Abstract
Angiotensin (Ang)-converting-enzyme (ACE) 2 converts Ang II into Ang (1–7), which in turn acts on MAS receptors (ACE2/Ang (1–7)/MAS receptors pathway). This pathway has neuroprotective properties, making it a potential therapeutic target for psychiatric disorders such as depression. Thus, we examined the effects of diminazene aceturate (DIZE), an ACE2 activator, on depressive-like behavior using behavioral, pharmacological, and biochemical assays. To determine whether DIZE or Ang (1–7) produce antidepressant-like effects, we measured the duration of immobility of mice in the tail suspension test following their intracerebroventricular administration. Next, we measured the levels of ACE2 activation in the cerebral cortex, prefrontal cortex, hippocampus, and amygdala after DIZE injection, and examined which cell types, including neurons, microglia, and astrocytes, express ACE2 in the hippocampus using immunofluorescence. Administration of DIZE or Ang (1–7) significantly shortened the duration of immobility time in the tail suspension test, while this effect was inhibited by the co-administration of the MAS receptor antagonist A779. DIZE activated ACE2 in the hippocampus. ACE2 was localized to neurons, astrocytes, and microglia in the hippocampus. In conclusion, these results suggest that DIZE may act on ACE2-positive cells in the hippocampus where it increases the activity of ACE2, thereby enhancing signaling of the ACE2/Ang (1–7)/MAS receptor pathway and resulting in antidepressant-like effects.
Similar content being viewed by others
Introduction
The number of depressed patients in the world is expected to rise due to the impact of the COVID-19 pandemic [1,2,3]. Based on the monoamine hypothesis, which proposes a biochemical explanation for depression, drugs that increase synaptic levels of monoamines such as serotonin (5-HT) and noradrenalin have been used in the treatment of depression. Currently, selective serotonin reuptake inhibitors and serotonin noradrenalin reuptake inhibitors are the mainstay of drug therapy. However, it is estimated that about 30% of depressed patients do not improve when treated with current antidepressants [4]. It is clear that further elucidation of the mechanisms underlying the onset of depression is needed, as well as the development of new drugs and therapies.
The Renin-angiotensin (Ang) system (RAS) is a regulatory system for cardiovascular functions such as blood pressure, water and electrolyte balance [5, 6]. In the RAS, angiotensinogen (AGT) is metabolized to Ang I by renin, and Ang I is converted to Ang II by angiotensin-converting-enzyme (ACE). Ang II is the major active peptide of the RAS, and is involved in vasoconstriction [7], inflammatory responses [8], oxidative stress [9], and vasoconstriction via the Ang II Type 1 (AT1) receptor, a G protein-coupled receptor. In contrast, AT2 receptors play a protective role against the RAS [10, 11]. It has been shown that the above-mentioned hyperfunction of the ACE/Ang II/AT1 receptor system is involved in depression as well as in the cardiovascular system [12]. For example, administration of captopril, an ACE inhibitor, to depressed patients with concurrent hypertension improved their depressive symptoms [13, 14]. In parallel, the RAS has an intrinsic mechanism that counterbalances the ACE/Ang II/AT1 receptor system, and is referred to the ACE2/Ang (1–7)/MAS receptor system [15, 16]. Ang (1–7) is an N-terminal fragment of Ang II generated by ACE2 [17] that antagonizes the ACE/Ang II/AT1 receptor system by exerting physiological effects such as vasodilation [18, 19], anti-inflammatory effects [16], and antioxidant effects [20] via MAS receptors. Therefore, the inhibition of the ACE/Ang II/AT1 receptor system could potentially represent a novel therapeutic target for depression [21].
Diminazene aceturate (DIZE) is an aromatic diamidine approved by the U.S. Food and Drug Administration, and has been used in veterinary medicine for over 60 years to treat trypanosomiasis and babesiosis. Recent studies have reported that DIZE enhances the catalytic activity of ACE2 by direct binding [22]. Indeed, the administration of DIZE in a diabetic mouse model activated ACE2 and increased Ang (1–7) production [23]. Furthermore, the intracerebroventricular (i.c.v.) administration of Ang (1–7) has been observed to shorten immobility time in the forced swimming test [24]. Thus, DIZE activation of the ACE2/Ang (1–7)/MAS receptor system in the brain may represent an effective therapeutic strategy for depression.
In the present study, to investigate whether ACE2 activation in the brain could be a novel therapeutic target for depression, we examined the effects of DIZE on the duration of immobile behavior using a behavioral-pharmacological test, and explored some mechanisms of action using biochemical and histological assays.
Results
DIZE shows antidepressant-like effect in the tail suspension test
To determine whether DIZE, an ACE2 activator, exerts antidepressant effects, mice were administered DIZE i.c.v., and a tail suspension test was performed 60 min after administration. DIZE dose-dependently reduced immobility time in mice (Fig. 1A). This decrease in immobility time or anti-depressant-like effects was observed not only after 60 min but also 90 min following DIZE administration at a dose of 1nmol (Fig. 1B). The immobility time in the tail suspension test is influenced by locomotor activity. In other words, if locomotor activity is enhanced by a drug treatment then the immobility time may be shortened and lead to a misinterpretation of the drug’s effect on despair. Thus, we measured the change in spontaneous locomotor activity of mice treated with DIZE. No significant difference was observed between the vehicle- and DIZE-treated groups in locomotor activity at any time points (Supplementary Fig. S1A). The total locomotor activity of the mice calculated for the 90 min period also showed no significant difference between the vehicle and DIZE groups (Supplementary Fig. S1B). These results indicated that the reduction of the immobility time induced by DIZE (1 nmol) was not due to changes in spontaneous locomotor activity.
Effects of DIZE on the tail suspension test in mice. A: The immobility time was measured 60 min after the administration of vehicle or DIZE (0.01, 0.1, 1 nmol). B: Time courses of the DIZE (1 nmol)-induced antidepressant-like effect in mice. C: Effects of A779 on the DIZE-induced reduction in immobility time in mice. Vehicle, DIZE (1 nmol) or DIZE (1 nmol) in combination with A779 (0.3 or 3 nmol) were administered 60 min prior to measurements. One-way ANOVA: F (3, 42) = 21.24, p < 0.0001, Fig. 1(A), F (4, 52) = 15.96, p < 0.0001, Fig. 1(C). Two-way ANOVA: [time: F (4, 95) = 4.671, p = 0.0017; treatment: F (1, 95) = 51.07, p < 0.0001; time × treatment: F (4, 95) = 6.079, p = 0.0002, Fig. 1(B)]. Values represent the means ± SEM. Numbers in parentheses indicate the number of animals in each group. **p < 0.01 compared with vehicle-treated mice. ##p < 0.01 compared with DIZE (1 nmol)-treated mice
A779 abolishes DIZE-induced antidepressant-like effect in mice
By activating ACE2, DIZE may produce it’s antidepressant-like effects as a result of an increase in Ang (1–7) acting on MAS receptors. We tested this hypothesis using A779, an antagonist of the MAS receptor on which Ang (1–7) acts. The antidepressant-like effect of DIZE (1 nmol) was found to be completely abolished by the co-administration of A779 (0.3, 3 nmol) (Fig. 1C). These results indicated that the MAS receptor may be involved in the antidepressant-like effects of DIZE.
Ang (1–7) shows antidepressant-like effect in the tail suspension test
The antidepressant-like effects of DIZE were found to involve MAS receptors, indicating that Ang (1–7) may mediate these effects. Therefore, to clarify whether Ang (1–7) exhibits antidepressant-like effects, Ang (1–7) was administered i.c.v. at different doses, and the tail suspension test was performed 60 min after administration. Ang (1–7) (300 pmol) administration significantly decreased immobility time in mice (Fig. 2A). This decrease was inhibited by a co-administration with A779 (3 nmol) (Fig. 2A). Furthermore, Ang (1–7) (300 pmol) showed antidepressant-like effects as soon as 45 and 60 min after i.c.v. administration (Fig. 2B). These results indicated that the antidepressant-like effects of DIZE are mediated through the MAS receptor by Ang (1–7), which is increased by ACE2 activation.
Effects of Ang (1–7) on the tail suspension test in mice. A: The immobility time was measured 60 min after the administration of vehicle, Ang (1–7) (30 or 300 pmol) or Ang (1–7) (300 pmol) in combination with A779 (3 nmol). B: Time courses of the Ang (1–7) (300 pmol)-induced antidepressant-like effect in mice. One-way ANOVA: F (3, 37) = 5.265, p = 0.0040, Fig. 2(A). Two-way ANOVA: [time: F (2, 56) = 8.822, p = 0.0005; treatment: F (1, 56) = 26.82, p < 0.0001; time × treatment: F (2, 56) = 4.369, p = 0.0172, Fig. 2(B)]. Values represent the means ± SEM. Numbers in parentheses indicate the number of animals in each group. **p < 0.01 compared with vehicle-treated mice. #p < 0.05 compared with Ang (1–7) (300 pmol)-treated mice
Changes in brain ACE2 activity after DIZE administration
MAS receptor expression is known to be abundant in the hippocampus, cortex, and amygdala in both humans and mice (https://www.proteinatlas.org/ENSG00000130368-MAS1/brain). To determine in which of these brain regions DIZE activation of ACE2 occurs, brains were removed 60 min after DIZE (1 nmol) was administered i.c.v. then divided into 4 regions known to be involved in depression (hippocampus, cerebral cortex, amygdala, and prefrontal cortex). The treatment with DIZE significantly increased ACE2 activity in the hippocampus, while it did not affect the activity in other regions (Fig. 3).
Changes in ACE2 activity in the brain of naïve mice following the i.c.v. administration of DIZE. Sixty min after the administration, samples of the cerebral cortex, prefrontal cortex, hippocampus, and amygdala were collected from the brain and homogenized to determine ACE2 activity. Student’s t-test: t = 0.1171, df = 10, p = 0.9091, Fig. 3(A); t = 0.2676, df = 10, p = 0.7945, Fig. 3(B); t = 3.137, df = 10, p = 0.0106, Fig. 3(C); t = 1.22, df = 10, p = 0.2505, Fig. 3(D). Values represent the means ± SEM. Numbers in parentheses indicate the number of animals in each group. *p < 0.05 compared with vehicle-treated mice
Localization of ACE2 in the hippocampus of naïve mice
As DIZE increased ACE2 activity in the hippocampus of mice, we examined which cell types, whether neurons, microglia, or astrocytes, express ACE2 in the hippocampus. ACE2 labeling was found in NeuN-, GFAP-, and Iba1-positive cells in the hippocampal cornu ammonis (CA) 1 (Fig. 4).
Double immunofluorescence staining for ACE2 and cell specific markers for neuron (NeuN), microglia (Iba-1) or astrocyte (GFAP) in the hippocampus of naïve mice. Photomicrographs showing fluorescent labeling for ACE2 (red), NeuN (green), Iba-1 (green), GFAP (green) or nuclei with DAPI (blue) in the hippocampus of naïve mice
Discussion
In this study, we showed that the ACE2 activator DIZE exhibited an antidepressant-like effect in mice through activation of MAS receptors via Ang (1–7).
DIZE has been found to alter the conformation of ACE2 by targeting and directly binding to a specific structural pocket in the hinge-bending region of ACE2, thereby enhancing its catalytic activity [22] and the conversion of Ang II into Ang (1–7) [23]. Earlier findings in rats suggested that Ang (1–7) has antidepressant-like effects. For example, when Ang (1–7) was administered i.c.v. to TGR (ASrAOGEN) 680 rats, it reduced their brain AGT levels and depression-like phenotype as they exhibited a decreased immobility time in a forced swimming test [24]. The administration of Ang (1–7) was also shown to improve the anxiety- and depressive-like behavior of hypertensive transgenic (mRen2)27 rats [25]. Moreover, the ACE2/Ang (1–7)/MAS receptors pathway, which is activated by exercise, has also been reported to improve cardiovascular and mental status [26]. From these findings, we hypothesize that the activation of the ACE2/Ang (1–7)/MAS receptor pathway in the central nervous system may induce antidepressant effect. In the present study, we found that DIZE administration shortened the duration of the immobile behavior in mice in the tail suspension test (Fig. 1A), but did not affect their locomotion when compared to the vehicle-treated group (Supplementary Fig. S1). DIZE produced a significant effect 60–90 min after its administration (Fig. 1B) which led us to consider that DIZE has antidepressant-like properties in mice. Next, we investigated whether the DIZE-induced reduction in immobility time was mediated by the MAS receptors on which Ang (1–7) acts. We found that the co-administration of DIZE and A779, a MAS receptor blocker, significantly inhibited the antidepressant-like effect of DIZE (Fig. 1C). These results suggest that DIZE exerts its antidepressant-like effects by increasing the production of Ang (1–7) which in turn activates MAS receptor signaling. Moreover, we examined whether Ang (1–7), which is produced by the activation of ACE2, can affect the immobility time in the tail suspension test. We found that compared to the vehicle-treated group, the immobility time was significantly decreased in mice treated with Ang (1–7), an effect that could be inhibited by the co-administration of the MAS receptor antagonist A779 (Fig. 2A). Taken together, these results suggest that the antidepressant-like effect of DIZE may have resulted from the activation of MAS receptors by Ang (1–7), which increased as a result of ACE2 activation.
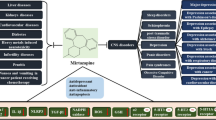
We then measured ACE2 activity in brain regions implicated in depression (hippocampus, cerebral cortex, prefrontal cortex, and amygdala) to determine those that show ACE2 activation after i.c.v. administration of DIZE. We found a marked increase in ACE2 activity in the hippocampus (Fig. 3C). We next identified the cell types in the hippocampus that showed expression of ACE2 by immunohistochemical staining. Neurons, astrocytes and microglia showed ACE2 expression in the hippocampal CA1 (Fig. 4). Moreover, similar co-localization results were observed in other hippocampal regions (CA2, CA3, and dentate gyrus) (Supplementary Fig. S2). A study using TGR (ASrAOGEN) 680 rats, which exhibit reduced levels of AGT in the brain and an anxiety-like or depression-like phenotype, showed that 5-HT and its metabolite 5-HIAA were reduced in brain regions that control emotion, such as the hippocampus and prefrontal cortex [27]. Other research groups have reported that when the Ang II precursor Ang (1–12) was administered intraperitoneally to rats, plasma Ang II and Ang (1–7) were markedly elevated 5 min after [28]. This report indicates that the conversion of Ang II to Ang (1–7) by ACE2 activation takes place in a very short time. Here, the antidepressant-like effect of Ang (1–7) was not observed immediately after its administration but 45 min later whereas the reduction in immobility time was similar to that observed 60 min after administration (Fig. 2B). Although in our previous studies we have confirmed that MAS receptors are localized to neurons and microglia of the spinal cord [29] another study has demonstrated that, while the depletion of ACE2 reduced serotonin levels in the brain, ACE2 activation is required for running-induced hippocampal neurogenesis [30]. Hippocampal neurogenesis and serotonin are known to play an important role in antidepressant effects [31, 32]. These findings suggest that the activation of MAS receptors may have mediated antidepressant-like effects as a result of the modulation of other neuronal systems such as serotonergic pathways. Together with the results of the previous report, the present study suggests that ACE2 activity in ACE2-positive cells of the hippocampus enhances the ACE2/Ang (1–7)/MAS receptor system, leading to antidepressant effects (Fig. 5).
The present study revealed that DIZE may have antidepressant-like effects in naïve mice. It has been reported that the activation of ACE2 increases alamandine [33], an Ang (1–7)-like protein which, interestingly and similar to Ang (1–7), has been reported to exhibit antidepressant effects via Mas receptors [34]. Therefore, we plan to continue our investigation on the antidepressant effects of DIZE by quantifying the levels of Ang (1–7) and alamandine to confirm whether Ang (1–7) or alamandine actually increase in the brain after DIZE administration, and by performing acute and chronic administration of DIZE using animal models of depression such as olfactory bulbectomy, social defeat, or chronic mild stress.
In conclusion, our results suggest that intracerebroventricular administration of DIZE reduces immobility time in mice via activation of MAS receptors by Ang (1–7), which is increased by ACE2 activation, suggesting the potential use of ACE2 activators or MAS receptor agonists for antidepressant therapy. We hope to clarify the possibility that the activation of ACE2 in the brain may be a useful target for the development of new treatments for depression.
Materials and methods
All experiments were performed in accordance with the Guide for the Care and Use of Laboratory Animals from the Tohoku Medical and Pharmaceutical University (Approval number: 20051-cn, 21035-cn) and the National Institutes of Health Guide for the Care and Use of Laboratory Animals. Efforts were made to minimize suffering and to reduce the number of animals used.
Animals
Male ddY mice (weighing 28–32 g; Japan SLC, Shizuoka, Japan) were used in all experiments (total: n = 345, behavioral test: n = 331, ACE2 assay: n = 12, immunohistochemical study: n = 2). The mice were housed in cages with free access to food and water under conditions of controlled temperature (22 ± 2 °C) and humidity (55 ± 5%) on a 12-h light-dark cycle (lights on: 07:00 to 19:00).
Drugs
DIZE (LKT Laboratories, Minnesota, USA), A779 (Bachem, Bubendorf, Switzerland), and Ang (1–7) (Peptide Institute, Osaka, Japan) were dissolved in Ringer’s solution. The compounds were administered i.c.v. alone or in combination to mice under diethyl ether inhalation anesthesia, at a volume of 5 µL using a 50 µL Hamilton microsyringe attached to a disposable 27-G needle. The dose for DIZE was obtained from a previous report [35].
Tail suspension test
The tail suspension test was performed as previously described [36,37,38], to evaluate the antidepressant-like effects of DIZE or Ang (1–7). Mice were suspended with their tails taped, such that they were 30 cm above the floor. An investigator blinded to the treatment assignments observed the immobility time for 10 min.
Locomotor activity
Locomotor activity was determined using the SUPERMEX monitoring system (Muromachi Kikai Co., Tokyo, Japan). The details of the apparatus have been previously described [36, 39, 40]. Briefly, the Supermex instrument can monitor minute movements in all three planes of motion (sagittal, coronal, and horizontal) as one movement owing to its infrared sensor with multiple Fresnel lenses, which can be moved close enough to the cage to capture multidirectional locomotor alterations in a single mouse. The Supermex instrument was connected to a behavioral analysis system (CompACT AMS, Muromachi Kikai) that can interpret each movement as one count. Locomotor activity was measured for 90 min during the light phase, between 11:00 am and 15:00 pm. Each mouse was placed in an activity box of SUPERMEX for 15 min for adaptation prior to injection with either vehicle or DIZE.
Measurement of ACE2 activity
ACE2 activity was measured using the SensoLyte 390 ACE2 activity assay kit (AnaSpec, CA, USA) according to the supplied manual. Mice were injected i.c.v. with vehicle or DIZE, and brains were removed 1 h later. Brain slices were prepared in the following manner. The excised brains were sectioned into the hippocampus, cerebral cortex, prefrontal cortex, and amygdala using a brain slicer (Muromachi Kikai) and thoroughly homogenized in 150 µL of assay buffer (Component C buffer) provided with the kit. Homogenized samples were incubated for 15 min at 4 °C. After centrifugation (20,000 × g, 10 min, 4 °C), 120 µL of the supernatant was collected and used as samples. The brain samples were reacted with ACE2 substrate for 30 min under light-shielded conditions at room temperature, and after addition of reaction stopper solution, ACE2 activity was measured by measuring fluorescence intensity (excitation: 330 nm /fluorescence: 390 nm) on a SoftMax Pro (Molecular Devices, CA, USA). Total protein concentration was quantified by the Bradford protein assay and used to normalize ACE2 activity.
Immunofluorescence study
Brain samples were collected as previously described [41, 42]. Mice were anesthetized by intraperitoneal administration of a combined triadic anesthesia: medetomidine (50 mg/kg; Nippon Zenyaku Kogyo Co., Ltd., Fukushima, Japan), midazolam (4 mg/kg; Sandoz, Tokyo, Japan), and butorphanol (5 mg/kg; Meiji Seika Pharma Co., Ltd., Tokyo, Japan), and perfused through the heart with ice-cold phosphate-buffered saline (PBS; pH 7.4), immediately followed by a fixative containing 4% paraformaldehyde in PBS. Brain were then postfixed with the same fixative solution at 4 °C for 1 h and then placed in a 20% sucrose-buffered solution at 4 °C for 12 h. The brains were sliced into 40-µm sections from -1.40 mm to -2.00 mm relative to bregma, using a cryostat (Cryostar NX70; Thermo Scientific, Waltham, MA, USA). Frozen sections were mounted on glass slides (Matsunami Glass, Osaka, Japan). After three 5-minute washes, the sections were incubated with PBS containing 1% normal goat serum and 0.3% Triton X-100 (PBSGT) at room temperature (23 ± 1 °C) for 2 h. They were then incubated overnight at 4 °C with a rabbit primary monoclonal antibody against ACE2 (1:100; Santa Cruz Biotechnology; Cat# sc-20,998), mouse anti-neuronal nuclear antigen antibody (NeuN; 1:200; Millipore, Burlington, USA; Cat# MAB377), mouse anti-glial fibrillary acidic protein antibody (GFAP; 1:200; Millipore; Cat# MAB360), and rabbit anti-ionized calcium binding adaptor molecule 1 antibodies (Iba-1; 1:200, Wako Pure Chemical Industries, Ltd., Osaka, Japan; Cat# 019–19741). After a 2-day incubation, the sections were washed twice with 0.1% PBS. When double labeling was performed using two primary antibodies from different host species (rabbit or mouse), sections were washed and incubated overnight at 4 °C with goat anti-rabbit IgG Alexa Fluor 568 (1:200; Molecular Probes, Eugene, USA; Cat# A11011) or goat anti-mouse IgG Alexa Fluor 488 (1:200; Molecular Probes; Cat# A11001) antibodies in PBSGT. When double labeling was performed using two primary antibodies from the same host species (rabbit anti-Iba-1 and rabbit anti-ACE2 antibodies), the detection of each antigen was performed sequentially and labeled goat anti-rabbit IgG Alexa Fluor 488 AffiniPure Fab fragment (1:80, Jackson ImmunoResearch Inc; Cat# 111-547-003), instead of whole antibodies, was used in the first detection (Iba-1). Thus, following the incubation with the rabbit anti-Iba-1 antibody, a goat monovalent Fab fragment tagged with fluorescent label 488 was added at a high concentration. This served two purposes: to label Iba-1 antibodies and to block/saturate their surface. The monovalent Fab fragment has only one antigen-binding site, which is used for binding to Iba-1, and therefore can not bind to the anti-ACE2 antibody used subsequently. The demonstration that the Fab fragment prevents binding of the secondary anti-rabbit Alexa 568 IgG (to detect the primary rabbit anti-ACE2 antibody on ACE2) to the primary anti-Iba-1 antibody is illustrated in Supplementary Fig. S3. In this control experiment, we show that there was no labeling by the anti-rabbit Alexa 568 IgG when immunostaining was performed without anti-ACE2 antibodies. The detailed Immunofluorescence staining methods for two primary antibodies from the same host species were carried out as previously described [43, 44]. DAPI (1:100; Wako Pure Chemical Industries) was used to stain and identify the nuclei. The sections were washed twice with 0.1% PBS and cover-slipped with fluorescent mounting medium (Dako, Carpinteria, CA, USA). The labeled sections were analyzed using a confocal laser scanning microscope (A1Rsi; Nikon, Tokyo, Japan).
Statistical analysis
The experimental results are presented as mean ± standard error of the mean (SEM). The statistical significance of differences was determined by Student’s t-test for two-group comparisons. Significant differences were determined using one- or two-way analysis of variance (ANOVA), followed by the Tukey-Kramer test or Bonferroni test for multiple group comparisons. The criterion for a significant difference was set at p < 0.05.
Data Availability
All data generated or analyzed during this study are included in this published article.
Abbreviations
- ACE:
-
Angiotensin-converting-enzyme
- Ang:
-
Angiotensin
- ANOVA:
-
Analysis of variance
- AGT:
-
Angiotensinogen
- AT1:
-
Ang II Type 1
- CA:
-
Cornu ammonis
- DG:
-
Dentate gyrus
- DIZE:
-
Diminazene aceturate
- GFAP:
-
Glial fibrillary acidic protein
- 5-HT:
-
Serotonin
- i.c.v.:
-
Intracerebroventricular
- Iba-1:
-
Ionized calcium binding adaptor molecule 1
- NeuN:
-
Neuronal nuclear antigen
- PBS:
-
Phosphate-buffered saline
- PBSGT:
-
PBS containing 1% normal goat serum and 0.3% Triton X-100
- RAS:
-
Renin-angiotensin system
- SEM:
-
Standard error of the mean
- TBST:
-
Tris-buffered saline supplemented with 0.01% Tween-20
References
Mazza MG, De Lorenzo R, Conte C, Poletti S, Vai B, Bollettini I, Melloni EMT, Furlan R, Ciceri F, Rovere-Querini P, Benedetti F. Anxiety and depression in COVID-19 survivors: role of inflammatory and clinical predictors. Brain Behav Immun. 2020;89:594–600.
Deng J, Zhou F, Hou W, Silver Z, Wong CY, Chang O, Huang E, Zuo QK. The prevalence of depression, anxiety, and sleep disturbances in COVID-19 patients: a meta-analysis. Ann N Y Acad Sci. 2021;1486:90–111.
Renaud-Charest O, Lui LMW, Eskander S, Ceban F, Ho R, Di Vincenzo JD, Rosenblat JD, Lee Y, Subramaniapillai M, McIntyre RS. Onset and frequency of depression in post-COVID-19 syndrome: a systematic review. J Psychiatr Res. 2021;144:129–37.
Krishnan V, Nestler EJ. The molecular neurobiology of depression. Nature. 2008;455:894–902.
Hall JE, Guyton AC, Mizelle HL. Role of the renin-angiotensin system in control of sodium excretion and arterial pressure. Acta Physiol Scand Suppl. 1990;591:48–62.
Leung PS. The physiology of a local renin-angiotensin system in the pancreas. J Physiol. 2007;580:31–7.
Touyz RM, Schiffrin EL. Signal transduction mechanisms mediating the physiological and pathophysiological actions of angiotensin II in vascular smooth muscle cells. Pharmacol Rev. 2000;52:639–72.
Suzuki Y, Ruiz-Ortega M, Lorenzo O, Ruperez M, Esteban V, Egido J. Inflammation and angiotensin II. Int J Biochem Cell Biol. 2003;35:881–900.
Chabrashvili T, Kitiyakara C, Blau J, Karber A, Aslam S, Welch WJ, Wilcox CS. Effects of ANG II type 1 and 2 receptors on oxidative stress, renal NADPH oxidase, and SOD expression. Am J Physiol Regul Integr Comp Physiol. 2003;285:R117–124.
Patel S, Hussain T. Dimerization of AT(2) and mas receptors in control of blood pressure. Curr Hypertens Rep. 2018;20:41.
Kukida M, Mogi M, Kan-No H, Tsukuda K, Bai HY, Shan BS, Yamauchi T, Higaki A, Min LJ, Iwanami J, et al. AT2 receptor stimulation inhibits phosphate-induced vascular calcification. Kidney Int. 2019;95:138–48.
Saavedra JM, Sánchez-Lemus E, Benicky J. Blockade of brain angiotensin II AT1 receptors ameliorates stress, anxiety, brain inflammation and ischemia: therapeutic implications. Psychoneuroendocrinology. 2011;36:1–18.
Deicken RF. Captopril treatment of depression. Biol Psychiatry. 1986;21:1425–8.
Germain L, Chouinard G. Treatment of recurrent unipolar major depression with captopril. Biol Psychiatry. 1988;23:637–41.
Santos RA, Ferreira AJ, Verano-Braga T, Bader M. Angiotensin-converting enzyme 2, angiotensin-(1–7) and mas: new players of the renin-angiotensin system. J Endocrinol. 2013;216:R1–r17.
Simões e Silva AC, Silveira KD, Ferreira AJ, Teixeira MM. ACE2, angiotensin-(1–7) and mas receptor axis in inflammation and fibrosis. Br J Pharmacol. 2013;169:477–92.
Santos RAS, Sampaio WO, Alzamora AC, Motta-Santos D, Alenina N, Bader M, Campagnole-Santos MJ. The ACE2/Angiotensin-(1–7)/MAS Axis of the renin-angiotensin system: focus on Angiotensin-(1–7). Physiol Rev. 2018;98:505–53.
Castro CH, Santos RA, Ferreira AJ, Bader M, Alenina N, Almeida AP. Evidence for a functional interaction of the angiotensin-(1–7) receptor mas with AT1 and AT2 receptors in the mouse heart. Hypertension. 2005;46:937–42.
Savergnini SQ, Beiman M, Lautner RQ, de Paula-Carvalho V, Allahdadi K, Pessoa DC, Costa-Fraga FP, Fraga-Silva RA, Cojocaru G, Cohen Y, et al. Vascular relaxation, antihypertensive effect, and cardioprotection of a novel peptide agonist of the MAS receptor. Hypertension. 2010;56:112–20.
Jiang T, Gao L, Shi J, Lu J, Wang Y, Zhang Y. Angiotensin-(1–7) modulates renin-angiotensin system associated with reducing oxidative stress and attenuating neuronal apoptosis in the brain of hypertensive rats. Pharmacol Res. 2013;67:84–93.
Vian J, Pereira C, Chavarria V, Köhler C, Stubbs B, Quevedo J, Kim SW, Carvalho AF, Berk M, Fernandes BS. The renin-angiotensin system: a possible new target for depression. BMC Med. 2017;15:144.
Kulemina LV, Ostrov DA. Prediction of off-target effects on angiotensin-converting enzyme 2. J Biomol Screen. 2011;16:878–85.
Zhang Y, Liu J, Luo JY, Tian XY, Cheang WS, Xu J, Lau CW, Wang L, Wong WT, Wong CM, et al. Upregulation of angiotensin (1–7)-Mediated signaling preserves endothelial function through reducing oxidative stress in diabetes. Antioxid Redox Signal. 2015;23:880–92.
Kangussu LM, Almeida-Santos AF, Bader M, Alenina N, Fontes MA, Santos RA, Aguiar DC, Campagnole-Santos MJ. Angiotensin-(1–7) attenuates the anxiety and depression-like behaviors in transgenic rats with low brain angiotensinogen. Behav Brain Res. 2013;257:25–30.
Almeida-Santos AF, Kangussu LM, Moreira FA, Santos RA, Aguiar DC, Campagnole-Santos MJ. Anxiolytic- and antidepressant-like effects of angiotensin-(1–7) in hypertensive transgenic (mRen2)27 rats. Clin Sci (Lond). 2016;130:1247–55.
De Sousa RAL, Improta-Caria AC, Aras-Júnior R, de Oliveira EM, Soci ÚPR, Cassilhas RC. Physical exercise effects on the brain during COVID-19 pandemic: links between mental and cardiovascular health. Neurol Sci. 2021;42:1325–34.
Voigt JP, Hörtnagl H, Rex A, van Hove L, Bader M, Fink H. Brain angiotensin and anxiety-related behavior: the transgenic rat TGR(ASrAOGEN)680. Brain Res. 2005;1046:145–56.
Moniwa N, Varagic J, Simington SW, Ahmad S, Nagata S, Voncannon JL, Ferrario CM. Primacy of angiotensin converting enzyme in angiotensin-(1–12) metabolism. Am J Physiol Heart Circ Physiol. 2013;305:H644–650.
Nemoto W, Yamagata R, Ogata Y, Nakagawasai O, Tadano T, Tan-No K. Inhibitory effect of angiotensin (1–7) on angiotensin III-induced nociceptive behaviour in mice. Neuropeptides. 2017;65:71–6.
Klempin F, Mosienko V, Matthes S, Villela DC, Todiras M, Penninger JM, Bader M, Santos RAS, Alenina N. Depletion of angiotensin-converting enzyme 2 reduces brain serotonin and impairs the running-induced neurogenic response. Cell Mol Life Sci. 2018;75:3625–34.
Santarelli L, Saxe M, Gross C, Surget A, Battaglia F, Dulawa S, Weisstaub N, Lee J, Duman R, Arancio O, et al. Requirement of hippocampal neurogenesis for the behavioral effects of antidepressants. Science. 2003;301:805–9.
Pehrson AL, Roberts D, Khawaja A, McNair R. The role of serotonin neurotransmission in rapid antidepressant actions. Psychopharmacology. 2022;239:1823–38.
Qaradakhi T, Gadanec LK, McSweeney KR, Tacey A, Apostolopoulos V, Levinger I, Rimarova K, Egom EE, Rodrigo L, Kruzliak P, et al. The potential actions of angiotensin-converting enzyme II (ACE2) activator diminazene aceturate (DIZE) in various diseases. Clin Exp Pharmacol Physiol. 2020;47:751–8.
Almeida-Santos AF, de Melo LA, Goncalves SCA, Oliveira Amaral LB, Santos RAS, Campagnole-Santos MJ, Kangussu LM. Alamandine through MrgD receptor induces antidepressant-like effect in transgenic rats with low brain angiotensinogen. Horm Behav. 2021;127:104880.
Mecca AP, Regenhardt RW, O’Connor TE, Joseph JP, Raizada MK, Katovich MJ, Sumners C. Cerebroprotection by angiotensin-(1–7) in endothelin-1-induced ischaemic stroke. Exp Physiol. 2011;96:1084–96.
Nakagawasai O, Takahashi K, Ambo A, Onuma K, Takahashi N, Nemoto W, Tan-No K. Antidepressant effect of intracerebroventricularly administered deltorphin analogs in the mouse tail suspension test. Biol Pharm Bull. 2022;45:538–41.
Nakagawasai O, Yamada K, Odaira T, Takahashi K, Nemoto W, Sakuma W, Wakou M, Lin JR, Tan-No K. Liver hydrolysate improves depressive-like behavior in olfactory bulbectomized mice: involvement of hippocampal neurogenesis through the AMPK/BDNF/CREB pathway. J Pharmacol Sci. 2020;143:52–5.
Nakagawasai O, Yamada K, Takahashi K, Odaira T, Sakuma W, Ishizawa D, Takahashi N, Onuma K, Hozumi C, Nemoto W, Tan-No K. Liver hydrolysate prevents depressive-like behavior in an animal model of colitis: involvement of hippocampal neurogenesis via the AMPK/BDNF pathway. Behav Brain Res. 2020;390:112640.
Nakagawasai O, Yamada K, Sakuma W, Takahashi K, Odaira T, Yamagata R, Nemoto W, Ejima A, Sato K, Tan-No K. A novel dipeptide derived from porcine liver hydrolysate induces recovery from physical fatigue in a mouse model. J Funct Foods 2021, 76.
Nakagawasai O, Takahashi K, Miura Y, Nemoto W, Obara Y, Tan-No K. ERK5 inhibitor BIX02189 attenuates methamphetamine-induced hyperactivity by modulating microglial activation in the striatum. J Pharmacol Sci. 2022;148:326–30.
Takahashi K, Kurokawa K, Hong L, Miyagawa K, Mochida-Saito A, Iwasa M, Iwasa H, Nakagawasai O, Tadano T, Takeda H, Tsuji M. Antidepressant effects of Enterococcus faecalis 2001 through the regulation of prefrontal cortical myelination via the enhancement of CREB/BDNF and NF-kappaB p65/LIF/STAT3 pathways in olfactory bulbectomized mice. J Psychiatr Res. 2022;148:137–48.
Nakagawasai O, Lin JR, Odaira T, Takahashi K, Nemoto W, Moriguchi S, Yabuki Y, Kobayakawa Y, Fukunaga K, Nakada M, Tan-No K. Scabronine G Methyl Ester improves memory-related behavior and enhances hippocampal cell proliferation and long-term potentiation via the BDNF-CREB pathway in olfactory Bulbectomized mice. Front Pharmacol. 2020;11:583291.
Takahashi K, Nakagawasai O, Nemoto W, Odaira T, Sakuma W, Onogi H, Nishijima H, Furihata R, Nemoto Y, Iwasa H, et al. Effect of Enterococcus faecalis 2001 on colitis and depressive-like behavior in dextran sulfate sodium-treated mice: involvement of the brain-gut axis. J Neuroinflammation. 2019;16:201.
Yamagata R, Nemoto W, Nakagawasai O, Takahashi K, Tan-No K. Downregulation of spinal angiotensin converting enzyme 2 is involved in neuropathic pain associated with type 2 diabetes mellitus in mice. Biochem Pharmacol. 2020;174:113825.
Acknowledgements
The authors would like to thank Dr. Wakana Sakuma of the Tohoku Medical and Pharmaceutical University for her technical assistance.
Funding
This study was supported in part by JSPS KAKENHI (grant numbers JP21K15351and JP22K06866).
Author information
Authors and Affiliations
Contributions
ON: Methodology, validation, conceptualization, project administration, writing - review and editing, funding acquisition. KT: Investigation, formal analysis, writing – original draft, funding acquisition, visualization. TK, RY, and WN: Investigation. KT-N: Supervision. All authors have critically reviewed the manuscript and approved the final version for publication.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interest.
Ethics approval and consent to participate
All experiments were performed in accordance with the Guide for the Care and Use of Laboratory Animals from the Tohoku Medical and Pharmaceutical University (Approval number: 20051-cn, 21035-cn) and the National Institutes of Health Guide for the Care and Use of Laboratory Animals. Efforts were made to minimize suffering and to reduce the number of animals used.
Consent for publication
Not applicable.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Nakagawasai, O., Takahashi, K., Koyama, T. et al. Activation of angiotensin-converting enzyme 2 produces an antidepressant-like effect via MAS receptors in mice. Mol Brain 16, 52 (2023). https://doi.org/10.1186/s13041-023-01040-y
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s13041-023-01040-y