involved in fatty acid and lipid metabolism (Fig. 3B). We then validated our RNA-seq data by quantitative reverse transcription (qRT)-PCR and confirmed that the genes involved in fatty acid oxidation (i.e., Ppara, Aldh1a1, Acaa1b, and Cyp2c55) have significantly reduced expression in ApcMin/+; p16cis/cis compared to ApcMin/+ mice (Fig. 3C). Interestingly, for the up-regulated DEGs, we detected striking enrichment (50-fold increase) in IFN-γ responsive genes (Fig. 3B). Indeed, qRT-PCR confirmed that expression levels of IFN-γ-stimulated genes, including Nos2, Bst2, Ifitm3, and Stat1, are significantly increased in colonic mucosa from ApcMin/+; p16cis/cis relative to ApcMin/+ mice (Fig. 3D).
p16 epimutation promotes inflammatory immune responses in Apc mutant colon tumors. A Heatmap of DEGs identified by RNA-seq in the normal-appearing colonic mucosa of ApcMin/+ ; p16cis/cis mice compared with ApcMin/+ mice. We used DESeq2 for DEG analysis with a fold change of > 2 and multiple-testing adjusted P value < 0.05. B DAVID functional GO analysis of down- and up-regulated DEGs. Significantly enriched terms (FDR < 0.05) are shown. C Down-regulation of genes involved in fatty acid and lipid metabolism was confirmed by qRT-PCR. D Up-regulation of IFN-γ-stimulated genes in colonic mucosa from ApcMin/+ ; p16cis/cis compared to ApcMin/+ mice. E Up-regulation of Ifng and Pdl1 in mouse colon tumors with p16 epimutation. F Flow cytometric analysis of tumor-infiltrating immune cells. CD4+ and CD8+ T cells, as well as monocytes (CD11b + Gr1-), increased in ApcMin/+ ; p16cis/cis colon tumors compared to those from ApcMin/+ mice. For figures (C–F), data are shown as mean ± SEM with individual values. P values were determined by a two-tailed Student’s t-test
Sustained IFN-γ signaling is known to upregulate expression of the immune checkpoint protein PD-L1, which suppresses T cell responses through binding to its ligand, programmed cell death protein 1 (PD-1). We therefore measured the expression levels of Ifng, Pdl1, and Pd1 in both colon tumors and adjacent normal mucosa from ApcMin/+ and ApcMin/+; p16cis/cis mice. Consistent with our RNA-seq data, both Ifng and Pdl1 were found to be specifically up-regulated in colon tumors from ApcMin/+; p16cis/cis mice (Fig. 3E). In contrast, no appreciable difference in Pd1 expression between the two groups was detected. To evaluate the clinical significance of these observations, we analyzed human CRC data from The Cancer Genome Atlas (TCGA) and found a weak but significant positive correlation between p16 epimutation and PDL1 mRNA expression (n = 633, R = 0.21, P = 0.00004 by Spearman’s rank correlation test) (Fig. S2A). Interestingly, the correlation becomes more prominent in KRAS WT CRCs (n = 329, R = 0.31, P = 0.000005 by Spearman’s rank correlation test) (Fig. S2B), as well as in a subset of KRAS WT CRCs with APC mutation (n = 161, R = 0.23, P = 0.003 by Spearman’s rank correlation test) (Fig. S2C).
We next compared the immune cell profiles in ApcMin/+ and ApcMin/+; p16cis/cis colon tumors using flow cytometry. Previous studies have reported that although ApcMin/+ mice have defects in hematopoietic stem cells, they show no intestinal inflammation or major alterations in intestinal immune function relative to WT mice [45, 46]. Consistent with the previous report [46], we detected similarly low frequencies of CD3+CD4+ and CD3+CD8+ T cells in ApcMin/+ colon tumors (average of 2.3% and 0.6%, respectively). Conversely, we found that both CD3+CD4+ and CD3+CD8+ T cells are significantly increased in ApcMin/+; p16cis/cis colon tumors (average of 17.8% and 7.5%, respectively) (Fig. 3F). In addition, frequencies of monocyte-derived myeloid-lineage cells (CD11b+Gr1+ and CD11b+Gr1−) were found to be increased approximately twofold in ApcMin/+; p16cis/cis colon tumors. Furthermore, we tested for MSI by PCR in colon tumors from ApcMin/+ and ApcMin/+; p16cis/cis mice. Using a set of five repeat markers, we found that all the tumors analyzed (n = 6) were microsatellite stable (Fig. S3). Thus, the immune phenotype in our mouse colon cancer model is independent of MSI. Together, these observations indicate that p16 epimutation may modulate TME by activating immune-related pathways to promote malignancy of Apc-mutant colon cancer.
scRNA-seq reveals immunosuppressive T cell subtypes during tumor development
Therefore, to comprehensively characterize immune function within the TME in these animals, we performed scRNA-seq on ApcMin/+; p16cis/cis colon tumors. Because tumor size is associated with malignant histology and nuclear grade, we divided the tumors into two groups: small, early-stage lesions (median diameter = 1.7 mm, n = 4) and larger, late-stage larger tumors (median diameter = 4.0 mm, n = 7). As a control, we also analyzed cells from adjacent normal colonic mucosa (n = 4). After quality filtering and doublet removal, a total of 85,347 cells were analyzed. Based on expression of canonical markers, we identified 11 transcriptionally distinct cell types, including epithelial cells, T cells, B cells, NK cells, neutrophils, monocytes, plasmacytoid (p)DCs, plasma cells, red blood cells, mast cells, and stromal cells (Fig. 4A and Fig. S4). As expected, we found that the expression of Ifng is largely restricted to T cells, whereas Pdl1 is expressed in multiple cell types, including neutrophils and monocytes (Fig. S5). We also detected almost all immune cell types within each individual tumor lesion; however, these immune cell clusters were found to be present in significantly different proportions in early- vs. late-stage tumor lesions (Fig. 4B). B cells are the most common cell type in early-stage tumors (56% of 27,945 cells analyzed) but markedly decreased in late-stage tumors (14% of 47,687 cells analyzed). In addition, we detected a rapid expansion of monocytes in late-stage tumors (30% in late-stage tumors vs. 10% in early-stage tumors). In contrast, T- cell abundance was found to be relatively unchanged during tumor progression (15% in early-stage tumors and 18% in late-stage tumors).
scRNA-seq reveals distinct immunosuppressive T-cell subtypes in ApcMin/+; p16cis/cis colon tumors during development and progression. A UMAP plot showing major cell populations identified from tumors and adjacent normal colon mucosa by canonical cell markers. B A bar plot of the proportion of each cell type in individual tumor tissues and adjacent normal colon mucosa. Individual tumors are ordered by diameter and binned into small or large tumors (T). C Sub-clustering analysis of CD3+ T cells. UMAP plot showing transcriptionally distinct T-cell, subtypes identified in both normal and tumor tissues. For individual normal and tumor samples, the cellular composition of T-cell subtypes is shown by a bar plot. D Violin plots showing expression levels of marker genes identifying CD8+ cells in each sub-cluster. Compared to the normal CD8+ T cells (CD8 + _N), tumor-associated CD8+ T cells (CD8 + _T) show decreased expression of the cytotoxic genes (Gzma and Gzmb) and increased expression of the exhaustion marker gene (Tcf7), indicating a dysfunctional state. E Violin plots showing expression levels of the marker genes for γδT17 cells. F GO analysis using ClusterProfiler of DEGs in γδT17 cells for early- vs. late-stage tumors for DEGs with a log-fold change ≥ 0.25. Correction for multiple testing in the Wilcoxon Rank Sum test and MAST was performed with the Benjamini–Hochberg method. G Category net (CNET) plot showing the top pathways and associated DEGs in γδT17 cells during tumor progression. The color of the dots represents the fold change in gene expression, and the size of the dots is proportional to the number of genes enriched with the GO term
We then explored T cell status during tumor initiation and progression. Sub-clustering of 13,996 CD3+ T cells identified five major clusters, which we annotated based on canonical T cell markers. Among them, we detected two clusters of CD8+ T cells: one preferentially present in adjacent normal tissues and another enriched in tumors (Fig. 4C and Fig. S6A). We found that genes associated with cytotoxic T cell functions (e.g., Gzma and Gzmb) are highly expressed in the normal CD8+ T cells. In contrast, tumor-associated CD8+ T cells show substantially decreased expression of cytotoxic genes and higher expression of the exhaustion marker gene Tcf7, indicating a dysfunctional state (Fig. 4D). Comparative transcriptome analysis of CD8+ T cells from late- vs. early-stage tumors further revealed exhaustion-driving transcriptional pathways in cells from late-stage tumors, including upregulation of inflammatory response and downregulation of ribosome biogenesis genes (e.g., ribosome assembly and ribonucleoprotein complex translation) (Fig. S6B). We also identified two clusters of tumor-specific CD4+ T cells, including a population of Tregs expressing the Treg-defining transcriptional factor Foxp3 (Fig. S7A). These Foxp3+ Tregs also exhibit high expression of interleukin 2 receptor α-chain (Il2ra), members of TNF receptor superfamily (Tnfrsf4 and Tnfrsf9), and genes involved in immune suppression (Lgals1, Areg, and Ctla4) (Fig. 4E and Fig. S7A). Moreover, GO analysis of DEGs from late- vs. early-stage Foxp3+ Tregs revealed downregulation of genes involved in adaptive immune response and regulation of leukocyte activation, which is consistent with the immunosuppressive functions of Tregs (Fig. S7B-C). Similarly, the CD4+ T cells showed reduced immune responses during tumor progression (Fig. S7D-E).
In addition, we identified a unique tumor-enriched population of CD4−CD8− T cells, exhibiting strong expression of T cell receptor γδ genes (Trdc and Trdv4) and the pro-inflammatory cytokine Il17; these were therefore annotated as γδT17 cells (Fig. 4E). Recent studies have reported that γδT17 cells can promote tumor growth by functioning as Treg-like cells [47, 48]. Indeed, we found that γδT17 cells in tumor tissue are characterized by elevated expression of Treg signature genes, such as Lgals1and Ctla4 (Fig. 4E). Notably, the immune checkpoint molecule Pd1 was also found to be highly expressed in γδT17 cells (Fig. 4E). Differential gene expression analysis further revealed that γδT17 cells possess both inflammatory and regulatory properties during tumor progression (Fig. 4F), with significant upregulation of genes related to chemotaxis (e.g., neutrophil migration and leukocyte chemotaxis) and downregulation of immune responses (e.g., cell recognition, cell activation, and cellular response to stimulus) (Fig. 4G). Taken together, these data show that, in the context of inflammation, dysfunctional CD8+ T cells, together with Foxp3+ Tregs and γδT17 cells, contribute to the immunosuppressive TME in colon tumors from ApcMin/+; p16cis/cis mice.
Combination therapy improves survival in mice with Apc mutation and p16 epimutation
Based on the above data showing an immunosuppressive phenotype in our unique mouse model of CRC, we next tested whether administration of single-agent anti-PD-L1 therapy at an early stage of tumor progression can effectively control intestinal tumor growth and improve survival of ApcMin/+; p16cis/cis mice. To this end, ApcMin/+; p16cis/cis mice (n = 28) at 12–14 wk of age were randomly divided into two groups and treated with 250 μg of either anti-PD-L1 or IgG2b isotype control. Treatments were given by intraperitoneal (i.p.) injection every 3 days for a total of six treatments (Fig. 5A). We found that both the drug dosage and frequency of administration were well tolerated in our mouse models. However, we observed no statistically significant difference in median survival between the two treatment groups (110 days vs. 133 days, P = 0.45 by the log–rank test; Fig. 5B). In addition, we found that PD-L1 blockade has no effect on tumor number or size, with profiles essentially identical to those in control-IgG treated mice.
A combined epigenetic and immune therapy improves survival in ApcMin/+; p16cis/cis mice. A Study design and treatment scheme for single anti-PD-L1 mAB therapy vs. a combination of the epigenetic hypomethylating agent DAC and anti-PD-L1 mAB. B Kaplan Meier survival analysis after anti-PD-L1 or control IgG treatments in ApcMin/+; p16cis/cis mice. C Kaplan Meier survival analysis of ApcMin/+; p16cis/cis mice treated with DAC plus control IgG vs. DAC plus PD-L1. Survival curves were compared using a log-rank test
We then tested whether combined epigenetic and immunotherapy is more efficacious than PD-L1 blockade alone. For these experiments, ApcMin/+; p16cis/cis mice (n = 44) were treated with DAC (1.0 mg/kg, i.p.) on Monday, followed by either anti-PD-L1 or control IgG on Thursday. This dosing schedule was based on the published works indicating that it is well tolerated in mice and rats [49,50,51] (i.e., resulting in no weight loss or premature mortality) and within the range being tested in human clinical trials. Because male mice at this age weigh more than female mice, we treated females for 6 consecutive weeks and males for an additional 4 weeks. We observed similar survival curves with DAC plus control IgG treatment compared to PD-L1 treatment alone, for both male and female mice, indicating no survival benefit for single-agent DAC therapy. In contrast, we found that DAC plus anti-PD-L1 treatment significantly prolongs survival in ApcMin/+; p16cis/cis mice, showing a similar effect in both sexes (Fig. 5C). Furthermore, when mice were euthanized at the end of study, we observed significant decreases in both tumor number and size in mice treated with DAC and anti-PD-L1 antibody (Fig. S8). Together, these results demonstrate that combination therapy is more effective than either DAC or anti-PD-L1 treatment alone in our mouse CRC model of Apc mutation and p16 epimutation.
Recent studies have suggested that the efficacy of PD-L1 blockade requires tumor cell-intrinsic cell-cycle arrest to achieve long-lasting responses [52, 53]. Therefore, based on our data showing that DAC can reactivate epigenetically silenced p16, we characterized the direct effects of DAC treatment on tumor cells using an ex vivo organoid system. To this end, we generated colonic tumor organoids from ApcMin/+; p16cis/cis mice and monitored the percentage of organoids surviving after DAC treatment at days 1, 3, 4, and 5. These time-points were chosen based on established kinetics in intestinal stem cell division and organoid growth [54]. As expected, we observed significantly decreased cell counts in response to DAC treatment, starting at day 3, in a dose-dependent manner (Fig. 6A). Interestingly, we further found that at day 5, DAC at the low dose of 0.5 μM is sufficient to stably inhibit cell proliferation (Fig. 6B). Moreover, consistent with the observed phenotype, we saw significantly decreased p16 promoter methylation, as well as restoration of p16 expression (Fig. 6C). On the other hand, DNA hypomethylating drugs can induce global hypomethylation and previous studies suggested that DAC treatment can enhance anti-tumor effects by reactivating transposable elements [26, 55]. To further determine the mechanisms of action for DAC, we additionally measured the expression levels of several murine endogenous retroviruses (ERVs), including LV30-2, MLV, and MuRRS. However, we observed no significant changes in ERV expression levels at the clinically relevant concentration of 0.5 μM (Fig. S9). Furthermore, we analyzed promoter methylation for additional genes that could play a similar tumor-suppressor role in CRC, including the mismatch repair gene Mlh1, the WNT signaling regulator Sfrp1, and the transcription factor Gata4. By comparing the changes of methylation among different promoters, we confirmed that the low dose DAC (0.5 μM) caused the most robust demethylation effect at the p16 promoter (Fig. S10). Thus, our findings indicate that reversal of epigenetic modification at the p16 locus suppresses intestinal tumor growth and promotes durable response to immune checkpoint blockade.
DAC treatment reactivates p16 and suppresses tumor growth in colon tumor organoids. A Images of colon tumor organoids derived from ApcMin/+; p16cis/cis mice after treatment with DAC at the concentrations shown on days 3 and 5. B A dose-dependent decrease in proliferation was observed in tumor organoids treated with DAC. C Low-dose DAC (0.5 μM) was sufficient to induce p16 promoter demethylation and gene reactivation. Error bars represent SEMs of three replicate experiments. Mean values were compared between the 0.5 μM DAC treatment and control (PBS) treatment groups. P values were determined by a two-tailed Student’s t-test
Discussion
The accumulation of epimutations in DNA methylation is a shared molecular phenomenon in both biological aging and cancer development; however, our understanding of how age-related epigenetic changes contribute to tumor evolution and response to therapies is limited. In this study, we have conducted the first functional characterization of age-dependent p16 epimutation in the adenoma–adenocarcinoma sequence of intestinal tumorigenesis that begins with Apc mutation. We chose the ApcMin/+ model because somatic inactivation of the WT Apc allele is necessary for tumorigenesis and occurs naturally in mice as they age. This therefore provides an excellent in vivo system to study the biological consequences of accumulated genetic and epigenetic defects, which are often present in human cancers. To elucidate the effect of p16 epimutation, we applied our previously established mouse model, in which targeted knock-in of the promethylation cis-element specifically induces p16 promoter hypermethylation, particularly in proliferative tissues during aging. Characterization of MEFs derived from this model revealed that induced p16 epimutation recapitulates key features of epigenetic silencing, resulting in downregulation of p16 expression. In addition, using pharmacological inhibition of DNA methylation and CRISPR-guided demethylation, we showed that engineered promoter methylation directly controls p16 gene activity, indicating that this system effectively models p16 epimutation. Critically, combining the genetically and epigenetically engineered mouse models revealed that p16 epimutation exacerbates the intestinal tumorigenesis initiated by Apc mutation, thus directly linking CRC development and progression to an epigenetic aging marker.
Previous studies [56, 57] have reported that p16 epimutation functions to control cell cycle progression in human fibroblasts and migration phenotype of cancer cell lines. Here, to investigate how this modification exacerbates intestinal tumorigenesis, we performed RNA-seq analysis of colon tumors from Apc-mutant mice with and without p16 epimutation. Our results showed that p16 epimutation is associated with increased expression of IFN-γ stimulated genes in colonic mucosa at early stage of tumorigenesis. It is well established that IFN-γ signaling critically regulates mucosal inflammatory processes. Further, although acute IFN-γ signaling is essential for anti-tumor immunity, previous studies have demonstrated that prolonged activation of the IFN-γ signaling pathway can upregulate expression of the immune checkpoint inhibitor PD-L1, leading to tumor immune evasion [58,59,60]. Indeed, we detected a significant positive correlation between p16 epimutation and PDL1 expression in both human and mouse CRCs. Moreover, the observation that p16 epimutation promotes tumor progression, despite an abundance of T cell infiltration, is consistent with a model in which the IFN-γ/PD-L1 axis can switch immune cell phenotypes from a pro-inflammatory to an immunosuppressive state. Our findings therefore suggest that p16 epimutation modulates the immune state of the TME.
We further explored this phenomenon using scRNA-seq, which revealed the striking observation that colon tumors with defined mutation (Apc) and p16 epimutation contain a high infiltration of T cell subtypes. Specifically, we detected the presence of Foxp3+ Tregs and γδT17 cells at the early phase of tumorigenesis. These Foxp3+ Tregs express high levels of Il2ra, T cell costimulatory receptors (Tnfrsf4 and Tnfrsf9), and regulatory molecules, such as Areg and Ctla4, all of which can contribute to the immunosuppressive activity of these cells. For example, IL-2R signaling is required for both Treg survival and suppression of CD8+ effector T cells [61], and CTLA-4 plays a key role in Treg-mediated suppression, in part, by inhibiting the activity of antigen-presenting cells via its interactions with both CD80 and CD86 [62]. Thus, elevated levels of Foxp3+ Tregs likely promote immunosuppression within the TME. In parallel, γδT17 cells may directly enhance tumor-elicited inflammation and colon cancer progression. In human CRCs, infiltration of γδT17 cells is positively correlated with advanced clinicopathological features, including TNM stage, tumor size, and both lymphatic and vascular invasion [48]. In addition, IL-17, a key cytokine produced by γδT17 cells, has been shown to promote tumor growth via the recruitment of myeloid-derived suppressor cells in the TME [47, 63]. Consistent with these findings, at the single-cell resolution, we found that inflammatory γδT17 cells specifically express Pd1, as well as key factors that stimulate granulocyte chemotaxis and promote recruitment of neutrophils and monocytes. Nevertheless, further studies utilizing in vitro functional assays, co-culture experiments, and in vivo selective ablations, are needed to uncover the precise roles of dysfunctional and immunosuppressive T cells in p16 epimutation-driven CRC progression.
Several epigenetic drugs, including DAC, are already in clinical use for treatment of CRC [64, 65]. In addition, anti-PD-1/PD-L1 therapy has emerged as one of the most promising immunotherapies for CRC patients with microsatellite instability (MSI) [66]. However, combinatorial epigenetic immunotherapy regimens have yielded limited response in advanced CRC patients with microsatellite stable (MSS) tumors [67]. Such approaches are also associated with numerous challenges, including a lack of predictive markers and uncertainty regarding the optimal timing for therapy. Notably, our mouse model of MSS tumors driven by defined mutation and epimutation provides an experimentally tractable system in which therapeutic effects can be studied in the natural tumor microenvironment with native stroma and intact immune system. We found that, despite high levels of Pdl1 expression and T cell infiltration, intestinal tumors from Apc-mutant mice with p16 epimutation are resistant to anti-PD-L1 treatment alone. Interestingly, however, delivery of DAC followed by anti-PD-L1 significantly improves animal survival. Mechanistically, we postulate that p16 may contribute to IFN-γ-dependent activation of tumor-intrinsic cell death, which is required for stable arrest of cancer cells that escape immune-mediated cytotoxicity and thus, long-term cancer control. Consistent with this, we found that p16 epimutation leads to senescence bypass in primary MEFs. Moreover, using colon tumor organoids generated from mice with p16 epimutation, we further confirmed that DAC treatment induces p16 promoter demethylation and restores tumor-intrinsic cell-cycle regulation. Interestingly, while we observed significant growth inhibitions in the tumor organoids in response to DAC, there is no survival benefit for DAC treatment alone in mice. It is known that the single-agent DAC therapy in vivo requires repetitive cycles for a long duration [68, 69]. However, to avoid the treatment-related toxicity, we stopped the drug administration after 6–10 cycles, which may explain the low efficacy in solid tumors. Nevertheless, our preclinical studies indicate that the epigenetic therapy works best when combines with the anti-PD-L1 immunotherapy. Although beyond the scope of this study, our established clinically relevant and immunocompetent mouse models will enable future studies to probe new and improved hypomethylating agents, such as GSK3685032 which selectively inhibits DNMT1 with lower toxicity [70].
Conclusions
In summary, we established a mouse model of CRC that combines Apc mutation with p16 epimutation, and we found that age-dependent p16 epimutation modulates tumor microenvironment to accelerate malignant transformation. At the molecular level, our mouse model closely resembles human CRC. From the therapeutic standpoint, epimutations are reversible, so our model has proven useful as an in vivo preclinical platform for developing novel therapeutics.
Availability of data and materials
The datasets generated and/or analyzed during the current study are available in the GEO database (GSE214032, GSE213568 and GSE213568).
Abbreviations
- CRC:
-
Colorectal cancer
- CDKN2A:
-
Cyclin-dependent kinase inhibitor 2A
- CGIs:
-
CpG islands
- MEF:
-
Mouse embryonic fibroblast
- DAC:
-
5-Aza-2’-deoxycytidine
- PD-L1:
-
Programmed death-ligand 1
- ERV:
-
Murine endogenous retroviruses
- TME:
-
Tumor microenvironment
- TNM:
-
The tumor, node, metastasis
- MSI:
-
Microsatellite instability
- MSS:
-
Microsatellite stable
- WGBS:
-
Whole genome bisulfite sequencing
- RNA-seq:
-
RNA sequencing
- scRNA-seq:
-
Single-cell RNA sequencing
- UMAP:
-
Uniform Manifold Approximation and Projection
- DEG:
-
Differentially expressed gene
- GO:
-
Gene Ontology
- KEGG:
-
Kyoto Encyclopedia of Genes and Genomes
- GEMS:
-
Gel Bead-in-Emulsions
- SEM:
-
Standard error of the mean
References
Holliday R. The inheritance of epigenetic defects. Science. 1987;238(4824):163–70.
Knudson AG Jr. Mutation and cancer: statistical study of retinoblastoma. Proc Natl Acad Sci U S A. 1971;68(4):820–3.
Ahuja N, Easwaran H, Baylin SB. Harnessing the potential of epigenetic therapy to target solid tumors. J Clin Invest. 2014;124(1):56–63.
Jones PA, Issa JP, Baylin S. Targeting the cancer epigenome for therapy. Nat Rev Genet. 2016;17(10):630–41.
Gonzalez-Zulueta M, Bender CM, Yang AS, Nguyen T, Beart RW, Van Tornout JM, Jones PA. Methylation of the 5’ CpG island of the p16/CDKN2 tumor suppressor gene in normal and transformed human tissues correlates with gene silencing. Cancer Res. 1995;55(20):4531–5.
Herman JG, Merlo A, Mao L, Lapidus RG, Issa JP, Davidson NE, Sidransky D, Baylin SB. Inactivation of the CDKN2/p16/MTS1 gene is frequently associated with aberrant DNA methylation in all common human cancers. Cancer Res. 1995;55(20):4525–30.
Chan AO, Issa JP, Morris JS, Hamilton SR, Rashid A. Concordant CpG island methylation in hyperplastic polyposis. Am J Pathol. 2002;160(2):529–36.
Petko Z, Ghiassi M, Shuber A, Gorham J, Smalley W, Washington MK, Schultenover S, Gautam S, Markowitz SD, Grady WM. Aberrantly methylated CDKN2A, MGMT, and MLH1 in colon polyps and in fecal DNA from patients with colorectal polyps. Clin Cancer Res. 2005;11(3):1203–9.
Belshaw NJ, Elliott GO, Foxall RJ, Dainty JR, Pal N, Coupe A, Garg D, Bradburn DM, Mathers JC, Johnson IT. Profiling CpG island field methylation in both morphologically normal and neoplastic human colonic mucosa. Br J Cancer. 2008;99(1):136–42.
Maegawa S, Hinkal G, Kim HS, Shen L, Zhang L, Zhang J, Zhang N, Liang S, Donehower LA, Issa JP. Widespread and tissue specific age-related DNA methylation changes in mice. Genome Res. 2010;20(3):332–40.
Campisi J. Aging, cellular senescence, and cancer. Annu Rev Physiol. 2013;75:685–705.
He S, Sharpless NE. Senescence in Health and Disease. Cell. 2017;169(6):1000–11.
Yu DH, Waterland RA, Zhang P, Schady D, Chen MH, Guan Y, Gadkari M, Shen L. Targeted p16(Ink4a) epimutation causes tumorigenesis and reduces survival in mice. J Clin Invest. 2014;124(9):3708–12.
Hanahan D, Weinberg RA. Hallmarks of cancer: the next generation. Cell. 2011;144(5):646–74.
Gibson SL, Boquoi A, Chen T, Sharpless NE, Brensinger C, Enders GH. p16(Ink4a) inhibits histologic progression and angiogenic signaling in min colon tumors. Cancer Biol Ther. 2005;4(12):1389–94.
Rad R, Cadinanos J, Rad L, Varela I, Strong A, Kriegl L, Constantino-Casas F, Eser S, Hieber M, Seidler B, et al. A genetic progression model of Braf(V600E)-induced intestinal tumorigenesis reveals targets for therapeutic intervention. Cancer Cell. 2013;24(1):15–29.
Tao Y, Kang B, Petkovich DA, Bhandari YR, In J, Stein-O'Brien G, Kong X, Xie W, Zachos N, Maegawa S et al: Aging-like Spontaneous Epigenetic Silencing Facilitates Wnt Activation, Stemness, and Braf(V600E)-Induced Tumorigenesis. Cancer Cell. 2019;35(2):315–328 e316.
Shen L, Issa JP. Epigenetics in colorectal cancer. Curr Opin Gastroenterol. 2002;18(1):68–73.
Moser AR, Pitot HC, Dove WF. A dominant mutation that predisposes to multiple intestinal neoplasia in the mouse. Science. 1990;247(4940):322–4.
Shen L, Kondo Y, Guo Y, Zhang J, Zhang L, Ahmed S, Shu J, Chen X, Waterland RA, Issa JP. Genome-wide profiling of DNA methylation reveals a class of normally methylated CpG island promoters. PLoS Genet. 2007;3(10):2023–36.
Shi J, Xu J, Chen YE, Li JS, Cui Y, Shen L, Li JJ, Li W. The concurrence of DNA methylation and demethylation is associated with transcription regulation. Nat Commun. 2021;12(1):5285.
Yu DH, Gadkari M, Zhou Q, Yu S, Gao N, Guan Y, Schady D, Roshan TN, Chen MH, Laritsky E, et al. Postnatal epigenetic regulation of intestinal stem cells requires DNA methylation and is guided by the microbiome. Genome Biol. 2015;16:211.
Xi Y, Li W. BSMAP: whole genome bisulfite sequence MAPping program. BMC Bioinformatics. 2009;10:232.
Morita S, Noguchi H, Horii T, Nakabayashi K, Kimura M, Okamura K, Sakai A, Nakashima H, Hata K, Nakashima K, et al. Targeted DNA demethylation in vivo using dCas9-peptide repeat and scFv-TET1 catalytic domain fusions. Nat Biotechnol. 2016;34(10):1060–5.
Shen L, Guo Y, Chen X, Ahmed S, Issa JP: Optimizing annealing temperature overcomes bias in bisulfite PCR methylation analysis. Biotechniques. 2007;42(1):48, 50, 52 passim.
Saito Y, Nakaoka T, Sakai K, Muramatsu T, Toshimitsu K, Kimura M, Kanai T, Sato T, Saito H. Inhibition of DNA Methylation Suppresses Intestinal Tumor Organoids by Inducing an Anti-Viral Response. Sci Rep. 2016;6:25311.
Yu DH, Ware C, Waterland RA, Zhang J, Chen MH, Gadkari M, Kunde-Ramamoorthy G, Nosavanh LM, Shen L. Developmentally programmed 3’ CpG island methylation confers tissue- and cell-type-specific transcriptional activation. Mol Cell Biol. 2013;33(9):1845–58.
Langmead B, Salzberg SL. Fast gapped-read alignment with Bowtie 2. Nat Methods. 2012;9(4):357–9.
Li B, Dewey CN. RSEM: accurate transcript quantification from RNA-Seq data with or without a reference genome. BMC Bioinformatics. 2011;12:323.
Love MI, Huber W, Anders S. Moderated estimation of fold change and dispersion for RNA-seq data with DESeq2. Genome Biol. 2014;15(12):550.
Bacher JW, Abdel Megid WM, Kent-First MG, Halberg RB. Use of mononucleotide repeat markers for detection of microsatellite instability in mouse tumors. Mol Carcinog. 2005;44(4):285–92.
Germano G, Lamba S, Rospo G, Barault L, Magri A, Maione F, Russo M, Crisafulli G, Bartolini A, Lerda G, et al. Inactivation of DNA repair triggers neoantigen generation and impairs tumour growth. Nature. 2017;552(7683):116–20.
Dharmat R, Kim S, Li Y, Chen R. Single-Cell Capture, RNA-seq, and Transcriptome Analysis from the Neural Retina. Methods Mol Biol. 2020;2092:159–86.
Hao Y, Hao S, Andersen-Nissen E, Mauck WM, 3rd, Zheng S, Butler A, Lee MJ, Wilk AJ, Darby C, Zager M et al: Integrated analysis of multimodal single-cell data. Cell. 2021;184(13):3573–3587 e3529.
McGinnis CS, Murrow LM, Gartner ZJ: DoubletFinder: Doublet Detection in Single-Cell RNA Sequencing Data Using Artificial Nearest Neighbors. Cell Syst. 2019;8(4):329–337 e324.
Xi NM, Li JJ: Benchmarking Computational Doublet-Detection Methods for Single-Cell RNA Sequencing Data. Cell Syst. 2021;12(2):176–194 e176.
Traag VA, Waltman L, van Eck NJ. From Louvain to Leiden: guaranteeing well-connected communities. Sci Rep. 2019;9(1):5233.
Haghverdi L, Lun ATL, Morgan MD, Marioni JC. Batch effects in single-cell RNA-sequencing data are corrected by matching mutual nearest neighbors. Nat Biotechnol. 2018;36(5):421–7.
Mclnnes LH, J.; Melville, J.: Umap: Uniform manifold approximation and projection for dimension reduction. arXiv preprint arXiv. 2018;1802.03426
Blondel VD, Guillaume, J.-L., Lambiotte, R. & Lefebvre, E. : Fast unfolding of communities in large networks. J Stat Mech Theory Exp. 2008;P10008.
Finak G, McDavid A, Yajima M, Deng J, Gersuk V, Shalek AK, Slichter CK, Miller HW, McElrath MJ, Prlic M, et al. MAST: a flexible statistical framework for assessing transcriptional changes and characterizing heterogeneity in single-cell RNA sequencing data. Genome Biol. 2015;16:278.
Ge X, Chen YE, Song D, McDermott M, Woyshner K, Manousopoulou A, Wang N, Li W, Wang LD, Li JJ. Clipper: p-value-free FDR control on high-throughput data from two conditions. Genome Biol. 2021;22(1):288.
Wu T, Hu E, Xu S, Chen M, Guo P, Dai Z, Feng T, Zhou L, Tang W, Zhan L et al: clusterProfiler 4.0: A universal enrichment tool for interpreting omics data. Innovation (Camb). 2021;2(3):100141.
Mahe MM, Aihara E, Schumacher MA, Zavros Y, Montrose MH, Helmrath MA, Sato T, Shroyer NF. Establishment of Gastrointestinal Epithelial Organoids. Curr Protoc Mouse Biol. 2013;3(4):217–40.
Kettunen HL, Kettunen AS, Rautonen NE. Intestinal immune responses in wild-type and Apcmin/+ mouse, a model for colon cancer. Cancer Res. 2003;63(16):5136–42.
Szeponik L, Akeus P, Rodin W, Raghavan S, Quiding-Jarbrink M. Regulatory T cells specifically suppress conventional CD8alphabeta T cells in intestinal tumors of APC(Min/+) mice. Cancer Immunol Immunother. 2020;69(7):1279–92.
Housseau F, Wu S, Wick EC, Fan H, Wu X, Llosa NJ, Smith KN, Tam A, Ganguly S, Wanyiri JW, et al. Redundant Innate and Adaptive Sources of IL17 Production Drive Colon Tumorigenesis. Cancer Res. 2016;76(8):2115–24.
Wu P, Wu D, Ni C, Ye J, Chen W, Hu G, Wang Z, Wang C, Zhang Z, Xia W, et al. gammadeltaT17 cells promote the accumulation and expansion of myeloid-derived suppressor cells in human colorectal cancer. Immunity. 2014;40(5):785–800.
Belinsky SA, Grimes MJ, Picchi MA, Mitchell HD, Stidley CA, Tesfaigzi Y, Channell MM, Liu Y, Casero RA Jr, Baylin SB, et al. Combination therapy with vidaza and entinostat suppresses tumor growth and reprograms the epigenome in an orthotopic lung cancer model. Cancer Res. 2011;71(2):454–62.
Belinsky SA, Klinge DM, Stidley CA, Issa JP, Herman JG, March TH, Baylin SB. Inhibition of DNA methylation and histone deacetylation prevents murine lung cancer. Cancer Res. 2003;63(21):7089–93.
Reed MD, Tellez CS, Grimes MJ, Picchi MA, Tessema M, Cheng YS, March TH, Kuehl PJ, Belinsky SA. Aerosolised 5-azacytidine suppresses tumour growth and reprogrammes the epigenome in an orthotopic lung cancer model. Br J Cancer. 2013;109(7):1775–81.
Brenner E, Schorg BF, Ahmetlic F, Wieder T, Hilke FJ, Simon N, Schroeder C, Demidov G, Riedel T, Fehrenbacher B, et al. Cancer immune control needs senescence induction by interferon-dependent cell cycle regulator pathways in tumours. Nat Commun. 2020;11(1):1335.
Deng J, Wang ES, Jenkins RW, Li S, Dries R, Yates K, Chhabra S, Huang W, Liu H, Aref AR, et al. CDK4/6 Inhibition Augments Antitumor Immunity by Enhancing T-cell Activation. Cancer Discov. 2018;8(2):216–33.
Sato T, Vries RG, Snippert HJ, van de Wetering M, Barker N, Stange DE, van Es JH, Abo A, Kujala P, Peters PJ, et al. Single Lgr5 stem cells build crypt-villus structures in vitro without a mesenchymal niche. Nature. 2009;459(7244):262–5.
Jones PA, Ohtani H, Chakravarthy A, De Carvalho DD. Epigenetic therapy in immune-oncology. Nat Rev Cancer. 2019;19(3):151–61.
Bernstein DL, Le Lay JE, Ruano EG, Kaestner KH. TALE-mediated epigenetic suppression of CDKN2A increases replication in human fibroblasts. J Clin Invest. 2015;125(5):1998–2006.
Cui C, Gan Y, Gu L, Wilson J, Liu Z, Zhang B, Deng D. P16-specific DNA methylation by engineered zinc finger methyltransferase inactivates gene transcription and promotes cancer metastasis. Genome Biol. 2015;16:252.
Garcia-Diaz A, Shin DS, Moreno BH, Saco J, Escuin-Ordinas H, Rodriguez GA, Zaretsky JM, Sun L, Hugo W, Wang X, et al. Interferon Receptor Signaling Pathways Regulating PD-L1 and PD-L2 Expression. Cell Rep. 2019;29(11):3766.
Havel JJ, Chowell D, Chan TA. The evolving landscape of biomarkers for checkpoint inhibitor immunotherapy. Nat Rev Cancer. 2019;19(3):133–50.
Spranger S, Spaapen RM, Zha Y, Williams J, Meng Y, Ha TT, Gajewski TF: Up-regulation of PD-L1, IDO, and T(regs) in the melanoma tumor microenvironment is driven by CD8(+) T cells. Sci Transl Med. 2013;5(200):200ra116.
Chinen T, Kannan AK, Levine AG, Fan X, Klein U, Zheng Y, Gasteiger G, Feng Y, Fontenot JD, Rudensky AY. An essential role for the IL-2 receptor in Treg cell function. Nat Immunol. 2016;17(11):1322–33.
Tekguc M, Wing JB, Osaki M, Long J, Sakaguchi S. Treg-expressed CTLA-4 depletes CD80/CD86 by trogocytosis, releasing free PD-L1 on antigen-presenting cells. Proc Natl Acad Sci U S A. 2021;118(30):e2023739118.
Ma S, Cheng Q, Cai Y, Gong H, Wu Y, Yu X, Shi L, Wu D, Dong C, Liu H. IL-17A produced by gammadelta T cells promotes tumor growth in hepatocellular carcinoma. Cancer Res. 2014;74(7):1969–82.
Bever KM, Thomas DL 2nd, Zhang J, Diaz Rivera EA, Rosner GL, Zhu Q, Nauroth JM, Christmas B, Thompson ED, Anders RA, et al. A feasibility study of combined epigenetic and vaccine therapy in advanced colorectal cancer with pharmacodynamic endpoint. Clin Epigenetics. 2021;13(1):25.
Jansen YJL, Verset G, Schats K, Van Dam PJ, Seremet T, Kockx M, Van Laethem JB, Neyns B. Phase I clinical trial of decitabine (5-aza-2’-deoxycytidine) administered by hepatic arterial infusion in patients with unresectable liver-predominant metastases. ESMO Open. 2019;4(2): e000464.
Andre T, Shiu KK, Kim TW, Jensen BV, Jensen LH, Punt C, Smith D, Garcia-Carbonero R, Benavides M, Gibbs P, et al. Pembrolizumab in Microsatellite-Instability-High Advanced Colorectal Cancer. N Engl J Med. 2020;383(23):2207–18.
Kuang C, Park Y, Augustin RC, Lin Y, Hartman DJ, Seigh L, Pai RK, Sun W, Bahary N, Ohr J, et al. Pembrolizumab plus azacitidine in patients with chemotherapy refractory metastatic colorectal cancer: a single-arm phase 2 trial and correlative biomarker analysis. Clin Epigenetics. 2022;14(1):3.
Ahuja N, Sharma AR, Baylin SB. Epigenetic Therapeutics: A New Weapon in the War Against Cancer. Annu Rev Med. 2016;67:73–89.
Azad N, Zahnow CA, Rudin CM, Baylin SB. The future of epigenetic therapy in solid tumours–lessons from the past. Nat Rev Clin Oncol. 2013;10(5):256–66.
Pappalardi MB, Keenan K, Cockerill M, Kellner WA, Stowell A, Sherk C, Wong K, Pathuri S, Briand J, Steidel M, et al. Discovery of a first-in-class reversible DNMT1-selective inhibitor with improved tolerability and efficacy in acute myeloid leukemia. Nat Cancer. 2021;2(10):1002–17.
Acknowledgements
We would like to thank the shared resources provided by the Cellular and Molecular Morphology core at Texas Medical Center Digestive Disease Center (NIH/NIDDK P30 DK056338), the Single-Cell Genomics Core at Baylor College of Medicine (S10OD023469, S10OD025240, P30EY002520, and CPRIT RP200504), and the Cancer Center Core Grant (NIH/NCI P30CA125123). Fluorescence activated sorting was performed in the Texas Children’s Cancer and Hematology Core Flow Laboratory with the expert assistance of Tatiana Goltsova.
Funding
This research was supported by grants from US Department of Agriculture (CRIS 3092–51000-060 to L.S.) and National Institutes of Health (R21CA229727, R01HD100914, and R01CA233472 to L.S.).
Author information
Authors and Affiliations
Contributions
Study conception and funding: L.S. Study design: L.Y., X.C., L.Z., Y.L., N.G., S.Y.J., Y.L., L.S. Data acquisition and analysis: L.Y., X.C., C.L., J.S., C.J.C., J.J.L., Y.C., Y.L., W.L. Technical or material support: E.B.L., Y.L., N.G., S.Y.J., S.A., Drafting of the manuscript: L.Y., C.L., L.S. Critical revisions to the manuscript: All. Final decision to submit: The author(s) read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
This study was approved and supervised by the Baylor College of Medicine. All animal research was performed in accordance with the NIH Guide for Care and Use of Laboratory Animals and approved by the Baylor College of Medicine Animal Care and Use Committee.
Consent for publication
All author consent to publication.
Competing interests
The authors have declared that no conflict of interest exists.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Additional file 1: Figure S1.
p16 epimutation accelerates malignant transformation of ApcMin-mediated adenomatous polyps. Figure S2. Positive correlation between p16 promoter methylation and PDL1 expression in the TCGA-CRC dataset. Figure S3. Mouse colon tumors with combined Apc mutation and p16 epimutation are microsatellite stable (MSS). Figure S4. Dot plot visualization of representative marker genes in each cell type. Figure S5. UMAP plots showing abundant Pdl1 expression in the Cd45+ tumor-infiltrating immune cells. Figure S6. scRNA-seq analysis of CD8+ T cells during tumor development and progression. Figure S7. scRNA-seq analysis of CD4+ T cells, including Tregs, during tumor development and progression. Figure S8. Number of intestinal tumors in mice at the end of study at ~30 wk of age. Figure S9. Expression of murine ERVs in colon tumor organoids after DAC treatment at day 5. Figure S10. The impact of DAC treatment on other CRC-related tumor suppressor genes.
Additional file 2:
Supplementary Table 1. Primers and PCR conditions for genotyping assays. Supplementary Table 2. Sequences of gRNAs for CRISPR-mediated targeted demethylation. Supplementary Table 3. Bisulfite-pyrosequencing PCR primers and sequenced regions for quantitative DNA methylation analysis. Supplementary Table 4. TaqMan assays and SYBR green primers for qRT-PCR. Supplementary Table 5. Microsatellite markers for detection of MSI in mouse tumors.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Yang, L., Chen, X., Lee, C. et al. Functional characterization of age-dependent p16 epimutation reveals biological drivers and therapeutic targets for colorectal cancer. J Exp Clin Cancer Res 42, 113 (2023). https://doi.org/10.1186/s13046-023-02689-y
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s13046-023-02689-y