Abstract
Background
Thiotepa-busulfan-fludarabine (TBF) is a widely used conditioning regimen in single umbilical cord blood transplantation (SUCBT). More recently, it was introduced in the setting of non-T cell depleted haploidentical stem cell transplantation (NTD-Haplo). Whether TBF based conditioning provides additional benefit in transplantation from a particular alternative donor type remains to be established.
Methods
This was a retrospective study based on an international European registry. We compared outcomes of de-novo acute myeloid leukemia patients in complete remission receiving NTD-Haplo (n = 186) vs. SUCBT (n = 147) following myeloablative conditioning (MAC) with TBF. Median follow-up was 23 months. Treatment groups resembled in baseline characteristics.
Results
SUCBT was associated with delayed engraftment and higher graft failure. In multivariate analysis no statistically significant differences were observed between the two groups in terms of acute or chronic graft-versus-host disease (GvHD) (HR = 1.03, p = 0.92 or HR = 1.86, p = 0.21) and relapse incidence (HR = 0.8, p = 0.65). Non-relapse mortality (NRM) was significantly higher in SUCBT as compared to NTD-Haplo (HR = 2.63, p = 0.001); moreover, SUCBT did worse in terms of overall survival (HR = 2.18, p = 0.002), leukemia-free survival (HR = 1.94, p = 0.007), and GvHD relapse-free survival (HR = 2.38, p = 0.0002).
Conclusions
Our results suggest that TBF-MAC might allow for a potent graft-versus-leukemia, regardless of the alternative donor type. Furthermore, in patients receiving TBF-MAC, survival with NTD-Haplo may be better compared to SUCBT due to decreased NRM.
Similar content being viewed by others
Background
Allogeneic hematopoietic stem cell transplantation (HSCT) is a potential curative treatment for patients with acute myeloid leukemia (AML) [1]. The introduction of transplantation from alternative donors, i.e., unrelated umbilical cord blood transplantation (UCBT) and haploidentical transplantation (Haplo), has increased the availability of this treatment. UCBT and Haplo are considered a valid option for patients with acute leukemia lacking a human leukocyte antigen (HLA) matched sibling or unrelated donor, or when transplantation cannot be delayed [2,3,4,5,6]. Stem cells from both types of donors are readily available. In the UCBT setting the process of stem cell collection is risk-free to the donor, and the graft is relatively permissive to HLA incompatibility [7,8,9,10,11]. Contemporary transplantation practice involving the use of double cord blood units in case that there are not enough stem cells in a single cord, flexible conditioning regimens, effective graft-versus-host disease (GvHD) prophylaxis platforms with non-T cell depleted (NTD) Haplo, and improved management of post-transplant complications, have brought improvement in outcomes of alternative donor transplantations [3, 7, 12]. Several studies have reported that results with UCBT and Haplo are comparable with those of transplants from HLA identical or matched unrelated donors [13,14,15,16,17,18,19,20,21,22].
Conditioning regimens are administered as part of the transplant procedure to prevent graft rejection by immunoablation and in order to reduce the tumor burden. As the graft versus tumor effect was recognized to contribute to the effectiveness of HSCT, reduced-intensity and nonmyeloablative conditioning regimens have been developed, making HSCT applicable to older or unfit patients [23]. Still, myeloablative conditioning (MAC) regimens remain the preferred option in adult patients (age ≤ 55 years) with high-risk acute leukemia [24]. Despite the availability of various effective conditioning protocols, standard regimens have yet to be established for the different types of HSCT in the various malignancies, leading to high heterogeneity in clinical practice [25]. Therefore, characterizing the effects of a specific regimen in a particular disease category is of major clinical importance.
The use of thiotepa–IV busulfan–fludarabine (TBF) at a myeloablative dose in single unit UCBT (SUCBT) was pioneered by the Valencia group, which reported high rates of engraftment and long-term disease-free survival in patients transplant at early disease stage of hematological malignancies [26]. TBF is widely applied in UCBT and its efficacy is well established [27]. Conditioning protocols in the Haplo setting are more heterogeneous and often determined according to institutional policies [2, 28,29,30,31,32]. More recently, TBF has been increasingly employed in Haplo transplantation with favorable outcomes [31, 32]. Comparing the outcome between patients receiving an allogeneic HSCT from alternative donors is an unmet need. Therefore, we retrospectively analyzed and compared the results of allogeneic HSCT with myeloablative TBF-based conditioning, in a homogeneous population of AML adult patients in complete remission (CR) receiving either NTD-Haplo (n = 186) or SUCBT (n = 147). The analysis was based on data reported to the European Society for Blood and Marrow Transplantation (EBMT) Acute Leukemia Working Party (ALWP), Cellular Therapy and Immunobiology Working Party, and the Eurocord registry.
Methods
Study design and definition
We retrospectively analyzed patients aged ≥18 years diagnosed with de novo AML, who received a first HSCT either from an NTD haploidentical-related donor (recipient-donor number of mismatches ≥ 2) (n = 186) or an unmanipulated single cord blood unit (n = 147). Data were reported by the ALWP of the EBMT and EUROCORD, between January 2007 and December 2015. Minimal HLA typing requirements for UCBT followed the current practice of antigen level typing for HLA-A and -B and allele-level typing of HLA-DRB1. For patients receiving Haplo, peripheral blood or bone marrow was used as a stem cell source, without ex vivo T cell depletion. Transplants were performed in 75 EBMT transplant centers: 17 performed only SUCBT, 44 only Haplo, and 14 centers performed both procedures. All patients were given a myeloablative reduced toxicity conditioning regimen consisting of thiotepa, IV busulfan, and fludarabine. TBF-MAC was defined as a regimen containing a total dose of IV busulfan ≥ 9.6 mg/kg [33]. Cytogenetic risk groups were defined according to the Medical Research Council (MRC) classification system [34].
All patients provided informed consent for transplants according to the Declaration of Helsinki. The Review Boards of the ALWP of EBMT, and Eurocord approved this study.
Endpoints
The primary endpoint was leukemia-free survival (LFS). LFS was defined as survival without leukemia or relapse following transplantation. GvHD-free relapse-free survival (GRFS) events were defined as grade 3–4 acute GvHD, extensive chronic GvHD, disease relapse, or death from any cause [35]. Overall survival (OS) was calculated from the date of transplant until death from any cause or last observation alive. Relapse incidence (RI) was defined as the occurrence of disease after transplantation, determined by morphological evidence of the disease in bone marrow, blood, or extramedullary organs. Non-relapse mortality (NRM) was defined as death without prior relapse.
Neutrophil recovery was defined as achieving absolute neutrophil count of 0.5 × 109/l for three consecutive days. Acute and chronic GvHD was defined using the standard criteria [36, 37].
Statistical analysis
Median values and ranges were used for continuous variables and percentages for categorical variables. For each continuous variable, the study population was initially split into quartiles and into two groups by the median. Patient-, disease-, and transplant-related variables of the groups were compared using chi-square or Fischer’s exact test for categorical variables, and the Mann–Whitney test for continuous variables. The probabilities of OS, LFS, and GRFS were calculated using the Kaplan–Meier method and the log-rank test for univariate comparisons [38]. The probabilities of neutrophil engraftment, grade II–IV acute and chronic GvHD, relapse, and NRM were calculated with the cumulative incidence method and Gray test for comparisons. Multivariate analyses adjusted for differences between the groups were performed using the Cox proportional hazards regression model for LFS and OS, and for engraftment, GvHD, NRM, and relapse [39].
The final model was adjusted for the following variables: transplant strategy (Haplo or SUCBT), disease status at HSCT (first or second CR), time from diagnosis to HSCT, age at transplant, year of HSCT, donor/recipient sex match, Karnofsky performance status (KPS), and center effect. p values were two-sided. Statistical analyses were performed with the SPSS 22 (SPSS Inc./IBM, Armonk, NY, USA) and R 3.0 (R Development Core Team, Vienna, Austria) software packages.
Results
Patients, disease, and transplant characteristics
Patient and disease characteristics are summarized in Table 1. Per protocol, all patients received a TBF-MAC-based regimen. The two populations were overall homogeneous in terms of patients and disease characteristics, except for median age at transplant which was older for NTD-Haplo (44 [range, 19–66] vs. 42 [range, 18–68], p = 0.046). Most patients were in first CR (NTD-Haplo, 70% vs. SUCBT, 77% p = 0.14); median interval from diagnosis to transplant was also similar (176 vs. 194 days; p = 0.09). Cytogenetic risk groups were alike between the Haplo and SUCBT groups (p = 0.76), with intermediate risk being most prevalent (36% vs. 41%, respectively). Haplo transplantations were performed in more recent years (median year of transplantation was 2014 vs. 2011; p < 0.001). As expected, anti-thymocyte globulin (ATG) was mostly used in SUCBT (91% vs. 29% in NTD-Haplo; p < 0.001). For SUCBT, the median dose of total nucleated cells at collection was 3.3 × 107/kg (range, 1.7–8.4), and 80% of the patients received ≥ 2.5 × 107/kg. Cord blood units were HLA matched with the recipient at a level of at least 4/6 in 68% of the cases. Among NTD-Haplo patients, 80% received bone marrow as stem cell source, and post-transplant cyclophosphamide (PTCY) was administrated in 71% of the cases (Additional file 1: Table S1). Further details about transplant procedures and GvHD prophylaxis are provided in (Additional file 1: Tables S2, S3). The median follow-up was 22 (range, 1–96) and 24 (range, 1–83) months for NTD-Haplo and SUCBT, respectively.
Engraftment
The cumulative incidence of neutrophil engraftment at day 60 after NTD-Haplo and SUCBT was 96% vs. 86% (p < 0.001), respectively. The median time for neutrophil recovery was 18 (range − 8-38) days for Haplo and 21 (range 11–57) days for SUCBT, (p < 0.001). Twenty patients did not engraft after SUCBT; of these, two are alive at 10 and 62 months, respectively, both after salvage with a second transplant from a haploidentical-related donor. The remaining 18 patients died in a median time of 1 month (range, 0–7), one patient after an autologous back-up. Among the seven patients who did not engraft after NTD-Haplo, none are alive, with a median time to death of 1.74 months (range, 0.3–17.22). Three of these patients received a second allogeneic transplantation, and only one engrafted, surviving more than 1 year.
Acute and chronic GvHD
The cumulative incidence of day 100 grade II–IV acute GvHD was 26% and 29% after NTD-Haplo and SUCBT (p = 0.85), respectively (Table 2). Cumulative incidence of grade III–IV acute GvHD was 7% in both groups (p = 0.99). The cumulative incidence of chronic GvHD was 33% after NTD-Haplo and 37% after SUCBT (p = 0.49). In the multivariate analysis (Table 3), no significant difference was found between the two groups in terms of acute or chronic GvHD (hazard ratio (HR) = 1.03, p = 0.92; HR = 1.86, p = 0.92, respectively). A center effect was found for chronic GvHD (p < 0.001).
Relapse and NRM
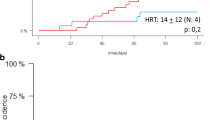
The 2-year RI was 17% for NTD-Haplo vs. 12% for SUCBT (p = 0.7) (Fig. 1a, Table 2). In the multivariate analysis (Table 3), relapse was not statistically different between the two groups of patients (HR = 0.8, p = 0.65). However, it was lower in patients who had a good KPS (≥ 90) at transplant (HR = 0.35, p = 0.01). NRM at 2 years was 21% and 48% for NTD-Haplo and SUCBT (p < 0.001), respectively (Fig. 1b, Table 2). The causes of death are listed in Additional file 1: Table S4. The multivariate model confirmed a significantly higher risk of NRM in the SUCBT group (HR = 2.63, p = 0.002). Also, NRM was higher in male recipients receiving a female donor (HR 1.84, p = 0.015), independently of the stem cell source. Infections and GvHD were the most common causes of transplant-related deaths in both groups (NTD-Haplo vs. SUCBT, infections 35% vs. 45%; GvHD 20% vs. 19%). Disease recurrence accounted for 27% and 16% of deaths after NTD-Haplo and SUCBT, respectively.
OS, LFS, and GRFS
The probability of 2-year OS, LFS, and GRFS in the NTD-Haplo vs. SUCBT groups were 69% vs. 42% (p < 0.001), 63% vs. 40% (p < 0.001), and 56% vs. 30% (p < 0.001), respectively (Table 2). The benefit of NTD-Haplo was maintained in a sub-analysis restricted to patients with intermediate cytogenetic risk (Additional file 1: Table S5). In the multivariate analysis (Table 3), the type of donor had a statistically significant impact on OS, LFS, and GRFS, which were significantly lower in SUCBT as compared to NTD-Haplo (OS, HR = 2.18, p = 0.003; LFS, HR = 1.93, p = 0.007; GRFS, HR = 2.38, p = 0.0002). The use of female donors for male recipients was independently associated with lower OS, LFS overall survival (HR = 2.18, p = 0.002) and GRFS (HR = 1.67, p = 0.02; HR = 1.67, p = 0.014; and HR = 1.54, p = 0.026), while a KPS ≥ 90 at transplant was associated with higher LFS and GRFS (HR = 0.5, p = 0.004 and HR = 0.57, p = 0.02) Fig. 2.
The estimated probability of overall survival (a), leukemia-free survival (b), and GRFS (c) by donor type. The numbers of patients at risk for the event are included below each graph. Graft-versus-host disease-free relapse-free survival (GRFS), haploidentical transplantation (Haplo), and single umbilical cord blood transplantation (CBT)
Discussion
TBF is a well-established conditioning regimen in SUCBT and has more recently brought into use in Haplo transplantations [26, 27, 31, 32]. In this retrospective analysis, we compare outcomes of NTD-Haplo and SUCBT in a population of AML patients conditioned with TBF at a myeloablative dose. Overall, the treatment groups resembled with regard to baseline characteristics. The risk for relapse and acute and chronic GvHD were similar regardless of donor type. Engraftment was faster with a Haplo donor. Importantly, the risk of NRM, death, or having a GRFS-related event was all higher in UCBT patients.
Differences in OS and LFS in favor of Haplo transplantation are the results of increased NRM with SUCBT. The difference in NRM was mainly driven by infectious complications since the incidence of acute and chronic GvHD was similar between groups. Several factors might have contributed to the excess NRM observed in the SUCBT. First, consistent with previous publication, engraftment with CB was inferior [40]. These issues could translate to an incidence of life-threatening infections. Indeed, infection accounted for 45% of deaths in the SUCBT vs. 35% in NTD-Haplo transplantation. Novel strategies for CB stem cell expansion and others facilitating engraftment kinetics equivalent to other graft sources may help to overcome this limitation [41]. Another important difference is the use of ATG, mainly in patients undergoing SUCBT. Retrospective analyses have found ATG to be associated with worse outcomes after myeloablative and reduced intensity conditioning in the setting of UCBT [42,43,44]; possibly due to a delayed immune recovery with ATG and increased incidence of post-transplantation lymphoproliferative disorder and infections [43, 45,46,47,48].
Finally, Haplo transplantations were performed more recently compared to SUCBT (median year of transplantation 2014 vs. 2011, p < 0.001), possibly accounting for lower rates of NRM in the former (20.6% vs. 48.4%, p < 0.001) due to improvements in supportive care. Still, one would not expect such a major difference solely on the basis of year of transplantation. Furthermore, in a multivariable analysis, adjusting for transplantation year, the benefit of Haplo was maintained.
TBF is widely used in the setting of SUCBT. Sanz et al. reported in 2012 on a single center experience of 88 patients with hematologic malignancies, who were treated with a SUCBT after conditioning with TBF-MAC [26]. Over 90% of patients engrafted at a median of 19 days. Furthermore, the 5-year cumulative incidence of NRM and relapse were 44% and 18%, respectively. Ruggeri et al. have found a similar incidence of relapse and lower NRM (33%) in acute leukemia patients treated with SUCBT and TBF-MAC [27]; outcomes were evaluated at 2 years following transplantation. The rather low relapse rates reported in these two analyses have paved the way for the increasing use of TBF-MAC for SUCBT. Indeed, our results further support the anti-leukemia effect of TBF not only in SUCBT but also with Haplo transplantations, both groups experiencing relatively low relapse rates. The effectiveness of the TBF regimen may be related to the combination of two alkylating agents, as shown in other regimens (e.g., busulfan and melphalan or carmustine [BCNU] and melphalan) [49]. More recently, our group compared TBF to a fludarabine-busulfan protocol in AML patients. Relapse rate was lower in the former, suggesting a stronger anti-leukemic effect with two alkylating agents [33]. In an additional study, the likelihood of relapse was lower with TBF compared to busulfan-cyclophosphamide, indicating that even within possible combinations of alkylating agents, thiotepa confers an additional anti-leukemia advantage [50].
Aside from donor type, additional prognostic factors were observed in our population. Low-performance status was a major predictor of relapse, decreased LFS and GRFS. Poor performance status may be a confounder of disease aggressiveness and exposure to multiple treatments. Therefore, it is difficult to determine its independent merit. Our analysis was not designed to study the importance of donor–recipient sex mismatch in the alternative donor setting. However, we found that transplantation from a female donor to male recipients was associated with an increase in NRM risk, without an apparent reduction of relapse. The results are in line with findings described by Wang and others [51,52,53].
The current study has several limitations. First, being a retrospective registry-based study, unknown or unmeasured factors could influence the results. However, such studies provide useful guidelines for clinical practice while waiting for randomized trials comparing specific conditionings in defined transplant settings Second, the GvHD prophylaxis strategy varied within each transplantation type. Nonetheless, both the ATG and PTCY are established options in the setting of NTD-Haplo [31, 32, 54]. Finally, a minority of patients in the SUCBT group received grafts with a total nucleated cell dose below 3 × 107/kg, thereby, possibly contributing to the higher incidence of NRM in this group [55]. Yet, since only 20% grafts with less than 2.5 × 107/kg, it is unlikely that changes in NRM can be entirely attributed to the cell dose.
The results of the current analysis validate the effectiveness of TBF-MAC as a potent conditioning platform allowing for graft-versus-leukemia, regardless of the type of alternative donor. While 2-year relapse risk was similar between NTD-Haplo and SUCBT in the current analysis, OS, GRFS, and NRM were superior with the former. Efforts to decrease toxicity and transplant-related mortality needs to be done to further improve outcomes. Nonetheless, a decisive conclusion that NTD-Haplo is preferable is still premature. Prospective trials comparing the two donor types are currently ongoing, and the results will help to clarify the place of the type of graft in the algorithm of donor selection. Second, UCBT safety is likely to improve with the introduction of novel technologies for stem cell expansion and better graft selection. Third, the selection of an alternative donor is mostly dependent on center preference. Currently, most institutions performing these types of transplantation are highly experienced.
Conclusion
This retrospetive analysis suggest that TBF conditioning at a myeloablative dose enables a potent graft-versus-leukemia, regardless of the alternative donor type. Furthermore, in patients receiving TBF, survival with NTD-Haplo may be better compared to SUCBT due to decreased NRM.
Abbreviations
- ALWP:
-
Acute Leukemia Working Party
- AML:
-
Acute myeloid leukemia
- ATG:
-
Anti-thymocyte globulin
- CBUs:
-
Cord blood units
- CR:
-
Complete remission
- EBMT:
-
European Society for Blood and Marrow Transplantation
- GRFS:
-
GvHD-free relapse-free survival
- GvHD:
-
Graft-versus-host disease
- Haplo:
-
Haploidentical stem cell transplantation
- HLA:
-
Human leukocyte antigen
- HR:
-
Hazard ratio
- HSCT:
-
Hematopoietic stem cell transplantation
- KPS:
-
Karnofsky performance status
- LFS:
-
Leukemia-free survival
- MAC:
-
Myeloablative conditioning
- NRM:
-
Non-relapse mortality
- NTD:
-
Non-T cell depleted
- OS:
-
Overall survival
- PTCY:
-
Post-transplant cyclophosphamide
- RI:
-
Relapse incidence
- SUCBT:
-
Single unit umbilical cord blood transplantation
- TBF:
-
Thiotepa–IV busulfan–fludarabine
- UCBT:
-
Unrelated umbilical cord blood transplantation
References
Cornelissen JJ, Blaise D. Hematopoietic stem cell transplantation for patients with AML in first complete remission. Blood. 2016;127(1):62–70.
Aversa F, Terenzi A, Tabilio A, Falzetti F, Carotti A, Ballanti S, et al. Full haplotype-mismatched hematopoietic stem-cell transplantation: a phase II study in patients with acute leukemia at high risk of relapse. J Clin Oncol. 2005;23(15):3447–54.
Ballen KK, Spitzer TR. The great debate: haploidentical or cord blood transplant. Bone Marrow Transplant. 2011;46(3):323–9.
Ciceri F, Labopin M, Aversa F, Rowe JM, Bunjes D, Lewalle P, et al. A survey of fully haploidentical hematopoietic stem cell transplantation in adults with high-risk acute leukemia: a risk factor analysis of outcomes for patients in remission at transplantation. Blood. 2008;112(9):3574–81.
Gluckman E, Rocha V, Arcese W, Michel G, Sanz G, Chan KW, et al. Factors associated with outcomes of unrelated cord blood transplant: guidelines for donor choice. Exp Hematol. 2004;32(4):397–407.
Rocha V, Labopin M, Sanz G, Arcese W, Schwerdtfeger R, Bosi A, et al. Transplants of umbilical-cord blood or bone marrow from unrelated donors in adults with acute leukemia. N Engl J Med. 2004;351(22):2276–85.
Shouval R, Nagler A. From patient centered risk factors to comprehensive prognostic models: a suggested framework for outcome prediction in umbilical cord blood transplantation. Stem Cell Investig. 2017;4:39.
Ruggeri A. Alternative donors: cord blood for adults. Semin Hematol. 2016;53(2):65–73.
Querol S, Rubinstein P, Marsh SG, Goldman J, Madrigal JA. Cord blood banking: “providing cord blood banking for a nation”. Br J Haematol. 2009;147(2):227–35.
MacMillan ML, Weisdorf DJ, Brunstein CG, Cao Q, DeFor TE, Verneris MR, et al. Acute graft-versus-host disease after unrelated donor umbilical cord blood transplantation: analysis of risk factors. Blood. 2009;113(11):2410–5.
Rocha V, Wagner JE Jr, Sobocinski KA, Klein JP, Zhang MJ, Horowitz MM, et al. Graft-versus-host disease in children who have received a cord-blood or bone marrow transplant from an HLA-identical sibling. Eurocord and International Bone Marrow Transplant Registry Working Committee on Alternative Donor and Stem Cell Sources. N Engl J Med. 2000;342(25):1846–54.
Bregante S, Dominietto A, Ghiso A, Raiola AM, Gualandi F, Varaldo R, et al. Improved outcome of alternative donor transplantations in patients with myelofibrosis: from unrelated to haploidentical family donors. Biol Blood Marrow Transplant. 2016;22(2):324–9.
Laughlin MJ, Eapen M, Rubinstein P, Wagner JE, Zhang MJ, Champlin RE, et al. Outcomes after transplantation of cord blood or bone marrow from unrelated donors in adults with leukemia. N Engl J Med. 2004;351(22):2265–75.
Lu DP, Dong L, Wu T, Huang XJ, Zhang MJ, Han W, et al. Conditioning including antithymocyte globulin followed by unmanipulated HLA-mismatched/haploidentical blood and marrow transplantation can achieve comparable outcomes with HLA-identical sibling transplantation. Blood. 2006;107(8):3065–73.
Eapen M, Rocha V, Sanz G, Scaradavou A, Zhang MJ, Arcese W, et al. Effect of graft source on unrelated donor haemopoietic stem-cell transplantation in adults with acute leukaemia: a retrospective analysis. Lancet Oncol. 2010;11(7):653–60.
Brunstein CG, Eapen M, Ahn KW, Appelbaum FR, Ballen KK, Champlin RE, et al. Reduced-intensity conditioning transplantation in acute leukemia: the effect of source of unrelated donor stem cells on outcomes. Blood. 2012;119(23):5591–8.
Bashey A, Zhang X, Sizemore CA, Manion K, Brown S, Holland HK, et al. T-cell-replete HLA-haploidentical hematopoietic transplantation for hematologic malignancies using post-transplantation cyclophosphamide results in outcomes equivalent to those of contemporaneous HLA-matched related and unrelated donor transplantation. J Clin Oncol. 2013;31(10):1310–6.
Raiola AM, Dominietto A, di Grazia C, Lamparelli T, Gualandi F, Ibatici A, et al. Unmanipulated haploidentical transplants compared with other alternative donors and matched sibling grafts. Biol Blood Marrow Transplant. 2014;20(10):1573–9.
Wang Y, Liu QF, Xu LP, Liu KY, Zhang XH, Ma X, et al. Haploidentical vs identical-sibling transplant for AML in remission: a multicenter, prospective study. Blood. 2015;125(25):3956–62.
Wang Y, Liu QF, Xu LP, Liu KY, Zhang XH, Ma X, et al. Haploidentical versus matched-sibling transplant in adults with Philadelphia-negative high-risk acute lymphoblastic leukemia: a biologically phase III randomized study. Clin Cancer Res. 2016;22(14):3467–76.
Wang Y, Wang HX, Lai YR, Sun ZM, Wu DP, Jiang M, et al. Haploidentical transplant for myelodysplastic syndrome: registry-based comparison with identical sibling transplant. Leukemia. 2016;30(10):2055–63.
Mo XD, Tang BL, Zhang XH, Zheng CC, Xu LP, Zhu XY, et al. Comparison of outcomes after umbilical cord blood and unmanipulated haploidentical hematopoietic stem cell transplantation in children with high-risk acute lymphoblastic leukemia. Int J Cancer. 2016;139(9):2106–15.
Ringden O, Labopin M, Ehninger G, Niederwieser D, Olsson R, Basara N, et al. Reduced intensity conditioning compared with myeloablative conditioning using unrelated donor transplants in patients with acute myeloid leukemia. J Clin Oncol. 2009;27(27):4570–7.
Scott BL, Pasquini MC, Logan BR, Wu J, Devine SM, Porter DL, et al. Myeloablative versus reduced-intensity hematopoietic cell transplantation for acute myeloid leukemia and myelodysplastic syndromes. J Clin Oncol. 2017;35(11):1154–61.
Gyurkocza B, Sandmaier BM. Conditioning regimens for hematopoietic cell transplantation: one size does not fit all. Blood. 2014;124(3):344–53.
Sanz J, Boluda JC, Martin C, Gonzalez M, Ferra C, Serrano D, et al. Single-unit umbilical cord blood transplantation from unrelated donors in patients with hematological malignancy using busulfan, thiotepa, fludarabine and ATG as myeloablative conditioning regimen. Bone Marrow Transplant. 2012;47(10):1287–93.
Ruggeri A, Sanz G, Bittencourt H, Sanz J, Rambaldi A, Volt F, et al. Comparison of outcomes after single or double cord blood transplantation in adults with acute leukemia using different types of myeloablative conditioning regimen, a retrospective study on behalf of Eurocord and the Acute Leukemia Working Party of EBMT. Leukemia. 2014;28(4):779–86.
Huang XJ, Liu DH, Liu KY, Xu LP, Chen H, Han W, et al. Treatment of acute leukemia with unmanipulated HLA-mismatched/haploidentical blood and bone marrow transplantation. Biol Blood Marrow Transplant. 2009;15(2):257–65.
Lee KH, Lee JH, Lee JH, Kim DY, Kim SH, Shin HJ, et al. Hematopoietic cell transplantation from an HLA-mismatched familial donor is feasible without ex vivo-T cell depletion after reduced-intensity conditioning with busulfan, fludarabine, and antithymocyte globulin. Biol Blood Marrow Transplant. 2009;15(1):61–72.
Luznik L, Bolanos-Meade J, Zahurak M, Chen AR, Smith BD, Brodsky R, et al. High-dose cyclophosphamide as single-agent, short-course prophylaxis of graft-versus-host disease. Blood. 2010;115(16):3224–30.
Di Bartolomeo P, Santarone S, De Angelis G, Picardi A, Cudillo L, Cerretti R, et al. Haploidentical, unmanipulated, G-CSF-primed bone marrow transplantation for patients with high-risk hematologic malignancies. Blood. 2013;121(5):849–57.
Raiola AM, Dominietto A, Ghiso A, Di Grazia C, Lamparelli T, Gualandi F, et al. Unmanipulated haploidentical bone marrow transplantation and posttransplantation cyclophosphamide for hematologic malignancies after myeloablative conditioning. Biol Blood Marrow Transplant. 2013;19(1):117–22.
Saraceni F, Labopin M, Hamladji RM, Mufti G, Socie G, Shimoni A, et al. Thiotepa-busulfan-fludarabine compared to busulfan-fludarabine for sibling and unrelated donor transplant in acute myeloid leukemia in first remission. Oncotarget. 2018;9(3):3379–93.
Grimwade D, Walker H, Oliver F, Wheatley K, Harrison C, Harrison G, et al. The importance of diagnostic cytogenetics on outcome in AML: analysis of 1,612 patients entered into the MRC AML 10 trial. The Medical Research Council Adult and Children’s Leukaemia Working Parties. Blood. 1998;92(7):2322–33.
Ruggeri A, Labopin M, Ciceri F, Mohty M, Nagler A. Definition of GvHD-free, relapse-free survival for registry-based studies: an ALWP-EBMT analysis on patients with AML in remission. Bone Marrow Transplant. 2016;51(4):610–1.
Glucksberg H, Storb R, Fefer A, Buckner CD, Neiman PE, Clift RA, et al. Clinical manifestations of graft-versus-host disease in human recipients of marrow from HL-A-matched sibling donors. Transplantation. 1974;18(4):295–304.
Terwey TH, Vega-Ruiz A, Hemmati PG, Martus P, Dietz E, le Coutre P, et al. NIH-defined graft-versus-host disease after reduced intensity or myeloablative conditioning in patients with acute myeloid leukemia. Leukemia. 2012;26(3):536–42.
Kaplan EL, Meier P. Nonparametric estimation from incomplete observations. J Am Stat Assoc. 1958;53(282):457–81.
Cox DR. Regression models and life-tables. In Breakthroughs in statistics. New York: Springer; 1992. p. 527–41.
Ruggeri A, Labopin M, Sanz G, Piemontese S, Arcese W, Bacigalupo A, et al. Comparison of outcomes after unrelated cord blood and unmanipulated haploidentical stem cell transplantation in adults with acute leukemia. Leukemia. 2015;29(9):1891–900.
Baron F, Ruggeri A, Nagler A. Methods of ex vivo expansion of human cord blood cells: challenges, successes and clinical implications. Expert Rev Hematol. 2016;9(3):297–314.
Shouval R, Ruggeri A, Labopin M, Mohty M, Sanz G, Michel G, et al. An integrative scoring system for survival prediction following umbilical cord blood transplantation in acute leukemia. Clin Cancer Res. 2017;23(21):6478–86.
Pascal L, Tucunduva L, Ruggeri A, Blaise D, Ceballos P, Chevallier P, et al. Impact of ATG-containing reduced-intensity conditioning after single- or double-unit allogeneic cord blood transplantation. Blood. 2015;126(8):1027–32.
Pascal L, Mohty M, Ruggeri A, Tucunduva L, Milpied N, Chevallier P, et al. Impact of rabbit ATG-containing myeloablative conditioning regimens on the outcome of patients undergoing unrelated single-unit cord blood transplantation for hematological malignancies. Bone Marrow Transplant. 2015;50(1):45–50.
Admiraal R, Lindemans CA, van Kesteren C, Bierings MB, Versluijs AB, Nierkens S, et al. Excellent T-cell reconstitution and survival depend on low ATG exposure after pediatric cord blood transplantation. Blood. 2016;128(23):2734–41.
Sanz J, Arango M, Senent L, Jarque I, Montesinos P, Sempere A, et al. EBV-associated post-transplant lymphoproliferative disorder after umbilical cord blood transplantation in adults with hematological diseases. Bone Marrow Transplant. 2014;49(3):397–402.
Dumas PY, Ruggeri A, Robin M, Crotta A, Abraham J, Forcade E, et al. Incidence and risk factors of EBV reactivation after unrelated cord blood transplantation: a Eurocord and Societe Francaise de Greffe de Moelle-Therapie Cellulaire collaborative study. Bone Marrow Transplant. 2013;48(2):253–6.
Brunstein CG, Weisdorf DJ, DeFor T, Barker JN, Tolar J, van Burik JA, et al. Marked increased risk of Epstein-Barr virus-related complications with the addition of antithymocyte globulin to a nonmyeloablative conditioning prior to unrelated umbilical cord blood transplantation. Blood. 2006;108(8):2874–80.
Bertz H, Potthoff K, Finke J. Allogeneic stem-cell transplantation from related and unrelated donors in older patients with myeloid leukemia. J Clin Oncol. 2003;21(8):1480–4.
Saraceni F, Beohou E, Labopin M, Arcese W, Bonifazi F, Stepensky P, et al. Thiotepa, busulfan and fludarabine compared to busulfan and cyclophosphamide as conditioning regimen for allogeneic stem cell transplant from matched siblings and unrelated donors for acute myeloid leukemia. Am J Hematol. 2018. https://doi.org/10.1002/ajh.25225. [Epub ahead of print].
Wang Y, Chang YJ, Xu LP, Liu KY, Liu DH, Zhang XH, et al. Who is the best donor for a related HLA haplotype-mismatched transplant? Blood. 2014;124(6):843–50.
Luo Y, Xiao H, Lai X, Shi J, Tan Y, He J, et al. T-cell-replete haploidentical HSCT with low-dose anti-T-lymphocyte globulin compared with matched sibling HSCT and unrelated HSCT. Blood. 2014;124(17):2735–43.
McCurdy SR, Kanakry JA, Showel MM, Tsai HL, Bolanos-Meade J, Rosner GL, et al. Risk-stratified outcomes of nonmyeloablative HLA-haploidentical BMT with high-dose posttransplantation cyclophosphamide. Blood. 2015;125(19):3024–31.
Bacigalupo A, Dominietto A, Ghiso A, Di Grazia C, Lamparelli T, Gualandi F, et al. Unmanipulated haploidentical bone marrow transplantation and post-transplant cyclophosphamide for hematologic malignanices following a myeloablative conditioning: an update. Bone Marrow Transplant. 2015;50(Suppl 2):S37–9.
Barker JN, Scaradavou A, Stevens CE. Combined effect of total nucleated cell dose and HLA match on transplantation outcome in 1061 cord blood recipients with hematologic malignancies. Blood. 2010;115(9):1843–9.
Acknowledgements
The authors thank Emmanuelle Polge from the ALWP EBMT and Chantal Kenzey from Eurocord for data management, and all participating centres and patients.
Availability of data and materials
The data that support the findings of this study are available from EBMT and Eurocord, but restrictions apply to the availability of these data, which were used under license for the current study, and so are not publicly available. Data are however available from the authors upon reasonable request and with permission of EBMT and EUROCORD.
Author information
Authors and Affiliations
Contributions
FG and ML designed the study. FG, RoS, AR, and AN wrote the manuscript. ML performed the statistical analysis. JaS, WA, EA, JoS. JMRS, SS, BB, AR, RiS, and DB provided cases for the study. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
The Review Boards of the ALWP of EBMT and Eurocord approved this study.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Additional file
Additional file 1:
Table S1. HLA haploidentical transplantation strategy. Table S2. GvHD prophylaxis in patients receiving NTD-Haplo. Table S3. GvHD prophylaxis in patients receiving single umbilical cord blood transplantation. Table S4. Causes of Death. Table S5. The impact of MRC cytogenetic risk groups. (DOCX 19 kb)
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated.
About this article
Cite this article
Giannotti, F., Labopin, M., Shouval, R. et al. Haploidentical transplantation is associated with better overall survival when compared to single cord blood transplantation: an EBMT-Eurocord study of acute leukemia patients conditioned with thiotepa, busulfan, and fludarabine. J Hematol Oncol 11, 110 (2018). https://doi.org/10.1186/s13045-018-0655-8
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s13045-018-0655-8