Abstract
In recent years, neutrophils have attracted increasing attention because of their cancer-promoting effects. An elevated neutrophil-to-lymphocyte ratio is considered a prognostic indicator for patients with cancer. Neutrophils are no longer regarded as innate immune cells with a single function, let alone bystanders in the pathological process of cancer. Their diversity and plasticity are being increasingly recognized. This review summarizes previous studies assessing the roles and mechanisms of neutrophils in cancer initiation, progression, metastasis and relapse. Although the findings are controversial, the fact that neutrophils play a dual role in promoting and suppressing cancer is undeniable. The plasticity of neutrophils allows them to adapt to different cancer microenvironments and exert different effects on cancer. Given the findings from our own research, we propose a reasonable hypothesis that neutrophils may be reprogrammed into a cancer-promoting state in the cancer microenvironment. This new perspective indicates that neutrophil reprogramming in the course of cancer treatment is a problem worthy of attention. Preventing or reversing the reprogramming of neutrophils may be a potential strategy for adjuvant cancer therapy.
Similar content being viewed by others
Background
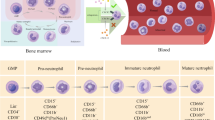
Neutrophils have been recognized as the most abundant innate immune cells in both bone marrow and peripheral blood [1]. They are rapidly recruited into sterile or infected inflammation sites and show high plasticity and a strong effector response. Perhaps to avoid unnecessary tissue damage, neutrophils possess a short lifespan [2]. Therefore, the abundance of neutrophils relies on constant replenishment via granulopoiesis in the bone marrow. Their origin is hematopoietic stem cells, which give rise to lymphoid-primed multipotent progenitors (LMPPs). Neutrophils are derived from the early committed neutrophil progenitor (proNeu1), a subtype of granulocyte–monocyte myeloid progenitor (GMP) that develops from LMPPs [3, 4]. Classically, as determined based on nuclear morphology, neutrophils mature through the following sequence: GMPs, myeloblasts, promyelocytes, myelocytes, metamyelocytes, banded neutrophils and segmented neutrophils [1]. According to recent studies, the neutrophil developmental pathway mapped based on single-cell analyses is proNeu1, intermediate progeny (proNeu2), preneutrophil (preNeu), immature neutrophils and, finally, mature neutrophils [4]. Transcription factors, such as C/EBPα and C/EBPε, exquisitely control neutrophil development [5,6,7]. During neutrophil maturation, migration and immune response functions gradually overtake proliferation. Both microbial and cancer stresses trigger preNeu expansion and immature neutrophil release from bone marrow [8]. Moreover, extramedullary granulopoiesis commonly occurs in the spleen under pathological states [9].
Neutrophils play various roles in different diseases, including infectious diseases, metabolic diseases, autoimmune diseases and aging-associated diseases. On the one hand, neutrophils exert positive functions in host defense, including antibacterial [10], antifungal [11] and antiviral [12] functions. In addition, they eliminate apoptotic cell debris, which is beneficial for tissue regeneration and angiogenesis after tissue damage [13]. On the other hand, neutrophils are involved in pathogenesis through diverse mechanisms. First, neutrophils recruited to the lesion site release proteases and produce a large amount of reactive oxygen species (ROS), resulting in tissue damage, rendering the tissue more susceptible to pathogens and even the development of chronic inflammation [14]. This pathological effect on many infectious diseases and pulmonary diseases, including severe cases of coronavirus disease 2019 (COVID-19), has frequently been observed [15]. In addition, neutrophil elastase (NE) causes insulin resistance during the development of obesity and type 2 diabetes [16]. Second, neutrophils may shift their function to immunosuppression characterized by a lower response to chemokines and inhibition of T cell immunity. In sepsis, this functional change is life-threatening [17]. Third, neutrophil extracellular traps (NETs) extruded by activated neutrophils have been reported to participate in the occurrence and development of a wide range of diseases. NETs are large extracellular complexes composed of cytosolic and granule proteins and chromatin [18]. In individuals with atherosclerosis, NETs result in the destabilization of atherosclerotic plaques through the lysis of smooth muscle cells [19]. NETs are also the major inducers of thrombosis [20]. In autoimmune diseases, such as systemic lupus erythematosus, rheumatoid arthritis and ANCA-associated vasculitis, NETs are recognized as antigens that contribute to the production of anti-self-antibodies [21]. In general, neutrophils are a double-edged sword in diseases with both defensive and harmful functions.
Neutrophils, the most dominant immune cells [22], also play complex and important roles in cancer. Many studies have reported elevated peripheral blood counts of neutrophils in patients with different cancers. The neutrophil-to-lymphocyte ratio (NLR) has been shown to be an independent prognostic indicators for patients with cancer [23]. This review will describe the multifaceted roles of neutrophils in cancer initiation, growth and metastasis, thereby revealing the heterogeneity and high plasticity of neutrophils in cancer. Based on these findings and those from our own studies, we attempted to analyze the possible mechanism of neutrophil heterogeneity from the perspective of cell reprogramming.
Neutrophils in carcinogenesis
Cancer initiation
Inflammation plays an essential role in cancer initiation by damaging tissues, and neutrophils are a crucial component of this process. Thus, neutrophils provide a link between inflammation and cancer. Cancer that develops in various mouse models of KRAS-driven ovarian cancer exhibits upregulated levels of neutrophil-related chemokines and an expansion of neutrophils. These phenotypes may result from direct upregulation of neutrophil-related cytokines such as GM-CSF and CXCL8 [24, 25]. In a zebrafish model of HRASG12V-driven melanoma, wounding-induced inflammation with elevated levels of prostaglandin E2 increase the formation of cancer in a neutrophil-dependent manner [26]. Depletion of the entire neutrophil population using anti-Ly6G antibodies impairs carcinogenesis in both chemically induced and spontaneous cancer models. Neutrophils overexpressing CXCR2 are attracted to cancer-prone tissues via the cytokine IL-8 and chemokine ligands CXCL1, CXCL2 and CXCL5. The application of chemical carcinogens in CXCR2-deficient mice, which show impaired neutrophil trafficking, prevents papilloma or adenoma formation [27, 28]. CXCR2-mediated neutrophil trafficking from bone marrow into peripheral blood is antagonized by CXCR4 expression due to the retention of neutrophils by CXCL12-expressing bone marrow stromal cells mediated retention. Bone marrow macrophages subsequently eliminate the retained neutrophils in a rhythmic manner.
Neutrophils induce DNA damage
The evidence described above has indicated that neutrophils are crucial for carcinogenesis, but the exact mechanisms by which neutrophils foster carcinogenesis require further elucidation. Neutrophils produce and release genotoxic DNA substances that increase DNA instability. In an in vitro coculture model mimicking intestinal inflammation in ulcerative colitis, neutrophils increase errors in the replication of colon epithelial cells. In individuals with chronic colon inflammation, activated neutrophils cause an accumulation of target cells in G2/M phase, consistent with the installation of a DNA damage checkpoint [29]. Neutrophil-derived elastase, neutrophil production of ROS, reactive nitrogen species (RNS) and angiogenic factors such as MMP-9 and the immunosuppressive ability of neutrophils may be associated with this process. ROS released by neutrophils during chronic inflammation, such as hypochlorous acid (HOCl, formed by myeloperoxidase (MPO)), cause DNA damage and are mutagenic in lung cells in vitro. HOCl is a major neutrophil oxidant. MPO-catalyzed formation of HOCl during lung inflammation is an important source of neutrophil-induced genotoxicity. Neutrophils cause DNA damage by releasing ROS and inducing gene mutations in premalignant epithelial cells, thus driving oncogenic transformation in lung cancer. Additionally, at physiological concentrations, HOCl induces mutations in the hypoxanthine phosphoribosyl transferase (HPRT) gene, inducing three major types of DNA lesions [30]. Haqqani and coworkers analyzed a mouse model of subcutaneous cancer and showed that inducible nitric oxide synthase (iNOS) and nitric oxide synthase (NOS) contents and neutrophil infiltration were significantly correlated with the number of mutations in the Hprt locus [31]. However, a new mechanism that does not rely on ROS was also recently identified. In clinical samples from patients with inflammatory bowel disease and injury models, activated tissue-infiltrating neutrophils release particles carrying proinflammatory microRNAs, including miR-23a and miR-155, which increase DNA double-strand breaks and genomic instability [32]. miR-155 is also responsible for neutrophils-induced DNA damage and DNA repair landscape in acute colon injury, resulting in colorectal cancer initiation even shaping the progression [33].
Neutrophils promote angiogenesis and immunosuppression
Coussens et al. documented that MMP-9 supplied by bone marrow-derived neutrophils and other hematopoietic cells contributes to squamous carcinogenesis [34]. MMP-9 produced by neutrophils also contributes to the carcinogenesis of pancreatic islet carcinoma and lung cancer accelerating angiogenesis [35]. NETs promote inflammation in subjects with nonalcoholic steatohepatitis, resulting in the development of hepatocellular carcinoma, which is inhibited by deoxyribonuclease treatment or peptidyl arginine deaminase type IV knockout, decreasing NET formation [36]. Furthermore, NETs positively correlate with the increased number of regulatory T cells (Tregs) in cancer by facilitating naïve CD4+ T cell metabolic reprogramming. Therapies targeting the interaction between these two cell types or inhibiting Treg activity may promote cancer immunosurveillance and prevent hepatocellular carcinoma formation [37].
In summary, neutrophils recruited to inflammatory sites promote cancer initiation mainly by increasing DNA damage, angiogenesis and immunosuppression. However, the mechanism underlying neutrophil-dependent carcinogenesis is complicated and cannot be reduced to one specific molecule. Even the same molecule often exerts different effects on diverse stages. Although CXCR2 promotes neutrophil migration into pro-cancer sites, knockdown of CXCR2 in neutrophils increases ROS production and exerts pro-cancer effect [38]. Thus, in future studies, genetically engineered mouse models (GEMMs) will be extremely valuable for research in the field of cancer-related neutrophil biology, as they enable neutrophils and neutrophil-derived factors to be manipulated as cancer arises de novo.
Neutrophils in cancer progression
More than two decades ago, neutrophils were presumed to cause cancer xenograft rejection in mice [39, 40]. Just a few years later, the opposite result was reported: depletion of neutrophils reduced the growth of transplanted cancer [41]. Since then, reports of neutrophils promoting cancer progression have vastly outpaced those of neutrophils inhibiting cancer.
Neutrophils promote cancer growth
The mechanisms by which neutrophils promote cancer growth are diverse. Neutrophils are characterized by rich granules, which perform different functions (Table 1). Some granule proteins (MMP-9 and ARG-1) released by activated neutrophils are associated with cancer progression. For example, MMP-9 released by neutrophils degrades the extracellular matrix, which in turn releases vascular endothelial growth factor (VEGF) and promotes angiogenesis [42]. Depletion of neutrophils or blockade of CXCR2 signaling to affect neutrophil recruitment inhibits cancer growth and reduces angiogenesis [43]. In contrast, an injection of cancer cells with neutrophils from cancer-bearing mice increases cancer growth and angiogenesis. In addition, the release of ARG-1 from neutrophils depletes arginine in T cells, causing the downregulation of CD3ζ. This process inhibits CD3-mediated T cell activation and proliferation, creating an immunosuppressive environment that also contributes to cancer growth [44]. In addition, the H+-pumping ATPase on tertiary granules causes cancer acidosis when it is mobilized to the cell surface, which may lead to cancer progression. Furthermore, an acidic pH inhibits the anticancer activity of T cells and natural killer (NK) cells, resulting in immune escape. Neutrophils also promote cancer growth and progression by recruiting macrophages and Tregs [45]. The structure of NETs formed by granule proteins and DNA induces the proliferation of cancer cells through high mobility group protein B1 (HMGB1) and NE [46,47,48]. In hematological malignancies, levels of NETs are found to positively correlated with lymphoma progression or childhood acute leukemia development [49, 50].
In addition to granular proteins, neutrophils also play a role in promoting cancer growth by releasing growth factors, including epidermal growth factor, hepatocyte growth factor (HGF) and platelet-derived growth factor. Another study has shown that neutrophils eliminate senescence through IL-1 receptor antagonist (IL-1RA) and thus promote the progression of prostate cancer. Based on cancer promotion effect of neutrophil in pancreatic ductal adenocarcinoma (PDAC), lorlatinib inhibiting FES kinase, which is activated in neutrophils by PDAC cells, can attenuate cancer growth [104] (Fig. 1A).
Dual roles and plasticity of neutrophils in cancer. A Neutrophils with cancer-promoting effects. Neutrophils promote cancer initiation, progression and metastasis: (1) Neutrophils cause DNA damage and gene mutation through ROS produced by MPO, NO produced by iNOS, microRNAs and MMP9, which induce carcinogenesis. (2) Neutrophils eliminate senescence through IL-1RA and thus promote cancer progression. (3) Immunosuppression mediated by the release of Arg-1 from neutrophils to inhibit CD3-mediated T cell activation and proliferation. An acidic pH inhibits the anticancer activity of T cells and NK cells. (4) The acidic pH, cytokines and NETs can increase cancer cell proliferation. (5) Neutrophils promote each step of cancer metastasis. Cytokines released by neutrophils prepare the premetastatic niche in distant organs. MMP9 induces angiogenesis by releasing VEGF from degraded ECM. HMGB1 and TNF promote the migration of cancer cells toward blood vessels. Cathepsin G promotes intravasation through the activation of IGF-1. NETs and the interaction between neutrophils and cancer cells promote cancer cell survival in the peripheral blood. NETs also facilitate extravasation. MMP9 and NE in NETs waken up dormant cancer cells in distant organs causing the formation of metastasis. B Neutrophils with anti-cancer effect. Neutrophils exert a cytotoxic effect via H2O2 and NO production induced by MET-mediated iNOS. ADCC during antibody therapy may be another mechanism by which neutrophils kill cancer cells. Chemokines produced by neutrophils recruit T cells and other leukocytes and indirectly kill cancer cells. C Reprogramming between protumor neutrophils and antitumor neutrophils. Generally, in the process of cancer progression, various cytokines released from cancer cells and stromal cells around them may transform anticancer neutrophils into protumor ones. Additionally, many experiments proved that protumor ones or normal neutrophils can be trained to function as anticancer neutrophils. The plasticity of neutrophils has been confirmed based on concrete evidence and should be considered in cancer therapy
Neutrophils inhibit cancer growth
Although fewer studies have assessed the inhibitory effects of neutrophils on cancer, very interesting data have been reported. For example, in models transplanted with different cancer cell lines or spontaneous cancer models, changes in neutrophil recruitment induced by specifically knocking out neutrophil MET, the HGF receptor, increase cancer growth [105, 106]. In mice transplanted with mouse mammary cancer virus promoter-driven polyomavirus middle T antigen (MMTV-PyMT) or MMTV-myc mammary cancer, neutrophils may exert a cytotoxic effect by producing H2O2 and subsequently inhibit cancer growth. Antibody-dependent cellular cytotoxicity (ADCC) during antibody therapy may be another mechanism by which neutrophils kill cancer cells [107, 108]. Neutrophils express Fcγ receptors, which mediate cancer cell elimination through ADCC. Depletion of neutrophils reduces the efficacy of treatment with anti-CD52 mAb (alemtuzumab) and anti-CD20 mAb (rituximab) in mouse lymphomas [109]. IgA induces the killing of cancer cells by neutrophils much more strongly than IgG [110]. In addition, neutrophils slow cancer growth by controlling microbial populations and cancer-associated inflammation [111]. However, since endogenous antibodies usually activate the anticancer effects of neutrophils via Fc receptors, researchers have not determined whether ADCC occurs in vivo in the absence of exogenous antibodies (Fig. 1B).
Remarkably, many studies using the same transplanted cell lines reported the opposite results. This discrepancy may be caused by the use of different experimental methods or sampling times in each experiment. For example, the different antibodies used to deplete neutrophils have different corresponding targets and efficiencies. Neutrophils will have different functions in different stages of cancer progression and will gradually transform from exerting anticancer effects to producing cancer-promoting effects. All of these factors may have led to inconsistent conclusions. Therefore, future studies should focus on how the context affects neutrophil function.
Neutrophils in cancer metastasis
In recent years, most studies examining the role of neutrophils in cancer have been related to metastasis. Combined intravenous injection of cancer cells and neutrophils from cancer-bearing rodents was found to increase the incidence of lung metastases as early as the late 1980s [112]. Subsequent studies have shown that the increased levels of neutrophils induced by the IL-17/G-CSF axis or the cholesterol metabolite 27-hydroxycholesterol promote cancer metastasis [113, 114], and the concentration of β2-integrin (CD18) in the intracellular granules of neutrophils is positively correlated with liver metastasis of colorectal cancer in mice [115]. Increased NETs also facilitate hepatocellular carcinoma cell metastasis by activating TLR4/9-COX2 signaling. NET-enabled metastatic activity is abrogated by inhibiting this signaling pathway [116]. Neutrophils are actively involved in each step of the metastatic cascade: formation of the premetastatic niche, cancer cell escape from the primary tumor, intravasation into the blood and/or the lymphatic vascular system, survival in the circulation, extravasation into distant organs, awakening of dormant cancer cells and outgrowth of metastases.
Neutrophils promote cancer cell migration and intravasation
In the early stages of metastasis, neutrophils release MMP-9 to promote angiogenesis, playing an important role again by not only facilitating cancer growth but also providing more routes for cancer cells to escape. Neutrophils also direct cancer cells to endothelial cells, prompting them to enter the bloodstream. One mouse model of melanoma showed that cancer cells clustered around blood vessels and increased lung metastasis but had no effect on the growth of the primary tumor. In this model, cell damage increased HMGB1 levels, leading to the recruitment of neutrophils that subsequently promoted the migration of cancer cells toward blood vessels [117,118,119]. In vitro, neutrophil-derived tumor necrosis factor (TNF) stimulates melanoma cell migration, suggesting that TNF is one of the factors related to neutrophil-induced metastasis.
Next, neutrophils guide cancer cells into blood vessels. Cathepsin G, a neutrophil-derived serine protease, induces cell migration, activates insulin-like growth factor 1, increases E-cadherin-mediated intercellular adhesion and cancer cell aggregation, and promotes cancer cell entry into blood vessels [120]. NETs trap circulating cancer cells (CTCs), helping them spread to distant sites and promoting their adhesion to distant sites [121, 122]. The interaction between neutrophils and CTCs promotes cell cycle progression in the blood and expands the metastatic potential of CTCs [123]. According to a recent study, ROS produced by neutrophils increase NETs, especially in obese cancer-bearing mice, which weakens endothelial junctions and promotes the extravasation of cancer cells[124]. In addition, several studies have shown that direct interaction between neutrophils and cancer cells activates neutrophils, increases the migration of cancer cells, promotes the anchoring of cancer cells to endothelial cells, and ultimately helps cancer cells exit blood vessels [123, 125].
Neutrophils facilitate cancer cell extravasation
Finally, metastatic cancer cells in distant tissues typically remain dormant for an extended period, during which infiltrating neutrophils release MMP-9 to promote angiogenesis, triggering the growth of dormant metastases. In addition, continued inflammation induces the formation of NETs, which are needed to wake dormant cancer cells. A mechanistic analysis has shown that two NEs and MMP-9, which are associated with NETs, cleave laminin. Cleaved laminin induces the proliferation of dormant cancer cells by activating α3β1-integrin signaling [72].
A related interesting phenomenon has been observed. Before disseminated cancer cells arrive, neutrophils accumulate in distant organs, forming the premetastatic niche. Neutrophils have been observed to aggregate in the lungs prior to the occurrence of metastasis in mouse models of MMTV-PyMT mammary cancer, breast cancer with nicotine exposure and melanoma, all of which are closely associated with the occurrence of pulmonary metastasis [79, 126, 127]. Neutrophils also contribute to ovarian cancer metastasis to the omentum by premetastic niche formation [128]. In cancer-bearing mice, cancer tissues modulate the microenvironment in the distal organ by releasing various cytokines, including vascular endothelial growth factor A (VEGFA), TNF, transforming growth factor-β (TGFβ), and G-CSF, to prepare for subsequent cancer metastasis [126, 129]. Blockage of neutrophil recruitment to the premetastatic sites or NET formation often prevents metastasis. However, whether targeting this phenomenon can prevent cancer metastasis into other organs or tissues, which are common as metastatic sites such as brain, breast, and lymph nodes, remains to be further investigated.
Neutrophils inhibit cancer metastasis
In contrast, other researchers have shown that neutrophil depletion facilitates metastasis. CCL2 and G-CSF secreted by the primary tumor activate the cytotoxic functions of these antimetastatic neutrophils mediated by H2O2. The type of tumor-entrained neutrophils is only observed in patients with cancer and not in healthy people; neutrophils migrate from primary breast tumor sites into the lung before metastatic cancer cells and then exert an inhibitory effect on metastatic colonization [130]. Neutrophils produce chemokines that recruit T cells and other leukocytes to indirectly kill cancer cells [131]. In a mouse model of breast cancer cell metastasis to the lung, the inhibitory effect of neutrophils required the presence of NK cells. In the absence of NK cells, the tumoricidal activity of neutrophils switched into metastatic facilitation [132]. Moreover, neutrophil expression of thrombospondin 1, IL-1β and the receptor tyrosine-protein kinase MET limit the formation of metastases by blocking the cancer cell mesenchymal-to-epithelial transition and releasing NO individually [69, 105, 133]. Neutrophils acquire the characteristics of antigen-presenting cells (APCs) in the early stage and thus might stimulate the proliferation of T cells to protect against tumor metastasis [134].
Neutrophils in cancer recurrence
According to clinical data, the NLR predicts the prognostic outcome and the absolute neutrophil counts are considered independent prognostic factors for cervical cancer relapse and postoperative recurrence of intrahepatic cholangiocarcinoma [135, 136]. Although the underlying mechanism remains unclear, the interaction between neutrophils and cancer cells may play a role in cancer recurrence. In a zebrafish melanoma model, neutrophils were recruited to the inflammatory site of postoperative trauma and interacted with precancerous cells, providing them with environmental conditions that support their proliferation, and these interactions may be associated with postoperative cancer relapse. In ovarian and lung cancer, stress hormone-induced neutrophil activation reactivates dormant cancer cells and leads to early recurrence. Neutrophil activation is based on the release of S100A8/A9 proteins, myeloperoxidase activation and oxidized lipid accumulation, which finally activate the fibroblast growth factor-related signaling pathway in dormant cancer cells and push them to exit from dormancy [76]. In patients with breast cancer diagnosed with COVID-19, emerging reports show that dormant cancer cells are reawakened by factors released during lung inflammation, including NETs. Severe acute respiratory syndrome coronavirus 2 infection of airway epithelial cells first releases damage-associated molecular patterns followed by inflammatory cytokines and chemokines, which further recruit and activate neutrophils to release NETs [137].
Taken together, these findings show that the premetastatic behavior of neutrophils can be switched in vivo, providing possible opportunities for therapeutic intervention (Table 2). Although cancer recurrence is currently proposed to increase in the presence of neutrophils, our understanding of the role of neutrophils might be altered as this field advances.
Neutrophil plasticity and the cancer microenvironment
Cancer microenvironment mediates dual roles of neutrophils
In cancer, neutrophils exert both pro-cancer and anticancer effects. The diversity of neutrophils is very common in cancer. A transcriptomic analysis revealed that tumor-associated neutrophils (TANs) and neutrophils from patients with cancer or cancer-bearing mice, which showed a higher proportion of neutrophil progenitors and a tendency toward immunosuppressive properties, differed significantly from those from healthy people or mice [166]. This diversity results from the high plasticity of neutrophils due to the effects of complex cancer microenvironments. The cancer and tissue microenvironments, conventional therapies and immunotherapy shape neutrophil function.
In a GEMM of lung adenocarcinoma, TGFβ polarized neutrophils in a cancer-promoting direction, and TGFβ blockade reversed the neutrophil protumor phenotype to an antitumor phenotype. These two types of neutrophils with opposite functions are named N2 and N1, respectively, which are similar and comparable to tumor-associated macrophages, such as M2 and M1 [167]. In the early stage of non-small cell lung cancer, the anticancer state of neutrophils is also induced by interferon-γ (IFNγ) and GM-CSF. Induced neutrophils indeed develop from immature progenitors through the negative regulation of the transcription factor Ikaros and acquire APCs properties, which as APC-like hybrid cells, promote T cell antitumor responses [152]. Another study has shown that hypoxia is a potent determinant of the TAN phenotype and direct neutrophil-cancer cell interactions. After the removal of hypoxia, the number of neutrophils recruited by the cancer decreased significantly, but the recruited cells were more effective at killing the cancer cells. This activity is mediated by the production of NADPH oxidase-derived ROS and MMP-9. At the same time, the ability of neutrophils to promote cancer cell proliferation, which appears to be mediated by their production of NE, is also reduced [155]. The general trend is that TANs belong to a network of anticancer cells in the early stages of carcinogenesis, but with cancer progression, neutrophil function shifts to immunosuppressive and cancer-promoting states.
Metabolic reprogramming of neutrophils
Neutrophils among TANs with proven immunosuppressive function have been extensively studied and have been named granulocytic myeloid-derived suppressor cells (G-MDSCs) or polymorphonuclear myeloid-derived suppressive cells. G-MDSCs appear as neutrophils at different stages of maturation [168]. G-MDSCs flexibly adapt to the cancer microenvironment. The most important of these adaptations is the metabolic shift, which exerts a substantial effect on cell function.
Metabolic features include the upregulation of fatty acid transport protein 2 (FATP2) [143], increased levels of arginase I [169], high NADPH oxidase activity [155] and active NOS [170]. These factors have all been shown to inhibit T cell function. Regarding the accumulation of high lipids in cancer microenvironment, G-MDSCs increase the uptake of exogenous fatty acids through STAT3- or STAT5-mediated upregulation of lipid transport receptors. Increased fatty acid oxidation induces G-MDSCs to undergo metabolic reprogramming from glycolysis and become immunosuppressive [171]. Accordingly, inhibition of the neutrophil metabolic reprogramming by blocking fatty acid oxidation can synergize with the immunotherapeutic effect of T cells. Thus, neutrophils not only utilize diverse metabolic strategies to meet the energy requirements for survival but also exhibit functional alterations in cancer based on changes in the cancer microenvironment, such as decreased glucose levels, low oxygen pressure and low pH values [172]. Cancer can produce many factors such as IL-1β, CCL2, TGF-β, G-CSF and GM-CSF influencing innate immune cells, including neutrophils [173]. In particular, G-CSF, GM-CSF and IL-6 secreted by cancer and/or by stromal cells surrounding cancer cells induce potent activation of G-MDCs by activating the myeloid transcription factor C/EBPβ.
Neutrophil subset identification and markers
Researchers have attempted to identify neutrophil subsets. Specific surface markers proposed to identify neutrophil subsets in cancer include CD101 and CD177 [174, 175], which are associated with cancer regression, and CD117, PDL1, CD170, LOX1, CD84 and JAML [176], which are associated with T cell immunosuppression and cancer progression. In PDAC, the purinergic receptor P2RX-negative neutrophil subset exhibits immunosuppressive role with enhanced PD-L1 expression and mitochondrial metabolism [177]. However, an unequivocal method to detect immunosuppressive neutrophils and other neutrophil subsets using flow cytometry or other strategies remains to be developed. Since the subsets of neutrophils show continuous changes and are highly phenotypically and morphologically similar (even between MDSCs and other cells), a reasonable assumption is that these hypothetical subsets are actually the same type of cells, with larger or smaller changes induced by different local environments. These neutrophils are a single cell type with many different functional phenotypes. The high plasticity of neutrophils enables them to respond quickly to external stimuli, leading to their heterogeneity. Because different stimuli mobilize different cytoplasmic granules, different degrees of exposure of the membrane proteins of each granule to the cell surface can change the cell surface composition of neutrophils, potentially leading to the misidentification of new cell types. Taken together, TANs appear to be more flexible than circulating neutrophils, which enables them to adapt to diverse cancer microenvironments.
Moreover, TANs or normal neutrophils have consistently been shown to be trained to become anticancer neutrophils through various methods to achieve the goal of killing cancer cells. For example, PPM1D/Wip1 is a negative regulator of the cancer suppressor p53 and is overexpressed in several human solid cancers. Ppm1d knockout or chemical inhibition of Wip1 in human or mouse neutrophils exacerbates anticancer phenotypes and increases p53-dependent expression of costimulatory ligands and the proliferation of cocultured cytotoxic T cells [178]. Another study showed that exposure to β-glucan [179], a fungal-derived prototype agonist of trained immunity, trained neutrophils in mice to enhance the anticancer activity of neutrophils. These results, in turn, prove that neutrophils are highly plastic (Fig. 1C).
Interaction between neutrophils and other microenvironmental cells
Cancer is highly heterogeneous and is considered one of its hallmarks. The tumor contains cancer cells and noncancerous cells such as neutrophils, macrophages, T cells, adipocytes, stromal cells and others constituting the microenvironment. All these cells communicate directly or indirectly. Thus, neutrophils in cancer not only have a relationship with the T cells mentioned above but also affect or are affected by other cells. During advanced colorectal cancer progression, cancer stem cell-derived exosomes containing triphosphate RNAs prime neutrophils for cancer development and depletion of neutrophils with antibodies attenuate the tumorigenicity of these cancer stem cells [180]. In obese patients with pancreatic cancer, crosstalk among pancreatic stellate cells, neutrophils and adipocytes mediated by IL1β promotes PDAC. Genetic or pharmacological targeting of this circuit provides a potential method for pancreatic cancer treatment [181]. Cancer-associated fibroblasts are considered one of the important stromal cells contributing to cancer development. A recent report identified that one of the underlying mechanisms as NET induction. This induction is driven by increased amyloid and β-secretase expression in fibroblasts [182].
Discussion and perspectives
We speculate that the cancer microenvironment may reprogram neutrophils to achieve conversion between anticancer polarity and cancer-promoting one. First, as previously described, neutrophils are heterogeneous in patients with cancer, which may result from the reprogramming of mature neutrophils. Many data indicate that neutrophil precursors support cancer growth and metastatic progression. Second, cancer cells functionally shape the cancer microenvironment by secreting various cytokines, chemokines and other factors, which provides the necessary environmental conditions for the reprogramming of surrounding neutrophils. Neutrophils acquiring new transcriptional activity, which could be characterized as diverse neutrophil subsets, based on single cell RNA sequencing analysis under specific microenvironment support the hypothesis [183]. Our previous review also stated that cancer cells undergo cellular reprogramming either spontaneously or after anticancer treatment [184]. All of these findings suggest the possibility of reprogramming both cancer cells and neutrophils in the cancer microenvironment. Third, our experiments show that mature neutrophils are reprogrammed into multipotent progenitors in the presence of a chemical cocktail [185]. In other words, neutrophils have the potential to undergo cell reprogramming.
More evidence of neutrophil reprogramming is illustrated below. Neutrophils transdifferentiate into other cell types. One study has shown that human postmitotic neutrophils are reprogrammed into macrophages via growth factors. The molecular mechanisms underlying functional changes in neutrophils has been discovered that GM-CSF controls the overexpression of FATP2 in neutrophils through the activation of the STAT5 transcription factor, thereby enabling neutrophils to obtain immunosuppressive activity and promote cancer progression in mice [143]. In addition, metabolic reprogramming of neutrophils leads to functional changes, as a metabolic shift of innate immune cells, including neutrophils, is observed in pulmonary diseases, accompanied by an impaired normal immune function of these cells.
In conclusion, neutrophils exert both pro-cancer and anticancer effects on the initiation, growth and metastasis of cancer, and these different functions are accompanied by the existence of different neutrophil subpopulations. Because neutrophils normally possess antimicrobial and anticancer functions, functional transformation or abnormal cell differentiation must occur. Here, we propose a hypothesis that the cancer microenvironment or clinical treatment may induce the reprogramming of neutrophils. In clinical practice, an elevated NLR serves as a prognostic indicator and the inhibition or reversal of neutrophil reprogramming can also be employed as a potential therapeutic strategy, e.g., conversion of neutrophils into antigen-presenting cells by FcγR engagement can exhibit immunotherapeutic effect on cancer [186].
Conclusions
Neutrophils would be a promising cell target population for anticancer therapy, although their roles in cancer are dual and remain to be further investigated. Direct target neutrophils or indirect target microenvironment factors reprogramming neutrophil plasticity might be potential therapeutic strategies.
Availability of data and materials
Not applicable.
Abbreviations
- ADCC:
-
Antibody-dependent cellular cytotoxicity
- COVID-19:
-
Coronavirus disease 2019
- CTCs:
-
Circulating cancer cells
- FATP2:
-
Fatty acid transport protein 2
- GEMMs:
-
Genetically engineered mouse models
- G-MDSCs:
-
Granulocytic myeloid-derived suppressor cells
- GMP:
-
Granulocyte–monocyte myeloid progenitor
- HGF:
-
Hepatocyte growth factor
- HMGB1:
-
High mobility group protein B1
- HOCl:
-
Hypochlorous acid
- HPRT:
-
Hypoxanthine phosphoribosyl transferase
- IFNγ:
-
Interferon-γ
- IL-1RA:
-
IL-1 receptor antagonist
- iNOS:
-
Inducible nitric oxide synthase
- LMPPs:
-
Lymphoid-primed multipotent progenitors
- MPO:
-
Myeloperoxidase
- NE:
-
Neutrophil elastase
- NETs:
-
Neutrophil extracellular traps
- NK:
-
Natural killer
- NLR:
-
Neutrophil-to-lymphocyte ratio
- NOS:
-
Nitric oxide synthase
- PDAC:
-
Pancreatic ductal adenocarcinoma
- proNeu1:
-
Early committed neutrophil progenitor
- proNeu2:
-
Intermediate progeny
- preNeu:
-
Preneutrophil
- RNS:
-
Reactive nitrogen species
- ROS:
-
Reactive oxygen species
- TANs:
-
Tumor-associated neutrophils
- TGFβ:
-
Transforming growth factor-β
- TNF:
-
Tumor necrosis factor
- VEGF:
-
Vascular endothelial growth factor
References
Coffelt SB, Wellenstein MD, de Visser KE. Neutrophils in cancer: neutral no more. Nat Rev Cancer. 2016;16(7):431–46.
Ballesteros I, Rubio-Ponce A, Genua M, Lusito E, Kwok I, Fernández-Calvo G, Khoyratty TE, van Grinsven E, González-Hernández S, Nicolás-Ávila J, et al. Co-option of neutrophil fates by tissue environments. Cell. 2020;183(5):1282-1297.e1218.
Drissen R, Buza-Vidas N, Woll P, Thongjuea S, Gambardella A, Giustacchini A, Mancini E, Zriwil A, Lutteropp M, Grover A, et al. Distinct myeloid progenitor-differentiation pathways identified through single-cell RNA sequencing. Nat Immunol. 2016;17(6):666–76.
Kwok I, Becht E, Xia Y, Ng M, Teh YC, Tan L, Evrard M, Li JLY, Tran HTN, Tan Y, et al. Combinatorial Single-cell analyses of granulocyte-monocyte progenitor heterogeneity reveals an early uni-potent neutrophil progenitor. Immunity. 2020;53(2):303-318.e305.
Avellino R, Delwel R. Expression and regulation of C/EBPα in normal myelopoiesis and in malignant transformation. Blood. 2017;129(15):2083–91.
Muraoka M, Akagi T, Ueda A, Wada T, Koeffler HP, Yokota T, Yachie A. C/EBPε ΔRS derived from a neutrophil-specific granule deficiency patient interacts with HDAC1 and its dysfunction is restored by trichostatin A. Biochem Biophys Res Commun. 2019;516(1):293–9.
Avellino R, Havermans M, Erpelinck C, Sanders MA, Hoogenboezem R, van de Werken HJ, Rombouts E, van Lom K, van Strien PM, Gebhard C, et al. An autonomous CEBPA enhancer specific for myeloid-lineage priming and neutrophilic differentiation. Blood. 2016;127(24):2991–3003.
Danek P, Kardosova M, Janeckova L, Karkoulia E, Vanickova K, Fabisik M, Lozano-Asencio C, Benoukraf T, Tirado-Magallanes R, Zhou Q, et al. β-Catenin-TCF/LEF signaling promotes steady-state and emergency granulopoiesis via G-CSF receptor upregulation. Blood. 2020;136(22):2574–87.
Mumau MD, Vanderbeck AN, Lynch ED, Golec SB, Emerson SG, Punt JA. Identification of a multipotent progenitor population in the spleen that is regulated by NR4A1. J Immunol (Baltimore, Md:1950). 2018;200(3):1078–87.
Thanabalasuriar A, Scott BNV, Peiseler M, Willson ME, Zeng Z, Warrener P, Keller AE, Surewaard BGJ, Dozier EA, Korhonen JT, et al. Neutrophil extracellular traps confine pseudomonas aeruginosa ocular biofilms and restrict brain invasion. Cell Host Microbe. 2019;25(4):526-536.e524.
Drummond RA, Lionakis MS. measuring in vivo neutrophil trafficking responses during fungal infection using mixed bone marrow chimeras. Methods Mol Biol (Clifton, NJ). 2021;2260:179–96.
Iversen MB, Reinert LS, Thomsen MK, Bagdonaite I, Nandakumar R, Cheshenko N, Prabakaran T, Vakhrushev SY, Krzyzowska M, Kratholm SK, et al. An innate antiviral pathway acting before interferons at epithelial surfaces. Nat Immunol. 2016;17(2):150–8.
Castanheira FVS, Kubes P. Neutrophils and NETs in modulating acute and chronic inflammation. Blood. 2019;133(20):2178–85.
El-Benna J, Hurtado-Nedelec M, Marzaioli V, Marie JC, Gougerot-Pocidalo MA, Dang PM. Priming of the neutrophil respiratory burst: role in host defense and inflammation. Immunol Rev. 2016;273(1):180–93.
Laforge M, Elbim C, Frère C, Hémadi M, Massaad C, Nuss P, Benoliel JJ, Becker C. Tissue damage from neutrophil-induced oxidative stress in COVID-19. Nat Rev Immunol. 2020;20(9):515–6.
Talukdar S, Oh DY, Bandyopadhyay G, Li D, Xu J, McNelis J, Lu M, Li P, Yan Q, Zhu Y, et al. Neutrophils mediate insulin resistance in mice fed a high-fat diet through secreted elastase. Nat Med. 2012;18(9):1407–12.
Venet F, Monneret G. Advances in the understanding and treatment of sepsis-induced immunosuppression. Nat Rev Nephrol. 2018;14(2):121–37.
Papayannopoulos V. Neutrophil extracellular traps in immunity and disease. Nat Rev Immunol. 2018;18(2):134–47.
Döring Y, Soehnlein O, Weber C. Neutrophil extracellular traps in atherosclerosis and atherothrombosis. Circ Res. 2017;120(4):736–43.
Perdomo J, Leung HHL, Ahmadi Z, Yan F, Chong JJH, Passam FH, Chong BH. Neutrophil activation and NETosis are the major drivers of thrombosis in heparin-induced thrombocytopenia. Nat Commun. 2019;10(1):1322.
Lood C, Blanco LP, Purmalek MM, Carmona-Rivera C, De Ravin SS, Smith CK, Malech HL, Ledbetter JA, Elkon KB, Kaplan MJ. Neutrophil extracellular traps enriched in oxidized mitochondrial DNA are interferogenic and contribute to lupus-like disease. Nat Med. 2016;22(2):146–53.
Kargl J, Busch SE, Yang GH, Kim KH, Hanke ML, Metz HE, Hubbard JJ, Lee SM, Madtes DK, McIntosh MW, et al. Neutrophils dominate the immune cell composition in non-small cell lung cancer. Nat Commun. 2017;8:14381.
Templeton AJ, McNamara MG, Šeruga B, Vera-Badillo FE, Aneja P, Ocaña A, Leibowitz-Amit R, Sonpavde G, Knox JJ, Tran B, et al. Prognostic role of neutrophil-to-lymphocyte ratio in solid tumors: a systematic review and meta-analysis. J Natl Cancer Inst. 2014;106(6):dju124.
Yoshida M, Taguchi A, Kawana K, Adachi K, Kawata A, Ogishima J, Nakamura H, Fujimoto A, Sato M, Inoue T, et al. Modification of the tumor microenvironment in KRAS or c-MYC-induced ovarian cancer-associated peritonitis. PLoS ONE. 2016;11(8):e0160330.
Powell D, Lou M, Barros Becker F, Huttenlocher A. Cxcr1 mediates recruitment of neutrophils and supports proliferation of tumor-initiating astrocytes in vivo. Sci Rep. 2018;8(1):13285.
Antonio N, Bønnelykke-Behrndtz ML, Ward LC, Collin J, Christensen IJ, Steiniche T, Schmidt H, Feng Y, Martin P. The wound inflammatory response exacerbates growth of pre-neoplastic cells and progression to cancer. EMBO J. 2015;34(17):2219–36.
Jamieson T, Clarke M, Steele CW, Samuel MS, Neumann J, Jung A, Huels D, Olson MF, Das S, Nibbs RJ, et al. Inhibition of CXCR2 profoundly suppresses inflammation-driven and spontaneous tumorigenesis. J Clin Investig. 2012;122(9):3127–44.
Gong L, Cumpian AM, Caetano MS, Ochoa CE, De la Garza MM, Lapid DJ, Mirabolfathinejad SG, Dickey BF, Zhou Q, Moghaddam SJ. Promoting effect of neutrophils on lung tumorigenesis is mediated by CXCR2 and neutrophil elastase. Mol Cancer. 2013;12(1):154.
Campregher C, Luciani MG, Gasche C. Activated neutrophils induce an hMSH2-dependent G2/M checkpoint arrest and replication errors at a (CA)13-repeat in colon epithelial cells. Gut. 2008;57(6):780–7.
Güngör N, Knaapen AM, Munnia A, Peluso M, Haenen GR, Chiu RK, Godschalk RW, van Schooten FJ. Genotoxic effects of neutrophils and hypochlorous acid. Mutagenesis. 2010;25(2):149–54.
Sandhu JK, Privora HF, Wenckebach G, Birnboim HC. Neutrophils, nitric oxide synthase, and mutations in the mutatect murine tumor model. Am J Pathol. 2000;156(2):509–18.
Butin-Israeli V, Bui TM, Wiesolek HL, Mascarenhas L, Lee JJ, Mehl LC, Knutson KR, Adam SA, Goldman RD, Beyder A, et al. Neutrophil-induced genomic instability impedes resolution of inflammation and wound healing. J Clin Investig. 2019;129(2):712–26.
Bui TM, Butin-Israeli V, Wiesolek HL, Zhou M, Rehring JF, Wiesmüller L, Wu JD, Yang GY, Hanauer SB, Sebag JA, et al. Neutrophils alter DNA repair landscape to impact survival and shape distinct therapeutic phenotypes of colorectal cancer. Gastroenterology. 2021;161(1):225-238.e215.
Coussens LM, Tinkle CL, Hanahan D, Werb Z. MMP-9 supplied by bone marrow-derived cells contributes to skin carcinogenesis. Cell. 2000;103(3):481–90.
Deryugina EI, Zajac E, Juncker-Jensen A, Kupriyanova TA, Welter L, Quigley JP. Tissue-infiltrating neutrophils constitute the major in vivo source of angiogenesis-inducing MMP-9 in the tumor microenvironment. Neoplasia. 2014;16(10):771–88.
van der Windt DJ, Sud V, Zhang H, Varley PR, Goswami J, Yazdani HO, Tohme S, Loughran P, O’Doherty RM, Minervini MI, et al. Neutrophil extracellular traps promote inflammation and development of hepatocellular carcinoma in nonalcoholic steatohepatitis. Hepatology (Baltimore, MD). 2018;68(4):1347–60.
Wang H, Zhang H, Wang Y, Brown ZJ, Xia Y, Huang Z, Shen C, Hu Z, Beane J, Ansa-Addo EA, Huang H, Tian D, Tsung A. Regulatory T-cell and neutrophil extracellular trap interaction contributes to carcinogenesis in non-alcoholic steatohepatitis. J Hepatol. 2021. https://doi.org/10.1016/j.jhep.2021.07.032
Timaxian C, Vogel CFA, Orcel C, Vetter D, Durochat C, Chinal C, NGuyen P, Aknin ML, Mercier-Nome F, Davy M, et al. Pivotal role for Cxcr2 in regulating tumor-associated neutrophil in breast cancer. Cancers. 2021;13(11):2584.
Provinciali M, Argentati K, Tibaldi A. Efficacy of cancer gene therapy in aging: adenocarcinoma cells engineered to release IL-2 are rejected but do not induce tumor specific immune memory in old mice. Gene Ther. 2000;7(7):624–32.
Shimizu M, Fontana A, Takeda Y, Yagita H, Yoshimoto T, Matsuzawa A. Induction of antitumor immunity with Fas/APO-1 ligand (CD95L)-transfected neuroblastoma neuro-2a cells. J immunol (Baltimore, Md:1950). 1999;162(12):7350–7.
Nozawa H, Chiu C, Hanahan D. Infiltrating neutrophils mediate the initial angiogenic switch in a mouse model of multistage carcinogenesis. Proc Natl Acad Sci USA. 2006;103(33):12493–8.
Christoffersson G, Vågesjö E, Vandooren J, Lidén M, Massena S, Reinert RB, Brissova M, Powers AC, Opdenakker G, Phillipson M. VEGF-A recruits a proangiogenic MMP-9-delivering neutrophil subset that induces angiogenesis in transplanted hypoxic tissue. Blood. 2012;120(23):4653–62.
Purohit A, Saxena S, Varney M, Prajapati DR, Kozel JA, Lazenby A, Singh RK. Host Cxcr2-dependent regulation of pancreatic cancer growth, angiogenesis, and metastasis. Am J Pathol. 2021;191(4):759–71.
Romano A, Parrinello NL, Vetro C, Tibullo D, Giallongo C, La Cava P, Chiarenza A, Motta G, Caruso AL, Villari L, et al. The prognostic value of the myeloid-mediated immunosuppression marker Arginase-1 in classic Hodgkin lymphoma. Oncotarget. 2016;7(41):67333–46.
Zhou SL, Zhou ZJ, Hu ZQ, Huang XW, Wang Z, Chen EB, Fan J, Cao Y, Dai Z, Zhou J. Tumor-associated neutrophils recruit macrophages and T-regulatory cells to promote progression of hepatocellular carcinoma and resistance to Sorafenib. Gastroenterology. 2016;150(7):1646-1658.e1617.
Zha C, Meng X, Li L, Mi S, Qian D, Li Z, Wu P, Hu S, Zhao S, Cai J, et al. Neutrophil extracellular traps mediate the crosstalk between glioma progression and the tumor microenvironment via the HMGB1/RAGE/IL-8 axis. Cancer Biol Med. 2020;17(1):154–68.
Yang R, Zhong L, Yang XQ, Jiang KL, Li L, Song H, Liu BZ. Neutrophil elastase enhances the proliferation and decreases apoptosis of leukemia cells via activation of PI3K/Akt signaling. Mol Med Rep. 2016;13(5):4175–82.
Lerman I, Ma X, Seger C, Maolake A, Garcia-Hernandez ML, Rangel-Moreno J, Ackerman J, Nastiuk KL, Susiarjo M, Hammes SR. Epigenetic suppression of SERPINB1 promotes inflammation-mediated prostate cancer progression. Mol Cancer Res MCR. 2019;17(4):845–59.
Nie M, Yang L, Bi X, Wang Y, Sun P, Yang H, Liu P, Li Z, Xia Y, Jiang W. Neutrophil extracellular traps induced by IL8 promote diffuse large B-cell lymphoma progression via the TLR9 signaling. Clin Cancer Res. 2019;25(6):1867–79.
Ostafin M, Ciepiela O, Pruchniak M, Wachowska M, Ulińska E, Mrówka P, Głodkowska-Mrówka E, Demkow U. Dynamic changes in the ability to release neutrophil extracellular traps in the course of childhood acute leukemias. Int J Mol Sci. 2021;22(2):821.
Xue Y, Li J, Lu X. A novel immune-related prognostic signature for thyroid carcinoma. Technol Cancer Res Treat. 2020;19:1533033820935860.
Husi H, Fernandes M, Skipworth RJ, Miller J, Cronshaw AD, Fearon KCH, Ross JA. Identification of diagnostic upper gastrointestinal cancer tissue type-specific urinary biomarkers. Biomed Rep. 2019;10(3):165–74.
Cassatella MA, Östberg NK, Tamassia N, Soehnlein O. Biological roles of neutrophil-derived granule proteins and cytokines. Trends Immunol. 2019;40(7):648–64.
Mayer P, Dinkic C, Jesenofsky R, Klauss M, Schirmacher P, Dapunt U, Hackert T, Uhle F, Hänsch GM, Gaida MM. Changes in the microarchitecture of the pancreatic cancer stroma are linked to neutrophil-dependent reprogramming of stellate cells and reflected by diffusion-weighted magnetic resonance imaging. Theranostics. 2018;8(1):13–30.
Jingushi K, Uemura M, Ohnishi N, Nakata W, Fujita K, Naito T, Fujii R, Saichi N, Nonomura N, Tsujikawa K, et al. Extracellular vesicles isolated from human renal cell carcinoma tissues disrupt vascular endothelial cell morphology via azurocidin. Int J Cancer. 2018;142(3):607–17.
Zhou M, Kong Y, Wang X, Li W, Chen S, Wang L, Wang C, Zhang Q. LC-MS/MS-based quantitative proteomics analysis of different stages of non-small-cell lung cancer. Biomed Res Int. 2021;2021:5561569.
Sasani N, Roghanian R, Emtiazi G, Aghaie A. A novel approach on leukodepletion filters: investigation of synergistic anticancer effect of purified α-defensins and nisin. Adv Pharmaceut Bull. 2021;11(2):378–84.
Berghmans E, Jacobs J, Deben C, Hermans C, Broeckx G, Smits E, Maes E, Raskin J, Pauwels P, Baggerman G. Mass spectrometry imaging reveals neutrophil defensins as additional biomarkers for anti-PD-(L)1 immunotherapy response in NSCLC patients. Cancers. 2020;12(4):863.
Xu D, Lu W. Defensins: a double-edged sword in host immunity. Front Immunol. 2020;11:764.
Kolonin MG, Sergeeva A, Staquicini DI, Smith TL, Tarleton CA, Molldrem JJ, Sidman RL, Marchiò S, Pasqualini R, Arap W. Interaction between tumor cell surface receptor RAGE and proteinase 3 mediates prostate cancer metastasis to bone. Can Res. 2017;77(12):3144–50.
Wang MM, Zhuang LK, Zhang YT, Xia D, Pan XR, Tong JH. A novel specific cleavage of IκBα protein in acute myeloid leukemia cells involves protease PR3. Exp Cell Res. 2019;382(1):111441.
Yang TH, St John LS, Garber HR, Kerros C, Ruisaard KE, Clise-Dwyer K, Alatrash G, Ma Q, Molldrem JJ. Membrane-associated proteinase 3 on granulocytes and acute myeloid leukemia inhibits T cell proliferation. J Immunol (Baltimore, Md:1950). 2018;201(5):1389–99.
Schoeps B, Eckfeld C, Prokopchuk O, Böttcher J, Häußler D, Steiger K, Demir IE, Knolle P, Soehnlein O, Jenne DE, et al. TIMP1 triggers neutrophil extracellular trap formation in pancreatic cancer. Can Res. 2021;81(13):3568–79.
Grünwald B, Harant V, Schaten S, Frühschütz M, Spallek R, Höchst B, Stutzer K, Berchtold S, Erkan M, Prokopchuk O, et al. Pancreatic premalignant lesions secrete tissue inhibitor of metalloproteinases-1, which activates hepatic stellate cells via CD63 signaling to create a premetastatic niche in the liver. Gastroenterology. 2016;151(5):1011-1024.e1017.
Guan X, Lu Y, Zhu H, Yu S, Zhao W, Chi X, Xie C, Yin Z. The crosstalk between cancer cells and neutrophils enhances hepatocellular carcinoma metastasis via neutrophil extracellular traps-associated Cathepsin G component: a potential therapeutic target. J Hepatocell Carcinoma. 2021;8:451–65.
Morimoto-Kamata R, Tsuji D, Yui S. Cathepsin G-induced insulin-like growth factor (IGF) elevation in MCF-7 medium is caused by proteolysis of IGF binding protein (IGFBP)-2 but not of IGF-1. Biol Pharm Bull. 2020;43(11):1678–86.
Sionov RV, Fainsod-Levi T, Zelter T, Polyansky L, Pham CT, Granot Z. Neutrophil cathepsin G and tumor cell RAGE facilitate neutrophil anti-tumor cytotoxicity. Oncoimmunology. 2019;8(9):e1624129.
Valayer A, Brea D, Lajoie L, Avezard L, Combes-Soia L, Labas V, Korkmaz B, Thibault G, Baranek T, Si-Tahar M. Neutrophils can disarm NK cell response through cleavage of NKp46. J Leukoc Biol. 2017;101(1):253–9.
El Rayes T, Catena R, Lee S, Stawowczyk M, Joshi N, Fischbach C, Powell CA, Dannenberg AJ, Altorki NK, Gao D, et al. Lung inflammation promotes metastasis through neutrophil protease-mediated degradation of Tsp-1. Proc Natl Acad Sci USA. 2015;112(52):16000–5.
Cui C, Chakraborty K, Tang XA, Zhou G, Schoenfelt KQ, Becker KM, Hoffman A, Chang YF, Blank A, Reardon CA, et al. Neutrophil elastase selectively kills cancer cells and attenuates tumorigenesis. Cell. 2021;184(12):3163-3177.e3121.
Lee J, Lee D, Lawler S, Kim Y. Role of neutrophil extracellular traps in regulation of lung cancer invasion and metastasis: structural insights from a computational model. PLoS Comput Biol. 2021;17(2):e1008257.
Albrengues J, Shields MA, Ng D, Park CG, Ambrico A, Poindexter ME, Upadhyay P, Uyeminami DL, Pommier A, Küttner V, et al. Neutrophil extracellular traps produced during inflammation awaken dormant cancer cells in mice. Science (New York, NY). 2018;361:6409.
Lane AA, Ley TJ. Neutrophil elastase cleaves PML-RARalpha and is important for the development of acute promyelocytic leukemia in mice. Cell. 2003;115(3):305–18.
Lerman I, Hammes SR. Neutrophil elastase in the tumor microenvironment. Steroids. 2018;133:96–101.
Yee PP, Wei Y, Kim SY, Lu T, Chih SY, Lawson C, Tang M, Liu Z, Anderson B, Thamburaj K, et al. Neutrophil-induced ferroptosis promotes tumor necrosis in glioblastoma progression. Nat Commun. 2020;11(1):5424.
Perego M, Tyurin VA, Tyurina YY, Yellets J, Nacarelli T, Lin C, Nefedova Y, Kossenkov A, Liu Q, Sreedhar S, et al. Reactivation of dormant tumor cells by modified lipids derived from stress-activated neutrophils. Sci Transl Med. 2020;12:572.
Gu QQ, He SW, Liu LH, Wang GH, Hao DF, Liu HM, Wang CB, Li C, Zhang M, Li NQ. A teleost bactericidal permeability-increasing protein-derived peptide that possesses a broad antibacterial spectrum and inhibits bacterial infection as well as human colon cancer cells growth. Dev Compar Immunol. 2021;118:103.
Zhao T, Su Z, Li Y, Zhang X, You Q. Chitinase-3 like-protein-1 function and its role in diseases. Signal Transduct Target Ther. 2020;5(1):201.
Tyagi A, Sharma S, Wu K, Wu SY, Xing F, Liu Y, Zhao D, Deshpande RP, D’Agostino RB Jr, Watabe K. Nicotine promotes breast cancer metastasis by stimulating N2 neutrophils and generating pre-metastatic niche in lung. Nat Commun. 2021;12(1):474.
Olson B, Zhu X, Norgard MA, Levasseur PR, Butler JT, Buenafe A, Burfeind KG, Michaelis KA, Pelz KR, Mendez H, et al. Lipocalin 2 mediates appetite suppression during pancreatic cancer cachexia. Nat Commun. 2021;12(1):2057.
Nelson AM, Zhao W, Gilliland KL, Zaenglein AL, Liu W, Thiboutot DM. Neutrophil gelatinase-associated lipocalin mediates 13-cis retinoic acid-induced apoptosis of human sebaceous gland cells. J Clin Investig. 2008;118(4):1468–78.
Hao L, Shan Q, Wei J, Ma F, Sun P. Lactoferrin: Major Physiological Functions and Applications. Curr Protein Pept Sci. 2019;20(2):139–44.
Ueda K, Shimizu M, Ohashi A, Murata D, Suzuki T, Kobayashi N, Baba J, Takeuchi T, Shiga Y, Nakamura M, et al. Albumin fusion at the N-terminus or C-terminus of human lactoferrin leads to improved pharmacokinetics and anti-proliferative effects on cancer cell lines. Eur J Pharmaceut Sci. 2020;155:105551.
Dong H, Yang Y, Gao C, Sun H, Wang H, Hong C, Wang J, Gong F, Gao X. Lactoferrin-containing immunocomplex mediates antitumor effects by resetting tumor-associated macrophages to M1 phenotype. J Immunother Cancer. 2020;8(1):10.
Elzoghby AO, Abdelmoneem MA, Hassanin IA, Abd Elwakil MM, Elnaggar MA, Mokhtar S, Fang JY, Elkhodairy KA. Lactoferrin, a multi-functional glycoprotein: active therapeutic, drug nanocarrier & targeting ligand. Biomaterials. 2020;263:120355.
Ahn GO, Brown JM. Matrix metalloproteinase-9 is required for tumor vasculogenesis but not for angiogenesis: role of bone marrow-derived myelomonocytic cells. Cancer Cell. 2008;13(3):193–205.
Bergers G, Brekken R, McMahon G, Vu TH, Itoh T, Tamaki K, Tanzawa K, Thorpe P, Itohara S, Werb Z, et al. Matrix metalloproteinase-9 triggers the angiogenic switch during carcinogenesis. Nat Cell Biol. 2000;2(10):737–44.
Mizuno R, Kawada K, Itatani Y, Ogawa R, Kiyasu Y, Sakai Y. The role of tumor-associated neutrophils in colorectal cancer. Int J Mol Sci. 2019;20(3):10.
Sokołowska A, Świerzko AS, Gajek G, Gołos A, Michalski M, Nowicki M, Szala-Poździej A, Wolska-Washer A, Brzezińska O, Wierzbowska A, et al. Associations of ficolins and mannose-binding lectin with acute myeloid leukaemia in adults. Sci Rep. 2020;10(1):10561.
Świerzko AS, Michalski M, Sokołowska A, Nowicki M, Szala-Poździej A, Eppa Ł, Mitrus I, Szmigielska-Kapłon A, Sobczyk-Kruszelnicka M, Michalak K, et al. Associations of ficolins with hematological malignancies in patients receiving high-dose chemotherapy and autologous hematopoietic stem cell transplantations. Front Immunol. 2019;10:3097.
Rasmussen LJH, Schultz M, Gaardsting A, Ladelund S, Garred P, Iversen K, Eugen-Olsen J, Helms M, David KP, Kjaer A, et al. Inflammatory biomarkers and cancer: CRP and suPAR as markers of incident cancer in patients with serious nonspecific symptoms and signs of cancer. Int J Cancer. 2017;141(1):191–9.
Sainz B Jr, Alcala S, Garcia E, Sanchez-Ripoll Y, Azevedo MM, Cioffi M, Tatari M, Miranda-Lorenzo I, Hidalgo M, Gomez-Lopez G, et al. Microenvironmental hCAP-18/LL-37 promotes pancreatic ductal adenocarcinoma by activating its cancer stem cell compartment. Gut. 2015;64(12):1921–35.
Chen X, Zou X, Qi G, Tang Y, Guo Y, Si J, Liang L. Roles and mechanisms of human cathelicidin LL-37 in cancer. Cell Physiol Biochem Int J Exp Cell Physiol Biochem Pharmacol. 2018;47(3):1060–73.
Scheenstra MR, van Harten RM, Veldhuizen EJA, Haagsman HP, Coorens M. Cathelicidins modulate TLR-activation and inflammation. Front Immunol. 2020;11:1137.
Chen J, Shin VY, Ho JC, Siu MT, Cheuk IW, Kwong A. Functional implications of cathelicidin antimicrobial protein in breast cancer and tumor-associated macrophage microenvironment. Biomolecules. 2020;10(5):10.
Juurikka K, Butler GS, Salo T, Nyberg P, Åström P. The role of MMP8 in cancer: a systematic review. Int J Mol Sci. 2019;20(18):10.
Juurikka K, Dufour A, Pehkonen K, Mainoli B, Campioni Rodrigues P, Solis N, Klein T, Nyberg P, Overall CM, Salo T, et al. MMP8 increases tongue carcinoma cell-cell adhesion and diminishes migration via cleavage of anti-adhesive FXYD5. Oncogenesis. 2021;10(5):44.
Acharyya S, Oskarsson T, Vanharanta S, Malladi S, Kim J, Morris PG, Manova-Todorova K, Leversha M, Hogg N, Seshan VE, et al. A CXCL1 paracrine network links cancer chemoresistance and metastasis. Cell. 2012;150(1):165–78.
Oh P, Testa JE, Borgstrom P, Witkiewicz H, Li Y, Schnitzer JE. In vivo proteomic imaging analysis of caveolae reveals pumping system to penetrate solid tumors. Nat Med. 2014;20(9):1062–8.
Vacchelli E, Ma Y, Baracco EE, Sistigu A, Enot DP, Pietrocola F, Yang H, Adjemian S, Chaba K, Semeraro M, et al. Chemotherapy-induced antitumor immunity requires formyl peptide receptor 1. Science (New York, NY). 2015;350(6263):972–8.
Le Naour J, Liu P, Zhao L, Adjemian S, Sztupinszki Z, Taieb J, Mulot C, Silvin A, Dutertre CA, Ginhoux F, et al. A TLR3 ligand reestablishes chemotherapeutic responses in the context of FPR1 deficiency. Cancer Discov. 2021;11(2):408–23.
Gastardelo TS, Cunha BR, Raposo LS, Maniglia JV, Cury PM, Lisoni FC, Tajara EH, Oliani SM. Inflammation and cancer: role of annexin A1 and FPR2/ALX in proliferation and metastasis in human laryngeal squamous cell carcinoma. PLoS ONE. 2014;9(12):e111317.
Liang Z, Li X. Identification of ANXA1 as a potential prognostic biomarker and correlating with immune infiltrates in colorectal cancer. Autoimmunity. 2021;54(2):76–87.
Nielsen SR, Strøbech JE, Horton ER, Jackstadt R, Laitala A, Bravo MC, Maltese G, Jensen ARD, Reuten R, Rafaeva M, et al. Suppression of tumor-associated neutrophils by lorlatinib attenuates pancreatic cancer growth and improves treatment with immune checkpoint blockade. Nat Commun. 2021;12(1):3414.
Finisguerra V, Di Conza G, Di Matteo M, Serneels J, Costa S, Thompson AA, Wauters E, Walmsley S, Prenen H, Granot Z, et al. MET is required for the recruitment of anti-tumoural neutrophils. Nature. 2015;522(7556):349–53.
MET Promotes Antitumor Neutrophil Recruitment and Cytotoxicity. Cancer Discovery 2015, 5(7):689.
Matlung HL, Babes L, Zhao XW, van Houdt M, Treffers LW, van Rees DJ, Franke K, Schornagel K, Verkuijlen P, Janssen H, et al. Neutrophils kill antibody-opsonized cancer cells by trogoptosis. Cell Rep. 2018;23(13):3946-3959.e3946.
Hubert P, Heitzmann A, Viel S, Nicolas A, Sastre-Garau X, Oppezzo P, Pritsch O, Osinaga E, Amigorena S. Antibody-dependent cell cytotoxicity synapses form in mice during tumor-specific antibody immunotherapy. Can Res. 2011;71(15):5134–43.
van Egmond M, Bakema JE. Neutrophils as effector cells for antibody-based immunotherapy of cancer. Semin Cancer Biol. 2013;23(3):190–9.
Brandsma AM, Bondza S, Evers M, Koutstaal R, Nederend M, Jansen JHM, Rösner T, Valerius T, Leusen JHW, Ten Broeke T. Potent Fc receptor signaling by IgA leads to superior killing of cancer cells by neutrophils compared to IgG. Front Immunol. 2019;10:704.
Triner D, Devenport SN, Ramakrishnan SK, Ma X, Frieler RA, Greenson JK, Inohara N, Nunez G, Colacino JA, Mortensen RM, et al. Neutrophils restrict tumor-associated microbiota to reduce growth and invasion of colon tumors in mice. Gastroenterology. 2019;156(5):1467–82.
Pekarek LA, Starr BA, Toledano AY, Schreiber H. Inhibition of tumor growth by elimination of granulocytes. J Exp Med. 1995;181(1):435–40.
Coffelt SB, Kersten K, Doornebal CW, Weiden J, Vrijland K, Hau CS, Verstegen NJM, Ciampricotti M, Hawinkels L, Jonkers J, et al. IL-17-producing γδ T cells and neutrophils conspire to promote breast cancer metastasis. Nature. 2015;522(7556):345–8.
Baek AE, Yu YA, He S, Wardell SE, Chang CY, Kwon S, Pillai RV, McDowell HB, Thompson JW, Dubois LG, et al. The cholesterol metabolite 27 hydroxycholesterol facilitates breast cancer metastasis through its actions on immune cells. Nat Commun. 2017;8(1):864.
Benedicto A, Marquez J, Herrero A, Olaso E, Kolaczkowska E, Arteta B. Decreased expression of the β(2) integrin on tumor cells is associated with a reduction in liver metastasis of colorectal cancer in mice. BMC Cancer. 2017;17(1):827.
Yang LY, Luo Q, Lu L, Zhu WW, Sun HT, Wei R, Lin ZF, Wang XY, Wang CQ, Lu M, et al. Increased neutrophil extracellular traps promote metastasis potential of hepatocellular carcinoma via provoking tumorous inflammatory response. J Hematol Oncol. 2020;13(1):3.
Wang Z, Yang C, Li L, Jin X, Zhang Z, Zheng H, Pan J, Shi L, Jiang Z, Su K, et al. Tumor-derived HMGB1 induces CD62L(dim) neutrophil polarization and promotes lung metastasis in triple-negative breast cancer. Oncogenesis. 2020;9(9):82.
Zhang X, Shi H, Yuan X, Jiang P, Qian H, Xu W. Tumor-derived exosomes induce N2 polarization of neutrophils to promote gastric cancer cell migration. Mol Cancer. 2018;17(1):146.
Bald T, Quast T, Landsberg J, Rogava M, Glodde N, Lopez-Ramos D, Kohlmeyer J, Riesenberg S, van den Boorn-Konijnenberg D, Hömig-Hölzel C, et al. Ultraviolet-radiation-induced inflammation promotes angiotropism and metastasis in melanoma. Nature. 2014;507(7490):109–13.
Morimoto-Kamata R, Yui S. Insulin-like growth factor-1 signaling is responsible for cathepsin G-induced aggregation of breast cancer MCF-7 cells. Cancer Sci. 2017;108(8):1574–83.
Cools-Lartigue J, Spicer J, McDonald B, Gowing S, Chow S, Giannias B, Bourdeau F, Kubes P, Ferri L. Neutrophil extracellular traps sequester circulating tumor cells and promote metastasis. J Clin Investig. 2013;123(8):3446–58.
Najmeh S, Cools-Lartigue J, Rayes RF, Gowing S, Vourtzoumis P, Bourdeau F, Giannias B, Berube J, Rousseau S, Ferri LE, et al. Neutrophil extracellular traps sequester circulating tumor cells via β1-integrin mediated interactions. Int J Cancer. 2017;140(10):2321–30.
Szczerba BM, Castro-Giner F, Vetter M, Krol I, Gkountela S, Landin J, Scheidmann MC, Donato C, Scherrer R, Singer J, et al. Neutrophils escort circulating tumour cells to enable cell cycle progression. Nature. 2019;566(7745):553–7.
McDowell SAC, Luo RBE, Arabzadeh A, Doré S, Bennett NC, Breton V, Karimi E, Rezanejad M, Yang RR, Lach KD, et al. Neutrophil oxidative stress mediates obesity-associated vascular dysfunction and metastatic transmigration. Nat Cancer. 2021;2(5):545–62.
Saini M, Szczerba BM, Aceto N. Circulating tumor cell-neutrophil tango along the metastatic process. Can Res. 2019;79(24):6067–73.
Wculek SK, Malanchi I. Neutrophils support lung colonization of metastasis-initiating breast cancer cells. Nature. 2015;528(7582):413–7.
Liu Y, Gu Y, Han Y, Zhang Q, Jiang Z, Zhang X, Huang B, Xu X, Zheng J, Cao X. Tumor exosomal RNAs promote lung pre-metastatic niche formation by activating alveolar epithelial TLR3 to recruit neutrophils. Cancer Cell. 2016;30(2):243–56.
Lee W, Ko SY, Mohamed MS, Kenny HA, Lengyel E, Naora H. Neutrophils facilitate ovarian cancer premetastatic niche formation in the omentum. J Exp Med. 2019;216(1):176–94.
Kaplan RN, Riba RD, Zacharoulis S, Bramley AH, Vincent L, Costa C, MacDonald DD, Jin DK, Shido K, Kerns SA, et al. VEGFR1-positive haematopoietic bone marrow progenitors initiate the pre-metastatic niche. Nature. 2005;438(7069):820–7.
Granot Z, Henke E, Comen EA, King TA, Norton L, Benezra R. Tumor entrained neutrophils inhibit seeding in the premetastatic lung. Cancer Cell. 2011;20(3):300–14.
Oberg HH, Wesch D, Kalyan S, Kabelitz D. Regulatory interactions between neutrophils, tumor cells and T cells. Front Immunol. 2019;10:1690.
Li P, Lu M, Shi J, Hua L, Gong Z, Li Q, Shultz LD, Ren G. Dual roles of neutrophils in metastatic colonization are governed by the host NK cell status. Nat Commun. 2020;11(1):4387.
Castano Z, San Juan BP, Spiegel A, Pant A, DeCristo MJ, Laszewski T, Ubellacker JM, Janssen SR, Dongre A, Reinhardt F, et al. IL-1beta inflammatory response driven by primary breast cancer prevents metastasis-initiating cell colonization. Nat Cell Biol. 2018;20(9):1084–97.
Vono M, Lin A, Norrby-Teglund A, Koup RA, Liang F, Loré K. Neutrophils acquire the capacity for antigen presentation to memory CD4(+) T cells in vitro and ex vivo. Blood. 2017;129(14):1991–2001.
Carus A, Ladekarl M, Hager H, Nedergaard BS, Donskov F. Tumour-associated CD66b+ neutrophil count is an independent prognostic factor for recurrence in localised cervical cancer. Br J Cancer. 2013;108(10):2116–22.
Watanabe A, Harimoto N, Araki K, Kubo N, Igarashi T, Tsukagoshi M, Ishii N, Yamanaka T, Yoshizumi T, Shirabe K. Absolute neutrophil count predicts postoperative prognosis in mass-forming intrahepatic cholangiocarcinoma. Anticancer Res. 2019;39(2):941–7.
Francescangeli F, De Angelis ML, Zeuner A. COVID-19: a potential driver of immune-mediated breast cancer recurrence? Breast Cancer Res. 2020;22(1):117.
Houghton AM, Rzymkiewicz DM, Ji H, Gregory AD, Egea EE, Metz HE, Stolz DB, Land SR, Marconcini LA, Kliment CR, et al. Neutrophil elastase-mediated degradation of IRS-1 accelerates lung tumor growth. Nat Med. 2010;16(2):219–23.
Demers M, Wong SL, Martinod K, Gallant M, Cabral JE, Wang Y, Wagner DD. Priming of neutrophils toward NETosis promotes tumor growth. Oncoimmunology. 2016;5(5):e1134073.
Guglietta S, Chiavelli A, Zagato E, Krieg C, Gandini S, Ravenda PS, Bazolli B, Lu B, Penna G, Rescigno M. Coagulation induced by C3aR-dependent NETosis drives protumorigenic neutrophils during small intestinal tumorigenesis. Nat Commun. 2016;7:11037.
Engblom C, Pfirschke C, Zilionis R, Da Silva Martins J, Bos SA, Courties G, Rickelt S, Severe N, Baryawno N, Faget J, et al. Osteoblasts remotely supply lung tumors with cancer-promoting SiglecF(high) neutrophils. Science (New York, NY). 2017;358:6367.
Glodde N, Bald T, van den Boorn-Konijnenberg D, Nakamura K, O’Donnell JS, Szczepanski S, Brandes M, Eickhoff S, Das I, Shridhar N, et al. Reactive neutrophil responses dependent on the receptor tyrosine kinase c-MET limit cancer immunotherapy. Immunity. 2017;47(4):789-802.e789.
Veglia F, Tyurin VA, Blasi M, De Leo A, Kossenkov AV, Donthireddy L, To TKJ, Schug Z, Basu S, Wang F, et al. Fatty acid transport protein 2 reprograms neutrophils in cancer. Nature. 2019;569(7754):73–8.
Gillette MA, Satpathy S, Cao S, Dhanasekaran SM, Vasaikar SV, Krug K, Petralia F, Li Y, Liang WW, Reva B, et al. Proteogenomic characterization reveals therapeutic vulnerabilities in lung adenocarcinoma. Cell. 2020;182(1):200-225.e235.
Kuang DM, Zhao Q, Wu Y, Peng C, Wang J, Xu Z, Yin XY, Zheng L. Peritumoral neutrophils link inflammatory response to disease progression by fostering angiogenesis in hepatocellular carcinoma. J Hepatol. 2011;54(5):948–55.
Steele CW, Karim SA, Leach JDG, Bailey P, Upstill-Goddard R, Rishi L, Foth M, Bryson S, McDaid K, Wilson Z, et al. CXCR2 inhibition profoundly suppresses metastases and augments immunotherapy in pancreatic ductal adenocarcinoma. Cancer Cell. 2016;29(6):832–45.
Park J, Wysocki RW, Amoozgar Z, Maiorino L, Fein MR, Jorns J, Schott AF, Kinugasa-Katayama Y, Lee Y, Won NH, et al. Cancer cells induce metastasis-supporting neutrophil extracellular DNA traps. Sci Transl Med. 2016;8(361):361ra138.
Wellenstein MD, Coffelt SB, Duits DEM, van Miltenburg MH, Slagter M, de Rink I, Henneman L, Kas SM, Prekovic S, Hau CS, et al. Loss of p53 triggers WNT-dependent systemic inflammation to drive breast cancer metastasis. Nature. 2019;572(7770):538–42.
Yang L, Liu Q, Zhang X, Liu X, Zhou B, Chen J, Huang D, Li J, Li H, Chen F, et al. DNA of neutrophil extracellular traps promotes cancer metastasis via CCDC25. Nature. 2020;583(7814):133–8.
Eruslanov EB, Bhojnagarwala PS, Quatromoni JG, Stephen TL, Ranganathan A, Deshpande C, Akimova T, Vachani A, Litzky L, Hancock WW, et al. Tumor-associated neutrophils stimulate T cell responses in early-stage human lung cancer. J Clin Investig. 2014;124(12):5466–80.
Blaisdell A, Crequer A, Columbus D, Daikoku T, Mittal K, Dey SK, Erlebacher A. Neutrophils oppose uterine epithelial carcinogenesis via debridement of hypoxic tumor cells. Cancer Cell. 2015;28(6):785–99.
Singhal S, Bhojnagarwala PS, O’Brien S, Moon EK, Garfall AL, Rao AS, Quatromoni JG, Stephen TL, Litzky L, Deshpande C, et al. Origin and role of a subset of tumor-associated neutrophils with antigen-presenting cell features in early-stage human lung cancer. Cancer Cell. 2016;30(1):120–35.
Governa V, Trella E, Mele V, Tornillo L, Amicarella F, Cremonesi E, Muraro MG, Xu H, Droeser R, Däster SR, et al. The interplay between neutrophils and CD8(+) T cells improves survival in human colorectal cancer. Clin Cancer Res. 2017;23(14):3847–58.
Ponzetta A, Carriero R, Carnevale S, Barbagallo M, Molgora M, Perucchini C, Magrini E, Gianni F, Kunderfranco P, Polentarutti N, et al. Neutrophils driving unconventional T cells mediate resistance against murine sarcomas and selected human tumors. Cell. 2019;178(2):346-360.e324.
Mahiddine K, Blaisdell A, Ma S, Créquer-Grandhomme A, Lowell CA, Erlebacher A. Relief of tumor hypoxia unleashes the tumoricidal potential of neutrophils. J Clin Investig. 2020;130(1):389–403.
Cedervall J, Zhang Y, Huang H, Zhang L, Femel J, Dimberg A, Olsson AK. Neutrophil extracellular traps accumulate in peripheral blood vessels and compromise organ function in tumor-bearing animals. Can Res. 2015;75(13):2653–62.
Wolach O, Sellar RS, Martinod K, Cherpokova D, McConkey M, Chappell RJ, Silver AJ, Adams D, Castellano CA, Schneider RK, et al. Increased neutrophil extracellular trap formation promotes thrombosis in myeloproliferative neoplasms. Sci Transl Med. 2018;10:436.
Chao MP, Alizadeh AA, Tang C, Myklebust JH, Varghese B, Gill S, Jan M, Cha AC, Chan CK, Tan BT, et al. Anti-CD47 antibody synergizes with rituximab to promote phagocytosis and eradicate non-Hodgkin lymphoma. Cell. 2010;142(5):699–713.
Vincent J, Mignot G, Chalmin F, Ladoire S, Bruchard M, Chevriaux A, Martin F, Apetoh L, Rébé C, Ghiringhelli F. 5-Fluorouracil selectively kills tumor-associated myeloid-derived suppressor cells resulting in enhanced T cell-dependent antitumor immunity. Can Res. 2010;70(8):3052–61.
Alizadeh D, Trad M, Hanke NT, Larmonier CB, Janikashvili N, Bonnotte B, Katsanis E, Larmonier N. Doxorubicin eliminates myeloid-derived suppressor cells and enhances the efficacy of adoptive T-cell transfer in breast cancer. Can Res. 2014;74(1):104–18.
Bruchard M, Mignot G, Derangère V, Chalmin F, Chevriaux A, Végran F, Boireau W, Simon B, Ryffel B, Connat JL, et al. Chemotherapy-triggered cathepsin B release in myeloid-derived suppressor cells activates the Nlrp3 inflammasome and promotes tumor growth. Nat Med. 2013;19(1):57–64.
Takeshima T, Pop LM, Laine A, Iyengar P, Vitetta ES, Hannan R. Key role for neutrophils in radiation-induced antitumor immune responses: Potentiation with G-CSF. Proc Natl Acad Sci USA. 2016;113(40):11300–5.
Xue J, Zhao Z, Zhang L, Xue L, Shen S, Wen Y, Wei Z, Wang L, Kong L, Sun H, et al. Neutrophil-mediated anticancer drug delivery for suppression of postoperative malignant glioma recurrence. Nat Nanotechnol. 2017;12(7):692–700.
Chen HM, van der Touw W, Wang YS, Kang K, Mai S, Zhang J, Alsina-Beauchamp D, Duty JA, Mungamuri SK, Zhang B, et al. Blocking immunoinhibitory receptor LILRB2 reprograms tumor-associated myeloid cells and promotes antitumor immunity. J Clin Investig. 2018;128(12):5647–62.
Treffers LW, Ten Broeke T, Rösner T, Jansen JHM, van Houdt M, Kahle S, Schornagel K, Verkuijlen P, Prins JM, Franke K, et al. IgA-mediated killing of tumor cells by neutrophils is enhanced by CD47-SIRPα checkpoint inhibition. Cancer Immunol Res. 2020;8(1):120–30.
Shaul ME, Fridlender ZG. Tumour-associated neutrophils in patients with cancer. Nat Rev Clin Oncol. 2019;16(10):601–20.
Fridlender ZG, Sun J, Kim S, Kapoor V, Cheng G, Ling L, Worthen GS, Albelda SM. Polarization of tumor-associated neutrophil phenotype by TGF-beta: “N1” versus “N2” TAN. Cancer Cell. 2009;16(3):183–94.
Mehmeti-Ajradini M, Bergenfelz C, Larsson AM, Carlsson R, Riesbeck K, Ahl J, Janols H, Wullt M, Bredberg A, Källberg E, et al. Human G-MDSCs are neutrophils at distinct maturation stages promoting tumor growth in breast cancer. Life Sci Alliance. 2020;3(11):10.
Canè S, Bronte V. Detection and functional evaluation of arginase-1 isolated from human PMNs and murine MDSC. Methods Enzymol. 2020;632:193–213.
Raber PL, Thevenot P, Sierra R, Wyczechowska D, Halle D, Ramirez ME, Ochoa AC, Fletcher M, Velasco C, Wilk A, et al. Subpopulations of myeloid-derived suppressor cells impair T cell responses through independent nitric oxide-related pathways. Int J Cancer. 2014;134(12):2853–64.
Al-Khami AA, Zheng L, Del Valle L, Hossain F, Wyczechowska D, Zabaleta J, Sanchez MD, Dean MJ, Rodriguez PC, Ochoa AC. Exogenous lipid uptake induces metabolic and functional reprogramming of tumor-associated myeloid-derived suppressor cells. Oncoimmunology. 2017;6(10):e1344804.
Rogers T, DeBerardinis RJ. Metabolic plasticity of neutrophils: relevance to pathogen responses and cancer. Trends Cancer. 2021;7(8):700–13.
Galli SJ, Borregaard N, Wynn TA. Phenotypic and functional plasticity of cells of innate immunity: macrophages, mast cells and neutrophils. Nat Immunol. 2011;12(11):1035–44.
Evrard M, Kwok IWH, Chong SZ, Teng KWW, Becht E, Chen J, Sieow JL, Penny HL, Ching GC, Devi S, et al. Developmental analysis of bone marrow neutrophils reveals populations specialized in expansion, trafficking, and effector functions. Immunity. 2018;48(2):364-379.e368.
Zhou G, Peng K, Song Y, Yang W, Shu W, Yu T, Yu L, Lin M, Wei Q, Chen C, et al. CD177+ neutrophils suppress epithelial cell tumourigenesis in colitis-associated cancer and predict good prognosis in colorectal cancer. Carcinogenesis. 2018;39(2):272–82.
Jaillon S, Ponzetta A, Di Mitri D, Santoni A, Bonecchi R, Mantovani A. Neutrophil diversity and plasticity in tumour progression and therapy. Nat Rev Cancer. 2020;20(9):485–503.
Wang X, Hu LP, Qin WT, Yang Q, Chen DY, Li Q, Zhou KX, Huang PQ, Xu CJ, Li J, et al. Identification of a subset of immunosuppressive P2RX1-negative neutrophils in pancreatic cancer liver metastasis. Nat Commun. 2021;12(1):174.
Uyanik B, Goloudina AR, Akbarali A, Grigorash BB, Petukhov AV, Singhal S, Eruslanov E, Chaloyard J, Lagorgette L, Hadi T, et al. Inhibition of the DNA damage response phosphatase PPM1D reprograms neutrophils to enhance anti-tumor immune responses. Nat Commun. 2021;12(1):3622.
Kalafati L, Kourtzelis I, Schulte-Schrepping J, Li X, Hatzioannou A, Grinenko T, Hagag E, Sinha A, Has C, Dietz S, et al. Innate immune training of granulopoiesis promotes anti-tumor activity. Cell. 2020;183(3):771-785.e712.
Hwang WL, Lan HY, Cheng WC, Huang SC, Yang MH. Tumor stem-like cell-derived exosomal RNAs prime neutrophils for facilitating tumorigenesis of colon cancer. J Hematol Oncol. 2019;12(1):10.
Incio J, Liu H, Suboj P, Chin SM, Chen IX, Pinter M, Ng MR, Nia HT, Grahovac J, Kao S, et al. Obesity-induced inflammation and desmoplasia promote pancreatic cancer progression and resistance to chemotherapy. Cancer Discov. 2016;6(8):852–69.
Munir H, Jones JO, Janowitz T, Hoffmann M, Euler M, Martins CP, Welsh SJ, Shields JD. Stromal-driven and Amyloid beta-dependent induction of neutrophil extracellular traps modulates tumor growth. Nat Commun. 2021;12(1):683.
Grieshaber-Bouyer R, Radtke FA, Cunin P, Stifano G, Levescot A, Vijaykumar B, Nelson-Maney N, Blaustein RB, Monach PA, Nigrovic PA. The neutrotime transcriptional signature defines a single continuum of neutrophils across biological compartments. Nat Commun. 2021;12(1):2856.
Xiong S, Feng Y, Cheng L. Cellular reprogramming as a therapeutic target in cancer. Trends Cell Biol. 2019;29(8):623–34.
Zhou Y, Zhu X, Dai Y, Xiong S, Wei C, Yu P, Tang Y, Wu L, Li J, Liu D, et al. Chemical cocktail induces hematopoietic reprogramming and expands hematopoietic stem/progenitor cells. Adv Sci (Weinheim, Baden-Wurttemberg, Germany). 2020;7(1):190.
Mysore V, Cullere X, Mears J, Rosetti F, Okubo K, Liew PX, Zhang F, Madera-Salcedo I, Rosenbauer F, Stone RM, et al. FcγR engagement reprograms neutrophils into antigen cross-presenting cells that elicit acquired anti-tumor immunity. Nat Commun. 2021;12(1):4791.
Acknowledgements
The authors apologize to those works could not be fully detailed due to space limitations.
Funding
This work is supported by the National Natural Science Foundation of China (92068101, 31871498), the Shanghai Municipal Education Commission-Gaofeng Clinical Medicine Grant Support (828313), the Project from National Research Center for Translational Medicine at Shanghai (TMSK-2021–106), the Shanghai Collaborative Innovation Program on Regenerative Medicine and Stem Cell Research (2019CXJQ01), and Samuel Waxman Cancer Research Foundation.
Author information
Authors and Affiliations
Contributions
S.X. wrote the manuscript and prepared the figures and tables. L.D. helped writing the manuscript. L.C. reviewed and revised the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Xiong, S., Dong, L. & Cheng, L. Neutrophils in cancer carcinogenesis and metastasis. J Hematol Oncol 14, 173 (2021). https://doi.org/10.1186/s13045-021-01187-y
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s13045-021-01187-y