Abstract
Background
Although Philadelphia-negative myeloproliferative neoplasms (Ph-MPN) are usually not aggressive, the type and the number of molecular lesions impact greatly on leukemic transformation. Indeed, the molecular background underlying progression is still largely unexplored even though ASXL1, IDH1/2, SRSF2, and TP53 mutations, together with adverse karyotypic changes, place the patient at high risk of leukemic transformation.
Case presentation
Our patient, a 64-year old man with a diagnosis of JAK2 V617F primary myelofibrosis (PMF) had an unusually rapid leukemic transformation. Genomic profiling showed that TET2 and SRSF2 mutations were also present. At leukemic transformation, the patient developed a complex chromosome rearrangement producing a EWSR1-MYB fusion. Remarkably, the expression of MYB and of its target BCL2 was, respectively, ≥4.7 and ≥2.8 fold higher at leukemic transformation than after chemotherapy, when the patient obtained the hematological remission. At this time point, the EWSR1-MYB fusion disappeared while JAK2 V617F, TET2, and SRSF2 mutations, as well as PMF morphological features persisted.
Conclusions
Rapid leukemic transformation of JAK2 V617F PMF was closely linked to a previously undescribed putative EWSR1-MYB transcription factor which was detected only at disease evolution. We hypothesize that the EWSR1-MYB contributed to leukemia transformation through at least two mechanisms: 1) it sustained MYB expression, and consequently deregulated its target BCL2, a putative onco-suppressor gene; and 2) ectopic EWSR1-MYB expression probably fulfilled its own oncogenic potential as demonstrated for other MYB-fusions. As our study confirmed that MYB is recurrently involved in chronic as well as leukemic transformation of PMF, it appears to be a valid molecular marker for tailored treatments.
Similar content being viewed by others
Background
Ph-MPN are usually not aggressive malignancies. Evolution into Acute Myeloid Leukemia (AML) occurs in ~2 % of Polycythemia Vera (PV), 1 % of Essential Thrombocytopenia (ET), and 10–20 % of PMF, considering the first decade of disease. Although, transformation rates increase with genotoxic therapy, leukemic transformation is part of the natural history of these disorders as AML also occurs in treatment-naive patients [1, 2].
Genomic profiling of Ph-MPN is useful for understanding the pathobiology of leukemic transformation and improving prognostic stratification of patients and treatment. During leukemic transformation very few new mutations are acquired and the majority of somatic mutations are already present in the chronic phase. Two or more mutations affecting ASXL1, IDH1/2, SRSF2, and TP53, are considered high-risk events. Besides mutations, unfavourable (chromosome 3, 5 or 7 rearrangements), and very unfavourable (chromosome 17 abnormalities) karyotypic changes impact upon prognosis [3, 4]. In any case, the molecular mechanisms underlying the progression from Ph-MPN to AML are not yet completely understood [5–9].
Longitudinal genomic investigation into a unique case of JAK2 V617F positive PMF with rapid leukemic evolution, detected a leukemia-specific complex rearrangement involving chromosomes 6, 9 and 22 which produced an aberrant EWSR1-MYB transcription factor. EWSR1-MYB ectopic expression, as well as high expression of MYB and its target BCL2, likely contributed to the leukemia phenotype. It is worth mentioning that, high level of MYB expression blocks differentiation and confers self-renewal properties to leukemic cells, whereas the expression of the anti-apoptotic BCL2 protein inhibits leukemic cell death [10–12].
Case presentation
In January 2011, a 64-year-old man was referred to our Department with leukocytosis (WBC 20.100/uL with 71.8 % neutrophils), macrocytic anemia (Hb 11,5 g/dL; MCV 104 fl), splenomegaly, and hepatomegaly. Bone marrow (BM) biopsy revealed PMF with grade 1 fibrosis; karyotype was normal. The JAK2 V617F mutation was found at 60 % allelic burden. After 4 months the patient developed an acute myeloid leukemia (AML; M2 morphology) and acquired an ins(6;22)(q23.3;q11) in the 20 metaphases analyzed (Fig. 1a). He was treated according to the FLAI protocol [13] and obtained hematologic and cytogenetic remission. Myeloproliferative features, including JAK2 V617F, persisted. Demethylating treatment with 5-azacytidine (5-AZA) was administered for 8 cycles, reducing anemia and splenomegaly, but treatment was discontinued because the patient developed a gastric adenocarcinoma. He died 30 months after PMF was diagnosed.
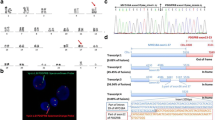
a) G-banded karyotype bone marrow cells at leukemic transformation showed ins(6;22)(q23.3;q11) and der(22q) (black arrows). b) Metaphase FISH experiment with Whole Chromosome Paint (WCP) 6 (Texas Red) and WCP 22 (FITC). Genomic material of chromosome 22 was present on der(6) and der(9) long arms. c) Schema of clones RP1-32B1 (MYB) (Spectrum Orange) and RP11-367E7 (EWSR1) (Spectrum Green) and their mapping. Normal and derivative chromosomes 6 and 22 hybridization patterns; a green/orange fusion signal was present on der(6). A fusion signal was found in interphase nuclei (yellow arrow). d) SNPa analysis detected loss at 22q (red triangle) in BM sample at leukemic transformation. e) Schema of the EWSR1-MYB fusion gene and putative protein. f) MYB expression analysis in four combined cases at chronic phase and leukemic transformation and in a positive control group (4 RUNX1-RUNX1T1 AML, 4 MLL-positive AML, and 4 BCR-ABL1-positive B-cell ALL). g) Longitudinal expression of MYB and BCL2 in the index case. Data are reported for one representative of three independent experiments. Abbreviations: der, derivative chromosome; nl, normal; TAD, transcriptional-activating domain; R1, R2, R3, imperfect aminoacidic repeats; NRD, negative regulatory domain; CP, chronic phase; LT, leukemic transformation; wt, wild-type; R, relapse
Methods
Molecular-cytogenetic and mutational analyses
All molecular and cytogenetic studies were carried out on BM samples. Multi-FISH (24XCyte Multi-colour probe kit, MetaSystems), whole chromosome paints for chromosomes 6, 9 and 22 (Cytocell, Milan, Italy), and metaphase FISH with DNA clones for MYB (G248P8686G9 and G248P89100B2), EWSR1 (G248P89991F7 and G248P80286D12), 9q33.1-q33.3, and 22q11-q12 regions, were done at leukemic transformation (Additional file 1 and Additional file 2: Tables S1, S2, S3). A EWSR1-MYB FISH assay (RP1-32B1 for MYB and RP11-367E7 for EWSR1) was performed at leukemic transformation, diagnosis and post-chemotherapy. The EWSR1-MYB assay was also applied to a cohort of 7 PMF and 3 leukemia groups (Additional file 1). Single Nucleotide Polymorphism array (SNPa) experiments were performed at initial PMF and at leukemic transformation; targeted mutational analysis of DNMT3A, SETBP1, EZH2, IDH1, IDH2, SRSF2, ASXL1, NRAS, TET2 and TERT promoter was done at diagnosis, leukemic transformation, and after chemotherapy by DHPLC and Sanger’s Sequencing. For details of experiments see Additional file 1 and Additional file 2: Table S4.
Reverse transcription-polymerase chain reaction (RT-PCR) and cloning of EWSR1-MYB
cDNA from each patient sample was amplified using primers EWSR1_400F (5′-GCCCACTCAAGGATATGCAC-3′) and MYB_561R (5′-TGCTTGGCAATAACAGACCA-3′), for the first amplification round; nested PCR was set up with primers EWSR1_515F (5′-CAGACCGCCTATGCAACTTC-3′) and MYB_433R (5′-GCACTGCACATCTGTTCGAT-3′). PCR products were cloned and sequenced (Additional file 1).
Quantitative reverse transcription PCR (qRT-PCR) for MYB and BCL2
MYB expression was investigated in our patient, at all time points, and in 7 PMF cases (four at leukemic transformation and three in paired chronic phase/leukemic transformation) (TaqMan assay probe Hs00920556_m1; Applied Biosystems, Foster City, CA). Negative controls were four healthy BM samples. Positive controls were 12 acute leukemias with high MYB expression (4 RUNX1-RUNX1T1 AML, 4 AML with MLL translocations, and 4 BCR-ABL1-positive B-cell ALL) [14]. Expression of BCL2, a known MYB target, was also investigated in our case (TaqMan assay probe Hs00608023_m1, Applied Biosystems). Amplifications were normalized to the endogenous reference control ABL1 (Hs00245445_m1, Applied Biosystems) (Additional file 1).
Results
Molecular cytogenetic and mutational analyses
Multi-FISH and whole chromosome paints showed chromosome 22q11-q12 material inserted into chromosome 6q23 and revealed an additional rearrangement between der(22) and an apparently normal chromosome 9 (Fig. 1b) (Additional file 3: Figure S1). The breakpoint occurred at 6q23 within the MYB oncogene (Additional file 3: Figure S2). Fosmids for EWSR1 and the EWSR1-MYB assay showed that the 5′EWSR1 was inserted into the MYB locus (Fig.1c) (Additional file 3: Figure S2). The EWSR1-MYB rearrangement was not detected at chronic phase or in 3 consecutive samples analyzed after chemotherapy and during treatment with 5-AZA (Table 1).
SNPa detected a 96 Mb copy neutral loss of heterozygosity (cnLOH) at 12q11-12q24.33 at chronic phase and leukemic transformation. Lack of germinal material precluded definition as a congenital or acquired event. Applying a 50 Kb filter revealed a 99 kb loss at 22q11.1 (cytostart 17585764 - cytoend 17684472) only at leukemic transformation (Fig. 1d) (Additional file 3: Figure S3). SRSF2 (c.284C > A; p.P95H) and TET2 (c.3781C > T; p.R1261C) (c.2732_2733insC; p.A912Cfs*12) mutations were found, and confirmed, at all disease stages (Table 1).
RT-PCR and qRT-PCR
At leukemic transformation an in-frame fusion transcript EWSR1-MYB with breakpoint in EWSR1 exon 7 (nt 927) (NM_013986.3) and MYB exon 2 (nt 223) (NM_001130173.1) was detected. The 954 aminoacids predicted fusion protein retained the EWSR1 transcriptional-activating domain (TAD) at the N-terminal and all MYB functional domains at the C-terminal (Fig. 1e). After chemotherapy, at hematological and cytogenetic remission, the EWSR1-MYB fusion disappeared. At leukemic phase, MYB and BCL2 expression was respectively ≥4.7 and ≥2.8 fold higher than at remission. High MYB expression was detected in 7 PMF and in the 3 groups of acute leukemia used as positive controls (Fig. 1f).
Discussion
This case of JAK2-positive PMF with very rapid evolution to AML had a specific mutational profile at diagnosis bearing mutations of JAK2, SRSF2 and TET2. A complex rearrangement involving chromosomes 6, 9, and 22, which produced a previously undescribed EWSR1-MYB fusion, underlies the leukemic transformation.
EWSR1 is a member of the TET (TLS, EWSR1, TAFII68) protein family which acts as multifunctional protein and regulates transcription and mRNA splicing to maintain cellular homeostasis. Although well known in soft tissue sarcoma, EWSR1 fusions have only occasionally been detected in leukemia [15–19]. Interestingly, all EWSR1 fusions retained the TAD portion at the N-terminal and the partner DNA Binding Domain (DBD) at the C-terminal. Since EWSR1-FLI1, the most frequent sarcoma-associated fusion, acted as an aberrant transcription factor, deregulating several targets [20], one might speculate that the EWSR1-MYB fusion in our patient also acted as an aberrant transcription factor, altering the MYB transcriptional program.
MYB is a leucin zipper transcription factor which plays a key role in cell proliferation and differentiation [21–23]. High level of MYB is common in AML associated with BCR-ABL1, RUNX1-RUNX1T1, MYST3-CREBBP, or MLL- rearrangements [11, 12, 24]. Chromosomal aberrations placing MYB under the promoter of a partner gene, such as TCRB-MYB in T-cell ALL [14, 25] or in close proximity of super-enhancer regions, such as MYB-NFIB in adenoid cystic carcinoma [26], caused MYB over-expression. It is worth noting, however, that besides promoting tumour growth through MYB over-expression, the MYB fusions might have their own oncogenic potential as demonstrated for the MYB-QKI in pediatric angiocentric glioma, which drives tumor development in vitro and in vivo models [27].
High MYB level confers self-renewal properties on leukemic cells and blocks differentiation, thus it has been regarded as a putative therapeutic target [11, 12]. Indeed, in vitro and in vivo studies showed that a slight MYB drop is enough to block leukemic cell proliferation without affecting normal haematopoiesis [28]. The present study confirmed two previous reports of high MYB expression in PMF [29, 30] as it was detected in all cases at chronic phase with a rising trend at leukemic transformation (Fig. 1f).
In determining whether and how the aberrant EWSR1-MYB transcription factor played a role in rapid disease progression, paired longitudinal studies showed that the EWSR1-MYB fusion was closely linked to leukemic transformation, as it was not detected at diagnosis and disappeared after treatment. As a functional consequence of MYB deregulation we investigated BCL2 expression as it is a well-known MYB target which acts as an anti-apoptotic onco-suppressor. Recent findings in leukemia xenograft models showed that sustained MYB expression maintained high BCL2 expression and consequently, inhibited leukemic cell death [10]. In line with these data, we found that MYB as well as BCL2 expression was higher at leukemic transformation than after treatment (Fig. 1g).
Our patient responded to treatment by regressing from AML to PMF maintaining JAK2, TET2 and SRSF2 mutations. Therefore, the JAK2-positive clone, showed a constant allele burden in all disease phases, marking the PMF stem line which persisted throughout disease, while the EWSR1-MYB fusion was the hallmark of the leukemic clone. Whether EWSR1-MYB affected a JAK2 V617F cell or developed as an independent clone, could not be established. Interestingly Engle E.K. et al. [31], identified MYB mutations in a complex PMF subclonal branching.
Conclusion
We report a unique case of JAK2 V617F PMF. Rapid leukemia transformation, due to complex cytogenetic rearrangement, produced a previously undescribed EWSR1-MYB fusion that appeared to act as an aberrant transcription factor deregulating BCL2. Our study provided new insights to point to MYB as a good molecular target in patients presenting with high-risk PMF [11, 32].
Abbreviations
- AML:
-
Acute myeloid leukemia
- BM:
-
Bone marrow
- DBD:
-
DNA binding domain
- DHPLC:
-
Denaturing high performance liquid chromatography
- ET:
-
Essential thrombocytopenia
- FISH:
-
Fluorescence in situ hybridization
- Hb:
-
Haemoglobin
- I-FISH:
-
Interphase-FISH
- MCV:
-
Mean corpuscular volume
- Ph-MPN:
-
Philadelphia negative myeloproliferative neoplasia
- PMF:
-
Primary myelofibrosis
- PV:
-
Polycythemia vera
- qRT-PCR:
-
Quantitative reverse transcription-polymerase chain reaction
- RT-PCR:
-
Reverse transcription-polymerase chain reaction
- SNPa:
-
Single nucleotide polymorphism array
- TAD:
-
Transcriptional-activating domain
- WBC:
-
White blood cell
References
Kennedy JA, Atenafu EG, Messner HA, et al. Treatment outcomes following leukemic transformation in Philadelphia-negative myeloproliferative neoplasms. Blood. 2013;121(14):2725–33.
Finazzi G, Caruso V, Marchioli R, et al. Acute leukemia in polycythemia vera: an analysis of 1638 patients enrolled in a prospective observational study. Blood. 2005;105(7):2664–70.
Tam CS, Abruzzo LV, Lin KI, et al. The role of cytogenetic abnormalities as a prognostic marker in primary myelofibrosis: applicability at the time of diagnosis and later during disease course. Blood. 2009;113(18):4171–8.
Tefferi A, Pardanani A, Gangat N, et al. Leukemia risk models in primary myelofibrosis: an International Working Group study. Leukemia. 2012;26(6):1439–41.
Abdel-Wahab O, Manshouri T, Patel J, et al. Genetic analysis of transforming events that convert chronic myeloproliferative neoplasms to leukemias. Cancer Res. 2010;70(2):447–52.
Harutyunyan A, Klampfl T, Cazzola M, Kralovics R. p53 lesions in leukemic transformation. N Engl J Med. 2011;364(5):488–90.
Klampfl T, Harutyunyan A, Berg T, et al. Genome integrity of myeloproliferative neoplasms in chronic phase and during disease progression. Blood. 2011;118(1):167–76.
Lundberg P, Karow A, Nienhold R, et al. Clonal evolution and clinical correlates of somatic mutations in myeloproliferative neoplasms. Blood. 2014;123(14):2220–8.
Quintás-Cardama A, Kantarjian H, Pierce S, Cortes J, Verstovsek S. Prognostic model to identify patients with myelofibrosis at the highest risk of transformation to acute myeloid leukemia. Clin Lymphoma Myeloma Leuk. 2013;13(3):315–18.
Jing D, Bhadri VA, Beck D, et al. Opposing regulation of BIM and BCL2 controls glucocorticoid-induced apoptosis of pediatric acute lymphoblastic leukemia cells. Blood. 2015;125(2):273–83.
Uttarkar S, Dassé E, Coulibaly A, et al. Targeting acute myeloid leukemia with a small molecule inhibitor of the Myb/p300 interaction. Blood. 2016;127(9):1173–82.
Lieu YK, Reddy EP. Conditional c-myb knockout in adult hematopoietic stem cells leads to loss of self-renewal due to impaired proliferation and accelerated differentiation. Proc Natl Acad Sci U S A. 2009;106(51):21689–94.
Russo D, Pricolo G, Michieli M, et al. Fludarabine, arabinosyl cytosine and idarubicin (FLAI) for remission induction in poor-risk acute myeloid leukemia. Leuk Lymphoma. 2001;40(3-4):335–43.
Lahortiga I, De Keersmaecker K, Van Vlierberghe P, et al. Duplication of the MYB oncogene in T cell acute lymphoblastic leukemia. Nat Genet. 2007;39(5):593–5.
Schlaak M, Renner R, Treudler R, et al. CD30+ anaplastic lymphoma kinase-positive anaplastic large cell lymphoma with an unusual translocation t(11;22). Br J Dermatol. 2008;159(1):240–2.
Martini A, La Starza R, Janssen H, et al. Recurrent rearrangement of the Ewing’s sarcoma gene, EWSR1, or its homologue, TAF15, with the transcription factor CIZ/NMP4 in acute leukemia. Cancer Res. 2002;62(19):5408–12.
Jakovljević G, Nakić M, Rogosić S, et al. Pre-B-cell acute lymphoblastic leukemia with bulk extramedullary disease and chromosome 22 (EWSR1) rearrangement masquerading as Ewing sarcoma. Pediatr Blood Cancer. 2010;54(4):606–9.
Hawkins JM, Craig JM, Secker-Walker LM, Prentice HG, Mehta AB. Ewing’s sarcoma t(11;22) in a case of acute nonlymphocytic leukemia. Cancer Genet Cytogenet. 1991;55(2):157–62.
Cantile M, Marra L, Franco R, et al. Molecular detection and targeting of EWSR1 fusion transcripts in soft tissue tumors. Med Oncol. 2013;30(1):412.
Uren A, Toretsky JA. Ewing’s sarcoma oncoprotein EWS-FLI1: the perfect target without a therapeutic agent. Future Oncol. 2005;1(4):521–8.
Sandberg ML, Sutton SE, Pletcher MT, et al. Myb and p300 regulate hematopoietic stem cell proliferation and differentiation. Dev Cell. 2005;8(2):153–66.
Mucenski ML, McLain K, Kier AB, et al. A functional c-myb gene is required for normal murine fetal hepatic hematopoiesis. Cell. 1991;65(4):677–89.
Malaterre J, Carpinelli M, Ernst M, et al. Myb is required for progenitor cell homeostasis in colonic crypts. Proc Natl Acad Sci U S A. 2007;104(10):3829–34.
Pattabiraman DR, McGirr C, Shakhbazov K, et al. Interaction of c-Myb with p300 is required for the induction of acute myeloid leukemia (AML) by human AML oncogenes. Blood. 2014;123(17):2682–90.
Clappier E, Cuccuini W, Kalota A, et al. The C-MYB locus is involved in chromosomal translocation and genomic duplications in human T-cell acute leukemia (T-ALL), the translocation defining a new T-ALL subtype in very young children. Blood. 2007;110(4):1251–61.
Drier Y, Cotton MJ, Williamson KE, et al. An oncogenic MYB feedback loop drives alternate cell fates in adenoid cystic carcinoma. Nat Genet. 2016;48(3):265–72.
Bandopadhayay P, Ramkissoon LA, Jain P, et al. MYB-QKI rearrangements in angiocentric glioma drive tumorigenicity through a tripartite mechanism. Nat Genet. 2016;48(3):273–82.
Zuber J, Rappaport AR, Luo W, et al. An integrated approach to dissecting oncogene addition implicates a Myb-coordinated self-renewal program as essential for leukemia maintenance. Genes Dev. 2011;25(15):1628–40.
Steensma DP, Pardanani A, Stevenson WS, et al. More on Myb in myelofibrosis: molecular analyses of MYB and EP300 in 55 patients with myeloproliferative disorders. Blood. 2006;107(4):1733–5.
Guglielmelli P, Tozzi L, Pancrazzi A, et al. MicroRNA expression profile in granulocytes from primary myelofibrosis patients. Exp Hematol. 2007;35(11):1708–18.
Engle EK, Fisher DA, Miller CA, et al. Clonal evolution revealed by whole genome sequencing in a case of primary myelofibrosis transformed to secondary acute myeloid leukemia. Leukemia. 2015;29(4):869–76.
Amaru Calzada A, Todoerti K, Donadoni L, et al. The HDAC inhibitor Givinostat modulates the hematopoietic transcription factors NFE2 and C-MYB in JAK2(V617F) myeloproliferative neoplasm cells. Exp Hematol. 2012;40(8):634–45.
Acknowledgements
The authors would like to thank Dr G A Boyd for assistance in preparing the manuscript.
Funding
This work was funded by Associazione Italiana per la Ricerca sul Cancro (AIRC, IG-15525), Fondo per gli Investimenti della Ricerca di Base (FIRB 2011 RBAP11TF7Z_005), Programmi di Ricerca scientifica di rilevante Interesse Nazionale (PRIN), Associazione Sergio Luciani, Fabriano, Italy and Fondazione Cassa di Risparmio di Perugia (2014.0265.021).
Availability of data and materials
Not applicable.
Authors’ contributions
RLS and CM conceived and designed the study, analyzed the data, and wrote the manuscript; TP and DDG wrote the paper; TP and VP performed and analyzed FISH experiments; GB performed and analyzed SNP array; DDG and PG performed and analyzed RT-PCR and qRT-PCR; AGLF, FP, TI performed targeted mutational analysis and analyzed results; FF determined JAK2 V617F allelic burden. All Authors approved the manuscript.
Competing interests
Authors declare that they have no competing interests.
Consent for publication
Written informed consent was obtained from the patient’s relatives for publication of this Case report and any accompanying images. A copy of the written consent is available for review by the Editor of this journal.
Author information
Authors and Affiliations
Corresponding author
Additional files
Additional file 1:
Detailed materials/methods and results. (DOC 68 kb)
Additional file 2: Table S1.
FISH clones for chromosome 6 long arm breakpoint characterization. Table S2. FISH clones for chromosome 22 long arm telomeric breakpoint characterization. Table S3. FISH clones for chromosome 9 long arm breakpoint characterization. Table S4. Genes, amplicons and primers for mutational analysis. (DOC 991 kb)
Additional file 3: Figure S1.
Multi-FISH analysis showed a complex rearrangement between chromosomes 6, 9 and 22 (yellow arrows). Figure S2. A) Chromosome 6q23 breakpoint within MYB. B) Chromosome 22q11.2 breakpoint within EWSR1. Figure S3. A) SNPa findings at PMF diagnosis: no CNV with 100 and 50 Kb filters (left panel) but LOH at 12q (right panel, black arrow). B) SNPa findings at leukemic transformation: a genomic loss, at 22q11.2, was identified with a 50 Kb filter (left panel, red arrow); LOH at 12q (right panel, red arrow). (DOC 3447 kb)
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated.
About this article
Cite this article
Pierini, T., Di Giacomo, D., Pierini, V. et al. MYB deregulation from a EWSR1-MYB fusion at leukemic evolution of a JAK2 V617F positive primary myelofibrosis. Mol Cytogenet 9, 68 (2016). https://doi.org/10.1186/s13039-016-0277-1
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s13039-016-0277-1