Abstract
Background
We aimed to determine the safety and efficacy of nintedanib, an oral anti-angiogenic tyrosine kinase inhibitor, in combination with pembrolizumab, an anti-PD1 immunotherapy, in patients with advanced solid tumors (PEMBIB trial; NCT02856425).
Methods
In this monocentric phase Ib dose escalation cohort, we evaluated escalating doses of nintedanib (Dose level 1 (DL1) = 150 mg bid [bis in die, as twice a day]; DL2 = 200 mg bid, oral delivery) in combination with pembrolizumab (200 mg Q3W, IV). Patients received a 1-week lead-in dose of nintedanib monotherapy prior starting pembrolizumab. The primary objective was to establish the maximum tolerated dose (MTD) of the combination based on dose limiting toxicity (DLT) occurrence during the first 4 weeks. Secondary objectives were to assess the anti-tumor efficacy and to identify the associated immune and angiogenic parameters in order to establish the recommended nintedanib dose for expansion cohorts. Flow cytometry (FC), Immuno-Histo-Chemistry (IHC) and electrochemiluminescence multi-arrays were prospectively performed on baseline & on-treatment tumor and blood samples to identify immune correlates of efficacy.
Results
A total of 12/13 patients enrolled were evaluable for DLT (1 patient withdrew consent prior receiving pembrolizumab). Three patients at 200 mg bid experienced a DLT (grade 3 liver enzymes increase). Four patients developed grade 1–2 immune related adverse events (irAE). Eight patients died because of cancer progression. Median follow-up was 23.7 months (95%CI: 5.55–40.5). Three patients developed a partial response (PR) (ORR = 25%) and five patients (42%) had durable clinical benefit (DCB), defined as PR or stable disease (SD) ≥ 6 months. At baseline, patients with DCB had higher plasma levels of Tie2, CXCL10, CCL22 and circulating CD4+ PD1+ OX40+ T cells than patients without DCB. Patients with DCB presented also with more DC-LAMP+ dendritic cells, CD3+ T cells and FOXP3+ Tregs in baseline tumor biopsies. For DCB patients, the nintedanib lead-in monotherapy resulted in higher blood CCL3, Tregs and CCR4+ CXCR3+ CXCR5− memory CD4 T cells. After the first pembrolizumab infusion, patients with DCB showed lower IL-6, IL-8, IL-27 plasma levels.
Conclusion
Nintedanib 150 mg bid is the recommended dose for combination with pembrolizumab and is currently investigated in multiple expansion cohorts. Early tumoral and circulating immune factors were associated with cancer outcome under nintedanib & pembrolizumab therapy.
Trial registration
ClinicalTrials.gov, NCT02856425. Registered August 4, 2016 — Prospectively registered.
Similar content being viewed by others
Background
Angiogenesis is a key mechanism in tumour growth and development of metastases [1]. Tumours induce blood vessel growth (angiogenesis) by secreting growth factors such as the Vascular Endothelial Growth Factor (VEGF), fibroblast growth factor (FGF) and platelet-derived growth factor (PDGF). VEGF and its high affinity receptor VEGFR-2 are crucial for the formation of new tumour vessels [2]. In addition, there is preclinical evidence that FGF, PDGF and their associated receptor tyrosine kinases substantially contribute to tumour angiogenesis. The VEGF/VEGFR-2 axis may also generate an autocrine loop which stimulates growth of tumour cells [3]. Therefore, suppression of neo-angiogenesis via inhibition of VEGFR-2 is a promising and efficient strategy for the treatment of solid tumors [4].
Immune checkpoint blockade is a new treatment strategy undergoing extensive investigation in multiple malignancies. Unlike standard chemotherapy or targeted therapy, the immune checkpoint blockade restores the immune system’s capacity to eradicate tumours [5]. Antibodies targeting the Programmed Death-1 (PD-1) receptor and its ligand (PD-L1) have been extensively investigated and are still in active development across malignancies and their different lines of treatment [6]. Combining anti-angiogenic drugs and anti-PD-(L)1 therapy has recently shown important synergistic results in renal cell carcinoma (RCC) and hepatocellular carcinoma (HCC) [7,8,9]. Indeed, anti-VEGF therapies may enhance anti–PD-(L)1 efficacy by reversing VEGF-mediated immunosuppression and promoting T-cell infiltration in tumours [10,11,12,13,14,15].
Although the combination of anti-angiogenics together with immune checkpoint blockade is becoming an attractive combination for the treatment of many cancers [16], the safety and activity of such combination is depending on the anti-angiogenic molecule tested. For instance, combinations of sunitinib and pazopanib with the anti-PD1 nivolumab were deemed too toxic with more than 70% grade 3/4 treatment related adverse events [17]. Therefore, novel anti-angiogenics should be tested in dedicated clinical trials to establish the dose, regimen, safety and activity when used in combination with anti-PD1 immunotherapies [18]. Nintedanib is an oral small-molecule tyrosine kinase inhibitor (TKI) that has been approved in 2014 by the EMA in combination with docetaxel for the treatment of adult patients with locally advanced, metastatic or locally recurrent non-small cell lung cancer (NSCLC) of adenocarcinoma histology after first-line chemotherapy. It acts as a triple angiokinase inhibitor blocking VEGF receptors (VEGFR 1–3), PDGF receptors (PDGFR α and β) and FGF receptors (FGFR 1–3) kinase activity.
We aimed to determine for the first time in humans the safety and efficacy of nintedanib, in combination with pembrolizumab, a humanized IgG4 anti-PD1 monoclonal antibody immunotherapy in patients with advanced solid tumors (NCT02856425).
Patients and methods
Study design
In this monocentric phase Ib dose escalation cohort (NCT02856425), we evaluated escalating doses of nintedanib (Dose level 1 (DL1) = 150 mg bid, DL2 = 200 mg bid) in combination with IV flat dose of pembrolizumab over 30 minutes (200 mg Q3W). Two dose-levels below the approved regimen of nintedanib (DL-1 & DL-2) could also be tested in case of unacceptable toxicities upon combination with pembrolizumab. Patients received a one-week lead-in course of nintedanib monotherapy prior starting pembrolizumab. The nintedanib monotherapy lead-in had two main objectives. First, based on the abundant literature on the positive immune modulatory effects of anti-angiogenic TKIs, we aimed at modifying the tumor microenvironment to maximize the potential effects of anti-PD1 immunnotherapy. Second, we wanted to be in a situation where we could assess separately the respective effects of nintedanib and pembrolizumab by comparing blood samples prior treatment (at D-7), after one week of nintedanib (D1) and upon addition of pembrolizumab (C2D1). The sample size of the dose escalation cohort was conducted according to the rolling 6 design [19]. Up to 6 patients evaluable for dose-limiting toxicity (DLT) could be exposed to a dose level. As soon as 3 patients were evaluable for DLT at a given dose level, dose escalation or de-escalation was permitted upon review of the safety data. The protocol was first approved by the Agence Nationale de Sécurité du Médicament (ANSM) on June 24th, 2016 (Ref #160371A-12) and by the Ethical Committee (Comité de Protection des Personnes Ile-de-France 1) on Jul 12th, 2016 (Ref #2016-mai-14236ND). The trial was first posted on clinicaltrials.gov on Aug 4th 2016 (NCT02856425).
Patients
Eligible patients had advanced, metastatic cancer which progressed after at least one line of standard therapy or were intolerant to standard therapy, naive to immune checkpoint blockade and nintedanib. Additional inclusion criteria included age ≥ 18 years, Eastern Cooperative Oncology Group (ECOG) performance status of 0–1, adequate organ function, measurable disease according to RECIST v1.1, and written informed consent. Key exclusion criteria were radiographic evidence of cavitary tumors, local invasion of major blood vessels and/or at risk for perforation, history of clinically significant hemoptysis within the past 3 months, history of clinically significant hemorrhagic or thromboembolic event in the past 6 months, history of significant cardiovascular diseases, prior treatment with anti PD-(L)1 agents, concurrent steroid medication, history of autoimmune and inflammatory disease. This study was conducted in compliance with the Declaration of Helsinki and the International Ethical Guidelines for Biomedical Research Involving Human Subjects.
Procedures
Screening procedures were performed up to 21 days (D-28) before Day − 7 (start of nintedanib). Patients continued treatment until disease progression, undue toxicity, or withdrawal of consent for a maximum duration of 24 months.
Adverse events were graded using National Cancer Institute (NCI) CTCAE Version 4.03. The occurrence of a non-hematological toxicity ≥ CTCAE Grade 3, a hematological toxicity ≥ CTCAE Grade 4, or an inability to resume nintedanib dosing within 7 days of stopping due to treatment related toxicity during the first 4 weeks were considered as a DLT, if judged by the Investigator to be possibly, probably, or related to study drug administration. Tumor evaluation was performed every 6 weeks based on Response Evaluation Criteria in Solid Tumors (RECIST) 1.1 and immune-related RECIST (irRECIST) [20, 21]. Details about screening exams, Nintedanib dose modification criteria, and other patient management rules are detailed in the protocol of the trial provided in the Supplementary information files.
Patients underwent tumor biopsies prior to the beginning of nintedanib and prior to the second injection of pembrolizumab in order to monitor the pharmacodynamic effects of the treatment and to identify factors associated with efficacy.
Outcomes
The primary objective was to establish the maximal tolerated dose (MTD) of nintedanib in combination with pembrolizumab based on the assessment of DLT occurrence during the first 4 weeks (28 days since C1D1). Secondary objectives were to determine the tolerability and safety of the Recommended Phase 2 Dose (RP2D) of nintedanib combined with pembrolizumab and to evaluate first anti-tumor activity of this combination in expansion cohorts. The aim of the ancillary studies was to identify factors associated with efficacy.
Tumor mutational burden by circulating tumor DNA sequencing
Plasma Cell-free circulating DNA samples were analysed using Oncomine™ Tumor Mutation Load Assay, according to the manufacturer recommendation, covering 1.65 Mb across 409 genes relevant cancer (Thermo Fisher Scientific, Les Ulis, France). Briefly, circulating the DNA were extracted by Maxwell® RSC ccfDNA Plasma Kit (Promega, Charbonnières-les-Bains, France). Libraries were performed using Ion Chef™ Instrument and Ion 550 Chip, then sequenced with Ion S5™ System (Thermo Fisher Scientific). The median coverage depth 751 X (from 636X to 817X) was obtained. Variant callings and Tumor mutational burden (TMB; count of mutations / megabase) were calculated using Ion reporter software package (version 5.14.1.0) with the pipeline: Oncomine Tumor Mutation Load - w3.1 - DNA - Single Sample (Thermo Fisher Scientific).
Immuno-Histo-chemistry staining
Multiple chromogenic and one fluorescent multiplex Immuno-Histo-Chemistry (IHC) stainings were performed on each biopsy. The Ventana Discovery Ultra platform was used for both PD-L1 and ICAM1 (DAB) single chromogenic staining, for FOXP3 (Purple)/CD31 (DAB) multiplex and CD3 (Purple)/CD8 (DAB) multiplex chromogenic staining and for a panel combining DC-LAMP (OPAL 620), CD68 (OPAL 570), IDO1 (OPAL 540), CD163 (OPAL 520) and DAPI nuclear staining for the identification of myeloid cells. The multispectral images were captured using the Vectra® microscope tunable filter that shines 35 incremental wave lengths of light from 300 nm to 750 nm. High-powered images were selected by a pathologist, matched to a Hematoxylin and Eosin Staining (HES) to validate tumor-associated tissue. The spectral library was synthesized using the Inform® v2.2 software.
Immune monitoring – fresh blood immune phenotype
For each patient, heparinized blood samples (30–40 mL) at day − 7 (baseline), C1D1 and C5D1 were collected whenever possible for monitoring circulating immune populations by flow cytometry. Fresh whole blood phenotyping of T-cell migration, T-cell polarization, T-cell activation, Treg function and myeloid cells was performed using 5 specific panels, as previously described [22]. Stained cells were acquired using a Gallios Cytometer (Beckman Coulter) and analyzed using Kaluza software (Beckman Coulter).
Cytokine, chemokine and soluble angiogenic factors measurements
Plasma samples were centrifuged for 15 min at 1000 g, diluted 1:4, then monitored using the Angiogenesis Panel 1 (human) (Meso Scale Discovery, ref.: K151P3S-1), Chemokines Panel 1, Proinflammatory Panel 1, Cytokine Panel 1 (Meso Scale Discovery, ref.: K151A9H-1), the ultra-sensitive assay S-plex Human IFNα2a kit (Meso Scale Discovery, ref.: K151P3S-1), Human PD-1 and PD-L1 antibody sets (Meso Scale Discovery, ref.: F214A-3 & F214C-3, respectively) following manufacturer’s instructions. Acquisitions and analyses were performed on a MESO™ QuickPlex SQ120 reader and the MSD’s Discovery Workbench 4.0. Each plasma sample was assayed twice with the average value taken as the result.
Statistical analysis and illustrations
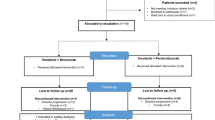
Clinical statistical analysis had been done using the SAS® statistical software version 9.4 (Cary, North Carolina, USA). Calculations and statistical tests for ancillary analyses were performed using R v3.4. Wilcoxon-Mann-Whitney test was used to assess differences between two patient’s groups. Data representation was performed with software R v3.3.3 using tidyverse, dplyr, ggplot2 and ggpubr packages. Figure’s aesthetics were worked with Affinity Designer® (v1.9.2.1035). Figure 1
Results
Safety of nintedanib dose escalation in combination with pembrolizumab
Thirteen patients were enrolled between Nov 24th, 2016, and Jul 24th, 2017, in the dose escalation cohort. Among them, one patient withdrew consent before C1D1. Consequently, 12 patients were evaluable for DLT. Six patients received nintedanib 150 mg bid per os (po) and 6 others consecutive patients received nintedanib 200 mg bid po. Their clinical characteristics are described in Table 1. Patients were treated for different tumor types: cervical cancer (n = 2), thymic carcinoma (n = 2), mesothelioma (n = 2), breast cancer (n = 1), colorectal adenocarcinoma (n = 1), gastric adenocarcinoma (n = 1), RCC (n = 1), neuroendocrine tumor of the caecum (n = 1) and undifferentiated carcinoma of nasopharyngeal type (UCNT) (n = 1). All patients received at least one previous line of treatment: chemotherapy (n = 12, 100%), immunotherapy (n = 1, 8%; intra-tumoral TLR7/8 agonist) and tyrosine kinase inhibitor (n = 1, 8%; pazopanib).
Patients treated with nintedanib 150 mg bid and 200 mg bid received a median of 9 (range: 4;34) and 3 (range: 2;16) complete cycles, respectively. Dose modifications of nintedanib occurred in 2 patients treated with nintedanib 150 mg bid (33%) and 4 patients (67%) and 200 mg bid, respectively. Three patients experienced DLT at 200 mg bid (nintedanib-related liver toxicities). The MTD of nintedanib with pembrolizumab 200 mg Q3W was 150 mg bid, based on DLT occurred in this cohort.
All patients presented at least one adverse event associated with one of both drugs. Four of them developed a defined immune related adverse event, a colitis, a nephritis and two thyroiditis (grade 3, 2 & 1 CTCAE v.4.03, respectively). Superior mesenteric artery occlusion was observed in one patient, concomitantly of a grade 3 colitis, confirmed by biopsies, and a thrombus on catheter in superior vena cava. These events were related to both nintedanib and pembrolizumab. One patient had an acute grade 4 pneumonitis related to Streptococcus pneumoniae infection, not related to the experimental combination. Listing of adverse events related to treatments are reported in Table 2.
Antitumoral activity upon nintedanib dose escalation in combination with pembrolizumab
Median follow up of the patients was 23.7 months (95% Confidence interval: [5.55; 40.5]). The best objective response was partial response (PR) for 3 patients (25%), stable disease (SD) for 4 patients (33%) and a primary progressive disease (PD) for 5 patients (42%) (Summarized in waterfall plot, Fig. 2A). Two patients with stable disease did not develop a tumor progression during follow up (Fig. 2B). Best responses were achieved at cycle 3 in most cases (58%). Five patients were alive at the end of the follow up period (Figs. 2C). Median Overall Survival was 16.3 months (95% Confidence Interval: [4.34; Not Reached]). Survival rates were 75% (95% CI: [46.8–91]), 64% (95% CI: [35.7–85.4]) and 32% (95% CI: [11.8–62.6]) at 6, 12 and 24 months, respectively. Eight patients died because of cancer progression and no treatment-related deaths were observed. We sequenced the circulating tumor DNA (ctDNA) of our patients in order to better characterize the genomic landscape of our patients’ cancers. No specific relationship could be found between efficacy and the somatic genomic alterations of our patients, including tumor mutational burden (TMB) (Table 3). Of note, TMB from ctDNA were low in this cohort of patients (median 4.19 mutations/Mb [3.35–5.05]) and did not discriminate the patients’ outcomes (Fig. 2C).
Preexisting immune & angiogenic characteristics were associated with antitumoral response upon immune checkpoint blockade in combination with antiangiogenic therapy
Patients with durable clinical benefit (DCB) defined as objective partial response or stable disease for at least 6 months after the beginning of treatment, presented higher CXCL10, CCL22/Macrophage-Derived Chemokine (MDC) and soluble Tie2 plasma levels before initiation of treatment than patients without DCB (Fig. 3A). Flow cytometry analyses on fresh whole blood highlighted that CD4+ PD1+ OX40+ and CD4+ α4β7+ among total CD4+ T cells were more present in patients with DCB than patients without DCB (Fig. 3B). IHC analyses on pre-treatment tumor biopsies highlighted that patient who developed DCB tended to have higher immune infiltration characterized by higher percentage of PDL1+ tumor cells, and higher densities of CD3+ T cells, FOXP3+ cells and DCLAMP+ dendritic cells than patients without DCB after treatment (Fig. 3C).
Increased tumor immune cell infiltrations in patients with durable clinical benefit (DCB) before initiation of the nintedanib and pembrolizumab association. A CXCL10, CCL22 and soluble Tie2 plasma levels were significantly higher in patients with DCB (1464 pg/mL [range: 409.8–4290.5], 1032.8 pg/mL [range: 810.5–1479.9] and 3796.6 pg/mL [range: 2710.9–4665.6], respectively) than patients without DCB (334.7 pg/mL [range: 81.1–767.6], 745.2 pg/mL [range: 396.8–1088.5] and 2896.5 pg/mL [range: 2230.4–4132.7]) (Wilcoxon rank sum test; p = 0.03, p = 0.03 and p = 0.04, respectively). B The percentage of α4β7+ CD4+ and PD1+ OX40+ CD4+ cells among total circulating CD4+ T cells was significantly higher in patients with DCB (28.7% [range: 22.4–42.6] and 43.1% [range: 25.6–78.9], respectively) than without DCB (17.7% [range: 13.1–23.9] and 25.9% [range: 14.5–55.4]) (Wilcoxon rank sum test; p = 0.024 and p = 0.03, respectively). C Patients with PR had PD-L1 expression on tumor cells and immune infiltration in tumor stroma, characterized by IHC (Wilcoxon rank sum test; non-significant). Abbreviations: PD = Progressive disease; SD = Stable disease; PR = Partial response; Ninte. = nintedanib; DCB = Durable clinical benefit; MDC = Macrophage Derived Chemokine; DC = dendritic cells
Specific angiogenic and immune changes occurring during nintedanib lead-in monotherapy could favor primary resistance to immune checkpoint blockade efficiency
During the first week of the clinical trial, patients received nintedanib monotherapy. The comparison of soluble factors in plasma and phenotype of fresh blood circulating lymphocytes between day - 7 and C1D1 allowed the identification of changes associated with DCB to treatment which could be induced by the lead-in nintedanib monotherapy.
Placental Growth Factor (PlGF) and VEGF-D plasma levels increased significantly between day − 7 and C1D1 only in patients without DCB (Fig. 4A; Supplementary Fig. 1), although other soluble angiogenic factors levels remained stable. In parallel, plasma levels of CCL3 / MIP-1α (Macrophage Inflammatory Protein 1-Alpha), another ligand for CCR4 with CCL22/MDC, tended to increase in patients with DCB and was significantly higher before pembrolizumab infusion in patients with DCB compared to patients without DCB (Fig. 4B). Also, patients who benefited from treatment had a higher percentage of circulating conventional CCR4+ CXCR3+ helper memory T cells and Tregs than patients without DCB (Fig. 4C).
Increased of circulating soluble PlGF, CCL3 and increase in specific T cells subsets during lead-in nintedanib monotherapy associated to clinical outcomes. A Plasma rate of Placental Growth Factor (PlGF) increased in patients without DCB between D-7 (13.4 pg/mL [range: 8.4–17.9]) and C1D1 (18.8 pg/mL [range: 10.8–30.3]) (Paired Wilcoxon signed rank test; p = 0.015). B Plasma rate of CCL3 were higher in patients with DCB (19.8 pg/mL [range: 11.7–26.1]) than without DCB (12.9 pg/mL [range: 9.9–17.8]) after 7 days of nintedanib monotherapy (non-parametric Wilcoxon rank sum test; p = 0.03). C Percentage of blood CCR4+ CXCR3+ T cells among effector memory CD4+ T cells and CD25high CD127low Tregs among total CD4+ T lymphocytes were higher at C1D1 in patients with DCB (25.3% [range: 21.2–35.8] and 10% [range: 6.4–15.4], respectively) than those without DCB (17.9% [range: 14.4–23.8] and 5.8% [range: 4.1–8.5], respectively) (non-parametric Wilcoxon rank sum test; p = 0.017 and p = 0.024, respectively). Abbreviations: PD = Progressive disease; SD = Stable disease; PR = Partial response; Ninte. = nintedanib; DCB = Durable clinical benefit; D-7 = day − 7; C1D1 = Cycle 1 day 1; MIP = Macrophage Inflammatory Protein
Early inflammatory changes occurring after immune checkpoint blockade were associated with resistance to treatment and progressive disease
Early inflammatory changes were observed in plasma of patients without DCB as opposed to patients with DCB. Before the second pembrolizumab infusion, patients without DCB presented an increasing plasmatic rate of TNF, compared to paired pre-pembrolizumab plasma (Fig. 5A), and also for CCL3, CCL4, IL-18, IL-10, IL-22 and VEGF-D (Supplementary Fig. 2). Patients without DCB also had higher plasmatic rate of inflammatory cytokines such as IL-6, IL-27 and CXCL8 than patients with DCB at C2D1 (Fig. 5B). IHC performed on tumor biopsies done before C2D1, showed that percentages of PDL1+ tumor cells, ratio of CD3+ on CD163+ cells and density of FOXP3+ cells were significantly higher in patients with DCB than without DCB (Fig. 5C). Tumor densities of CD68+ and CD163+ cells were not different in C2D1 biopsies (Supplementary Fig. 3); neither those of DC-LAMP+, IDO1+, ICAM1+ or CD31+ cells (data not shown).
After pembrolizumab infusion, in association with nintedanib treatment, circulating and tumor microenvironment changes were associated with distinct clinical outcomes. A Plasma levels of soluble TNF increased in patients without DCB between C1D1 (0.7 pg/mL [range: 0.4–1.1]) and C2D1 (1.8 pg/mL [range: 0.8–3.4]) (Paired Wilcoxon signed rank test; p = 0.015) instead of in patients with DCB. B Plasma rate of IL6, IL8 and IL27 were significantly higher at C2D1 in patients without DCB (9.6 pg/mL [range: 3.5–22.4], 10.8 pg/mL [range: 5.8–16.7] and 380.9 pg/mL [range: 181.8–845.2], respectively) than those with DCB (1.7 pg/mL [range: 0.6–4.14], 4.9 pg/mL [range: 1.7–6.8] and 163.9 pg/mL [range: 56.6–247.4], respectively) (non-parametric Wilcoxon rank sum test; p = 0.01, p = 0.01 and p = 0.03, respectively). C Percentage of PDL1+ tumor cells, ratio of CD3+ per CD163+ cells and FOXP3+ cells density in biopsies of patients with DCB (41.8% [range: 2–90], 1.9 [range: 0.8–3] and 221/mm2 [range: 116–320], respectively) was higher than patients without DCB (3% [range: 0–20], 0.5 [range: 0.06–1.4] and 49/mm2 [range: 1–158], respectively) at C2D1 (non-parametric Wilcoxon rank sum test; p = 0.009, p = 0.047 and p = 0.03, respectively). D Illustration of increased expression of PDL1 at tumor cell’s surface, CD3+ and FOXP3+ T cells infiltration in tumor microenvironment observed with IHC analysis (patient #3). Abbreviations: PD = Progressive disease; SD = Stable disease; PR = Partial response; Ninte. = nintedanib; DCB = Durable clinical benefit; C1 = Cycle 1; TNF = Tumor Necrosis Factor
Discussion
This phase 1b dose escalation cohort showed that the toxicity of the nintedanib and pembrolizumab combination was manageable and consistent with the safety profile of each drug and did not seem to generate higher toxicity than the cumulative impact of each compound. The RP2D for the combination was defined as nintedanib 150 mg bid and flat dose of pembrolizumab 200 mg IV and is currently being investigated in multiple expansion cohorts. The main toxicity of this combination therapy was liver enzymes increase, which is consistent with other combination of immune checkpoint inhibitors and antiangiogenic TKIs. The incidence of drug-related grade 3 liver enzymes elevation was 33% for alanine aminotransferase and 25% for aspartate aminotransferase in our study. Other combinations of anti PD-1 monoclonal antibody and antiangiogenic TKI reported variable liver toxicity: nivolumab/sunitinib, nivolumab/pazopanib, pembrolizumab/pazopanib and pembrolizumab/axitinib reported 18, 20%, 60–70 and 8% of grade 3–4 elevated alanine aminotransferase, respectively [17, 25, 26]. In the dose escalation trial of nintedanib monotherapy, the DLTs were grade 3 or 4 liver enzyme elevations (2–5%), all reversible upon drug interruption [27]. The adverse events reported in this study seem to be mostly related to nintedanib but programmed death 1 blockade could possibly enhance the on-target off-tumor side effects of the TKIs. The observed levels of immune related adverse events such as colitis (8%), nephritis (8%) and thyroiditis (25%) were in line with the expected irAE associated with pembrolizumab monotherapy. Thromboses were observed in two patients: one with superior mesenteric artery thrombus and both with thrombus on catheter in superior vena cava. These adverse events could be related to the combination of antiangiogenic TKI with immune checkpoint blockade and not to nintedanib alone. These two patients with thromboses obtained a durable clinical benefit (SD and PR, respectively during at least 6 months). Overall, the antitumor activity of the combination tested was promising with an ORR of 25% in this all-comer cohort and will be confirmed in the expansion cohorts of the study.
Baseline ancillary analyses highlighted those patients who developed DCB to this anti-angiogenic / ICB combination presented with both preexisting immune and angiogenic features. Higher expression of CXCL10 and immune infiltrate in biopsies were previously associated with ICB monotherapy efficiency in different tumor types [28, 29]. It was interesting to observe that soluble Tie2 was higher in plasma of patients with DCB. Tie2 is the receptor of angiopoietin-2, expressed at surface of endothelial cells but also circulating monocytes [30]. Studies highlighted that Tie2 production and membrane shedding are increased by hypoxia and induced by VEGF [31]. Here, Tie2 plasmatic rate could be a witness of hypoxia occurring in the tumor of patients who benefited from the treatment.
After seven days of nintedanib monotherapy, quick changes were observed in patients who benefited from the treatment. Although those could be spontaneous changes favored by different tumor changes, their rapid evolution suggest that they could be induced by nintedanib. Particularly, the increase of plasmatic CCL3/MIP-1α in patients with DCB could be a consequence of tumor hypoxia on chemokine production [32]. At baseline, a higher Tie2 plasmatic rate, along with higher proportions of circulating Tregs, and conventional helper memory T cells which co-expressed CCR4 and CXCR3 (receptors of CCL22/MDC, CCL3/MIP-1α and CXCL10 respectively) were also predictive of DCB. Blood Tregs constitutively express CCR4 on their surface [33]. CCL22 production by tumor cells was identified as a mechanism of immune escape in breast cancer, through CCR4+ Treg recruitment in the tumor microenvironment (TME), induced by IFNγ exposure [34, 35]. These observations led to the development of CCR4 antagonists as antitumoral immunotherapy [36, 37]. Our results suggest that Treg recruitment in the TME was associated with a higher infiltration of immune cells, particularly DC-LAMP+ dendritic cells and T cells, associated with a pre-existing anti-tumoral immune response, sensitive to immune checkpoint blockade.
However, patients without DCB developed others significant changes after one week of nintedanib monotherapy. They seemed to present an increasing plasma rate of PlGF as of patients with DCB. This soluble angiogenic factor was recently implicated in interactions between angiogenesis, Th17 polarization of CD4+ T cells and autoimmunity [38]. This observation should be associated with changes induced further by anti PD-1 blockade. Patients with tumor progression and resistance to treatment presented then with an increasing plasma rate of IL17A and TNF after the first infusion of pembrolizumab, in comparison to patients with DCB. Patients without DCB had also a higher plasma rate of IL-6, IL-8/CXCL8 and IL-27 at C2D1 than patients who had benefited from treatment. Increased rates of both IL-6 and IL-8/CXCL8 have been described to be associated with resistance to anti-PD1 blockade monotherapy [39, 40]. Of note, the baseline plasma rates of these cytokines were not significantly different between patients with or without DCB outcome.
Interestingly, VEGF-D levels were significantly increasing in patients with No DCB upon nintedanib monotherapy (between D-7 and D1; Supplementary Fig. 1) and upon addition of pembrolizumab (between C1D1 and C2D1; Supplementary Fig. 2). Patients with DCB did not show a specific change in VEGF-D levels upon treatment. This observation raises the question of potential differential sensitivity to angiogenic inhibitors between patients presenting DCB and No DCB via either variations in target receptor expressions or reactional feedback loops.
Conclusion
Nintedanib 150 mg bid is the RP2D for combination with pembrolizumab and is being currently investigated in multiple expansion cohorts. Early immune parameters in the tumor and in the blood were associated with the efficacy of the nintedanib + pembrolizumab combination therapy. Our biological findings at baseline and during the early phases of treatment, associated significantly with the clinical benefit to treatment or to primary tumor progression, suggesting that patients could be selected on their tumor biology rather than their cancer histology in order to benefit from such anti-angiogenic and immune targeted therapies.
Availability of data and materials
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Abbreviations
- ANSM:
-
Agence Nationale de Sécurité du Médicament
- DCB:
-
Durable clinical benefit
- DL1:
-
Dose level 1
- DLT:
-
Dose-limiting toxicity
- ECOG:
-
Eastern Cooperative Oncology Group
- FGF:
-
Fibroblast growth factor
- HES:
-
Hematoxylin and Eosin Staining
- HCC:
-
Hepatocellular carcinoma
- IHC:
-
Immuno-Histo-Chemistry
- irRECIST:
-
Immune-related RECIST
- MDC:
-
Macrophage-Derived Chemokine
- MIP-1α:
-
Macrophage Inflammatory Protein 1-Alpha
- MTD:
-
Maximal tolerated dose
- NCI:
-
National Cancer Institute
- NSCLC:
-
Non-small cell lung cancer
- PD:
-
Progressive disease
- PDGF:
-
Platelet-derived growth factor
- PD-1:
-
Programmed Death-1
- PlGF:
-
Placental Growth Factor
- Po:
-
Per os
- PR:
-
Partial response
- RCC:
-
Renal cell carcinoma
- RECIST:
-
Response Evaluation Criteria in Solid Tumors
- RP2D:
-
Recommended Phase 2 Dose
- SD:
-
Stable disease
- UCNT:
-
Undifferentiated carcinoma of nasopharyngeal type
- VEGF:
-
Vascular Endothelial Growth Factor
References
Potente M, Gerhardt H, Carmeliet P. Basic and therapeutic aspects of angiogenesis. Cell. 2011;146(6):873–87.
Vajkoczy P, Farhadi M, Gaumann A, Heidenreich R, Erber R, Wunder A, et al. Microtumor growth initiates angiogenic sprouting with simultaneous expression of VEGF, VEGF receptor-2, and angiopoietin-2. J Clin Invest. 2002;109(6):777–85.
De Palma M, Biziato D, Petrova TV. Microenvironmental regulation of tumour angiogenesis. Nat Rev Cancer. 2017;17(8):457–74.
Grothey A, Galanis E. Targeting angiogenesis: progress with anti-VEGF treatment with large molecules. Nat Rev Clin Oncol. 2009;6(9):507–18.
Ribas A, Wolchok JD. Cancer immunotherapy using checkpoint blockade. Science. 2018;359(6382):1350–5.
Hirsch L, Zitvogel L, Eggermont A, Marabelle A. PD-Loma: a cancer entity with a shared sensitivity to the PD-1/PD-L1 pathway blockade. Br J Cancer. 2019;120(1):3–5.
Finn RS, Qin S, Ikeda M, Galle PR, Ducreux M, Kim T-Y, et al. Atezolizumab plus Bevacizumab in Unresectable Hepatocellular Carcinoma. N Engl J Med. 2020;382(20):1894–905.
Rini BI, Plimack ER, Stus V, Gafanov R, Hawkins R, Nosov D, et al. Pembrolizumab plus Axitinib versus Sunitinib for Advanced Renal-Cell Carcinoma. N Engl J Med. 2019;380(12):1116–27.
Motzer RJ, Penkov K, Haanen J, Rini B, Albiges L, Campbell MT, et al. Avelumab plus Axitinib versus Sunitinib for Advanced Renal-Cell Carcinoma. N Engl J Med. 2019;380(12):1103–15.
Motz GT, Santoro SP, Wang L-P, Garrabrant T, Lastra RR, Hagemann IS, et al. Tumor endothelium FasL establishes a selective immune barrier promoting tolerance in tumors. Nat Med. 2014;20(6):607–15.
Roland CL, Dineen SP, Lynn KD, Sullivan LA, Dellinger MT, Sadegh L, et al. Inhibition of vascular endothelial growth factor reduces angiogenesis and modulates immune cell infiltration of orthotopic breast cancer xenografts. Mol Cancer Ther. 2009;8(7):1761–71.
Voron T, Colussi O, Marcheteau E, Pernot S, Nizard M, Pointet A-L, et al. VEGF-A modulates expression of inhibitory checkpoints on CD8+ T cells in tumors. J Exp Med. 2015;212(2):139–48.
Wallin JJ, Bendell JC, Funke R, Sznol M, Korski K, Jones S, et al. Atezolizumab in combination with bevacizumab enhances antigen-specific T-cell migration in metastatic renal cell carcinoma. Nat Commun. 2016;7:12624.
Hegde PS, Wallin JJ, Mancao C. Predictive markers of anti-VEGF and emerging role of angiogenesis inhibitors as immunotherapeutics. Semin Cancer Biol. 2018;52(Pt 2):117–24.
McDermott DF, Huseni MA, Atkins MB, Motzer RJ, Rini BI, Escudier B, et al. Clinical activity and molecular correlates of response to atezolizumab alone or in combination with bevacizumab versus sunitinib in renal cell carcinoma. Nat Med. 2018;24(6):749–57.
Khan KA, Kerbel RS. Improving immunotherapy outcomes with anti-angiogenic treatments and vice versa. Nat Rev Clin Oncol. 2018;15(5):310–24.
Amin A, Plimack ER, Ernstoff MS, Lewis LD, Bauer TM, McDermott DF, et al. Safety and efficacy of nivolumab in combination with sunitinib or pazopanib in advanced or metastatic renal cell carcinoma: the CheckMate 016 study. J Immunother Cancer. 2018;6(1):109.
Qin S, Li A, Yi M, Yu S, Zhang M, Wu K. Recent advances on anti-angiogenesis receptor tyrosine kinase inhibitors in cancer therapy. J Hematol Oncol. 2019;12(1):27.
Skolnik JM, Barrett JS, Jayaraman B, Patel D, Adamson PC. Shortening the timeline of pediatric phase I trials: the rolling six design. J Clin Oncol. 2008;26(2):190–5.
Schwartz LH, Litière S, de Vries E, Ford R, Gwyther S, Mandrekar S, et al. RECIST 1.1-update and clarification: from the RECIST committee. Eur J Cancer. 2016;62:132–7.
Seymour L, Bogaerts J, Perrone A, Ford R, Schwartz LH, Mandrekar S, et al. iRECIST: guidelines for response criteria for use in trials testing immunotherapeutics. Lancet Oncol. 2017;18(3):e143–52.
Pitoiset F, Cassard L, El Soufi K, Boselli L, Grivel J, Roux A, et al. Deep phenotyping of immune cell populations by optimized and standardized flow cytometry analyses. Cytometry A. 2018;93(8):793–802.
Mezquita L, Auclin E, Ferrara R, Charrier M, Remon J, Planchard D, et al. Association of the Lung Immune Prognostic Index With Immune Checkpoint Inhibitor Outcomes in Patients With Advanced Non-Small Cell Lung Cancer. JAMA Oncol. 2018;4(3):351–7.
Bigot F, Castanon E, Baldini C, Hollebecque A, Carmona A, Postel-Vinay S, et al. Prospective validation of a prognostic score for patients in immunotherapy phase I trials: the Gustave Roussy immune score (GRIm-score). Eur J Cancer. 2017;84:212–8.
Chowdhury S, Infante JR, Hawkins R, Voss MH, Perini R, Arkenau T, et al. A Phase I/II Study to Assess the Safety and Efficacy of Pazopanib and Pembrolizumab Combination Therapy in Patients with Advanced Renal Cell Carcinoma. Clin Genitourin Cancer. 2021;S1558–7673(21):00092–6.
Atkins MB, Plimack ER, Puzanov I, Fishman MN, McDermott DF, Cho DC, et al. Axitinib in combination with pembrolizumab in patients with advanced renal cell cancer: a non-randomised, open-label, dose-finding, and dose-expansion phase 1b trial. Lancet Oncol. 2018;19(3):405–15.
Mross K, Stefanic M, Gmehling D, Frost A, Baas F, Unger C, et al. Phase I study of the angiogenesis inhibitor BIBF 1120 in patients with advanced solid tumors. Clin Cancer Res. 2010;16(1):311–9.
Tumeh PC, Harview CL, Yearley JH, Shintaku IP, Taylor EJM, Robert L, et al. PD-1 blockade induces responses by inhibiting adaptive immune resistance. Nature. 2014;515(7528):568–71.
Herbst RS, Soria J-C, Kowanetz M, Fine GD, Hamid O, Gordon MS, et al. Predictive correlates of response to the anti-PD-L1 antibody MPDL3280A in cancer patients. Nature. 2014;515(7528):563–7.
Lewis CE, De Palma M, Naldini L. Tie2-expressing monocytes and tumor angiogenesis: regulation by hypoxia and angiopoietin-2. Cancer Res. 2007;67(18):8429–32.
Findley CM, Cudmore MJ, Ahmed A, Kontos CD. VEGF induces Tie2 shedding via a phosphoinositide 3-kinase/Akt dependent pathway to modulate Tie2 signaling. Arterioscler Thromb Vasc Biol. 2007;27(12):2619–26.
Korbecki J, Kojder K, Kapczuk P, Kupnicka P, Gawrońska-Szklarz B, Gutowska I, et al. The Effect of Hypoxia on the Expression of CXC Chemokines and CXC Chemokine Receptors-A Review of Literature. Int J Mol Sci. 2021;22(2):E843.
Iellem A, Mariani M, Lang R, Recalde H, Panina-Bordignon P, Sinigaglia F, et al. Unique chemotactic response profile and specific expression of chemokine receptors CCR4 and CCR8 by CD4(+)CD25(+) regulatory T cells. J Exp Med. 2001;194(6):847–53.
Gobert M, Treilleux I, Bendriss-Vermare N, Bachelot T, Goddard-Leon S, Arfi V, et al. Regulatory T cells recruited through CCL22/CCR4 are selectively activated in lymphoid infiltrates surrounding primary breast tumors and lead to an adverse clinical outcome. Cancer Res. 2009;69(5):2000–9.
Faget J, Biota C, Bachelot T, Gobert M, Treilleux I, Goutagny N, et al. Early detection of tumor cells by innate immune cells leads to T (reg) recruitment through CCL22 production by tumor cells. Cancer Res. 2011;71(19):6143–52.
Sun W, Li W-J, Wei F-Q, Wong T-S, Lei W-B, Zhu X-L, et al. Blockade of MCP-1/CCR4 signaling-induced recruitment of activated regulatory cells evokes an antitumor immune response in head and neck squamous cell carcinoma. Oncotarget. 2016;7(25):37714–27.
Marshall LA, Marubayashi S, Jorapur A, Jacobson S, Zibinsky M, Robles O, et al. Tumors establish resistance to immunotherapy by regulating Treg recruitment via CCR4. J Immunother Cancer. 2020;8(2):e000764.
Yoo S-A, Kim M, Kang M-C, Kong J-S, Kim K-M, Lee S, et al. Placental growth factor regulates the generation of TH17 cells to link angiogenesis with autoimmunity. Nat Immunol. 2019;20(10):1348–59.
Laino AS, Woods D, Vassallo M, Qian X, Tang H, Wind-Rotolo M, et al. Serum interleukin-6 and C-reactive protein are associated with survival in melanoma patients receiving immune checkpoint inhibition. J Immunother Cancer. 2020;8(1):e000842.
Schalper KA, Carleton M, Zhou M, Chen T, Feng Y, Huang S-P, et al. Elevated serum interleukin-8 is associated with enhanced intratumor neutrophils and reduced clinical benefit of immune-checkpoint inhibitors. Nat Med. 2020;26(5):688–92.
Acknowledgements
The authors would like to thank the patients who participated to this dose escalation part of the PEMBIB trial and their families. They also thank the investigators, nurses, study coordinators and all the staff involved in the PEMBIB trial, including collection and provision of patients’ data and samples, notably Ms. Delphine Vuillier, Dr. Emilie Lanoy, Ms. Camille Jannin, Mr. Dominique Schwob, Mr. Cedric Parlavecchio, Mr. Xavier Paoletti, Mr. Thibaud Motreff, Ms. Fouzia Azzouz, Ms. Nadia Zaghdoud, Ms. Siham Farhane, Dr. Eric Angevin, Dr. François Bidault. We also thank the Gustave Roussy team of Molecular Biopathology for their contribution to the sequencing and analysis of circulating tumor DNA notably Patrick Saulnier, Noemie Pata-Merci, Damien Vasseur, Nathalie Jacques, Etienne Rouleaux, and Aurelie Stourm. Last but not least, the authors would like to thank the teams from Boehringer Ingelheim (BI) and Merck Sharp & Dohme (MSD) for their support and contribution to the study, notably Dr. Claudia Nanette-Gann (BI), Dr. Rolf Kaiser (BI), Ms. Martine Vidal (BI), Ms. Sabrina Wiebe (BI), Dr. Christophe Maritaz (BI), Dr. Dejan Radonjic (BI), Ms. Isabelle Brassac (MSD), Dr. Rodolphe Montin (MSD), and Dr. Eric Rubin (MSD). BI had no role in the design, analysis or interpretation of the results in this study. BI was given the opportunity to review the manuscript for medical and scientific accuracy as it relates to BI substances, as well as intellectual property considerations.
Funding
This Gustave Roussy sponsored study benefited from financial support and nintedanib supply from Boehringer Ingelheim (BI) and pembrolizumab supply from Merck Sharp & Dohme (MSD). Most ancillary studies were funded by BI. Some technician time and reagents used for the ancillary studies were partly funded by a research grant from the Fondation MSD Avenir.
Author information
Authors and Affiliations
Contributions
AM & J-CS designed the study and wrote the PEMBIB protocol. CB, F-XD, and AM wrote the current manuscript. All authors were involved in the acquisition, analysis, or interpretation of the data and critical revisions of the manuscript. MT and FX-D performed to the statistical analysis. AP, SS and SF provided administrative, technical, or material support. AM supervised the study as principal investigator and national coordinator. All authors gave their final approval of the manuscript and agreed to be accountable for all aspects of the work.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Studies were conducted in accordance with the Declaration of Helsinki and approved by local and multicenter research ethical committees.
Consent for publication
Not applicable.
Competing interests
BI & MSD are partly funding the PEMBIB trial and its ancillary analysis. They had a right of first glance at the data reported but could not modify or influence the results reported in the manuscript neither their interpretation. Gustave Roussy as the sponsor and his investigators remain independent from BI & MSD for the data analysis, interpretation, and publication of the PEMBIB trial. Over the last 5 years, AM provided consulting work to BI for a project not related to the PEMBIB trial, participated to a scientific advisory board with MSD on a topic not related to the PEMBIB trial, has been a principal investigator for MSD-sponsored clinical trials not related to the PEMBIB trial and received a research grant from Fondation MSD Avenir that was partly funding some lab technician time and biological assays that were used for the ancillary analysis of the PEMBIB trial. JCS have shares in Gritstone bio and Relay Therapeutics, is Board of Director at Hookipa Pharmaceuticals and is Full time employee at Amgen since August 2021. As part of members of the DITEP CB, AV, SC, PV, PM-R, J-MM, RB, AG, VR, SP-V, AH and CM are Principal/sub-Investigator of Clinical Trials for Abbvie, Adaptimmune, Adlai Nortye USA Inc., Aduro Biotech, Agios Pharmaceuticals, Amgen, Argen-X Bvba, Astex Pharmaceuticals, Astra Zeneca Ab, Aveo, Basilea Pharmaceutica International Ltd., Bayer Healthcare Ag, Bbb Technologies Bv, Beigene, BicycleTx Ltd., Blueprint Medicines, Boehringer Ingelheim, Boston Pharmaceuticals, Bristol Myers Squibb, Ca, Celgene Corporation, Chugai Pharmaceutical Co, Clovis Oncology, Cullinan-Apollo, Curevac, Daiichi Sankyo, Debiopharm, Eisai, Eisai Limited, Eli Lilly, Exelixis, Faron Pharmaceuticals Ltd., Forma Tharapeutics, Gamamabs, Genentech, Glaxosmithkline, H3 Biomedicine, Hoffmann La Roche Ag, Imcheck Therapeutics, Innate Pharma, Institut De Recherche Pierre Fabre, Iris Servier, Iteos Belgium SA, Janssen Cilag, Janssen Research Foundation, Kura Oncology, Kyowa Kirin Pharm. Dev, Lilly France, Loxo Oncology, Lytix Biopharma As, Medimmune, Menarini Ricerche, Merck Sharp & Dohme Chibret, Merrimack Pharmaceuticals, Merus, Millennium Pharmaceuticals, Molecular Partners Ag, Nanobiotix, Nektar Therapeutics, Novartis Pharma, Octimet Oncology Nv, Oncoethix, Oncopeptides, Orion Pharma, Ose Pharma, Pfizer, Pharma Mar, Pierre Fabre, Medicament, Roche, Sanofi Aventis, Seattle Genetics, Sotio A. S, Syros Pharmaceuticals, Taiho Pharma, Tesaro, Turning Point Therapeutics, Xencor. They benefit from research Grants from Astrazeneca, BMS, Boehringer Ingelheim, GSK, INCA, Janssen Cilag, Merck, Novartis, Pfizer, Roche, Sanofi and non-financial support (drug supplied) from Astrazeneca, Bayer, BMS, Boringher Ingelheim, GSK, Medimmune, Merck, NH TherAGuiX, Pfizer, Roche. The other authors declare no conflicts of interest.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Additional file 1: Supplementary Fig. 1.
Evolution of plasma soluble angiogenic factors during lead-in nintedanib monotherapy. Tests were paired Wilcoxon signed rank test (paired samples) (representation of p-value: ns > 0.05, * ≤ 0.05). Abbreviations: Ninte. = nintedanib; PD = Progressive disease; SD = Stable disease; PR = Partial response; DCB = Durable clinical benefit; D-7 = day − 7; C1D1 = Cycle 1 day 1.
Additional file 2: Supplementary Fig. 2.
Evolution of plasma soluble cytokines between after the first pembrolizumab infusion, between cycle 1 and cycle 2. Tests were paired Wilcoxon signed rank test (paired samples) (representation of p-value: ns > 0.05, * ≤ 0.05). Abbreviations: PD = Progressive disease; SD = Stable disease; PR = Partial response; Ninte. = nintedanib; DCB = Durable clinical benefit; D-7 = day − 7; C1D1 = Cycle 1 day 1; MIP = Macrophage Inflammatory Protein; MCP1 = Monocyte Chemoattractant protein; MDC = Macrophage Derived Chemokine.
Additional file 3: Supplementary Fig. 3.
Illustration of multiplex chromogenic staining dedicated to myeloid cells. A Representative image displays a 500umx669um image after multispectral imaging and spectral unmixing (merged image). B All markers. C CD68 (pseudocoloured white). D DAPI nuclear marker (pseudocoloured blue). E CD163 (pseudocoloured green). F DC-LAMP (pseudocoloured magenta). G IDO1 (pseudocoloured yellow). H CD163 measurement was undertaken using Inform v2.2 software segmentation by pixel-based threshold. I CD163+ density in biopsies of patients with DCB or without DCB at baseline and C2D1 were not significantly different (Wilcoxon rank-sum test).
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Baldini, C., Danlos, FX., Varga, A. et al. Safety, recommended dose, efficacy and immune correlates for nintedanib in combination with pembrolizumab in patients with advanced cancers. J Exp Clin Cancer Res 41, 217 (2022). https://doi.org/10.1186/s13046-022-02423-0
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s13046-022-02423-0