Abstract
Background
One of the most malignant tumors in men is prostate cancer that is still incurable due to its heterogenous and progressive natures. Genetic and epigenetic changes play significant roles in its development. The RNA molecules with more than 200 nucleotides in length are known as lncRNAs and these epigenetic factors do not encode protein. They regulate gene expression at transcriptional, post-transcriptional and epigenetic levels. LncRNAs play vital biological functions in cells and in pathological events, hence their expression undergoes dysregulation.
Aim of review
The role of epigenetic alterations in prostate cancer development are emphasized here. Therefore, lncRNAs were chosen for this purpose and their expression level and interaction with other signaling networks in prostate cancer progression were examined.
Key scientific concepts of review
The aberrant expression of lncRNAs in prostate cancer has been well-documented and progression rate of tumor cells are regulated via affecting STAT3, NF-κB, Wnt, PI3K/Akt and PTEN, among other molecular pathways. Furthermore, lncRNAs regulate radio-resistance and chemo-resistance features of prostate tumor cells. Overexpression of tumor-promoting lncRNAs such as HOXD-AS1 and CCAT1 can result in drug resistance. Besides, lncRNAs can induce immune evasion of prostate cancer via upregulating PD-1. Pharmacological compounds such as quercetin and curcumin have been applied for targeting lncRNAs. Furthermore, siRNA tool can reduce expression of lncRNAs thereby suppressing prostate cancer progression. Prognosis and diagnosis of prostate tumor at clinical course can be evaluated by lncRNAs. The expression level of exosomal lncRNAs such as lncRNA-p21 can be investigated in serum of prostate cancer patients as a reliable biomarker.
Similar content being viewed by others
Background
Prostate is a walnut-sized reproductive organ located within the pelvic canal caudal to the urinary bladder and cranial to penis. The incidence of prostate cancer is high among men with 1 in 7 men in US and 1 in 25 worldwide diagnosed with this malignant condition in their lifetime [1, 2]. The enlargement of prostate that occurs with aging is called benign prostatic hyperplasia (BPH) and is associated with symptoms including polyuria observed in men over 60 years of age [3]. Due to similarities in histopathological and molecular presentations, BPH is considered as a phase in prostate tumor initiation. However, exact underlying mechanisms responsible for prostate tumor development from BPH have not been well understood [4, 5]. The incidence rate of prostate cancer is higher in developed countries due to availability of prostate specific antigen (PSA) testing for its diagnosis [6, 7]. Prostate tumor is among malignant tumors in men and newly published statistics demonstrate that it has an increase in incidence rate compared to 2020 with 248,530 people diagnosed resulting to 34,130 deaths [8]. Thanks to advancement in the field of medicine in recent years, particularly in developed countries, a significant improvement in survival and prognosis of prostate tumor patients has been observed. This can be observed in the 5-year survival rate of prostate tumor patients which stood at 97.8% in 2016, a significantly better record compared to 66.9% in 1975 [1]. Age, race, genetics, family history, obesity, and smoking, among the most common ones are risk factors of prostate tumor development [9,10,11]. If the treatment of prostate cancer fails, it progresses to a new form known as castration-resistant prostate cancer (CRPC) that is a problematic issue in clinical course and some major genes including androgen receptor (AR), TP53, RB1, PTEN and DNA damage repair (DDR) undergo mutations in this form of prostate cancer [12,13,14].
There are a variety of modalities in prostate tumor therapy. Surgery is beneficial in initial steps of prostate cancer. For advanced and metastatic forms of prostate cancer, chemotherapy and its combination with radiotherapy are utilized. Furthermore, due to dependence of prostate cancer cells on androgens, androgen-deprivation therapy (ADT) is extensively applied in its treatment. Immunotherapy including using immune checkpoint inhibitors, antibody-mediated radioimmunotherapy, antibody drug conjugates and bispecific antibodies is a new promising option in prostate cancer therapy [15,16,17,18,19,20,21]. However, due to the aggressive nature of prostate cancer cells, they acquire resistance to different therapies [22, 23]. They can activate tumor-promoting signaling pathways to induce chemoresistance, radio-resistance, ADT resistance and immune-resistance [24,25,26,27,28,29,30]. Therefore, strategies should be applied in reversing therapy resistance in prostate tumor, and this goal is achieved using pharmacological and genetic interventions [31,32,33,34,35]. Due to advances in field of genetics and bioinformatics, such molecular pathways have been recognized. Wnt, STAT3, Hedgehog (Hh), phosphatase and tensin homolog (PTEN), PI3K/Akt and NF-κB and SPOP are among the signaling networks undergoing abnormal expression in prostate cancer [36,37,38,39,40,41,42,43,44]. Noteworthy, non-coding RNAs (ncRNAs) are in special attention in prostate cancer due to their dual role in increasing/suppressing tumor progression [45,46,47,48,49,50].
Here, function of lncRNAs in prostate tumor is described in detail. It is started by an introduction about long non-coding RNAs (lncRNAs), their biogenesis and biological as well as their pathological functions. Then, we specifically discuss role of lncRNAs in progression rate (growth and migration), chemoresistance and radio-resistance of prostate tumor cells. Furthermore, role of lncRNAs as upstream mediators in regulation of major molecular pathways in prostate cancer is discussed. Finally, we describe currently applied therapeutics in targeting lncRNAs for prostate cancer therapy.
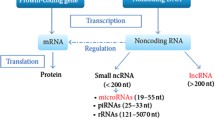
LncRNAs: Biogenesis and role in oncology
It has been reported that less than 2% of human genome is made up of genes encoding proteins, and other 98% of genome is transcribed to RNA without following the way to encoding proteins [51,52,53,54,55]. Although ncRNAs were considered as junk parts of genome, now it is obvious that ncRNAs possess functional roles in cells [56,57,58,59,60,61,62]. ncRNAs lack lengthy open reading frames and are divided according to their size. Small ncRNAs are non-coding transcripts with length less than 200 nucleotides and include miRNAs, siRNA and piRNA. On the other hand, RNA molecules with length more than 200 nucleotides are known as lncRNAs. Currently, up to 100,000 lncRNAs have been identified [63]. LncRNAs are uniquely expressed in various tissues and specific cancer types [64]. The inability of lncRNAs to encode proteins is due to lack of open reading frame (ORF) [65]. Mutations in ncRNAs are responsible for development of human cancer [66]. It appears that lncRNAs can be transcribed by RNA polymerase II, capped, polyadenylated and spliced [67]. The biogenesis of lncRNAs can be performed from promoter regions, exons, antisense sequences, enhancer sequences, untranslated regions (UTRs) such as 3/ and 5/, introns, intergenic and intragenic regions of genome. Furthermore, lncRNAs can affect expression of their target using different actions. LncRNAs are able to function as signal, decoy, guide, scaffold and miRNA modulator in affecting biological processes and preserving homeostasis [68]. Figure 1 provides a schematic representation of lncRNA function in cells.
The function of lncRNAs is dependent on their location in cytoplasm or nucleus of cells. Increasing evidence demonstrates that lncRNAs located in nucleus are involved in gene modulation at epigenetic and transcription levels including histone modification, DNA methylation, chromatin remodeling, and interacting with proteins and transcription factors in nucleus [69,70,71,72,73,74,75,76,77,78,79]. On the other hand, there are lncRNAs located in cytoplasm that transcriptionally and post-transcriptionally modulate gene expression. These kinds of lncRNAs can interact with miRNAs (acting as competitive endogenous RNA (ceRNA)), affecting proteins in cytoplasm and modulating RNA metabolism [80,81,82,83,84]. Due to these vital functional roles of lncRNAs in cells, lncRNAs regulate growth, invasion, and drug resistance of tumor [85,86,87,88,89,90,91]. Recent studies reveal that lncRNAs are master regulators of signaling networks in cancer [92,93,94,95]. The lncRNAs usually affect miRNAs in tumors, and by affecting miRNA expression, lncRNAs affect survival and migration of cancer cells [96,97,98]. Furthermore, lncRNAs with tumor-promoting role such as CCAT2 can prevent apoptosis in cancer cells [99]. Importantly, lncRNAs can promote infiltration of immune cells such as B cells, T cells (both CD8+ and CD4+ T cells), neutrophils and dendritic cells in promoting anti-tumor immunity against cancer cells [100].
LncRNAs in regulation of major molecular pathways
MicroRNAs
miRNAs are considered as short endogenous ncRNAs that can enhance or decrease expression of target messenger RNA (mRNA) by binding to 5/-UTR and 3/-UTR, respectively [101,102,103]. A miRNA can affect expression of different genes [104, 105]. Noteworthy, there are upstream mediators of miRNAs including lncRNAs that can reduce miRNA expression via sponging [106, 107]. Increasing evidence reveals dysregulation of miRNA expression in prostate cancer and association with malignant behavior of tumor cells [108,109,110,111,112]. In this section, we examine lncRNA impact on miRNAs in prostate tumor and its association with malignant behavior of cancer cells. Importantly, most of the works have focused on tumor-promoting lncRNAs. However, there are some studies evaluating role of tumor-suppressor lncRNAs in regulating miRNA expression in prostate cancer.
Tumor-promoting lncRNAs
LncRNA CCAT1 is considered as tumor-promoting factor that its role in various cancers have been discussed. CCAT1 increases endometrial cancer proliferation, while it down-regulates expression level of estrogen receptor-alpha (ERα) and its related molecular networks [113]. Increasing evidence demonstrates regulatory impact of lncRNA CCAT1 on miRNA expression in different cancers, so that CCAT1 can regulate miRNA-181a-5p and miRNA-138-5p in colorectal and pancreatic cancers, respectively for affecting progression [114, 115]. CCAT1 promotes tumor proliferation and progression in prostate tumor. For this purpose, CCAT1 interacts with miRNA-28-5p in cytoplasm (reduction in expression level) and paves the way for prostate cancer progression [116]. Noteworthy, lncRNAs can be affected by other upstream mediators in prostate cancer to mediate their regulatory impact on miRNAs. Such phenomenon occurs for lncRNA FOXP4-AS1 that prevents apoptosis in prostate tumor cells and significantly increases growth and metastasis. Paired box 5 (PAX5) is capable of triggering FOXP4-AS1 expression that in turn, functions as ceRNA for miRNA-3184-5p, leading to post-transcriptional regulation of FOXP4 and increasing its expression in favor of prostate cancer progression [117]. The regulation of lncRNAs by upstream mediators and its association with miRNA expression led to emergence of complicated molecular pathways, requiring more examination in further experiments.
LncRNA LINC00665 is a new emerging factor in cancer with crucial role in regulating various molecular pathways. Although there is evidence demonstrating that LINC00665 inhibits glioma progression via STAU1-mediated mRNA degradation [118], another experiment highlights that fact that LINC00665 overexpression is responsible for reduced overall survival of prostate cancer patients [119]. Therefore, LINC00665 possesses a tumor-promoting role of prostate cancer and can be considered as a prognostic and diagnostic tool. The overexpression of staphylococcal nuclease and Tudor domain containing 1 (SND1) is in favor of prostate cancer progression, and miRNA-1224-5p down-regulates SND1 expression in triggering cancer elimination. It has been reported that LINC00665 enhances tumor propagation, proliferation and metastasis via sponging miRNA-1224-5p and subsequent upregulation of SND1 [120]. Therefore, miRNAs are well-known downstream targets of lncRNAs, and tumor-promoting lncRNAs can affect their expression via sponging in mediating prostate cancer progression [117, 121].
LncRNA SNHG4 is an oncogenic factor in different cancers. LncRNA SNHG4 has multi-targeting ability and affects various mechanisms in promoting tumor malignancy. SNHG4 overexpression in gastric cancer leads to RRM2 upregulation via miRNA-204-5p down-regulation to prevent cell cycle arrest and to enhance growth and metastasis of tumor cells [122]. LncRNA SNHG4 is involved in increasing metastasis of gastric tumor cells via EMT induction by sponging miRNA-204-5p [123] and it also mediates immune evasion of cancer cells [124]. A same phenomenon occurs in prostate cancer and SNHG4 undergoes upregulation by an upstream mediator known as SP1. Then, SNHG4 promotes ZIC5 expression via miRNA-377 sponging to enhance survival of tumor cells and increase malignant behavior [125]. In case of recognizing a tumor-promoting lncRNA, the best strategy is its knock-down to diminish prostate cancer progression. For instance, silencing lncRNA TUG1 is beneficial in prostate cancer suppression and inducing radio-sensitivity via miRNA-139-5p overexpression and subsequent overexpression of SMC1A [126].
The capability of prostate tumor cells in mediating chemoresistance should be overcome [127]. LncRNA and miRNA interaction determines drug resistance in prostate tumor. The overexpression of lncRNA NEAT1 induces docetaxel resistance in prostate tumor. miRNA-34a-5p and miRNA-204-5p undergo down-regulation in prostate cancer and increasing their expression elevates chemosensitivity via preventing ACSL4 expression. As an upstream mediator, lncRNA NEAT1 down-regulates expression level of both miRNA-34a-5p and miRNA-204-5p to elevate ACSL4 expressions, leading to docetaxel resistance of prostate tumor cells [128].
Tumor-suppressor lncRNAs
LncRNA H19 is encoded by H19 gene located on chromosome 11q15.5 [129]. Except skeletal muscle, H19 demonstrates a decrease in expression in most of the tissues [130, 131]. H19 overexpression is in favor of tumor progression by enhancing metastasis, triggering EMT and regulating molecular pathways such as miRNAs [121, 132, 133]. However, H19 is an anti-tumor factor in prostate cancer. There is a positive relationship between H19 and miRNA-675 in prostate cancer. By promoting miRNA-675 expression, H19 reduces TGF-β levels, leading to metastasis suppression of prostate cancer cells [134]. LncRNA MEG3 is another factor that its role in regulating miRNA expression in prostate cancer has been investigated. MEG3 has a similar role in other cancers such as ovarian cancer that can suppress progression and promote drug sensitivity [135, 136]. In prostate tumor cells and tissues, MEG3 expression undergoes down-regulation. Increasing MEG3 expression is associated with miRNA-9-5p down-regulation and subsequent increase in expression level of QKI-5, as downstream of miRNA-9-5p. This axis significantly suppresses growth and invasion of prostate tumor cells and induces apoptotic cell death [137].
ZEB1 mediates malignant behavior of prostate cancer cells. ZEB1 down-regulation is associated with a reduction in stemness of prostate tumor [138]. Furthermore, overexpression of ZEB1 promotes growth and metastasis as well as induces drug resistance in prostate cancer [139]. LncRNA IUR appears to suppress metastasis of prostate cancer cells. For this purpose, lncRNA IUR decreases ZEB1 expression via miRNA-200 upregulation to impair prostate cancer progression [140]. Restoring expression level of tumor-suppressor lncRNAs stimulates apoptosis and interferes with proliferation of prostate cancer cells [141].
As more experiments are performed, more lncRNAs involved in prostate cancer progression/inhibition are identified. The interesting point is that lncRNA role is context-dependent and a certain lncRNA may possess various functions in different cancer types [142,143,144]. Hence, the exact role of each lncRNA in different cancers should be explored. LncRNA XIST is such factor that demonstrates tumor-promoting role in gastric and ovarian cancers via regulating miRNA expression [145, 146], while it has tumor-suppressor role in prostate cancer. Enhancing XIST expression diminishes miRNA-23a expression via sponging to upregulate RKIP expression at post-transcriptional level, resulting in reduced prostate cancer growth and migration [147]. These experiments clearly highlight role of lncRNAs in regulating miRNA expression and affecting prostate cancer progression [148]. However, we are still a long way from understanding the full potential of lncRNAs in prostate cancer progression/inhibition (Table 1 and Figure 2).
The lncRNAs regulating miRNAs in prostate cancer. LncRNAs reduce the expression level of target miRNAs via sponging. The tumor progression including proliferation and invasion, as well as drug resistance are modulated by lncRNA/miRNA axis in prostate cancer. Regulating expression level of lncRNAs or miRNAs is beneficial in impairing progression of prostate cancer cells
Wnt signaling
Another promising target in cancer suppression is Wnt/β-catenin [161,162,163]. Briefly, Wnt signaling activation occurs by attachment of Wnt ligand to cell membrane receptors, known as Frizzled (Fz). Besides, Wnt ligands can bind to LRP families on cell membrane to induce Wnt signaling. Upon activation, β-catenin translocates into nucleus to stimulate downstream targets involved in cancer progression. However, in normal conditions, GSK-3β participates in degrading β-catenin and translocation to nucleus is inhibited [164, 165]. Activation of Wnt signaling can mediate growth, metastasis and therapy resistance of prostate tumor [166,167,168]. LncRNAs have been shown to exert regulatory influence on Wnt signaling in prostate cancer. Wnt2B activation results in EMT induction in prostate cancer. miRNA-324-3p diminishes Wnt2B expression to inhibit EMT-mediated migration of prostate tumor. LncRNA SNHG7, owing to its tumor-promoting role, can reduce miRNA-324-3p expression to elevate Wnt2B expression, resulting in EMT and progression of prostate cancer cells. Silencing SNHG7 significantly impairs progression of prostate tumor, highlighting role of this lncRNA in metastasis via Wnt signaling activation [169].
LncRNA noncoding RNA activated by DNA damage (NORAD) is another factor capable of regulating Wnt signaling and prostate cancer progression. Overall, NORAD is involved in development of different cancers such as lung cancer, ovarian cancer and osteosarcoma [170,171,172]. It appears that NORAD is a critical regulator of miRNAs in different cancers [173]. In order to affect Wnt signaling in prostate cancer, NORAD targets miRNA-30a-5p. By binding to miRNA-30a-5p and acting as a ceRNA, NORAD upregulates expression level of RAB11A as a member of RAS oncogene family, resulting in Wnt/β-catenin activation and subsequent increase in metastasis of prostate cancer cells via EMT induction [174].
Androgen-independent prostate cancer (AIPC) is a complex condition in which prostate cancer cells do not depend on androgen for their progression and ADT is not effective [175]. It has been reported that genomic alterations and cellular events participate in development of AIPC [176, 177]. Recent study has shown that lncRNAs can regulate Wnt signaling to affect progression of AIPC cells. LncRNA LEF1-AS1 shows overexpression in APIC cells and tissues that subsequently promotes proliferation and invasion. In this way, lncRNA LEF1-AS1 increases expression level of FZD2 to activate Wnt signaling. Furthermore, LEF1-AS1 induces GSK-3β phosphorylation at Serine 9 to prevent β-catenin degradation [178].
The role of lncRNA/Wnt axis in therapy response and progression of prostate cancer cells has been examined. The sensitivity of prostate tumor to cisplatin diminishes upon Wnt stimulation. miRNA-425-5p upregulation can increase cisplatin-mediated apoptosis via β-catenin down-regulation [179]. LncRNA HOTTIP is capable of promoting proliferation of prostate tumor and triggering cisplatin resistance. Knock-down of lncRNA HOTTIP inhibits Wnt pathway, resulting in cell death, cell cycle arrest and cisplatin sensitivity of prostate cancer cells [180]. Therefore, lncRNAs are potent regulators of Wnt signaling in prostate cancer and identification of their interaction is of importance in understanding mechanisms involved in prostate cancer progression/inhibition. Furthermore, experiments have focused on tumor-promoting lncRNAs inducing Wnt signaling, and function of tumor-suppressor lncRNAs in Wnt modulation should be explored [181,182,183,184,185].
STAT3 signaling
STAT3 protein has 770 amino acids with 6 functionally conserved domains mediating its biological roles [186,187,188]. A variety of ligands have been identified for STAT3 signaling including Janus kinase (JAK), tyrosine kinases and cytokines that can result in STAT3 phosphorylation at tyrosine 705 and serine 727, leading to nuclear translocation, DNA binding and affecting downstream targets [189,190,191]. Upregulation of STAT3 promotes metastasis of prostate tumor to bone [192]. STAT3 signaling activation elevates CRPC cell viability and metastasis [193]. Exposing CRPC cells to enzalutamide (Enz) elevates lncRNA-p21 expression that is required for neuroendocrine differentiation (NED). Enz induces AR signaling to promote lncRNA-p21 expression that in turn, upregulates expression level of EZH2 which is required for suppressing STAT3 signaling by lncRNA-p21. In this way, lncRNA-p21 changes EZH2 function from histone-methyltransferase to non-histone methyltransferase to induce STAT3 methylation, leading to NED and CRPC suppression [194]. This study demonstrates that lncRNAs can indirectly affect STAT3 expression by targeting their upstream mediators. miRNAs are other upstream mediators of STAT3 in cancer [195, 196]. LINC00473 reduces expression level of miRNA-195-5p to enhance expression level of SEPT2 in prostate cancer. In turn, SEPT2 induces JAK/STAT3 signaling to dually increase growth and viability of prostate tumor [197].
PTEN/PI3K/Akt/mTOR signaling
PTEN is a tumor-suppressor located on chromosome 10 with mutation in various cancers [198,199,200]. Owing to its lipid-phosphatase activity, PTEN diminishes cellular levels of phosphatidylinositol-3,4,5-phosphate (PIP3) that is considered as a seconder messenger in different biological and molecular mechanisms [201]. By reducing PIP3 levels, PTEN inhibits PI3K signaling and its downstream axis Akt/mTOR that is responsible for cancer progression [196, 202]. Increasing evidence has confirmed role of PTEN signaling in prostate cancer. Polymorphisms in PTEN gene is responsible for extracapsular extension in prostate cancer [203]. In CRPC cells, the phosphorylation of PTEN by LIMK2 results in its degradation, paving the way for cancer progression [204]. Besides, activation of PI3K/Akt axis prevents ferroptosis in prostate tumor [205], and mediates therapy resistance [206]. LncRNAs are potent modulators of PTEN and PI3K/Akt in prostate tumor. Noteworthy, for promoting progression of prostate cancer, lncRNAs should be capable of decreasing PTEN expression. LncRNA MCM3AP-AS1 has overexpression in prostate tumor and its knockdown prevents tumor progression. Mechanistically, MCM3AP-AS1 down-regulates miRNA-543-3p to inhibit PTEN, resulting in Akt signaling activation and further promotion in progression of prostate cancer cells [207]. Decreasing expression level of tumor-promoting lncRNAs such as PlncRNA-1 enhances PTEN expression to suppress Akt signaling and prostate cancer progression [208]. By inducing PI3K/Akt/mTOR axis, lncRNA LINC01296 enhances proliferation and survival. This axis can be considered as a biomarker in prostate cancer, in which its activation provides poor prognosis in prostate cancer [209].
Similar to other molecular pathways discussed before, activation of PI3K/Akt signaling is responsible for drug resistance trait of prostate cancer [210]. Overexpression of lncRNA PCAT6 occurs in prostate cancer cells resistant to 5-flourouracil (5-FU). In this way, PCAT6 down-regulates miRNA-204 expression to induce HMGA2/PI3K axis, resulting in drug resistance [211]. As miRNAs play a remarkable role in PI3K/Akt regulation in cancer [212], their regulation by lncRNAs occurs in prostate cancer. It has been reported that lncRNA HCG11 overexpression significantly stimulates apoptosis and simultaneously, inhibits prostate tumor progression. HCG11 is capable of miRNA-543 down-regulation to inhibit PI3K/Akt signaling in impairing prostate cancer growth [213]. The impact of lncRNA/PI3K/Akt axis on prostate cancer progression is attributed to downstream targets of this signaling network. The expression level of lncRNA DANCR enhances in prostate cancer and induces EMT-mediated metastasis. By reducing expression level of miRNA-185-5p, DANCR increases LIM and SH3 protein 1 (LASP1), resulting in FAK/PI3K/Akt axis induction. Then, Akt phosphorylates GSK-3β to stimulate Snail expression in promoting prostate tumor progression [214]. Overall, modulation of PI3K/Akt signaling by lncRNAs occurs in prostate cancer [215], and therapeutic targeting of lncRNAs, using pharmacological or genetic interventions, can result in cancer inhibition.
Notch signaling
Notch signaling is a new emerging target in prostate cancer due to its tumor-promoting function. Notch1 can promote expression levels of MMP-2 and MMP-9 in increasing progression and metastasis of prostate cancer cells. As anti-cancer agent, rubimaillin suppresses Notch signaling to down-regulate MMP-2 and MMP-9 expressions in inhibiting growth and invasion of prostate cancer cells [216]. Aspartate β-hydroxylase is involved in castration-resistant prostate cancer via activation of Notch signaling [217]. Overexpression of Notch1 is linked to EMT stimulation in enhancing metastasis of prostate tumor cells [218]. Furthermore, Notch signaling stimulates drug resistance in prostate cancer and its inhibition is of importance in reversing chemoresistance [219]. Studies have demonstrated interaction between lncRNAs and Notch signaling in regulating prostate cancer progression. HIF-1α functions as upstream mediator to stimulate Notch1 signaling in prostate cancer. LncRNA GHET1 reduces KLF2 expression to trigger HIF-1α/Notch1 signaling in increasing prostate cancer progression. Notably, silencing GHET1 promotes KLF2 expression, leading to HIF-1α/Notch1 inhibition and subsequent decrease in prostate cancer progression [220]. Future studies will shed more light on the interaction between lncRNAs and Notch signaling in prostate cancer.
NF-κB signaling
NF-κB contains five subunits such as NF-κB1, NF-κB2, c-Rel, RelA and RelB [221, 222]. It has two main pathways including classical pathway for which RelA and cRel play critical role, and alternative pathway that applies to RelB containing dimers [223, 224]. Due to tumor-promoting role of NF-κB signaling in cancer, its synthetic and natural inhibitors have been developed [225, 226]. ncRNAs are considered as potent regulators of NF-κB signaling in cancer [227]. The increasing evidence demonstrates that NF-κB signaling activation can significantly promote progression of prostate cancer cells and induces their resistance to therapy [193, 228, 229]. In this section, we provide a discussion of lncRNAs role in NF-κB regulation in prostate cancer.
The activation of NF-κB signaling is mediated via cytokines such as tumor necrosis factor-a (TNF-α) and interleukin-1 (IL-1), among others [230, 231]. These factors stimulate IκB kinase complex (IKK), consisting of the catalytic IKKα and IKKβ subunits [232, 233]. IKK complex induces proteasomal degradation of IκBα protein via phosphorylation to release NF-κB, resulting in its nuclear translocation and activation of downstream targets [234,235,236]. As a tumor-suppressor factor, lncRNA DRAIC inhibits capacity of IKK complex in phosphorylating IκBα, resulting in NF-κB signaling inhibition and decreased progression of prostate cancer cells [237]. On the other hand, there are lncRNAs capable of inducing NF-κB signaling. It has been reported that lncRNA cardiac hypertrophy-related factor (CHRF) can upregulate miRNA-10b expression to induce NF-κB signaling and promote progression of prostate cancer cells. Silencing lncRNA CHRF significantly inhibits metastasis (EMT) and proliferation [238]. For activation of NF-κB signaling in prostate cancer, a complex containing different factors should be formed or disrupted. PH and leucine-rich repeat protein phosphatase (PHLPP) can interact with FKBP51 in regulating IKKα level. LncRNA PCAT1 induces NF-κB signaling to enhance CRPC progression via dissecting PHLPP from FKBP51/IKKα complex [239]. To date, a few experiments have explored role of lncRNAs in regulating NF-κB signaling in prostate cancer. However, these studies are in agreement with the fact that NF-κB and its components such as IKKα are regulated by lncRNAs and this axis affects both metastasis and growth of prostate cancer cells. Future studies can focus on the role of lncRNA/NF-κB axis in therapy response of prostate cancer. Figure 3 provides a summary of molecular pathways regulated by lncRNAs in prostate cancer therapy.
LncRNAs and molecular mechanisms
Role in proliferation
Cancer cells demonstrate rapid proliferation that requires high amount of energy provided by glucose uptake and consumption [240]. One of the distinct differences between normal and cancer cells is their way of energy production, in that cancer cells depends on glucose metabolism instead of oxidative phosphorylation in mitochondria [241]. Therefore, suppressing glycolysis or Warburg effect is a promising strategy in cancer therapy [242]. Glucose transporter-1 (GLUT-1) mediates translocation of glucose across cell membrane and its upregulation is associated with enhanced cancer progression, particularly prostate cancer [101, 243]. The glucose metabolism is affected by lncRNAs in prostate cancer. LncRNA SNHG16 possesses a tumor-promoting role that its overexpression stimulates glucose uptake and metabolism, leading to increased prostate cancer proliferation. Knock-down of SNHG16 significantly reduces GLUT-1 expression and prevents prostate cancer proliferation [244].
LncRNAs can regulate apoptosis in prostate cancer. Toll-like receptor (TLR) is an apoptosis-related pathway that its induction occurs in tumor microenvironment [245]. The activation of TLR signaling pathway occurs in prostate cancer to promote its progression [246]. LncRNA PART1 is capable of inducing TLR signaling and its downstream targets including TLR3, TNFSF10 and CXCL13 in apoptosis inhibition in prostate cancer. Silencing PART1 is associated with a decrease in prostate cancer proliferation and apoptosis induction [247]. Both in vitro and in vivo experiments have shown that overexpression of tumor-promoting lncRNAs can enhance prostate cancer proliferation and prevents apoptosis. By reducing miRNA-15a-5p expression, lncRNA PVT1 promotes KIF23 expression to prevent apoptosis in prostate cancer. Knock-down of PVT1 is correlated with apoptosis induction [248]. Overall, experiments have evaluated role of lncRNAs in regulating prostate cancer proliferation via affecting molecular pathways [249,250,251] that the major ones discussed in previous sections.
Role in metastasis
A high number of prostate cancer-related mortality arises from metastasis that is due to dissemination of cancer cells to distant organs including lung, liver, bone, and lymph nodes [252]. Bone metastasis is the most common complication of prostate cancer which subsequently, is associated with osteoblastic and osteolytic lesions [253]. Therefore, it is vital to identify factors involved in prostate cancer metastasis for the management of this malignant condition. Furthermore, the molecular pathways related to prostate cancer metastasis can be considered as biomarkers for prostate cancer prognosis [254, 255]. One of the molecular pathways involved in regulating prostate cancer metastasis is NDRG1 gene that its down-regulation results in increased migration [256]. As a tumor-suppressor factor, lncRNA LINC00844 undergoes down-regulation in metastatic prostate cancer cells and is associated with poor prognosis. Mechanistically, LINC00844 mediates AR binding to chromatin and its expression is vital for promoting NDRG1 gene expression in suppressing prostate cancer migration and invasion [257]. Increasing evidence has revealed role of transforming growth factor-beta (TGF-β) in mediating bone metastasis of prostate cancer cells via EMT induction [258, 259]. LncRNA prostate cancer-associated transcript 7 (PCAT7) is also called PCAN-R2 and located on chromosome 9q22.32. LncRNA PCAT7 is suggested to be involved in cancer progression [260, 261]. In prostate cancer, upregulation of PCAT7 enhances bone metastasis and aggressive behavior of prostate cancer cells via EMT induction. In this way, PCAT7 reduces miRNA-324-5p expression via sponging to enhance TGFBR1 expression, resulting in TGF-β/Smad axis stimulation. Furthermore, TGF-β signaling can form a positive feedback loop with PCAT7 to enhance its expression, resulting in EMT induction and bone metastasis of prostate cancer cells [262].
Another factor responsible for bone metastasis of prostate cancer is C-X-C chemokine receptor type 4 (CXCR-4) [263, 264]. The overexpression of CXCR4 occurs in different cancers and mediates their aggressive behavior [265,266,267,268]. In prostate cancer, CXCR4 upregulation is associated with poor prognosis and induces lymph node and bone metastasis [269]. LncRNA UCA1 can regulate CXCR4 expression in prostate cancer cells to affect their progression. By sponging miRNA-204, lncRNA UCA1 promotes expression level of CXCR4 to enhance metastasis of prostate cancer cells [270]. As it was mentioned, EMT induction is responsible for increased prostate cancer migration and invasion. EMT includes both morphological and cellular alterations [271]. At morphological level, epithelial cells that have low mobility, are transformed to mesenchymal cells with high migratory ability. At cellular level, a decrease occurs in E-cadherin level, while levels of N-cadherin and vimentin increase [55, 272]. In prostate cancer, STAT5A activates both lncRNA SNHG17 and SNORA71B to induce EMT and promote metastasis [273]. The same function is mediated by SNHG15 in prostate cancer that its overexpression significantly increases prostate cancer metastasis via EMT induction. Mechanistically, SNHG15 down-regulates miRNA-338-3p by acting as ceRNA to upregulate KBP prolyl isomerase 1A (FKBP1A), leading to EMT-mediated metastasis of prostate cancer [274]. Overall, lncRNAs are critical modulators of prostate cancer metastasis and more studies are needed to highlight other lncRNAs involved in promoting migration and invasion [275, 276]. Figure 4 highlights role of lncRNAs in regulating proliferation and migration of prostate cancer cells.
Role of lncRNAs in proliferation and metastasis of prostate cancer cells. EMT is responsible for increasing migration and invasion of prostate cancer cells. LncRNA SNH17 and PCAT7 are among the lncRNAs inducing EMT in increasing prostate cancer metastasis. Apoptosis induction and transfer of glucose into prostate cancer cells (GLUT1) are also modulated by lncRNAs
Role in therapy response
Although ADT is applied in prostate cancer therapy, it seems that these malignant cells can promote their progression via androgen-independent manner. Other kinds of therapies such as chemotherapy regimen with docetaxel and cabazitaxel and antiandrogens such as abiraterone and Enz are utilized in prostate cancer therapy [277,278,279,280,281,282]. However, it has been shown that prostate cancer cells can trigger chemoresistance [283, 284]. In respect to role of lncRNAs in regulating various molecular pathways in prostate cancer, these ncRNAs can affect drug resistance feature. Furthermore, prostate cancer cells can obtain resistance to radiotherapy [285]. The aim of this section is to examine role of lncRNAs in regulating therapy response of prostate cancer cells.
HOXD-AS1 is encoded by HOXD cluster gene and a recent experiment has evaluated its role in cancers. Overexpression of HOXD-AS1 enhances cyclin D1 expression via miRNA-526b-3p down-regulation, resulting in proliferation and metastasis of colorectal cancer cells [286]. By acting as ceRNA, lncRNA HOXD-AS1 promotes expression level of fibroblast growth factor 2 (FGF2) in mediating cervical cancer progression [287]. On the other hand, WD repeat domain 5 (WDR5) interacts with lncRNAs in maintaining chromatin activation [288]. In CRPC, silencing HOXD-AS1 impairs proliferation and increases sensitivity to chemotherapy. HOXD-AS1 recruits WDR5 to trigger histone H3 lysine 4 tri-methylation of target genes such as PLK1, AURKA, CDC25C, FOXM1 and UBE2C, leading to chemoresistance induction in prostate cancer [289]. Doxorubicin (DOX) is a well-known chemotherapeutic agent applied in cancer therapy. DOX administration stimulates apoptosis and cell cycle arrest via inhibiting topoisomerase activity [272, 290]. Prostate cancer cells have demonstrated DOX resistance by affecting various molecular pathways. p53 down-regulation and retinoic acid-related orphan nuclear receptor γ (RORγ) upregulation are among the factors involved in DOX resistance in prostate cancer [291, 292]. LncRNA LOXL1-AS1 is capable of promoting epidermal growth factor receptor (EGFR) in prostate cancer via miRNA-3et-7a-5p down-regulation to mediate DOX resistance. Silencing LOXL1-AS1 impairs proliferation and sensitizes prostate cancer cells to DOX-mediated apoptosis [293].
Paclitaxel (PTX) is another chemotherapy regimen used in cancer therapy including that of prostate. In respect to PTX resistance of prostate cancer cells, polymeric nanoparticles have been applied for targeted delivery of PTX [294]. Furthermore, activation of molecular mechanisms such as EMT stimulates PTX resistance [295]. LncRNA CCAT1 undergoes overexpression in PTX resistant-prostate cancer cells and prevents apoptosis. In this way, CCAT1 reduces miRNA-24-3p expression to upregulate fascin1 (FSCN1) expression, leading to prostate cancer proliferation, survival and PTX resistance [296]. Overall, drug resistance is a common feature of prostate cancer cells that is attributed to their aggressive behavior. Identification of lncRNAs and their downstream targets can pave the way to effective prostate cancer chemotherapy [297].
Radio-resistance is another problematic issue in prostate cancer therapy [298]. One of the molecular mechanisms involved in radio-resistance is autophagy. Briefly, autophagy is responsible for providing energy during starvation via degradation of amino acids and macromolecules. Furthermore, autophagy degrades aged organelles in cells. AMP-activated protein kinase (AMPK) and Beclin-1 are considered as inducers of autophagy, while mTOR signaling suppresses autophagy [299]. Recently, attention has been directed towards role of autophagy in cancer progression. Autophagy plays like a double-edged sword in cancer and can increase cancer malignancy [103]. Recently published experiments demonstrated that autophagy activation by upstream mediators such as Wnt, miRNA-129-5p and AMPK can result in radio-resistance [300,301,302]. On the other hand, there are studies showing that autophagy activation promotes radio-sensitivity [303, 304]. Therefore, more experiments are required to reveal exact role of autophagy in caner. LncRNA highly upregulated in liver cancer (HULC) has shown a tumor-promoting role in prostate cancer. The overexpression of HULC induces radio-resistance in prostate cancer and its silencing is correlated with cell cycle arrest at G0/G1 phase. HULC can inhibit autophagy via Beclin-1 down-regulation and triggering mTOR signaling. The autophagy inhibition by HULC sensitizes prostate cancer cells to irradiation by apoptosis induction through enhancing caspase-3 and Bax levels [305].
Role in immune regulation
Cancer cells are able to regulate various intrinsic and extrinsic biological pathways to ensure their adaptation to host defense. These adaptations include stimulation of tumor-promoting mechanisms, preventing cell death, angiogenesis induction, promoting migration and finally, triggering immune evasion [306]. Generally, natural killer (NK) and cytotoxic T cells (CTLs) are involved in anti-tumor immunity via apoptosis induction and mediating cell lysis [307]. However, cancer cells have obtained resistance to immune surveillance, and they are no longer responsive to immune system-mediated lysis. They can form an immunosuppressive microenvironment to escape anti-tumor immunity [308]. Immune evasion commonly occurs in prostate cancer, threatening efficacy of immunotherapy. In CRPC, Dickkopf-1 (DKK1) induces Wnt signaling, resulting in immune evasion [309]. It is worth mentioning that EMT induction and increased N-cadherin levels can reduce levels of cytotoxic T cells (CD8+), while they promote level of immunosuppressive regulatory T cells (CD4+/FOXP3+), triggering immune evasion of prostate cancer [310]. In this section, the regulatory impact of lncRNAs on immune system in prostate cancer is discussed.
One of the most well-known molecular pathways involved in immune evasion is programmed death-1 (PD-1) and its ligand, PD-L1. The tumor-suppressor factors are capable of regulating PD-L1 expression in prostate cancer. Retinoblastoma protein RB decreases expression level of PD-L1 to promote anti-tumor immunity and potential of radiotherapy in prostate cancer treatment [311]. The cyclin D-CDK4 can induce proteasomal degradation of PD-L1 in preventing immune evasion of prostate cancer [312]. Noteworthy, lncRNAs are considered as potent modulators of PD-L1 in cancer [313]. A recent experiment has shown that lncRNA KCNQ1QT1 induces escape of prostate cancer cells from immune surveillance. Normally, miRNA-15a binds to 3/-UTR of PD-L1 to reduce its expression, preventing apoptosis in CD8+ T cells and increasing their proliferation. Furthermore, miRNA-15a/PD-L1 axis enhances apoptosis induction in prostate cancer cells and impairs their proliferation and migration. It has been reported that lncRNA KCNQ1QT1 down-regulates miRNA-15a expression via sponging to induce PD-L1 signaling, increasing immune evasion of prostate cancer [314].
The signaling networks involved in regulating PD-L1 expression in prostate cancer is of importance for developing novel therapeutics in near future. LIF is a pleiotropic cytokine with physiological functions in embryonic development [315]. Increasing evidence demonstrates tumor-promoting role of LIF in cancer and its potential in mediating therapy resistance and increasing self-renewal capacity of cancer-initiating cells [316, 317]. LIF can function as upstream mediator of JAK1/STAT3 signaling in preventing differentiation of cancer cells [318]. A recent experiment has shown how lncRNAs can regulate LIF/STAT3 axis in affecting immune response of prostate cancer cells. Upregulation of lncRNA lncAMPC enhances metastasis and immune evasion. The process is started from cytoplasm, where lncAMPC reduces expression level of miRNA-637 via sponging to enhance LIF expression. lncAMPC then translocates into nucleus to promote LIFR expression via decoying histone H1.2. The activation of LIF/LIFR axis stimulates JAK1/STAT3 signaling to preserve PD-L1 expression, leading to immune evasion of prostate cancer [319]. PD-1 inhibitors are of interest in cancer immunotherapy. However, upregulation of LIF can prevent infiltration of CD8+ T cells, impairing efficacy of anti-PD-1 therapy [320]. It appears that lncRNAs can affect infiltration of immune cells. LncRNA SNHG9 is considered as a tumor-promoting factor in prostate cancer that diminishes infiltration of T central memory (Tcm) cells and T helper cells, while it promotes infiltration of plasmacytoid dendritic cells (pDCs) and NK CD56 bright cells. Furthermore, overexpression of SNHG9 mediates poor prognosis of prostate cancer patients, showing its role in immune evasion [321]. Figure 5 demonstrates how lncRNAs participate in regulating therapy response and immune system in prostate cancer with an emphasis on molecular pathways.
Exosomal lncRNAs
Recently, special attention has been directed towards extracellular vesicles (EVs) obtained from cancer and non-cancer cells [322, 323]. Overall, there are three main categories of EVs including exosomes, microvesicles and apoptotic bodies with functional roles in physiological and pathological conditions [324,325,326]. As nano-extracellular vesicles, exosomes are present in TME and various body fluids such as blood, saliva, pancreatic duct fluid, and amniotic fluid can participate in their transportation to distant tissues and organs [327]. Furthermore, they also function via autocrine and paracrine fluids [328]. Exosomes provide the communication among various cells and they contain various macromolecules such as proteins, lipids and most importantly, nucleic acids [329]. The exosomes originate from endosomal processing [330] and it has been reported that they contain ncRNAs, especially lncRNAs . Therefore, it is vital to reveal role of exosomal lncRNAs in cancer and in this section, we provide a description of exosome-mediated lncRNA delivery in prostate cancer and its association with malignant behavior [331].
It is worth mentioning that exosomal lncRNAs can be utilized for distinguishing prostate cancer and BPH. A clinical study collected urine samples from 30 prostate cancer patients and 49 BPH patients to examine potential of lncRNAs GAS5 and lincRNA-p21 in prostate cancer diagnosis. The expression level of exosomal GAS5 demonstrates no difference among prostate cancer and BPH. However, exosomal lincRNA-p21 lncRNA was different among patients with prostate cancer and BPH with more expression level in prostate cancer [332]. Another experiment investigated expression level of two exosomal lncRNAs including SAP30L-AS1 and SChLAP1 in prostate cancer and BPH. The results reveal high expression of exosomal lncRNA SAP30L-AS1 in BPH, while SChLAP1 shows more expression in prostate cancer compared to BPH [333]. Therefore, by developing novel imaging methods for tracing exosomes such as Antares2-mediated bioluminescence resonance energy transfer (BRET), a revolution can be made in cancer diagnosis [334].
LncRNAs are potent modulators of different molecular pathways in prostate cancer and microRNAs (miRNAs) are among the most common downstream targets of lncRNAs [335]. An interesting experiment has revealed that certain lncRNAs are enriched in prostate cancer exosomes and lncRNAs regulating miRNA expression are among them. Exosomal lncRNAs ELAVL1 and RBMX are enriched in prostate cancer due to their capacity in regulating expression level of miRNAs such as miRNA-17, miRNA-18a, miRNA-20a, miRNA-93 and miRNA-106b [336]. In fact, exosomes accelerate transfer of lncRNAs into extracellular milieu and based on the role of lncRNA as tumor-suppressor or tumor-promoting factor, it affects proliferation and invasion of prostate cancer cells [337]. Although a few studies have evaluated role of exosomal lncRNAs in prostate cancer, it appears that these kinds of lncRNAs can be considered as novel diagnostic and prognostic factors in prostate cancer and their expression level is of importance for distinguishing among BPH and prostate cancer. Furthermore, more diagnostic tools should be developed for detecting exosomes in prostate cancer. Table 2
Therapeutic targeting of lncRNAs
As lncRNAs are considered as critical regulators of molecular pathways and mechanisms in prostate cancer, it is of importance to regulate their expression level to affect progression of prostate cancer cells. As it was discussed, most of the experiments have focused on revealing role of tumor-promoting lncRNAs in prostate cancer. Therefore, decreasing expression of such lncRNAs can pave the way to effective treatment of prostate cancer. In this section, our aim is to show currently applied therapeutic strategies in regulating expression levels of lncRNAs in prostate cancer.
Genetic intervention
RNA interference (RNAi) was first discovered in 1998 and it is a biological mechanism occurring in most eukaryotic cells, when double-stranded RNA (dsRNA) induces biochemical events. RNAi leads to sequence-specific inhibition of target gene expression [354]. The first clinical application of RNAi was in 2004, when a naked siRNA, called Bevasiranib was utilized for topical intravitreal injection for treatment of age-related diseases [355]. siRNA and short-hairpin RNA (shRNA) are among the most common genetic tools applied in disease therapy. shRNA is a potent genetic tool applied in basic research and genome engineering, while siRNA has opened its way in clinical course [356]. siRNA is considered as a synthetic short non-coding RNA that is inactive in cells until it is loaded into Argonaute (Ago2) via RNA-binding protein (TRBP). Then, passenger or sense stranded is eliminated, while guide or antisense stranded remains attached to catalytic Ago2. At the next step, guide strand of siRNA binds to seed region of messenger RNA (mRNA) and then, Ago2 cleaves it, resulting in expression suppression [357,358,359,360]. However, siRNA has a variety of impediments before targeting genes and reducing their expression level. It has been reported that siRNA can be degraded by endogenous ribonuclease enzymes in plasma, and it can undergo clearance by kidney filtration. Furthermore, siRNA should effectively penetrate into cancer cells and escape endosome-mediated degradation [361]. In order to overcome such challenges, nanocarriers have been developed for targeted delivery of siRNA into cancer cells, protecting against RNase degradation, and mediating endosomal escape [52, 362,363,364]. Noteworthy, siRNA can be applied for downregulating lncRNA expression in cancer therapy, and subsequent inhibition of proliferation and migration of cancer cells [365, 366].
The newly conducted experiments have exploited siRNA in affecting lncRNA expression in prostate cancer therapy. The expression level of lncRNA MNX1-AS1 undergoes upregulation in prostate cancer cells and tissues to mediate their growth and metastasis. Silencing lncRNA MNX1-AS1 by siRNA is correlated with suppressing prostate cancer migration via reducing N-cadherin and vimentin levels and increasing E-cadherin levels [367]. Besides, potential of prostate cancer cells in colony formation and proliferation can be suppressed using siRNA for lncRNA down-regulation [368]. Using siRNA for targeting lncRNAs can affect downstream molecular pathways involved in prostate cancer progression. LncRNA plasmacytoma variant translocation 1 (PVT1) is a tumor-promoting factor located on chromosome 8q24 adjacent to MYC [369]. In prostate cancer, lncRNA PVT1 induces phosphorylation of p38 to promote both proliferation and invasion. Silencing PVT1 using siRNA is associated with a significant decrease in survival and invasion of prostate cancer cells via preventing p38 phosphorylation [370]. It is worth mentioning that siRNA is beneficial in revealing role of lncRNAs in prostate cancer. For instance, lncRNA GAS5 is a tumor-suppressor factor in prostate cancer and its overexpression decreases miRNA-103 to inhibit Akt/mTOR signaling, leading to a significant decrease in proliferation and metastasis. In this case, siRNA application diminishes GAS5 expression in increasing prostate cancer progression, revealing anti-tumor activity of GAS5 [371].
The potential involvement of lncRNAs in drug resistance feature of prostate cancer cells has made them as ideal candidates for therapeutic targeting. Recently, we have shown that lncRNA HORAS5 overexpression triggers resistance of CRPC cells to taxane chemotherapy. This is mediated via upregulation of BCL2A1 that induces resistance of cancer cells to chemotherapy-mediated apoptosis. Silencing lncRNA HORAS5 via siRNA significantly reduces IC50 of cabazitaxel, enhancing efficacy of chemotherapy in prostate cancer therapy [372]. Although studies have clearly showed role of siRNA in reducing expression level of tumor-promoting lncRNAs and suppressing prostate cancer progression [220], there are some limitations that should be addressed. As it was mentioned, siRNA delivery is a vital requirement due to protecting against degradation and providing targeted delivery. However, experiments have just focused on using siRNA for downregulating lncRNAs in prostate cancer therapy. Therefore, future experiments can focus on using nanoarchitectures for siRNA delivery in prostate cancer therapy. Another limitation is that experiments have just used siRNA for lncRNA regulation. There are other genetic tools such as shRNA and CRISPR/Cas9 that their potential in lncRNA expression modulation should be explored.
Pharmacological intervention
In addition to genetic tools, anti-tumor compounds can also be utilized for targeting lncRNAs in prostate cancer. However, anti-tumor compounds targeting lncRNAs are mostly phytochemicals and suffer from poor bioavailability and for introducing them to clinic, strategies such as application of drug delivery systems should be considered to improve their potency [373]. Quercetin is a plant derived-natural compound that is extensively applied in prostate cancer therapy. Quercetin can suppress proliferation and migration of prostate cancer cells, and significantly enhances their response to chemotherapy. Furthermore, in order to improve anti-tumor activity of quercetin against prostate cancer, nanoparticles have been developed for its delivery [374]. LncRNAs are targets of quercetin in prostate cancer therapy. In this way, quercetin down-regulates expression level of MALAT1 in a concentration- and time-dependent manner. In addition to in vitro experiment, in vivo experiment on xenograft tumors has shown role of quercetin in suppressing prostate cancer progression. By downregulating lncRNA MALAT1, quercetin inhibits metastasis via EMT suppression. Furthermore, quercetin inhibits PI3K/Akt pathway to suppress proliferation [375]. Curcumin is another well-known anti-tumor agent, isolated from rhizome and root of Curcuma longa that can suppress prostate cancer progression via inducing apoptosis and cell cycle arrest, down-regulating NF-κB signaling and inhibiting angiogenesis [376]. Curcumin administration negatively affects prostate cancer stem cells and suppresses their growth and migration. LncRNA ROR functions as ceRNA to reduce miRNA-145, leading to prostate cancer progression. Curcumin administration reduces ROR expression, while it promotes miRNA-145 expression to effectively suppress prostate cancer progression [377]. Figure 6 depicts a summary of genetic and pharmacological interventions for regulating lncRNA expression in prostate cancer.
Biomarker role and clinical application
With respect to high incidence rate and death resulting from prostate cancer, it is vital to translate pre-clinical findings to clinic for treatment of prostate cancer patients. LncRNAs can be considered as prognostic and diagnostic tools in prostate cancer. LncRNA ATB is a tumor-promoting factor capable of promoting both growth and invasion (EMT) of prostate cancer cells. The overexpression of lncRNA ATB is correlated with undesirable prognosis in prostate cancer patients [378]. As lncRNAs can affect immune system in providing immune evasion of prostate cancer cells, their expression level can determine response to immunotherapy [379]. In contrast to tumor-promoting lncRNAs that demonstrate high expression in prostate cancer, tumor-suppressor lncRNAs undergo significant down-regulation. It has been reported that lncRNA TINCR has close association with clinical T stage, lymph node and distant metastasis in prostate cancer. The expression level of TINCR is important in clinical course that its low expression shows poor prognosis [380]. The downregulation of tumor-suppressor lncRNAs such as DGCR5 reduces survival of prostate cancer patients [304]. Therefore, identification of these lncRNAs and investigating their expression level can be utilized as a reliable and potent prognostic tool [381]. Furthermore, it was discussed in previous section that expression level of exosomal lncRNAs can be examined in serum of prostate cancer patients as diagnostic and prognostic tools [332].
Conclusion and remarks
The present review article investigated role of lncRNAs in prostate cancer [382,383,384,385]. The expression level of lncRNAs is different among prostate cancer patients and BPH patients, so they can be considered as reliable biomarkers. LncRNAs are capable of regulating proliferation and metastasis of prostate cancer cells. Furthermore, autophagy and apoptosis as two major arms of programmed cell death, are modulated by lncRNAs in prostate cancer. A variety of downstream targets of lncRNAs have been identified that among them, STAT3, NF-κB, PTEN, PI3K/Akt and miRNAs are the most important ones. The tumor-promoting lncRNAs demonstrate an increase in expression in prostate cancer, while expression level of tumor-suppressor lncRNAs undergoes down-regulation. In addition to proliferation and migration, lncRNAs can regulate response of prostate cancer cells to chemotherapy and radiotherapy. Based on pre-clinical studies, lncRNAs induce resistance to PTX and DOX chemotherapy. Therefore, for providing effective cancer chemotherapy, lncRNAs involved in DOX and PTX resistance should be suppressed. Furthermore, lncRNAs can inhibit autophagy in mediating radio-resistance. However, lncRNA and autophagy interaction should be evaluated with more details due to pro-survival and pro-death functions of autophagy in prostate cancer.
To suppress prostate cancer progression, anti-tumor immunity is activated, and cytotoxic T cells are vital for this purpose. However, lncRNAs can induce PD-1 expression in preventing proliferation of cytotoxic T cells and mediating their apoptosis, leading to immune evasion of prostate cancer. Therefore, for effective immunotherapy, it is necessary to identify such lncRNAs to improve potential of immunotherapy. In respect to vital role of lncRNAs, pharmacological and genetic interventions have been performed to target lncRNAs in favor of prostate cancer suppression. For clinical course, lncRNAs can be utilized as diagnostic and prognostic tools for prostate cancer patients. Future experiments can focus on discovering more lncRNAs involved in prostate cancer progression/inhibition to pave the way for treatment of this malignant condition.
Availability of data and materials
Not applicable
Abbreviations
- BPH:
-
Benign prostatic hyperplasia
- PSA:
-
Prostate specific antigen
- CRPC:
-
Castration-resistant prostate cancer
- AR:
-
Androgen receptor
- DDR:
-
DNA damage repair
- ADT:
-
Androgen-deprivation therapy
- STAT3:
-
Signal transducer and activator of transcription 3
- Hh:
-
Hedgehog
- PTEN:
-
Phosphatase and tensin homolog
- PI3K:
-
Phosphatidylinositol 3-kinase
- Akt:
-
Protein kinase-B
- NF-kB:
-
Nuclear factor-kappaB
- ncRNAs:
-
Non-coding RNAs
- lncRNAs:
-
Long non-coding RNAs
- siRNA:
-
Small interfering RNA
- piRNA:
-
Piwi-interacting RNA
- ORF:
-
Open reading frame
- UTRs:
-
Untranslated regions
- ceRNA:
-
Competing endogenous RNA
- 5/-UTR:
-
5/-Untranslated region
- mRNA:
-
Messenger RNA
- CCAT1:
-
Colon cancer associated transcript-1
- ER α:
-
Estrogen receptor- α
- FOXP4:
-
Forkhead box P4 antisense RNA 1
- PAX5:
-
Paired box 5
- TGF-β:
-
Transforming growth factor-beta
- Fz:
-
Frizzled
- LRP:
-
Low-density lipoprotein receptor-related protein
- GSK-3 β:
-
Glycogen synthase kinase-3beta
- NORAD:
-
Noncoding RNA activated by DNA damage
- AIPC:
-
Androgen-independent prostate cancer
- JAK:
-
Janus kinase
- NED:
-
Neuroendocrine differentiation
- En:
-
Enzalutamide
- EZH2:
-
Enhancer of zeste homolog 2
- PIP3:
-
Phosphatidylinositol-3,4,5-phosphate
- mTOR:
-
Mammalian target of rapamycin
- 5-FU:
-
5-flourouracil
- LASP1:
-
LIM and SH3 protein 1
- TNF- α:
-
tumor necrosis factor-α
- IL-1:
-
Interleukin-1
- IKK:
-
IκB kinase
- CHRF:
-
Cardiac hypertrophy-related factor
- EMT:
-
Epithelial-to-mesenchymal transition
- PHLPP:
-
PH and leucine-rich repeat protein phosphatase
- GLUT-1:
-
Glucose transporter-1
- TLR:
-
Toll-like receptor
- PCAT7:
-
Prostate cancer-associated transcript 7
- CXCR-4:
-
C-X-C chemokine receptor type 4
- FGF2:
-
Fibroblast growth factor 2
- WDR5:
-
WD repeat domain 5
- DOX:
-
Doxorubicin
- RORγ:
-
Retinoic acid-related orphan nuclear receptor γ
- EGFR:
-
Epidermal growth factor receptor
- PTX:
-
Paclitaxel
- FSCN1:
-
Fascin1
- AMPK:
-
AMP-activated protein kinase
- HULC:
-
Highly upregulated in liver cancer
- NK:
-
Natural killer
- CTLs:
-
Cytotoxic T cells
- DKK1:
-
Dickkopf-1
- PD-1:
-
Programmed death-1
- PD-L1:
-
Programmed death-ligand 1
- Tcm:
-
T central memory
- pDCs:
-
Plasmacytoid dendritic cells
- EVs:
-
Extracellular vesicles
- BRET:
-
Bioluminescence resonance energy transfer
- miRNAs:
-
microRNAs
- RNAi:
-
RNA interference
- dsRNA:
-
Double-stranded RNA
- shRNA:
-
Short-hairpin RNA
- Ago2:
-
Argonaute 2
- PVT1:
-
Plasmacytoma variant translocation 1
References
Howlander N, Noone A, Krapcho M, Garshell J, Miller D, Altekruse S, et al. MD: Retrieved January: SEER Cancer Statistics Review, 1975–2012. National Cancer Inst. 2016:11.
Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018;68:394–424.
Langan RC. Benign Prostatic Hyperplasia. Prim Care. 2019;46:223–32.
McVary KT, Roehrborn CG, Avins AL, Barry MJ, Bruskewitz RC, Donnell RF, et al. Update on AUA guideline on the management of benign prostatic hyperplasia. J Urol. 2011;185:1793–803.
Miah S, Catto J. BPH and prostate cancer risk. Indian J Urol. 2014;30:214–8.
Cuzick J, Thorat MA, Andriole G, Brawley OW, Brown PH, Culig Z, et al. Prevention and early detection of prostate cancer. Lancet Oncol. 2014;15:e484–92.
Mohler J, Bahnson RR, Boston B, Busby JE, D'Amico A, Eastham JA, et al. NCCN clinical practice guidelines in oncology: prostate cancer. J Natl Compr Cancer Netw. 2010;8:162–200.
Siegel RL, Miller KD, Fuchs HE, Jemal AJCaCJfC: Cancer Statistics, 2021. 2021, 71:7-33.
Barsouk A, Padala SA, Vakiti A, Mohammed A, Saginala K, Thandra KC, et al. Barsouk AJMS: Epidemiology, staging and management of prostate cancer. 2020;8:28.
Sikka S, Chen L, Sethi G, Kumar AP. Targeting PPARγ Signaling Cascade for the Prevention and Treatment of Prostate Cancer. PPAR Res. 2012;2012:968040.
Shanmugam MK, Ong TH, Kumar AP, Lun CK, Ho PC, Wong PT, et al. Ursolic acid inhibits the initiation, progression of prostate cancer and prolongs the survival of TRAMP mice by modulating pro-inflammatory pathways. PLoS One. 2012;7:e32476.
Henzler C, Li Y, Yang R, McBride T, Ho Y, Sprenger C, et al. Truncation and constitutive activation of the androgen receptor by diverse genomic rearrangements in prostate cancer. 2016;7:1–12.
Antonarakis ES, Lu C, Wang H, Luber B, Nakazawa M, Roeser JC, et al. AR-V7 and resistance to enzalutamide and abiraterone in prostate cancer. 2014;371:1028–38.
Mateo J, Seed G, Bertan C, Rescigno P, Dolling D, Figueiredo I, Miranda S, Rodrigues DN, Gurel B, Clarke MJTJoci: Genomics of lethal prostate cancer at diagnosis and castration resistance. 2020, 130.
Handa S, Hans B, Goel S, Bashorun HO, Dovey Z, AJTAiU T. Immunotherapy in prostate cancer: current state and future perspectives. 2020;12:1756287220951404.
Nair SS, Weil R, Dovey Z, Davis A, AKJUC T. The Tumor Microenvironment and Immunotherapy in Prostate and Bladder Cancer. 2020;47:e17–54.
Montopoli M, Zumerle S, Vettor R, Rugge M, Zorzi M, Catapano CV, et al. Androgen-deprivation therapies for prostate cancer and risk of infection by SARS-CoV-2: a population-based study (N = 4532). Ann Oncol. 2020;31:1040–5.
Vale CL, Fisher D, Kneebone A, Parker C, Pearse M, Richaud P, et al. Adjuvant or early salvage radiotherapy for the treatment of localised and locally advanced prostate cancer: a prospectively planned systematic review and meta-analysis of aggregate data. Lancet. 2020;396:1422–31.
Conteduca V, Ku SY, Puca L, Slade M, Fernandez L, Hess J, et al. SLFN11 Expression in Advanced Prostate Cancer and Response to Platinum-based Chemotherapy. Mol Cancer Ther. 2020;19:1157–64.
Warrier VU, Makandar AI, Garg M, Sethi G, Kant R, Pal JK, et al. Engineering anti-cancer nanovaccine based on antigen cross-presentation. Biosci Rep. 2019:39.
Gupta B, Sadaria D, Warrier VU, Kirtonia A, Kant R, Awasthi A, et al. Plant lectins and their usage in preparing targeted nanovaccines for cancer immunotherapy. Semin Cancer Biol. 2020.
Liu Y, Xu X, Lin P, He Y, Zhang Y, Cao B, et al. Inhibition of the deubiquitinase USP9x induces pre-B cell homeobox 1 (PBX1) degradation and thereby stimulates prostate cancer cell apoptosis. J Biol Chem. 2019;294:4572–82.
Jung YY, Ko JH, Um JY, Chinnathambi A, Alharbi SA, Sethi G, et al. LDL cholesterol promotes the proliferation of prostate and pancreatic cancer cells by activating the STAT3 pathway. J Cell Physiol. 2021;236:5253–64.
Liu F, Wang C, Huang H, Yang Y, Dai L, Han S, et al. SEMA3A-mediated crosstalk between prostate cancer cells and tumor-associated macrophages promotes androgen deprivation therapy resistance. Cell Mol Immunol. 2021;18:752–4.
Hussain Y, Mirzaei S, Ashrafizadeh M, Zarrabi A, Hushmandi K, Khan H, et al. Quercetin and Its Nano-Scale Delivery Systems in Prostate Cancer Therapy: Paving the Way for Cancer Elimination and Reversing Chemoresistance. Cancers (Basel). 2021;13.
Chen X, Chen F, Ren Y, Weng G, Keng PC, Chen Y, et al. Glucocorticoid receptor upregulation increases radioresistance and triggers androgen independence of prostate cancer. Prostate. 2019;79:1386–98.
Crane CA, Panner A, Murray JC, Wilson SP, Xu H, Chen L, et al. PI (3) kinase is associated with a mechanism of immunoresistance in breast and prostate cancer. Oncogene. 2009;28:306–12.
Sethi G, Shanmugam MK, Arfuso F, Kumar AP. Role of RNF20 in cancer development and progression - a comprehensive review. Biosci Rep. 2018;38.
Puar YR, Shanmugam MK, Fan L, Arfuso F, Sethi G, Tergaonkar V. Evidence for the Involvement of the Master Transcription Factor NF-κB in Cancer Initiation and Progression. Biomedicines. 2018;6.
Loh CY, Arya A, Naema AF, Wong WF, Sethi G, Looi CY. Signal Transducer and Activator of Transcription (STATs) Proteins in Cancer and Inflammation: Functions and Therapeutic Implication. Front Oncol. 2019;9:48.
Wang K, Chen Z, Shi J, Feng Y, Yu M, Sun Y, et al. Resveratrol inhibits the tumor migration and invasion by upregulating TET1 and reducing TIMP2/3 methylation in prostate carcinoma cells. Prostate. 2020;80:977–85.
Ashrafizadeh M, Hushmandi K, Rahmani Moghadam E, Zarrin V, Hosseinzadeh Kashani S, Bokaie S, et al. Progress in Delivery of siRNA-Based Therapeutics Employing Nano-Vehicles for Treatment of Prostate Cancer. Bioengineering (Basel). 2020;7.
Zaffaroni N, Beretta GL. Resveratrol and prostate cancer: the power of phytochemicals. Curr Med Chem. 2020.
Zhang J, Ahn KS, Kim C, Shanmugam MK, Siveen KS, Arfuso F, et al. Nimbolide-Induced Oxidative Stress Abrogates STAT3 Signaling Cascade and Inhibits Tumor Growth in Transgenic Adenocarcinoma of Mouse Prostate Model. Antioxid Redox Signal. 2016;24:575–89.
Ranaware AM, Banik K, Deshpande V, Padmavathi G, Roy NK, Sethi G, et al. Magnolol: A Neolignan from the Magnolia Family for the Prevention and Treatment of Cancer. Int J Mol Sci. 2018;19.
Leibold J, Ruscetti M, Cao Z, Ho YJ, Baslan T, Zou M, et al. Somatic Tissue Engineering in Mouse Models Reveals an Actionable Role for WNT Pathway Alterations in Prostate Cancer Metastasis. Cancer Discov. 2020;10:1038–57.
Ma JB, Bai JY, Zhang HB, Jia J, Shi Q, Yang C, et al. KLF5 inhibits STAT3 activity and tumor metastasis in prostate cancer by suppressing IGF1 transcription cooperatively with HDAC1. Cell Death Dis. 2020;11:466.
Ishii A, Shigemura K, Kitagawa K, Sung SY, Chen KC, Yi-Te C, et al. Anti-tumor Effect of Hedgehog Signaling Inhibitor, Vismodegib, on Castration-resistant Prostate Cancer. Anticancer Res. 2020;40:5107–14.
Zhao D, Cai L, Lu X, Liang X, Li J, Chen P, et al. Chromatin Regulator CHD1 Remodels the Immunosuppressive Tumor Microenvironment in PTEN-Deficient Prostate Cancer. Cancer Discov. 2020;10:1374–87.
Torrealba N, Vera R, Fraile B, Martínez-Onsurbe P, Paniagua R, Royuela M. TGF-β/PI3K/AKT/mTOR/NF-kB pathway. Clinicopathological features in prostate cancer. Aging Male. 2020;23:801–11.
Thomas-Jardin SE, Dahl H, Nawas AF, Bautista M, Delk NA. NF-κB signaling promotes castration-resistant prostate cancer initiation and progression. Pharmacol Ther. 2020;211:107538.
Wang Z, Song Y, Ye M, Dai X, Zhu X, WJNRU W. The diverse roles of SPOP in prostate cancer and kidney cancer. Nat Rev Urol. 2020;17:339–50.
Zhang J, Sikka S, Siveen KS, Lee JH, Um JY, Kumar AP, et al. Cardamonin represses proliferation, invasion, and causes apoptosis through the modulation of signal transducer and activator of transcription 3 pathway in prostate cancer. Apoptosis. 2017;22:158–68.
Lee JH, Kim C, Baek SH, Ko JH, Lee SG, Yang WM, et al. Capsazepine inhibits JAK/STAT3 signaling, tumor growth, and cell survival in prostate cancer. Oncotarget. 2017;8:17700–11.
Deng ZH, Yu GS, Deng KL, Feng ZH, Huang Q, Pan B, et al. Hsa_circ_0088233 Alleviates Proliferation, Migration, and Invasion of Prostate Cancer by Targeting hsa-miR-185-3p. Front Cell Dev Biol. 2020;8:528155.
Zhang S, Zhang X, Chen G, Zheng X, Zhu X, Shan L. Hsa_circ_0007494 suppresses prostate cancer progression via miR-616/PTEN axis. Exp Cell Res. 2020;395:112233.
Zhang Y, Shi Z, Li Z, Wang X, Zheng P, Li H. Circ_0057553/miR-515-5p Regulates Prostate Cancer Cell Proliferation, Apoptosis, Migration, Invasion and Aerobic Glycolysis by Targeting YES1. Onco Targets Ther. 2020;13:11289–99.
Guan H, Peng R, Fang F, Mao L, Chen Z, Yang S, et al. Tumor-associated macrophages promote prostate cancer progression via exosome-mediated miR-95 transfer. J Cell Physiol. 2020;235:9729–42.
Liu Y, Yang HZ, Jiang YJ, Xu LQ. miR-451a is downregulated and targets PSMB8 in prostate cancer. Kaohsiung J Med Sci. 2020;36:494–500.
Urabe F, Kosaka N, Sawa Y, Yamamoto Y, Ito K, Yamamoto T, et al. miR-26a regulates extracellular vesicle secretion from prostate cancer cells via targeting SHC4, PFDN4, and CHORDC1. Sci Adv. 2020;6(eaay3051).
McCabe EM, Rasmussen TP. lncRNA involvement in cancer stem cell function and epithelial-mesenchymal transitions. In Seminars in Cancer Biology Elsevier. 2020.
Mirzaei S, Mahabady MK, Zabolian A, Abbaspour A, Fallahzadeh P, Noori M, et al. Small interfering RNA (siRNA) to target genes and molecular pathways in glioblastoma therapy: Current status with an emphasis on delivery systems. Life Sci. 2021:119368.
Mishra S, Verma SS, Rai V, Awasthee N, Chava S, Hui KM, et al. Long non-coding RNAs are emerging targets of phytochemicals for cancer and other chronic diseases. Cell Mol Life Sci. 2019;76:1947–66.
Ma Z, Wang YY, Xin HW, Wang L, Arfuso F, Dharmarajan A, et al. The expanding roles of long non-coding RNAs in the regulation of cancer stem cells. Int J Biochem Cell Biol. 2019;108:17–20.
Ashrafizadeh M, Hushmandi K, Hashemi M, Akbari ME, Kubatka P, Raei M, et al. Role of microRNA/Epithelial-to-Mesenchymal Transition Axis in the Metastasis of Bladder Cancer. Biomolecules. 2020;10.
Shen C, Yang C, Xia B, You MJCL. Long non-coding RNAs: Emerging regulators for chemo/immunotherapy resistance in cancer stem cells. Cancer Lett. 2021;500:244–52.
Wu M, Zhang X, Han X, Pandey V, Lobie PE, TJCL Z. The potential of long noncoding RNAs for precision medicine in human cancer. Cancer Lett. 2020.
Bhardwaj V, Tan YQ, Wu MM, Ma L, Zhu T, Lobie PE, et al. Long non-coding RNAs in recurrent ovarian cancer. Theranostic perspectives. 2021.
Mirzaei S, Zarrabi A, Hashemi F, Zabolian A, Saleki H, Ranjbar A, et al. Regulation of Nuclear Factor-KappaB (NF-κB) signaling pathway by non-coding RNAs in cancer: Inhibiting or promoting carcinogenesis? Cancer Lett. 2021.
Ashrafizaveh S, Ashrafizadeh M, Zarrabi A, Husmandi K, Zabolian A, Shahinozzaman M, et al. Long non-coding RNA in the doxorubicin resistance of cancer cells. Cancer Lett. 2021.
Ong MS, Cai W, Yuan Y, Leong HC, Tan TZ, Mohammad A, et al. 'Lnc'-ing Wnt in female reproductive cancers: therapeutic potential of long non-coding RNAs in Wnt signalling. Br J Pharmacol. 2017;174:4684–700.
Cheng JT, Wang L, Wang H, Tang FR, Cai WQ, Sethi G, Xin HW, Ma Z: Insights into Biological Role of LncRNAs in Epithelial-Mesenchymal Transition. Cells 2019, 8.
Heery R, Finn SP, Cuffe S, SGJC G. Long non-coding RNAs: key regulators of epithelial-mesenchymal transition, tumour drug resistance and cancer stem cells. Cancers (Basel). 2017;9:38.
Xing C, Sun S-g, Yue Z-Q, Bai FJB. Pharmacotherapy: Role of lncRNA LUCAT1 in cancer. Biomed Pharmacother. 2021;134(111158).
Geisler S, JJNrMcb C. RNA in unexpected places: long non-coding RNA functions in diverse cellular contexts. Nat Rev Mol Cell Biol. 2013;14:699–712.
Maurano MT, Humbert R, Rynes E, Thurman RE, Haugen E, Wang H, et al. Systematic localization of common disease-associated variation in regulatory DNA. Science. 2012;337:1190–5.
Derrien T, Johnson R, Bussotti G, Tanzer A, Djebali S, Tilgner H, et al. The GENCODE v7 catalog of human long noncoding RNAs: analysis of their gene structure, evolution, and expression. Genome Res. 2012;22:1775–89.
Pandya G, Kirtonia A, Sethi G, Pandey AK, Garg MJBBA-RC. The implication of long non-coding RNAs in the diagnosis, pathogenesis and drug resistance of pancreatic ductal adenocarcinoma and their possible therapeutic potential. Biochim Biophys Acta Rev Cancer. 2020;188423.
da Rocha ST, Boeva V, Escamilla-Del-Arenal M, Ancelin K, Granier C, Matias NR, et al. Jarid2 is implicated in the initial Xist-induced targeting of PRC2 to the inactive X chromosome. Mol Cell. 2014;53:301–16.
Li L, Liu B, Wapinski OL, Tsai M-C, Qu K, Zhang J, et al. Targeted disruption of Hotair leads to homeotic transformation and gene derepression. Cell Rep. 2013;5:3–12.
Marín-Béjar O, Marchese FP, Athie A, Sánchez Y, González J, Segura V, et al. Pint lincRNA connects the p53 pathway with epigenetic silencing by the Polycomb repressive complex. Genome Biol. 2013;2(14):1–17.
Venkatraman A, He XC, Thorvaldsen JL, Sugimura R, Perry JM, Tao F, et al. Maternal imprinting at the H19–Igf2 locus maintains adult haematopoietic stem cell quiescence. Nature. 2013;500:345–9.
Zhang J, Zhang P, Wang L, H-l P, Ma LJABBS. Long non-coding RNA HOTAIR in carcinogenesis and metastasis. Acta Biochim Biophys Sin Shanghai. 2014;46:1–5.
Chen D, Zhang Z, Mao C, Zhou Y, Yu L, Yin Y, et al. ANRIL inhibits p15INK4b through the TGFβ1 signaling pathway in human esophageal squamous cell carcinoma. Cell Immunol. 2014;289:91–6.
Hasegawa Y, Brockdorff N, Kawano S, Tsutui K, Tsutui K, Nakagawa SJD. The matrix protein hnRNP U is required for chromosomal localization of Xist RNA. Dev Cell. 2010;19:469–76.
Hacisuleyman E, Goff LA, Trapnell C, Williams A, Henao-Mejia J, Sun L, et al. biology m: Topological organization of multichromosomal regions by the long intergenic noncoding RNA Firre. Nat Struct Mol Biol. 2014;21:198.
Jiang W, Liu Y, Liu R, Zhang K, Zhang YJC. The lncRNA DEANR1 facilitates human endoderm differentiation by activating FOXA2 expression. Cell Rep. 2015;11:137–48.
Kurian L, Aguirre A, Sancho-Martinez I, Benner C, Hishida T, Nguyen TB, et al. Identification of novel long noncoding RNAs underlying vertebrate cardiovascular development. Circulation. 2015;131:1278–90.
Li M, Gou H, Tripathi BK, Huang J, Jiang S, Dubois W, Waybright T, Lei M, Shi J, Zhou MJCsc: An Apela RNA-containing negative feedback loop regulates p53-mediated apoptosis in embryonic stem cells. Cell Stem Cell. 2015, 16:669-683.
Chu C, Zhang QC, Da Rocha ST, Flynn RA, Bharadwaj M, Calabrese JM, et al. Systematic discovery of Xist RNA binding proteins. Cell. 2015;161:404–16.
Cooper DR, Carter G, Li P, Patel R, Watson JE, Patel NAJG. Long non-coding RNA NEAT1 associates with SRp40 to temporally regulate PPARγ2 splicing during adipogenesis in 3T3-L1 cells. Genes (Basel). 2014;5:1050–63.
Tripathi V, Ellis JD, Shen Z, Song DY, Pan Q, Watt AT, et al. The nuclear-retained noncoding RNA MALAT1 regulates alternative splicing by modulating SR splicing factor phosphorylation. Mol Cell. 2010;39:925–38.
Tay Y, Rinn J, Pandolfi PPJN. The multilayered complexity of ceRNA crosstalk and competition. Nature. 2014;505:344–52.
Karreth FA, Tay Y, Perna D, Ala U, Tan SM, Rust AG, et al. In vivo identification of tumor-suppressive PTEN ceRNAs in an oncogenic BRAF-induced mouse model of melanoma. Cell. 2011;147:382–95.
Shi Q, Li Y, Li S, Jin L, Lai H, Wu Y, et al. LncRNA DILA1 inhibits Cyclin D1 degradation and contributes to tamoxifen resistance in breast cancer. Nat Commun. 2020;11:5513.
Hong J, Guo F, Lu SY, Shen C, Ma D, Zhang X, et al. F. nucleatum targets lncRNA ENO1-IT1 to promote glycolysis and oncogenesis in colorectal cancer. Gut. 2020.
Xu W, Zhou G, Wang H, Liu Y, Chen B, Chen W, et al. Circulating lncRNA SNHG11 as a novel biomarker for early diagnosis and prognosis of colorectal cancer. Int J Cancer. 2020;146:2901–12.
Li X, Li Y, Yu X, Jin F. Identification and validation of stemness-related lncRNA prognostic signature for breast cancer. J Transl Med. 2020;18:331.
Hu B, Xu L, Liang D, Qi W, Fu X. LncRNA STARD13-AS Expression in Gastric Cancer and its Significance. Clin Lab. 2020;66.
Zhang W, Chen Q, Lei C. lncRNA MIAT promotes cell invasion and migration in esophageal cancer. Exp Ther Med. 2020;19:3267–74.
Sun C, Wang P, Dong W, Liu H, Sun J, Zhao L. LncRNA PVT1 promotes exosome secretion through YKT6, RAB7, and VAMP3 in pancreatic cancer. Aging (Albany NY). 2020;12:10427–40.
Hasan MF, Ganapathy K, Sun J, Khatib A, Andl T, Soulakova JN, et al. LncRNA PAINT is associated with aggressive prostate cancer and dysregulation of cancer hallmark genes. Int J Cancer. 2021.
Yang X, Wang L, Li R, Zhao Y, Gu Y, Liu S, et al. The long non-coding RNA PCSEAT exhibits an oncogenic property in prostate cancer and functions as a competing endogenous RNA that associates with EZH2. Biochem Biophys Res Commun. 2018;502:262–8.
Pandya G, Kirtonia A, Sethi G, Pandey AK, Garg M. The implication of long non-coding RNAs in the diagnosis, pathogenesis and drug resistance of pancreatic ductal adenocarcinoma and their possible therapeutic potential. Biochim Biophys Acta Rev Cancer. 2020;1874:188423.
Kansara S, Pandey V, Lobie PE, Sethi G, Garg M, Pandey AK. Mechanistic Involvement of Long Non-Coding RNAs in Oncotherapeutics Resistance in Triple-Negative Breast Cancer. Cells. 2020;9.
Yang TJ, Wang L, Zhang Y, Zheng JD, Liu L. LncRNA UCA1 regulates cervical cancer survival and EMT occurrence by targeting miR-155. Eur Rev Med Pharmacol Sci. 2020;24:9869–79.
Cui Y, Pu R, Ye J, Huang H, Liao D, Yang Y, et al. LncRNA FAM230B Promotes Gastric Cancer Growth and Metastasis by Regulating the miR-27a-5p/TOP2A Axis. Dig Dis Sci. 2020.
Dai G, Huang C, Yang J, Jin L, Fu K, Yuan F, et al. LncRNA SNHG3 promotes bladder cancer proliferation and metastasis through miR-515-5p/GINS2 axis. J Cell Mol Med. 2020;24:9231–43.
Gao P, Sun D, Guo H, Wu Z, Chen J. LncRNA CCAT2 promotes proliferation and suppresses apoptosis of colorectal cancer cells. J buon. 2020;25:1840–6.
Zhang Y, Li Z, Chen M, Chen H, Zhong Q, Liang L, et al. lncRNA TCL6 correlates with immune cell infiltration and indicates worse survival in breast cancer. Breast Cancer. 2020;27:573–85.
Mao Y, Chen W, Wu H, Liu C, Zhang J, Chen SJO. therapy: Mechanisms and Functions of MiR-200 Family in Hepatocellular Carcinoma. Onco Targets Ther. 2020;13(13479).
Huang Z, Xu Y, Wan M, Zeng X, Wu JJIJBS. miR-340: A multifunctional role in human malignant diseases. Int J Biol Sci. 2021;17(236).
Ashrafizadeh M, Paskeh MDA, Mirzaei S, Gholami MH, Zarrabi A, Hashemi F, et al. Targeting autophagy in prostate cancer: preclinical and clinical evidence for therapeutic response. J Exp Clin Cancer Res. 2022;41:105.
Lin S, Gregory RI. MicroRNA biogenesis pathways in cancer. Nat Rev Cancer. 2015;15:321–33.
Yuan Y, Anbalagan D, Lee LH, Samy RP, Shanmugam MK, Kumar AP, et al. ANXA1 inhibits miRNA-196a in a negative feedback loop through NF-kB and c-Myc to reduce breast cancer proliferation. Oncotarget. 2016;7:27007–20.
Zhou C, Yi C, Yi Y, Qin W, Yan Y, Dong X, et al. LncRNA PVT1 promotes gemcitabine resistance of pancreatic cancer via activating Wnt/β-catenin and autophagy pathway through modulating the miR-619-5p/Pygo2 and miR-619-5p/ATG14 axes. Mol Cancer. 2020;19:118.
Mirzaei S, Gholami MH, Hushmandi K, Hashemi F, Zabolian A, Canadas I, et al. The long and short non-coding RNAs modulating EZH2 signaling in cancer. J Hematol Oncol. 2022;15:18.
Kalogirou C, Linxweiler J, Schmucker P, Snaebjornsson MT, Schmitz W, Wach S, et al. MiR-205-driven downregulation of cholesterol biosynthesis through SQLE-inhibition identifies therapeutic vulnerability in aggressive prostate cancer. Nat Commun. 2021;12:5066.
Liu Y, Zhao S, Wang J, Zhu Z, Luo L, Xiang Q, et al. MiR-629-5p Promotes Prostate Cancer Development and Metastasis by Targeting AKAP13. Front Oncol. 2021;11:754353.
Chen Y, Sun F, Zhang L, Zhou J, Hou J. miR-499a inhibits the proliferation and apoptosis of prostate cancer via targeting UBE2V2. World J Surg Oncol. 2021;19:250.
Zhao W, Wang X, Jiang Y, Jia X, Guo Y. miR-217-5p Inhibits Invasion and Metastasis of Prostate Cancer by Targeting Clusterin. Mamm Genome. 2021;32:371–80.
Huang Q, Peng L, Sun Y, Huang J, Han T, Li Y, et al. miR-593-3p Promotes Proliferation and Invasion in Prostate Cancer Cells by Targeting ADIPOR1. Onco Targets Ther. 2021;14:3729–37.
Treeck O, Skrzypczak M, Schüler-Toprak S, Weber F, Ortmann O. Long non-coding RNA CCAT1 is overexpressed in endometrial cancer and regulates growth and transcriptome of endometrial adenocarcinoma cells. Int J Biochem Cell Biol. 2020;122:105740.
Han W, Sulidankazha Q, Nie X, Yilidan R, Len K. Pancreatic cancer cells-derived exosomal long non-coding RNA CCAT1/microRNA-138-5p/HMGA1 axis promotes tumor angiogenesis. Life Sci. 2021;119495.
Shang A, Wang W, Gu C, Chen W, Lu W, Sun Z, et al. Long non-coding RNA CCAT1 promotes colorectal cancer progression by regulating miR-181a-5p expression. Aging (Albany NY). 2020;12:8301–20.
You Z, Liu C, Wang C, Ling Z, Wang Y, Wang Y, et al. LncRNA CCAT1 Promotes Prostate Cancer Cell Proliferation by Interacting with DDX5 and MIR-28-5P. Mol Cancer Ther. 2019;18:2469–79.
Wu X, Xiao Y, Zhou Y, Zhou Z, Yan W. lncRNA SNHG20 promotes prostate cancer migration and invasion via targeting the miR-6516-5p/SCGB2A1 axis. Am J Transl Res. 2019;11:5162–9.
Ruan X, Zheng J, Liu X, Liu Y, Liu L, Ma J, et al. lncRNA LINC00665 Stabilized by TAF15 Impeded the Malignant Biological Behaviors of Glioma Cells via STAU1-Mediated mRNA Degradation. Mol Ther Nucleic Acids. 2020;20:823–40.
Eke I, Bylicky MA, Sandfort V, Chopra S, Martello S, Graves EE, et al. The lncRNAs LINC00261 and LINC00665 are upregulated in long-term prostate cancer adaptation after radiotherapy. Mol Ther Nucleic Acids. 2021;24:175–87.
Chen W, Yu Z, Huang W, Yang Y, Wang F, Huang H. LncRNA LINC00665 Promotes Prostate Cancer Progression via miR-1224-5p/SND1 Axis. Onco Targets Ther. 2020;13:2527–35.
Zhang Y, Huang W, Yuan Y, Li J, Wu J, Yu J, et al. Long non-coding RNA H19 promotes colorectal cancer metastasis via binding to hnRNPA2B1. J Exp Clin Cancer Res. 2020;39:141.
Cheng XB, Zhang T, Zhu HJ, Ma N, Sun XD, Wang SH, et al. Knockdown of lncRNA SNHG4 suppresses gastric cancer cell proliferation and metastasis by targeting miR-204-5p. Neoplasma. 2021;68:546–56.
Wang S, Zhu W, Qiu J, Chen F. lncRNA SNHG4 promotes cell proliferation, migration, invasion and the epithelial-mesenchymal transition process via sponging miR-204-5p in gastric cancer. Mol Med Rep. 2021;23.
Zhou N, Chen Y, Yang L, Xu T, Wang F, Chen L, et al. LncRNA SNHG4 promotes malignant biological behaviors and immune escape of colorectal cancer cells by regulating the miR-144-3p/MET axis. Am J Transl Res. 2021;13:11144–61.
Wang ZY, Duan Y, Wang P. SP1-mediated upregulation of lncRNA SNHG4 functions as a ceRNA for miR-377 to facilitate prostate cancer progression through regulation of ZIC5. J Cell Physiol. 2020;235:3916–27.
Xiu D, Liu L, Cheng M, Sun X, Ma X. Knockdown of lncRNA TUG1 Enhances Radiosensitivity of Prostate Cancer via the TUG1/miR-139-5p/SMC1A Axis. Onco Targets Ther. 2020;13:2319–31.
Quintanal-Villalonga Á, Chan JM, Yu HA, Pe'er D, Sawyers CL, Sen T, et al. Lineage plasticity in cancer: a shared pathway of therapeutic resistance. Nat Rev Clin Oncol. 2020;17:360–71.
Jiang X, Guo S, Zhang Y, Zhao Y, Li X, Jia Y, et al. LncRNA NEAT1 promotes docetaxel resistance in prostate cancer by regulating ACSL4 via sponging miR-34a-5p and miR-204-5p. Cell Signal. 2020;65:109422.
Shermane Lim YW, Xiang X, Garg M, Le MTN, Li-Ann Wong A, Wang L, et al. The double-edged sword of H19 lncRNA: Insights into cancer therapy. Cancer Lett. 2021;500:253–62.
Gabory A, Jammes H, Dandolo LJB. The H19 locus: role of an imprinted non-coding RNA in growth and development. 2010;32:473–80.
Pachnis V, Belayew A, SMJPotNAoS T. Locus unlinked to alpha-fetoprotein under the control of the murine raf and Rif genes. 1984;81:5523–7.
Wang D, Xing N, Yang T, Liu J, Zhao H, He J, et al. Exosomal lncRNA H19 promotes the progression of hepatocellular carcinoma treated with Propofol via miR-520a-3p/LIMK1 axis. Cancer Med. 2020;9:7218–30.
Li A, Mallik S, Luo H, Jia P, Lee DF, Zhao Z. H19, a Long Non-coding RNA, Mediates Transcription Factors and Target Genes through Interference of MicroRNAs in Pan-Cancer. s. 2020;21:180–91.
Zhu M, Chen Q, Liu X, Sun Q, Zhao X, Deng R, et al. lncRNA H19/miR-675 axis represses prostate cancer metastasis by targeting TGFBI. FEBS J. 2014;281:3766–75.
El-Khazragy N, Mohammed HF, Yassin M, Elghoneimy KK, Bayoumy W, Hewety A, et al. Tissue-based long non-coding RNAs "PVT1, TUG1 and MEG3" signature predicts Cisplatin resistance in ovarian Cancer. Genomics. 2020;112:4640–6.
Liu Y, Xu Y, Ding L, Yu L, Zhang B, Wei D. LncRNA MEG3 suppressed the progression of ovarian cancer via sponging miR-30e-3p and regulating LAMA4 expression. Cancer Cell Int. 2020;20:181.
Wu M, Huang Y, Chen T, Wang W, Yang S, Ye Z, et al. LncRNA MEG3 inhibits the progression of prostate cancer by modulating miR-9-5p/QKI-5 axis. J Cell Mol Med. 2019;23:29–38.
Pérez G, López-Moncada F, Indo S, Torres MJ, Castellón EA, Contreras HR. Knockdown of ZEB1 reverses cancer stem cell properties in prostate cancer cells. Oncol Rep. 2021;45.
Ma Z, Gu G, Pan W, Chen X. LncRNA PCAT6 Accelerates the Progression and Chemoresistance of Cervical Cancer Through Up-Regulating ZEB1 by Sponging miR-543. Onco Targets Ther. 2020;13:1159–70.
Sun L, Chen T, Li T, Yu J. LncRNA IUR downregulates ZEB1 by upregulating miR-200 to inhibit prostate carcinoma. Physiol Genomics. 2019;51:607–11.
Chen Z, Zhen M, Zhou J. LncRNA BRE-AS1 interacts with miR-145-5p to regulate cancer cell proliferation and apoptosis in prostate carcinoma and has early diagnostic values. Biosci Rep. 2019;39.
Shen C, Yang C, Xia B, You M. Long non-coding RNAs: Emerging regulators for chemo/immunotherapy resistance in cancer stem cells. Cancer Lett. 2021;500:244–52.
Wu M, Zhang X, Han X, Pandey V, Lobie PE, Zhu T. The potential of long noncoding RNAs for precision medicine in human cancer. Cancer Lett. 2021;501:12–9.
Robless EE, Howard JA, Casari I, Falasca M. Exosomal long non-coding RNAs in the diagnosis and oncogenesis of pancreatic cancer. Cancer Lett. 2021;501:55–65.
Li P, Wang L, Li P, Hu F, Cao Y, Tang D, et al. Silencing lncRNA XIST exhibits antiproliferative and proapoptotic effects on gastric cancer cells by up-regulating microRNA-132 and down-regulating PXN. Aging (Albany NY). 2020;12.
Zhao Y, Yu Z, Ma R, Zhang Y, Zhao L, Yan Y, et al. lncRNA-Xist/miR-101-3p/KLF6/C/EBPα axis promotes TAM polarization to regulate cancer cell proliferation and migration. Mol Ther Nucleic Acids. 2021;23:536–51.
Du Y, Weng XD, Wang L, Liu XH, Zhu HC, Guo J, et al. LncRNA XIST acts as a tumor suppressor in prostate cancer through sponging miR-23a to modulate RKIP expression. Oncotarget. 2017;8:94358–70.
Sun T, Du SY, Armenia J, Qu F, Fan J, Wang X, et al. Expression of lncRNA MIR222HG co-transcribed from the miR-221/222 gene promoter facilitates the development of castration-resistant prostate cancer. Oncogenesis. 2018;7:30.
Li G, Zhang Y, Mao J, Hu P, Chen Q, Ding W, et al. LncRNA TUC338 is overexpressed in prostate carcinoma and downregulates miR-466. Gene. 2019;707:224–30.
Xiao S, Song B. LncRNA HOXA-AS2 promotes the progression of prostate cancer via targeting miR-509-3p/PBX3 axis. Biosci Rep. 2020;40.
Liu HT, Fang L, Cheng YX, Sun Q. LncRNA PVT1 regulates prostate cancer cell growth by inducing the methylation of miR-146a. Cancer Med. 2016;5:3512–9.
Hu M, Yang J. Down-regulation of lncRNA UCA1 enhances radiosensitivity in prostate cancer by suppressing EIF4G1 expression via sponging miR-331-3p. Cancer Cell Int. 2020;20:449.
Li J, Zhang Z, Xiong L, Guo C, Jiang T, Zeng L, et al. SNHG1 lncRNA negatively regulates miR-199a-3p to enhance CDK7 expression and promote cell proliferation in prostate cancer. Biochem Biophys Res Commun. 2017;487:146–52.
Yan K, Hou L, Liu T, Jiao W, Ma Q, Fang Z, et al. lncRNA OGFRP1 functions as a ceRNA to promote the progression of prostate cancer by regulating SARM1 level via miR-124-3p. Aging (Albany NY). 2020;12:8880–92.
Hao H, Chen H, Xie L, Liu H, Wang D. LncRNA KCNQ1OT1 Promotes Proliferation, Invasion and Metastasis of Prostate Cancer by Regulating miR-211-5p/CHI3L1 Pathway. Onco Targets Ther. 2021;14:1659–71.
Dai X, Liu L, Liang Z, Guo K, Xu S, Wang H. Silencing of lncRNA MALAT1 inhibits cell cycle progression via androgen receptor signaling in prostate cancer cells. Pathol Res Pract. 2019;215:712–21.
Liu B, Qian D, Zhou W, Jiang H, Xiang Z, Wu D. A Novel Androgen-Induced lncRNA FAM83H-AS1 Promotes Prostate Cancer Progression via the miR-15a/CCNE2 Axis. Front Oncol. 2020;10:620306.
Zhao B, Lu YL, Yang Y, Hu LB, Bai Y, Li RQ, et al. Overexpression of lncRNA ANRIL promoted the proliferation and migration of prostate cancer cells via regulating let-7a/TGF-β1/ Smad signaling pathway. Cancer Biomark. 2018;21:613–20.
Zheng XY, Cao MZ, Ba Y, Li YF, Ye JL. LncRNA testis-specific transcript, Y-linked 15 (TTTY15) promotes proliferation, migration and invasion of colorectal cancer cells via regulating miR-29a-3p/DVL3 axis. Cancer Biomark. 2020.
Liao B, Chen S, Li Y, Yang Z, Yang Y, Deng X, et al. LncRNA BLACAT1 Promotes Proliferation, Migration and Invasion of Prostate Cancer Cells via Regulating miR-29a-3p/DVL3 Axis. Technol Cancer Res Treat. 2021;20:1533033820972342.
González-Sancho JM, Larriba MJ, Muñoz AJC. Wnt and vitamin D at the crossroads in solid cancer. 2020;12:3434.
Bhuvanalakshmi G, Gamit N, Patil M, Arfuso F, Sethi G, Dharmarajan A, et al. Stemness, Pluripotentiality, and Wnt Antagonism: sFRP4, a Wnt antagonist Mediates Pluripotency and Stemness in Glioblastoma. Cancers (Basel). 2018;11.
Bhuvanalakshmi G, Basappa RKS, Dharmarajan A, Sethi G, Kumar AP, Warrier S. Breast Cancer Stem-Like Cells Are Inhibited by Diosgenin, a Steroidal Saponin, by the Attenuation of the Wnt β-Catenin Signaling via the Wnt Antagonist Secreted Frizzled Related Protein-4. Front Pharmacol. 2017;8:124.
Ashrafizadeh M, Ahmadi Z, Farkhondeh T. Samarghandian SJJocp: Resveratrol targeting the Wnt signaling pathway: A focus on therapeutic activities. 2020;235:4135–45.
Hwang ST, Yang MH, Kumar AP, Sethi G, Ahn KS. Corilagin Represses Epithelial to Mesenchymal Transition Process Through Modulating Wnt/β-Catenin Signaling Cascade. Biomolecules. 2020;10.
Luo J, Wang D, Wan X, Xu Y, Lu Y, Kong Z, et al. Crosstalk Between AR and Wnt Signaling Promotes Castration-Resistant Prostate Cancer Growth. Onco Targets Ther. 2020;13:9257–67.
Lin SR, Mokgautsi N, Liu YN. Ras and Wnt Interaction Contribute in Prostate Cancer Bone Metastasis. Molecules. 2020:25.
Bian P, Dou Z, Jia Z, Li W, Pan D. Activated Wnt/β-Catenin signaling contributes to E3 ubiquitin ligase EDD-conferred docetaxel resistance in prostate cancer. Life Sci. 2020;254:116816.
Han Y, Hu H, Zhou J. Knockdown of LncRNA SNHG7 inhibited epithelial-mesenchymal transition in prostate cancer though miR-324-3p/WNT2B axis in vitro. Pathol Res Pract. 2019;215:152537.
Gao W, Weng T, Wang L, Shi B, Meng W, Wang X, et al. Long non-coding RNA NORAD promotes cell proliferation and glycolysis in non-small cell lung cancer by acting as a sponge for miR-136-5p. Mol Med Rep. 2019;19:5397–405.
Wang X, Zou J, Chen H, Zhang P, Lu Z, You Z, et al. Long noncoding RNA NORAD regulates cancer cell proliferation and migration in human osteosarcoma by endogenously competing with miR-199a-3p. IUBMB Life. 2019;71:1482–91.
Tong L, Ao Y, Zhang H, Wang K, Wang Y, Ma Q. Long noncoding RNA NORAD is upregulated in epithelial ovarian cancer and its downregulation suppressed cancer cell functions by competing with miR-155-5p. Cancer Med. 2019;8:4782–91.
Wang J, Sun Y, Zhang X, Cai H, Zhang C, Qu H, et al. Oxidative stress activates NORAD expression by H3K27ac and promotes oxaliplatin resistance in gastric cancer by enhancing autophagy flux via targeting the miR-433-3p. Cell Death Dis. 2021;12:90.
Zhang Y, Li Y. Long non-coding RNA NORAD contributes to the proliferation, invasion and EMT progression of prostate cancer via the miR-30a-5p/RAB11A/WNT/β-catenin pathway. Cancer Cell Int. 2020;20:571.
Shanmugam MK, Manu KA, Ong TH, Ramachandran L, Surana R, Bist P, et al. Inhibition of CXCR4/CXCL12 signaling axis by ursolic acid leads to suppression of metastasis in transgenic adenocarcinoma of mouse prostate model. Int J Cancer. 2011;129:1552–63.
Heidenreich A, Aus G, Bolla M, Joniau S, Matveev VB, Schmid HP, et al. EAU guidelines on prostate cancer. Eur Urol. 2008;53:68–80.
Klein EA, Silverman R. Inflammation, infection, and prostate cancer. Curr Opin Urol. 2008;18:315–9.
Li W, Yang G, Yang D, Li D, Sun Q. LncRNA LEF1-AS1 promotes metastasis of prostatic carcinoma via the Wnt/β-catenin pathway. Cancer Cell Int. 2020;20:543.
Liu S, Wang Q, Liu Y, Xia ZY. miR-425-5p suppresses tumorigenesis and DDP resistance in human-prostate cancer by targeting GSK3β and inactivating the Wnt/β-catenin signaling pathway. J Biosci. 2019;44.
Jiang H, Xiong W, Chen L, Lv Z, Yang C, Li Y. Knockdown of the long noncoding RNA HOTTIP inhibits cell proliferation and enhances cell sensitivity to cisplatin by suppressing the Wnt/β-catenin pathway in prostate cancer. J Cell Biochem. 2019;120:8965–74.
Chen J, Wang F, Xu H, Xu L, Chen D, Wang J, et al. Long Non-Coding RNA SNHG1 Regulates the Wnt/β-Catenin and PI3K/AKT/mTOR Signaling Pathways via EZH2 to Affect the Proliferation, Apoptosis, and Autophagy of Prostate Cancer Cell. Front Oncol. 2020;10:552907.
Song X, Wang H, Wu J, Sun Y. Long Noncoding RNA SOX2-OT Knockdown Inhibits Proliferation and Metastasis of Prostate Cancer Cells Through Modulating the miR-452-5p/HMGB3 Axis and Inactivating Wnt/β-Catenin Pathway. Cancer Biother Radiopharm. 2020;35:682–95.
Song J, Wu X, Ma R, Miao L, Xiong L, Zhao W. Long noncoding RNA SNHG12 promotes cell proliferation and activates Wnt/β-catenin signaling in prostate cancer through sponging microRNA-195. J Cell Biochem. 2019;120:13066–75.
Li J, Liu Y, Li P, Guo Y, Liu Y, Ren Y. Long noncoding RNA CCAT2 promotes proliferation and metastasis in non-small cell lung cancer through the Wnt pathway. Int J Clin Exp Pathol. 2017;10:7983–90.
He P, Xiong G, Guo W, Jiang G, Li Y, Li H. Long non-coding RNA CCAT2 promotes prostate cancer cell proliferation and invasion by regulating the Wnt/β-catenin signaling pathway. Oncol Lett. 2020;20:97.
Zou S, Tong Q, Liu B, Huang W, Tian Y, Fu X. Targeting STAT3 in Cancer Immunotherapy. Mol Cancer. 2020;19:145.
Garg M, Shanmugam MK, Bhardwaj V, Goel A, Gupta R, Sharma A, et al. The pleiotropic role of transcription factor STAT3 in oncogenesis and its targeting through natural products for cancer prevention and therapy. Med Res Rev. 2020.
Lee JH, Chiang SY, Nam D, Chung WS, Lee J, Na YS, et al. Capillarisin inhibits constitutive and inducible STAT3 activation through induction of SHP-1 and SHP-2 tyrosine phosphatases. Cancer Lett. 2014;345:140–8.
Sgrignani J, Garofalo M, Matkovic M, Merulla J, Catapano CV, Cavalli A. Structural Biology of STAT3 and Its Implications for Anticancer Therapies Development. Int J Mol Sci. 2018;19.
Li F, Shanmugam MK, Chen L, Chatterjee S, Basha J, Kumar AP, et al. Garcinol, a polyisoprenylated benzophenone modulates multiple proinflammatory signaling cascades leading to the suppression of growth and survival of head and neck carcinoma. Cancer Prev Res (Phila). 2013;6:843–54.
Kim SM, Lee JH, Sethi G, Kim C, Baek SH, Nam D, et al. Bergamottin, a natural furanocoumarin obtained from grapefruit juice induces chemosensitization and apoptosis through the inhibition of STAT3 signaling pathway in tumor cells. Cancer Lett. 2014;354:153–63.
Thulin MH, Määttä J, Linder A, Sterbova S, Ohlsson C, Damber JE, et al. Inhibition of STAT3 prevents bone metastatic progression of prostate cancer in vivo. Prostate. 2021;81:452–62.
Tan B, Chen X, Fan Y, Yang Y, Yang J, Tan L. STAT3 phosphorylation is required for the HepaCAM-mediated inhibition of castration-resistant prostate cancer cell viability and metastasis. Prostate. 2021.
Luo J, Wang K, Yeh S, Sun Y, Liang L, Xiao Y, et al. LncRNA-p21 alters the antiandrogen enzalutamide-induced prostate cancer neuroendocrine differentiation via modulating the EZH2/STAT3 signaling. Nat Commun. 2019;10:2571.
Huang H, Huang J, Yao J, Li N, Yang Z. miR-125a regulates HAS1 and inhibits the proliferation, invasion and metastasis by targeting STAT3 in non-small cell lung cancer cells. J Cell Biochem. 2020;121:3197–207.
Ashrafizadeh M, Zarrabi A, Samarghandian S, MJEJoP N. PTEN: What we know of the function and regulation of this onco-suppressor factor in bladder cancer? Eur J Pharmacol. 2020:173226.
Xing Z, Li S, Liu Z, Zhang C, Meng M, Bai Z. The long non-coding RNA LINC00473 contributes to cell proliferation via JAK-STAT3 signaling pathway by regulating miR-195-5p/SEPT2 axis in prostate cancer. Biosci Rep. 2020;40.
Aquila S, Santoro M, Caputo A, Panno ML, Pezzi V, De Amicis F. The Tumor Suppressor PTEN as Molecular Switch Node Regulating Cell Metabolism and Autophagy: Implications in Immune System and Tumor Microenvironment. Cells. 2020;9:1725.
Abadi AJ, Zarrabi A, Gholami MH, Mirzaei S, Hashemi F, Zabolian A, et al. Small in Size, but Large in Action: microRNAs as Potential Modulators of PTEN in Breast and Lung Cancers. Biomolecules. 2021;11.
Ashrafizadeh M, Najafi M, Ang HL, Moghadam ER, Mahabady MK, Zabolian A, et al. PTEN, a Barrier for Proliferation and Metastasis of Gastric Cancer Cells: From Molecular Pathways to Targeting and Regulation. Biomedicines. 2020;8.
Mighell TL, Evans-Dutson S, O'Roak BJ. A Saturation Mutagenesis Approach to Understanding PTEN Lipid Phosphatase Activity and Genotype-Phenotype Relationships. Am J Hum Genet. 2018;102:943–55.
Ashrafizadeh M, Najafi M, Ang HL, Moghadam ER, Mahabady MK, Zabolian A, et al. PTEN, a barrier for proliferation and metastasis of gastric cancer cells: from molecular pathways to targeting and regulation. Biomedicines. 2020;8:264.
Nóbrega M, Cilião HL, Souza MF, Souza MR, Serpeloni JM, Fuganti PE, et al. Association of polymorphisms of PTEN, AKT1, PI3K, AR, and AMACR genes in patients with prostate cancer. Genet Mol Biol. 2020;43:e20180329.
Nikhil K, Kamra M, Raza A, Shah K. Negative cross talk between LIMK2 and PTEN promotes castration resistant prostate cancer pathogenesis in cells and in vivo. Cancer Lett. 2021;498:1–18.
Yi J, Zhu J, Wu J, Thompson CB, Jiang X. Oncogenic activation of PI3K-AKT-mTOR signaling suppresses ferroptosis via SREBP-mediated lipogenesis. Proc Natl Acad Sci U S A. 2020;117:31189–97.
Chen X, Yu Q, Pan H, Li P, Wang X, Fu S. Overexpression of IGFBP5 Enhances Radiosensitivity Through PI3K-AKT Pathway in Prostate Cancer. Cancer Manag Res. 2020;12:5409–18.
Jia Z, Li W, Bian P, Liu H, Pan D, Dou Z. LncRNA MCM3AP-AS1 Promotes Cell Proliferation and Invasion Through Regulating miR-543-3p/SLC39A10/PTEN Axis in Prostate Cancer. Onco Targets Ther. 2020;13:9365–76.
Cui Z, Gao H, Yan N, Dai Y, Wang H, Wang M, et al. LncRNA PlncRNA-1 accelerates the progression of prostate cancer by regulating PTEN/Akt axis. Aging (Albany NY). 2021;13.
Wu J, Cheng G, Zhang C, Zheng Y, Xu H, Yang H, et al. Long noncoding RNA LINC01296 is associated with poor prognosis in prostate cancer and promotes cancer-cell proliferation and metastasis. Onco Targets Ther. 2017;10:1843–52.
Adelaiye-Ogala R, Gryder BE, Nguyen YTM, Alilin AN, Grayson AR, Bajwa W, et al. Targeting the PI3K/AKT Pathway Overcomes Enzalutamide Resistance by Inhibiting Induction of the Glucocorticoid Receptor. Mol Cancer Ther. 2020;19:1436–47.
Wu H, Zou Q, He H, Liang Y, Lei M, Zhou Q, et al. Long non-coding RNA PCAT6 targets miR-204 to modulate the chemoresistance of colorectal cancer cells to 5-fluorouracil-based treatment through HMGA2 signaling. Cancer Med. 2019;8:2484–95.
Chen L, Liu D, Yi X, Qi L, Tian X, Sun B, et al. The novel miR-1269b-regulated protein SVEP1 induces hepatocellular carcinoma proliferation and metastasis likely through the PI3K/Akt pathway. Cell Death Dis. 2020;11:320.
Wang YC, He WY, Dong CH, Pei L, Ma YL. lncRNA HCG11 regulates cell progression by targeting miR-543 and regulating AKT/mTOR pathway in prostate cancer. Cell Biol Int. 2019.
Sun W, Zu S, Shao G, Wang W, Gong F. LncRNA DANCR targets miR-185-5p to upregulate LIM and SH3 protein 1 (LASP1) promoting prostate cancer via the FAK/PI3K/AKT/GSK3β/Snail pathway. J Gene Med. 2021:e3344.
Ma T, Chen H, Wang P, Yang N, Bao J. Downregulation of lncRNA ZEB1-AS1 Represses Cell Proliferation, Migration, and Invasion Through Mediating PI3K/AKT/mTOR Signaling by miR-342-3p/CUL4B Axis in Prostate Cancer. Cancer Biother Radiopharm. 2020;35:661–72.
Cai F, Guo S, Huang S, Li J, Liu W. Rubimaillin suppresses proliferation, migration and invasion of prostate cancer cells via the Notch-1/MMP signaling pathway. Cell Mol Biol (Noisy-le-grand). 2020;66:130–4.
Barboro P, Benelli R, Tosetti F, Costa D, Capaia M, Astigiano S, et al. Aspartate β-hydroxylase targeting in castration-resistant prostate cancer modulates the NOTCH/HIF1α/GSK3β crosstalk. Carcinogenesis. 2020;41:1246–52.
Zhang L, Sha J, Yang G, Huang X, Bo J, Huang Y. Activation of Notch pathway is linked with epithelial-mesenchymal transition in prostate cancer cells. Cell Cycle. 2017;16:999–1007.
Farah E, Li C, Cheng L, Kong Y, Lanman NA, Pascuzzi P, et al. NOTCH signaling is activated in and contributes to resistance in enzalutamide-resistant prostate cancer cells. J Biol Chem. 2019;294:8543–54.
Zhu Y, Tong Y, Wu J, Liu Y, Zhao M. Knockdown of LncRNA GHET1 suppresses prostate cancer cell proliferation by inhibiting HIF-1α/Notch-1 signaling pathway via KLF2. Biofactors. 2019;45:364–73.
Eluard B, Thieblemont C, VJTic B. NF-κB in the new era of cancer therapy. Trends Cancer. 2020.
Morgan D, Garg M, Tergaonkar V, Tan SY, Sethi G. Pharmacological significance of the non-canonical NF-κB pathway in tumorigenesis. Biochim Biophys Acta Rev Cancer. 2020;1874:188449.
Oeckinghaus A, SJCSHpib G. The NF-κB family of transcription factors and its regulation. Cold Spring Harb Perspect Biol. 2009;1:a000034.
Liu L, Ahn KS, Shanmugam MK, Wang H, Shen H, Arfuso F, et al. Oleuropein induces apoptosis via abrogating NF-κB activation cascade in estrogen receptor-negative breast cancer cells. J Cell Biochem. 2019;120:4504–13.
Rasmi RR, Sakthivel KM. Guruvayoorappan CJB, Pharmacotherapy: NF-κB inhibitors in treatment and prevention of lung cancer. Biomed Pharmacother. 2020;130:110569.
Sethi G, Tergaonkar V. Potential pharmacological control of the NF-κB pathway. Trends Pharmacol Sci. 2009;30:313–21.
Mirzaei S, Zarrabi A, Hashemi F, Zabolian A, Saleki H, Ranjbar A, et al. Regulation of Nuclear Factor-KappaB (NF-κB) signaling pathway by non-coding RNAs in cancer: Inhibiting or promoting carcinogenesis? Cancer Lett. 2021;509:63–80.
Lv Z, Li W, Wei X. S100A9 promotes prostate cancer cell invasion by activating TLR4/NF-κB/integrin β1/FAK signaling. Onco Targets Ther. 2020;13:6443–52.
Yang B, Zhang D, Qian J, Cheng Y. Chelerythrine suppresses proliferation and metastasis of human prostate cancer cells via modulating MMP/TIMP/NF-κB system. Mol Cell Biochem. 2020;474:199–208.
Chen L, Yuan Y, Kar S, Kanchi MM, Arora S, Kim JE, et al. PPARγ Ligand-induced Annexin A1 Expression Determines Chemotherapy Response via Deubiquitination of Death Domain Kinase RIP in Triple-negative Breast Cancers. Mol Cancer Ther. 2017;16:2528–42.
Bist P, Phua QH, Shu S, Yi Y, Anbalagan D, Lee LH, et al. Annexin-A1 controls an ERK-RhoA-NFκB activation loop in breast cancer cells. Biochem Biophys Res Commun. 2015;461:47–53.
Hayden MS, Ghosh S. NF-κB, the first quarter-century: remarkable progress and outstanding questions. Genes Dev. 2012;26:203–34.
Manu KA, Shanmugam MK, Ramachandran L, Li F, Siveen KS, Chinnathambi A, et al. Isorhamnetin augments the anti-tumor effect of capecitabine through the negative regulation of NF-κB signaling cascade in gastric cancer. Cancer Lett. 2015;363:28–36.
Bonizzi G, Karin M. The two NF-kappaB activation pathways and their role in innate and adaptive immunity. Trends Immunol. 2004;25:280–8.
Liu B, Sun L, Liu Q, Gong C, Yao Y, Lv X, et al. A cytoplasmic NF-κB interacting long noncoding RNA blocks IκB phosphorylation and suppresses breast cancer metastasis. Cancer Cell. 2015;27:370–81.
Manu KA, Shanmugam MK, Li F, Chen L, Siveen KS, Ahn KS, et al. Simvastatin sensitizes human gastric cancer xenograft in nude mice to capecitabine by suppressing nuclear factor-kappa B-regulated gene products. J Mol Med (Berl). 2014;92:267–76.
Saha S, Kiran M, Kuscu C, Chatrath A, Wotton D, Mayo MW, et al. Long Noncoding RNA DRAIC Inhibits Prostate Cancer Progression by Interacting with IKK to Inhibit NF-κB Activation. Cancer Res. 2020;80:950–63.
Liu S, Wang L, Li Y, Cui Y, Wang Y, Liu C. Long non-coding RNA CHRF promotes proliferation and mesenchymal transition (EMT) in prostate cancer cell line PC3 requiring up-regulating microRNA-10b. Biol Chem. 2019.
Shang Z, Yu J, Sun L, Tian J, Zhu S, Zhang B, et al. LncRNA PCAT1 activates AKT and NF-κB signaling in castration-resistant prostate cancer by regulating the PHLPP/FKBP51/IKKα complex. Nucleic Acids Res. 2019;47:4211–25.
Vander Heiden MG, Plas DR, Rathmell JC, Fox CJ, Harris MH, Thompson CB. Growth factors can influence cell growth and survival through effects on glucose metabolism. Mol Cell Biol. 2001;21:5899–912.
Deberardinis RJ, Sayed N, Ditsworth D, Thompson CB. Brick by brick: metabolism and tumor cell growth. Curr Opin Genet Dev. 2008;18:54–61.
Brockmueller A, Sameri S, Liskova A, Zhai K, Varghese E, Samuel SM, et al. Resveratrol’s Anti-Cancer Effects through the Modulation of Tumor Glucose Metabolism. Cancers (Basel). 2021;13:188.
Meziou S, Ringuette Goulet C, Hovington H, Lefebvre V, Lavallée É, Bergeron M, et al. GLUT1 expression in high-risk prostate cancer: correlation with (18) F-FDG-PET/CT and clinical outcome. Prostate Cancer Prostatic Dis. 2020;23:441–8.
Shao M, Yu Z, Zou J. LncRNA-SNHG16 Silencing Inhibits Prostate Carcinoma Cell Growth, Downregulate GLUT1 Expression and Reduce Glucose Uptake. Cancer Manag Res. 2020;12:1751–7.
Zhao S, Zhang Y, Zhang Q, Wang F, DJFii Z. Toll-like receptors and prostate cancer. Front Immunol. 2014;5:352.
Galli R, Starace D, Busà R, Angelini DF, Paone A, De Cesaris P, et al. TLR stimulation of prostate tumor cells induces chemokine-mediated recruitment of specific immune cell types. J Immunol. 2010;184:6658–69.
Sun M, Geng D, Li S, Chen Z, Zhao W. LncRNA PART1 modulates toll-like receptor pathways to influence cell proliferation and apoptosis in prostate cancer cells. Biol Chem. 2018;399:387–95.
Wu H, Tian X, Zhu C. Knockdown of lncRNA PVT1 inhibits prostate cancer progression in vitro and in vivo by the suppression of KIF23 through stimulating miR-15a-5p. Cancer Cell Int. 2020;20:283.
Yu Y, Gao F, He Q, Li G, Ding G. lncRNA UCA1 Functions as a ceRNA to Promote Prostate Cancer Progression via Sponging miR143. Mol Ther Nucleic Acids. 2020;19:751–8.
Pan J, Xu X, Wang G. lncRNA ZFAS1 Is Involved in the Proliferation, Invasion and Metastasis of Prostate Cancer Cells Through Competitively Binding to miR-135a-5p. Cancer Manag Res. 2020;12:1135–49.
Beaver LM, Kuintzle R, Buchanan A, Wiley MW, Glasser ST, Wong CP, et al. Long noncoding RNAs and sulforaphane: a target for chemoprevention and suppression of prostate cancer. J Nutr Biochem. 2017;42:72–83.
Akoto T, SJIjoms S. Role of Exosomes in Prostate Cancer Metastasis. 2021;22:3528.
Keller ET, Brown J. Prostate cancer bone metastases promote both osteolytic and osteoblastic activity. J Cell Biochem. 2004;91:718–29.
Kretschmer A, Tilki D. Biomarkers in prostate cancer - Current clinical utility and future perspectives. Crit Rev Oncol Hematol. 2017;120:180–93.
Saini S. PSA and beyond: alternative prostate cancer biomarkers. Cell Oncol (Dordr). 2016;39:97–106.
Bandyopadhyay S, Pai SK, Gross SC, Hirota S, Hosobe S, Miura K, et al. The Drg-1 gene suppresses tumor metastasis in prostate cancer. 2003;63:1731–6.
Lingadahalli S, Jadhao S, Sung YY, Chen M, Hu L, Chen X, et al. Novel lncRNA LINC00844 Regulates Prostate Cancer Cell Migration and Invasion through AR Signaling. Mol Cancer Res. 2018;16:1865–78.
Tan E-J, Thuault S, Caja L, Carletti T, Heldin C-H, Moustakas AJJBC. Regulation of transcription factor Twist expression by the DNA architectural protein high mobility group A2 during epithelial-to-mesenchymal transition. 2012;287:7134–45.
Yu J, Lei R, Zhuang X, Li X, Li G, Lev S, et al. MicroRNA-182 targets SMAD7 to potentiate TGFβ-induced epithelial-mesenchymal transition and metastasis of cancer cells. 2016;7:1–12.
Liu Y, Tao Z, Qu J, Zhou X, Zhang CJB. communications br: Long non-coding RNA PCAT7 regulates ELF2 signaling through inhibition of miR-134-5p in nasopharyngeal carcinoma. 2017;491:374–81.
Liu Q, Wu Y, Xiao J, JJMsmimjoe Z. research c: Long non-coding RNA prostate cancer-associated transcript 7 (PCAT7) induces poor prognosis and promotes tumorigenesis by inhibiting mir-134-5p in non-small-cell lung (NSCLC). 2017;23:6089.
Lang C, Dai Y, Wu Z, Yang Q, He S, Zhang X, et al. SMAD3/SP1 complex-mediated constitutive active loop between lncRNA PCAT7 and TGF-β signaling promotes prostate cancer bone metastasis. Mol Oncol. 2020;14:808–28.
Shanmugam MK, Ahn KS, Hsu A, Woo CC, Yuan Y, Tan KHB, et al. Thymoquinone Inhibits Bone Metastasis of Breast Cancer Cells Through Abrogation of the CXCR4 Signaling Axis. Front Pharmacol. 2018;9:1294.
Shanmugam MK, Ahn KS, Lee JH, Kannaiyan R, Mustafa N, Manu KA, et al. Celastrol Attenuates the Invasion and Migration and Augments the Anticancer Effects of Bortezomib in a Xenograft Mouse Model of Multiple Myeloma. Front Pharmacol. 2018;9:365.
Furusato B, Mohamed A, Uhlén M, Rhim JS. CXCR4 and cancer. Pathol Int. 2010;60:497–505.
Zlotnik A. Ireland: New insights on the role of CXCR4 in cancer metastasis. J Pathol. 2008;215:211–3.
Vandercappellen J, Van Damme J, Struyf S. The role of CXC chemokines and their receptors in cancer. Cancer Lett. 2008;267:226–44.
Don-Salu-Hewage AS, Chan SY, McAndrews KM, Chetram MA, Dawson MR, Bethea DA, et al. Cysteine (C)-xC receptor 4 undergoes transportin 1-dependent nuclear localization and remains functional at the nucleus of metastatic prostate cancer cells. PLoS One. 2013;8:e57194.
Chen Q, Zhong T. The association of CXCR4 expression with clinicopathological significance and potential drug target in prostate cancer: a meta-analysis and literature review. Drug Des Devel Ther. 2015;9:5115–22.
He C, Lu X, Yang F, Qin L, Guo Z, Sun Y, et al. LncRNA UCA1 acts as a sponge of miR-204 to up-regulate CXCR4 expression and promote prostate cancer progression. Biosci Rep. 2019;39.
Ko JH, Nam D, Um JY, Jung SH, Sethi G, Ahn KS. Bergamottin Suppresses Metastasis of Lung Cancer Cells through Abrogation of Diverse Oncogenic Signaling Cascades and Epithelial-to-Mesenchymal Transition. Mol. 2018;23.
Ashrafizadeh M, Zarrabi A, Hashemi F, Zabolian A, Saleki H, Bagherian M, et al. Polychemotherapy with Curcumin and Doxorubicin via Biological Nanoplatforms: Enhancing Antitumor Activity. Pharmaceutics. 2020;12:1084.
Wu G, Hao C, Qi X, Nie J, Zhou W, Huang J, et al. LncRNA SNHG17 aggravated prostate cancer progression through regulating its homolog SNORA71B via a positive feedback loop. Cell Death Dis. 2020;11:393.
Zhang Y, Zhang D, Lv J, Wang S, Zhang Q. LncRNA SNHG15 acts as an oncogene in prostate cancer by regulating miR-338-3p/FKBP1A axis. Gene. 2019;705:44–50.
Zheng Z, Qiu K, Huang W. Long Non-Coding RNA (lncRNA) RAMS11 Promotes Metastatis and Cell Growth of Prostate Cancer by CBX4 Complex Binding to Top2α. Cancer Manag Res. 2021;13:913–23.
Shi X, Zhang W, Nian X, Lu X, Li Y, Liu F, et al. The previously uncharacterized lncRNA APP promotes prostate cancer progression by acting as a competing endogenous RNA. Int J Cancer. 2020;146:475–86.
Pagliarulo V, Bracarda S, Eisenberger MA, Mottet N, Schröder FH, Sternberg CN, et al. Contemporary role of androgen deprivation therapy for prostate cancer. Eur Urol. 2012;61:11–25.
Mansinho A, Macedo D, Fernandes I, Costa L. Castration-Resistant Prostate Cancer: Mechanisms, Targets and Treatment. Adv Exp Med Biol. 2018;1096:117–33.
Ryan CJ, Smith MR, de Bono JS, Molina A, Logothetis CJ, de Souza P, et al. Abiraterone in metastatic prostate cancer without previous chemotherapy. N Engl J Med. 2013;368:138–48.
Beer TM, Armstrong AJ, Rathkopf DE, Loriot Y, Sternberg CN, Higano CS, et al. Enzalutamide in metastatic prostate cancer before chemotherapy. N Engl J Med. 2014;371:424–33.
Petrylak DP, Tangen CM, Hussain MH, Lara PN Jr, Jones JA, Taplin ME, et al. Docetaxel and estramustine compared with mitoxantrone and prednisone for advanced refractory prostate cancer. N Engl J Med. 2004;351:1513–20.
de Wit R, de Bono J, Sternberg CN, Fizazi K, Tombal B, Wülfing C, et al. Cabazitaxel versus Abiraterone or Enzalutamide in Metastatic Prostate Cancer. N Engl J Med. 2019;381:2506–18.
Ehsani M, David FO, Baniahmad A. Androgen Receptor-Dependent Mechanisms Mediating Drug Resistance in Prostate Cancer. Cancers. 2021;13:1534.
Barth DA, Juracek J, Slaby O, Pichler M, Calin GA. lncRNA and Mechanisms of Drug Resistance in Cancers of the Genitourinary System. Cancers. 2020;12:2148.
Tsao T, Beretov J, Ni J, Bai X, Bucci J, Graham P, et al. Cancer stem cells in prostate cancer radioresistance. Cancer Lett. 2019;465:94–104.
Yan F, Ma Y, Liu L, Li L, Deng J, Sun J. Long Noncoding RNA HOXD-AS1 Promotes the Proliferation, Migration, and Invasion of Colorectal Cancer via the miR-526b-3p/CCND1 Axis. J Surg Res. 2020;255:525–35.
Chen S, Li K. HOXD-AS1 facilitates cell migration and invasion as an oncogenic lncRNA by competitively binding to miR-877-3p and upregulating FGF2 in human cervical cancer. BMC Cancer. 2020;20:924.
Yang YW, Flynn RA, Chen Y, Qu K, Wan B, Wang KC, et al. Essential role of lncRNA binding for WDR5 maintenance of active chromatin and embryonic stem cell pluripotency. Elife. 2014;3(e02046).
Gu P, Chen X, Xie R, Han J, Xie W, Wang B, et al. lncRNA HOXD-AS1 Regulates Proliferation and Chemo-Resistance of Castration-Resistant Prostate Cancer via Recruiting WDR5. Mol Ther. 2017;25:1959–73.
Mirzaei S, Zarrabi A, Hashemi F, Zabolian A, Saleki H, Azami N, et al. Nrf2 Signaling Pathway in Chemoprotection and Doxorubicin Resistance: Potential Application. Drug Des Discov. 2021;10(349).
Gao M, Guo L, Wang H, Huang J, Han F, Xiang S, et al. Orphan nuclear receptor RORγ confers doxorubicin resistance in prostate cancer. Cell Biol Int. 2020;44:2170–6.
Cheteh EH, Sarne V, Ceder S, Bianchi J, Augsten M, Rundqvist H, et al. Interleukin-6 derived from cancer-associated fibroblasts attenuates the p53 response to doxorubicin in prostate cancer cells. Cell Death Dis. 2020;6:42.
Bai T, Liu Y, Li B. LncRNA LOXL1-AS1/miR-let-7a-5p/EGFR-related pathway regulates the doxorubicin resistance of prostate cancer DU-145 cells. IUBMB Life. 2019;71:1537–51.
Faraji Dizaji B, Hasani Azerbaijan M, Sheisi N, Goleij P, Mirmajidi T, Chogan F, et al. Synthesis of PLGA/chitosan/zeolites and PLGA/chitosan/metal organic frameworks nanofibers for targeted delivery of Paclitaxel toward prostate cancer cells death. Int J Biol Macromol. 2020;164:1461–74.
da Fonseca LM, Calvalhan DM, Previato JO, Mendonça Previato L, Freire-de-Lima L. Resistance to paclitaxel induces glycophenotype changes and mesenchymal-to-epithelial transition activation in the human prostate cancer cell line PC-3. Tumour Biol. 2020;42:1010428320957506.
Li X, Han X, Wei P, Yang J, Sun J. Knockdown of lncRNA CCAT1 enhances sensitivity of paclitaxel in prostate cancer via regulating miR-24-3p and FSCN1. Cancer Biol Ther. 2020;21:452–62.
Leng W, Liu Q, Zhang S, Sun D, Guo Y. LncRNA AFAP1-AS1 modulates the sensitivity of paclitaxel-resistant prostate cancer cells to paclitaxel via miR-195-5p/FKBP1A axis. Cancer Biol Ther. 2020;21:1072–80.
Chaiswing L, Weiss HL, Jayswal RD, Clair DKS, Kyprianou N. Profiles of Radioresistance Mechanisms in Prostate Cancer. Crit Rev Oncog. 2018;23:39–67.
Piffoux M, Eriau E, Cassier PA. Autophagy as a therapeutic target in pancreatic cancer. Br J Cancer. 2021;124:333–44.
Jing Q, Li G, Chen X, Liu C, Lu S, Zheng H, et al. Wnt3a promotes radioresistance via autophagy in squamous cell carcinoma of the head and neck. J Cell Mol Med. 2019;23:4711–22.
Luo J, Chen J, He L. mir-129-5p Attenuates Irradiation-Induced Autophagy and Decreases Radioresistance of Breast Cancer Cells by Targeting HMGB1. Med Sci Monit. 2015;21:4122–9.
He Q, Li J, Dong F, Cai C, Zou X. LKB1 promotes radioresistance in esophageal cancer cells exposed to radiation, by suppression of apoptosis and activation of autophagy via the AMPK pathway. Mol Med Rep. 2017;16:2205–10.
Chang L, Huang Z, Li S, Yao Z, Bao H, Wang Z, et al. A low dose of AZD8055 enhances radiosensitivity of nasopharyngeal carcinoma cells by activating autophagy and apoptosis. Am J Cancer Res. 2019;9:1922–37.
Ho SY, Wu WS, Lin LC, Wu YH, Chiu HW, Yeh YL, et al. Cordycepin Enhances Radiosensitivity in Oral Squamous Carcinoma Cells by Inducing Autophagy and Apoptosis Through Cell Cycle Arrest. Int J Mol Sci. 2019;20.
Chen C, Wang K, Wang Q, Wang X. LncRNA HULC mediates radioresistance via autophagy in prostate cancer cells. Braz J Med Biol Res. 2018;51:e7080.
Eddy K, Chen S. Overcoming Immune Evasion in Melanoma. Int J Mol Sci. 2020;21:8984.
Terry S, Engelsen AST, Buart S, Elsayed WS, Venkatesh GH, Chouaib S. Hypoxia-driven intratumor heterogeneity and immune evasion. Cancer Lett. 2020;492:1–10.
Sharma P, Hu-Lieskovan S, Wargo JA, AJC R. Primary, adaptive, and acquired resistance to cancer immunotherapy. Cell. 2017;168:707–23.
Wise DR, Schneider JA, Armenia J, Febles VA, McLaughlin B, Brennan R, et al. Dickkopf-1 Can Lead to Immune Evasion in Metastatic Castration-Resistant Prostate Cancer. JCO Precis Oncol. 2020;4.
Kolijn K, Verhoef EI, Smid M, Böttcher R, Jenster GW, Debets R, et al. Epithelial-Mesenchymal Transition in Human Prostate Cancer Demonstrates Enhanced Immune Evasion Marked by IDO1 Expression. Cancer Res. 2018;78:4671–9.
Jin X, Ding D, Yan Y, Li H, Wang B, Ma L, et al. Phosphorylated RB Promotes Cancer Immunity by Inhibiting NF-κB Activation and PD-L1 Expression. Mol Cell. 2019;73:22–35.e26.
Zhang J, Bu X, Wang H, Zhu Y, Geng Y, Nihira NT, et al. Cyclin D-CDK4 kinase destabilizes PD-L1 via cullin 3-SPOP to control cancer immune surveillance. Nature. 2018;553:91–5.
Ashrafizadeh M, Zarrabi A, Hushmandi K, Zarrin V, Moghadam ER, Zabolian A, et al. PD-1/PD-L1 axis regulation in cancer therapy: The role of long non-coding RNAs and microRNAs. Life Sci. 2020;256:117899.
Chen QH, Li B, Liu DG, Zhang B, Yang X, Tu YL. LncRNA KCNQ1OT1 sponges miR-15a to promote immune evasion and malignant progression of prostate cancer via up-regulating PD-L1. Cancer Cell Int. 2020;20:394.
Mathieu ME, Saucourt C, Mournetas V, Gauthereau X, Thézé N, Praloran V, et al. LIF-dependent signaling: new pieces in the Lego. Stem Cell Rev Rep. 2012;8:1–15.
Peñuelas S, Anido J, Prieto-Sánchez RM, Folch G, Barba I, Cuartas I, et al. TGF-beta increases glioma-initiating cell self-renewal through the induction of LIF in human glioblastoma. Cancer Cell. 2009;15:315–27.
Yu H, Yue X, Zhao Y, Li X, Wu L, Zhang C, et al. LIF negatively regulates tumour-suppressor p53 through Stat3/ID1/MDM2 in colorectal cancers. Nat Commun. 2014;5:5218.
Zhang X, Hu B, Sun YF, Huang XW, Cheng JW, Huang A, et al. Arsenic trioxide induces differentiation of cancer stem cells in hepatocellular carcinoma through inhibition of LIF/JAK1/STAT3 and NF-kB signaling pathways synergistically. Clin Transl Med. 2021;11:e335.
Zhang W, Shi X, Chen R, Zhu Y, Peng S, Chang Y, et al. Novel Long Non-coding RNA lncAMPC Promotes Metastasis and Immunosuppression in Prostate Cancer by Stimulating LIF/LIFR Expression. Mol Ther. 2020;28:2473–87.
Pascual-García M, Bonfill-Teixidor E, Planas-Rigol E, Rubio-Perez C, Iurlaro R, Arias A, et al. LIF regulates CXCL9 in tumor-associated macrophages and prevents CD8(+) T cell tumor-infiltration impairing anti-PD1 therapy. Nat Commun. 2019;10:2416.
Li C, Hu J, Hu X, Zhao C, Mo M, Zu X, et al. LncRNA SNHG9 is a prognostic biomarker and correlated with immune infiltrates in prostate cancer. Transl Androl Urol. 2021;10:215–26.
Jabbari N, Akbariazar E, Feqhhi M, Rahbarghazi R, JJJocp R. Breast cancer-derived exosomes: Tumor progression and therapeutic agents. 2020;235:6345–56.
Weng J, Xiang X, Ding L, Wong AL, Zeng Q, Sethi G, et al. Extracellular vesicles, the cornerstone of next-generation cancer diagnosis? Semin Cancer Biol. 2021.
Hessvik NP, Llorente AJC, Sciences ML. Current knowledge on exosome biogenesis and release. Cell Mol Life Sci. 2018;75:193–208.
Jayasinghe MK, Tan M, Peng B, Yang Y, Sethi G, Pirisinu M, et al. New approaches in extracellular vesicle engineering for improving the efficacy of anti-cancer therapies. Semin Cancer Biol. 2021.
Wee I, Syn N, Sethi G, Goh BC, Wang L. Role of tumor-derived exosomes in cancer metastasis. Biochim Biophys Acta Rev Cancer. 2019;1871:12–9.
Sun W, Ren Y, Lu Z, XJMc Z. The potential roles of exosomes in pancreatic cancer initiation and metastasis. Mol Cancer. 2020;19:1–18.
Zhang W, Xia W, Lv Z, Ni C, Xin Y, Yang L. Liquid Biopsy for Cancer: Circulating Tumor Cells, Circulating Free DNA or Exosomes? Cell Physiol Biochem. 2017;41:755–68.
Tang Z, Li D, Hou S, XJIjoc Z. The cancer exosomes: clinical implications, applications and challenges. Int J Cancer. 2020;146:2946–59.
LeBleu VS, RJTic K. Exosomes as a multicomponent biomarker platform in cancer. Trends Cancer. 2020.
Ozgur E, Gezer U. Investigation of lncRNA H19 in prostate cancer cells and secreted exosomes upon androgen stimulation or androgen receptor blockage. Bratisl Lek Listy. 2020;121:362–5.
Işın M, Uysaler E, Özgür E, Köseoğlu H, Şanlı Ö, Yücel ÖB, et al. Exosomal lncRNA-p21 levels may help to distinguish prostate cancer from benign disease. Front Genet. 2015;6:168.
Wang YH, Ji J, Wang BC, Chen H, Yang ZH, Wang K, et al. Tumor-Derived Exosomal Long Noncoding RNAs as Promising Diagnostic Biomarkers for Prostate Cancer. Cell Physiol Biochem. 2018;46:532–45.
Hikita T, Miyata M, Watanabe R, Oneyama C. In vivo imaging of long-term accumulation of cancer-derived exosomes using a BRET-based reporter. Sci Rep. 2020;10:16616.
Liang Y, Zhang D, Zheng T, Yang G, Wang J, Meng F, et al. lncRNA-SOX2OT promotes hepatocellular carcinoma invasion and metastasis through miR-122-5p-mediated activation of PKM2. Oncogenesis. 2020;9:54.
Ahadi A, Brennan S, Kennedy PJ, Hutvagner G, Tran N. Long non-coding RNAs harboring miRNA seed regions are enriched in prostate cancer exosomes. Sci Rep. 2016;6:24922.
Wang J, Yang X, Li R, Wang L, Gu Y, Zhao Y, et al. Long non-coding RNA MYU promotes prostate cancer proliferation by mediating the miR-184/c-Myc axis. Oncol Rep. 2018;40:2814–25.
Tan SF, Ni JX, Xiong H. LncRNA UNC5B-AS1 promotes malignant progression of prostate cancer by competitive binding to caspase-9. Eur Rev Med Pharmacol Sci. 2020;24:2271–80.
Li Z, Liu H, Ju W, Xing Y, Zhang X, Yang J. LncRNA GASL1 inhibits growth and promotes expression of apoptosis-associated proteins in prostate carcinoma cells through GLUT-1. Oncol Lett. 2019;17:5327–34.
Hu J, Deng J, Cao R, Xiong S, Guo J. LncRNA GAS5 participates in the regulation of dexamethasone on androgen receptor -negative and -positive prostate cancer cell proliferation. Mol Cell Probes. 2020;53:101607.
Wang Z, Zhang C, Chang J, Tian X, Zhu C, Xu W. LncRNA EMX2OS, Regulated by TCF12, Interacts with FUS to Regulate the Proliferation, Migration and Invasion of Prostate Cancer Cells Through the cGMP-PKG Signaling Pathway. Onco Targets Ther. 2020;13:7045–56.
Zhao X, Wang Y, He J, Deng R, Huang X, Guo Y, et al. LncRNA UCA1 maintains the low-tumorigenic and nonmetastatic status by stabilizing E-cadherin in primary prostate cancer cells. Mol Carcinog. 2020;59:1174–87.
Huang W, Su X, Yan W, Kong Z, Wang D, Huang Y, et al. Overexpression of AR-regulated lncRNA TMPO-AS1 correlates with tumor progression and poor prognosis in prostate cancer. Prostate. 2018;78:1248–61.
Guan Z, Song Y, Ma J, Li F, Zhao X, Liang G, et al. Altered expression of lncRNA NCK1-AS1 distinguished patients with prostate cancer from those with benign prostatic hyperplasia. Oncol Lett. 2019;18:6379–84.
Fu X, Wang D, Shu T, Cui D, Fu Q. LncRNA NR2F2-AS1 positively regulates CDK4 to promote cancer cell proliferation in prostate carcinoma. Aging Male. 2020;23:1073–9.
Zhang Y, Su X, Kong Z, Fu F, Zhang P, Wang D, et al. An androgen reduced transcript of LncRNA GAS5 promoted prostate cancer proliferation. PLoS One. 2017;12:e0182305.
Misawa A, Takayama K, Urano T, Inoue S. Androgen-induced Long Noncoding RNA (lncRNA) SOCS2-AS1 Promotes Cell Growth and Inhibits Apoptosis in Prostate Cancer Cells. J Biol Chem. 2016;291:17861–80.
Chang YT, Lin TP, Tang JT, Campbell M, Luo YL, Lu SY, et al. HOTAIR is a REST-regulated lncRNA that promotes neuroendocrine differentiation in castration resistant prostate cancer. Cancer Lett. 2018;433:43–52.
Misawa A, Takayama KI, Fujimura T, Homma Y, Suzuki Y, Inoue S. Androgen-induced lncRNA POTEF-AS1 regulates apoptosis-related pathway to facilitate cell survival in prostate cancer cells. Cancer Sci. 2017;108:373–9.
Xing P, Wang Y, Zhang L, Ma C, Lu J. Knockdown of lncRNA MIR4435-2HG and ST8SIA1 expression inhibits the proliferation, invasion and migration of prostate cancer cells in vitro and in vivo by blocking the activation of the FAK/AKT/β-catenin signaling pathway. Int J Mol Med. 2021;47.
Tan X, Chen WB, Lv DJ, Yang TW, Wu KH, Zou LB, et al. LncRNA SNHG1 and RNA binding protein hnRNPL form a complex and coregulate CDH1 to boost the growth and metastasis of prostate cancer. Cell Death Dis. 2021;12:138.
Zhang Y, Pitchiaya S, Cieślik M, Niknafs YS, Tien JC, Hosono Y, et al. Analysis of the androgen receptor-regulated lncRNA landscape identifies a role for ARLNC1 in prostate cancer progression. Nat Genet. 2018;50:814–24.
Gu P, Chen X, Xie R, Xie W, Huang L, Dong W, et al. A novel AR translational regulator lncRNA LBCS inhibits castration resistance of prostate cancer. Mol Cancer. 2019;18:109.
Fire A, Xu S, Montgomery MK, Kostas SA, Driver SE, CCJn M. Potent and specific genetic interference by double-stranded RNA in Caenorhabditis elegans. 1998;391:806–11.
Garba AO, Mousa SAJO, diseases e: Bevasiranib for the treatment of wet, age-related macular degeneration. 2010, 2:OED. S4878.
Wu SY, Lopez-Berestein G, Calin GA, AKJStm S. RNAi therapies: drugging the undruggable. Sci Transl Med. 2014;6:240ps247.
Springer AD, SFJNat D. GalNAc-siRNA conjugates: leading the way for delivery of RNAi therapeutics. Nucleic Acid Ther. 2018;28:109–18.
Ohrt T, Muetze J, Svoboda P, Schwille P. Intracellular localization and routing of miRNA and RNAi pathway components. Curr Top Med Chem. 2012;12:79–88.
Schirle NT, MacRae IJ. The crystal structure of human Argonaute2. Science. 2012;336:1037–40.
Schirle NT, Sheu-Gruttadauria J, MacRae IJ. Structural basis for microRNA targeting. Science. 2014;346:608–13.
Wang T, Shigdar S, Al Shamaileh H, Gantier MP, Yin W, Xiang D, et al. Challenges and opportunities for siRNA-based cancer treatment. Cancer Lett. 2017;387:77–83.
Delfi M, Sartorius R, Ashrafizadeh M, Sharifi E, Zhang Y, De Berardinis P, et al. Self-assembled peptide and protein nanostructures for anti-cancer therapy: Targeted delivery, stimuli-responsive devices and immunotherapy. Nano Today. 2021;38:101119.
Mirzaei S, Gholami MH, Hashemi F, Zabolian A, Hushmandi K, Rahmanian V, et al. Employing siRNA tool and its delivery platforms in suppressing cisplatin resistance: Approaching to a new era of cancer chemotherapy. Life Sci. 2021;119430.
Ashrafizade M, Delfi M, Hashemi F, Zabolian A, Saleki H, Bagherian M, et al. Biomedical application of chitosan-based nanoscale delivery systems: Potential usefulness in siRNA delivery for cancer therapy. Carbohydr Polym. 2021;117809.
Zhang C, Ge S, Gong W, Xu J, Guo Z, Liu Z, et al. LncRNA ANRIL acts as a modular scaffold of WDR5 and HDAC3 complexes and promotes alteration of the vascular smooth muscle cell phenotype. Cell Death Dis. 2020;11:435.
Fu J, Zhao W, Guo D, Li Z. LncRNA E2F-Mediated Cell Proliferation Enhancing lncRNA Regulates Cancer Cell Behaviors and Affects Prognosis of Gastric Cancer. Dig Dis Sci. 2020;65:1348–54.
Li Z, Wang F, Zhang S. Knockdown of lncRNA MNX1-AS1 suppresses cell proliferation, migration, and invasion in prostate cancer. FEBS Open Bio. 2019;9:851–8.
Zhou X, Chen Q, Wang H, Zhang C, Fu B, Wang G. Specific expression of lncRNA RP13-650J16.1 and TCONS_00023979 in prostate cancer. Biosci Rep. 2018;38.
Luo X, Yang W, Ye DQ, Cui H, Zhang Y, Hirankarn N, et al. A functional variant in microRNA-146a promoter modulates its expression and confers disease risk for systemic lupus erythematosus. PLoS Genet. 2011;7:e1002128.
Wan B, Wu HY, Lv DJ, Zhou XM, Zhong LR, Lei B, et al. Downregulation of lncRNA PVT1 expression inhibits proliferation and migration by regulating p38 expression in prostate cancer. Oncol Lett. 2018;16:5160–6.
Xue D, Zhou C, Lu H, Xu R, Xu X, He X. LncRNA GAS5 inhibits proliferation and progression of prostate cancer by targeting miR-103 through AKT/mTOR signaling pathway. Tumour Biol. 2016.
Pucci P, Venalainen E, Alborelli I, Quagliata L, Hawkes C, Mather R, et al. LncRNA HORAS5 promotes taxane resistance in castration-resistant prostate cancer via a BCL2A1-dependent mechanism. Epigenomics. 2020;12:1123–38.
Kashyap D, Tuli HS, Yerer MB, Sharma A, Sak K, Srivastava S, et al. Natural product-based nanoformulations for cancer therapy: Opportunities and challenges. Semin Cancer Biol. 2021;69:5–23.
Hussain Y, Mirzaei S, Ashrafizadeh M, Zarrabi A, Hushmandi K, Khan H, et al. Quercetin and Its Nano-Scale Delivery Systems in Prostate Cancer Therapy: Paving the Way for Cancer Elimination and Reversing Chemoresistance. Cancers (Basel). 2021;13:1602.
Lu X, Chen D, Yang F, Xing N. Quercetin Inhibits Epithelial-to-Mesenchymal Transition (EMT) Process and Promotes Apoptosis in Prostate Cancer via Downregulating lncRNA MALAT1. Cancer Manag Res. 2020;12:1741–50.
Termini D, Den Hartogh DJ, Jaglanian A, Tsiani E. Curcumin against Prostate Cancer: Current Evidence. Biomolecules. 2020;10.
Liu T, Chi H, Chen J, Chen C, Huang Y, Xi H, et al. Curcumin suppresses proliferation and in vitro invasion of human prostate cancer stem cells by ceRNA effect of miR-145 and lncRNA-ROR. Gene. 2017;631:29–38.
Xu S, Yi XM, Tang CP, Ge JP, Zhang ZY, Zhou WQ. Long non-coding RNA ATB promotes growth and epithelial-mesenchymal transition and predicts poor prognosis in human prostate carcinoma. Oncol Rep. 2016;36:10–22.
Hu W, Wang Y, Fang Z, He W, Li S. Integrated Characterization of lncRNA-Immune Interactions in Prostate Cancer. Front Cell Dev Biol. 2021;9:641891.
Dong L, Ding H, Li Y, Xue D, Liu Y. LncRNA TINCR is associated with clinical progression and serves as tumor suppressive role in prostate cancer. Cancer Manag Res. 2018;10:2799–807.
Zhang X, Zhang Y, Mao Y, Ma X. The lncRNA PCAT1 is correlated with poor prognosis and promotes cell proliferation, invasion, migration and EMT in osteosarcoma. Onco Targets Ther. 2018;11:629–38.
Hua JT, Ahmed M, Guo H, Zhang Y, Chen S, Soares F, et al. Risk SNP-Mediated Promoter-Enhancer Switching Drives Prostate Cancer through lncRNA PCAT19. Cell. 2018;174:564–575.e518.
Wang D, Wan X, Zhang Y, Kong Z, Lu Y, Sun X, et al. A novel androgen-reduced prostate-specific lncRNA, PSLNR, inhibits prostate-cancer progression in part by regulating the p53-dependent pathway. Prostate. 2019;79:1362–77.
Yuan Q, Chu H, Ge Y, Ma G, Du M, Wang M, et al. LncRNA PCAT1 and its genetic variant rs1902432 are associated with prostate cancer risk. J Cancer. 2018;9:1414–20.
Yang J, Li C, Mudd A, Gu X. LncRNA PVT1 predicts prognosis and regulates tumor growth in prostate cancer. Biosci Biotechnol Biochem. 2017;81:2301–6.
Acknowledgements
EO is supported by a NUSMed Postdoctoral Fellowship funded by the Ministry of Education (MOE) and the Social Sciences Research Council (SSRC) of Singapore.
Funding
This research was supported in part by the Canadian Institutes of Health Research (#141635, #144159, #153081, #173338) (YW), Terry Fox Research Institute (#1062) (YW); Singapore Ministry of Education (MOE-T2EP30120-0016) (#180554) (APK).
Author information
Authors and Affiliations
Contributions
MA, APK, and YW conceptualized this topic; SM, MDAP, EO, MHG, KH, and MH performed the literature search, collated articles and wrote the first draft; AK, AZ, NN, and NR created the figures for the manuscript and assisted in editing the first draft; ES and HK-M further edited the draft manuscript; MA, APK, and YW edited the manuscript to its final form. The author(s) read and approved the final manuscript.
Corresponding authors
Ethics declarations
Ethics approval and consent to participate
Not applicable
Consent for publication
All authors have read the final version and given their consent
Competing interests
The authors declare no conflict of interest
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Mirzaei, S., Paskeh, M.D.A., Okina, E. et al. Molecular Landscape of LncRNAs in Prostate Cancer: A focus on pathways and therapeutic targets for intervention. J Exp Clin Cancer Res 41, 214 (2022). https://doi.org/10.1186/s13046-022-02406-1
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s13046-022-02406-1