Abstract
Introduction
COVID-19 has spread across the African continent, including Niger. Yet very little is known about the phenotype of people who tested positive for COVID-19. In this humanitarian crises region, we aimed at characterizing variation in clinical features among hospitalized patients with COVID-19-like syndrome and to determine predictors associated with COVID-19 mortality among those with confirmed COVID-19.
Methods
The study was a retrospective nationwide cohort of hospitalized patients isolated for COVID-19 infection, using the health data of the National Health Information System from 19 March 2020 (onset of the pandemic) to 17 November 2020. All hospitalized patients with COVID-19-like syndrome at admission were included. A Cox-proportional regression model was built to identify predictors of in-hospital death among patients with confirmed COVID-19.
Results
Sixty-five percent (472/729) of patients hospitalized with COVID-19 like syndrome tested positive for SARS-CoV-2 among which, 70 (15%) died. Among the patients with confirmed COVID-19 infection, age was significantly associated with increased odds of reporting cough (adjusted odds ratio [aOR] 1.02; 95% confidence interval [CI] 1.01–1.03) and fever/chills (aOR 1.02; 95% CI 1.02–1.04). Comorbidity was associated with increased odds of presenting with cough (aOR 1.59; 95% CI 1.03–2.45) and shortness of breath (aOR 2.03; 95% CI 1.27–3.26) at admission. In addition, comorbidity (adjusted hazards ratio [aHR] 2.04; 95% CI 2.38–6.35), shortness of breath at baseline (aHR 2.04; 95% CI 2.38–6.35) and being 60 years or older (aHR 5.34; 95% CI 3.25–8.75) increased the risk of COVID-19 mortality two to five folds.
Conclusion
Comorbidity, shortness of breath on admission, and being aged 60 years or older are associated with a higher risk of death among patients hospitalized with COVID-19 in a humanitarian crisis setting. While robust prospective data are needed to guide evidence, our data might aid intensive care resource allocation in Niger.
Similar content being viewed by others
Introduction
The novel coronavirus disease 2019 (COVID-19), a disease caused by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) has been declared a pandemic by the World Health Organization due to both the unprecedented levels of transmission and the magnitude of the epidemic [1]. As of 25 November 2020, 47 African countries were impacted with 1,451,296 total cases of COVID-19and 24,454 deaths reported [2]. Niger, one of the West African nations, announced its first case of COVID-19 in the capital city, Niamey, on 19 March 2020. As of 25 November 2020, there were 1,381 confirmed cases of the virus, including 70 deaths [3]. The pandemic in Niger, a Lake Chad region, is superimposed over various dynamics of insecurity and states of emergency due to constant attacks by armed groups, now lasting more than a decade [4]. The scale of this state of violence has been characterized by an ongoing humanitarian crisis in some areas, such as the Diffa region [5] that has weakened the health system in the country. Several health services have closed, and others have been overwhelmed by the high demand from locals as well as displaced and immigrant populations.
Although the literature on COVID-19 is growing at an exponential pace, there is still a shortage of observational studies from several African countries. However, local epidemiology is essential to enable the adaptation of the evidence-based response of COVID-19 at local levels as well as national and regional levels, once pooled [6, 7]. For example, in typical resource constrained settings, not every facility would have adequate intensive care beds, access to ventilators, continuous oxygen, CT scanners, or even basic biological testing kits. Thus, characterizing admitted patients who require an intensive care unit (ICI) bed, and using these characteristics in a risk assessment, can help to manage current resources, and plan for future needs [8].
In this particular context of Niger, we aimed to describe the clinical characteristic of patients hospitalized with COVID-19 like syndrome to compare the variation in clinical features among all hospital patients quarantined for COVID-19 infection and determine independent factors associated with COVID-19 mortality among the patients with confirmed COVID-19, to help inform triage decisions and proper allocation of medical resources.
Methods
Study design, setting and population
We conducted a retrospective cohort study in Niger among hospitalized patients quarantined for COVID-19 infection; from 19 March 2020 (onset of the pandemic) to 17 November 2020. Niger is a large nation in the heart of the Sahel region with a growing population estimated at 24,811,942 in 2020 [9]. Despite major measures taken over the last decade by the government of Niger to reduce poverty in the country, the severe poverty rate had remained considerable at 41.4% in 2019, impacting more than 9.5 million people. In recent years, Niger has also faced a major influx of refugees fleeing war in adjacent regions, especially from Nigeria and Mali (221,671 refugees and 196,717 displaced people, primarily in Diffa and Maradi in 2019) [10]. In Niger, the health sector is structured in three tiers: the central level determining the general plan and running national hospitals and health centers; the regional level, including the eight Directions Generales de la Santé Publique (DRSP), defined by the six regional hospitals and two comparison centers; and the third level includes 42 Equipes Cadres du District (ECD) in 42 district hospitals and the associated network of 578 centre de santé intégrés and 1201 aires de santé. All symptomatic COVID-19 patients are managed at level 1 and 2. In our study, we included all patients from all eight political regions (Niamey, Agadez, Diffa, Dosso, Maradi, Tahaoua, Tillaberi and Zinder) hospitalized in level 1 and 2 regardless of age or nationality, and whose health data were available in the National Health Information System (DHIS2). This information was supplemented as needed by consultation registers. All outpatients were excluded regardless of COVID-19 results. Hospitalized patients with missing data on the outcome of interest were also excluded.
Data collection procedures and variables definition
From the health data of the DHIS2, we collected explanatory variables in a Microsoft Excel sheet. These variables included socio-demographic data (age, sex, profession), travel information during the past 14 days, contact with a patient with confirmed COVID-19, history and type of comorbidity (cardiovascular and hypertension, obesity, diabetes, HIV-infection, tuberculosis, asthma and other chronic respiratory diseases, chronic kidney diseases and any type of cancer), symptoms at admission (fever/chills, sore throat, cough, rhinorrhea, shortness of breath, digestive signs, headache, asthenia/ fatigue, musculoskeletal pain and thoracic pain) as well as the time between first symptoms occurrence and the first visit at a recognized health center. We collected and used the result of SARS-CoV-2 RT-PCR testing to to distinguish between positive and negative isolated hospitalized patients with suspected COVID-19 infection. One dependent variable, hospitalization outcome (alive or dead or unknown), was also collected.
Data analysis
We described the data in terms of frequencies and percentages, means and standard deviations (SDs), or medians and interquartile ranges (IQRs). Chi-square (exact), t-tests and Wilcoxon rank sum tests were applied to test for associations, where applicable. To identify independent predictors associated with the risk of experiencing specific respiratory symptoms (cough, sore throat, shortness of breath, thoracic pain, and rhinorrhea) at admission, we built a multivariable logistic regression, using a backward stepwise approach, model. This model was complemented by manual selection of a priori variables of clinical or epidemiological relevance. The likelihood of overall survival between age groups was determined using the Kaplan–Meier method. We used univariate and multivariate Cox-proportional regression modeling to identify predictors independently associated with the odds of mortality among hospitalized patients with confirmed COVID-19 infection. The final model contained age (as continuous), sex and other predictors of significance (p < 0.05), defined by a stepwise model method. The variables included in the final model were checked for proportionality assumptions and no first-order interactions were found. The strength of the relationship was represented as adjusted odds ratios (aOR) for multivariate logistic regression or adjusted hazard ratio (aHR) for Cox-proportional regression and corresponding 95% confidence intervals (CI). Reported p-values are exact and two-tailed, and a value < 0.05 was considered statistically significant. Stata software version 14.1 (Stata, College Station, TX) was used for all data analysis.
Results
Characteristics of patients hospitalized for suspected COVID-19 infection
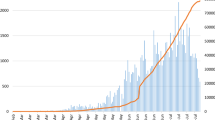
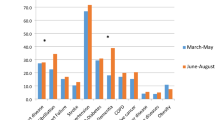
Of the 8393 persons screened during the study period, 1316 (16%) tested positive for COVID-19. Of the 729 hospitalized patients with COVID-19-like syndrome, 472 (65%) tested positive for the disease. Of these, 70 (15%) died with SARS-CoV-2 infection (Fig. 1). The details of the characteristics of the patients can be found in Table 1. Patients hospitalized for a suspected COVID-19 infection who remained negative for SARS-CoV-2 were significantly younger (median and interquartile range (IQR), 40 (27–60) years) compared to those who tested positive for SARS-CoV-2 but remained alive (median (IQR) 45 (29–59) years) and those who died (median (IQR) 65 (29–59) years). No such differences were observed in gender-based analysis, but patients hospitalized for suspected COVID-19 infection who tested positive were more likely to be working in private sectors. While only 120/729 (16%) of hospitalized patients with suspected COVID-19 indicated recent international travel within 14 days prior, 62/729 (13%) of these patients were quarantined (with suspected infection) at entry points and 125/729 (18%) were in contact with a person with confirmed COVID-19 infection. Among this cohort, a history of comorbidity was common (31%) and more prevalent among those who tested positive for COVID-19, or those who died; with cardiometabolic diseases and chronic respiratory illnesses being the most frequently reported. Overall, the time between showing first symptoms and the first hospital visit was shorter in patients who tested positive for COVID-19 than those who tested negative. The most frequent symptoms reported were cough (176/729; 24%), fever/chills (165/729; 23%) and shortness of breath (118/729; 16%). Half of patients who died had presented with fever/chills and/or shortness of breath; the mean time to death was just under five days.
Predictors of respiratory symptoms in in-patients with COVID-19 infection
Table 2 displays predictors of respiratory symptoms among hospitalized patients with confirmed SARS-CoV-2 infection. Every increase in year of age was significantly associated with increased odds of reporting cough (aOR 1.02; 95% CI 1.01–1.03), fever/chills (aOR 1.02; 95% CI 1.02–1.04) but was also associated with a slightly reduced in risk of reporting rhinorrhea (aOR 0.97; 95% CI 0.95–0.99). Men were more likely to report cough (aOR 1.55; 95% CI 1.0–2.50) and thoracic pain (aOR 2.26; 95% CI 0.90–5.65) compared to women but the difference was not statistically significant for thoracic pain. Patients with any history of comorbidity had 1.59 (95% CI 1.03–2.45) and 2.03 (95% CI: 1.27–3.26) higher odds of presenting cough and shortness of breath on admission, respectively. Every day delayed between the first symptom and first hospital visit was independently associated with higher odds of presenting fever/chills at admission (aOR 1.06; 95% CI 1.01–1.11).
Predictors of mortality in in-patients with COVID-19 infection
Table 3 and Fig. 2 depict predictors associated with death among hospitalized patients with confirmed COVID-19 infection. After holding constant other variables in the model, every one-year increase in age was independently associated with the hazard of death (aHR 1.05; 95% CI 1.03–1.08) among hospitalized patients with COVID-19 infection. In addition, compared to patients aged below 60 years, patients aged 60 years or above exhibited a more than fivefold increase in the hazard of death (aHR 5.34; 95% CI 3.25–8.75). No sex difference was observed in the hazards of death between males and females. The hazard of death among hospitalized patients with SARS-CoV-2 infection and a history of any comorbidity was more than double than that for those without history of any comorbidity (aHR 2.04; 95% CI 2.38–6.35). Similarly, patients admitted with baseline shortness of breath had significantly higher hazard of death than patients without baseline shortness of breath at admission (aHR 2.09; 95% CI 1.22–3.57).
Kaplan–Meier curves displaying the estimated survival time stratified by age of hospitalized patients with laboratory confirmed COVID-19 infection in Niger from March 2020 to November 2020. Note: Days since hospital admission is extended to 45 days corresponding to the longest hospitalization. The steps in the graph correspond to patient’s death-points. Discharged patients were censored at time of discharge
Discussion
In this first retrospective cohort study from Niger, we report the phenotype of patients hospitalized for COVID-19 suspicion, predictors associated with the odds of respiratory symptoms and predictors associated with the hazards of death among those who tested positive for COVID-19. We found that predictors independently associated with in-hospital mortality in relation to COVID-19 infection were age (mainly 60 years or older), history of comorbidity and baseline shortness of breath.
Socio-demographic and clinical parameters linked to the severity and mortality of COVID-19 have been widely reviewed [11,12,13,14,15]. Age is considered to be strongly correlated with COVID-19 outcomes globally [14, 16], but little is known about the situation on the African continent. A recent systematic review (n = 15 studies) reported that elderly adults and people with comorbidities suffer severe forms of COVID-19 and are at increased risk of hospitalization and death [17]. We found that patients hospitalized with a suspected COVID-19 infection who remained negative for SARS-CoV-2 were significantly younger than those who tested positive for SARS-CoV-2, as well as those who died. In addition, we found that every increase in year of age, was significantly associated with higher odds of reporting cough or fever/chills but was associated with a slightly reduced in risk of reporting rhinorrhea. Humans have developed natural immunity and immunological memory to withstand repeated infections. However, dysregulated adaptative immunity associated with the ageing process is known to increase the risk of morbidity following a decline in the immune system [18]. For example, roughly 90% of the excess deaths for seasonal influenza happen in elderly people. There is progressive lymphopenia with CD4 + T-cell attrition in the aging immune system, and reduced regulatory T-cell activity that contribute to homeostatic proliferation of lymphocytes with autoimmune tendency and unnecessary inflammatory responses [19]. Lymphopenia is a hallmark of SARS-CoV-2 infection [20] and is affected by several variables such as those that impact survival. Infection, such as with COVID-19, then exacerbates the imbalanced aged immune system, thereby exacerbating the loss of CD4 + T cells and inflammatory macrophage reaction [19].
In agreement with our findings, a study of 1028 confirmed cases of COVID-19 from Africa has also identified chronic diseases as an independent factor associated with death among patients infected with SARS-CoV-2 [21]. Chronic conditions are often associated with a sub-clinical level of inflammation, weakened innate immune responses and a strong ACE-2 receptor facilitating SARS-CoV-2 entry into the host cells [11, 13]; and correlating with COVID-19 severity [22]. These comorbidities carry the COVID-19 patient through a vicious infectious life cycle (amplification of cytokine storm) and are significantly correlated with severe morbidity and mortality [13, 23]. In our cohort, a history of comorbidity was common and more prevalent among those who tested positive for COVID-19; cardiometabolic diseases (cardiovascular diseases, hypertension, and diabetes) and chronic respiratory illnesses (COPD and asthma) were the most frequently reported. This is in line with previous data [24] (participants, n = 202,005 patients with COVID-19) showing any type of chronic comorbidity, hypertension/cardiovascular disease, diabetes, and respiratory diseases were the most prevalent chronic comorbid conditions. As in our study, polymorbidity was also common. However, the extent to which comorbidities influence the pandemic remains questionable. Previous research synthesis have shown some methodological limitations by using preprint data and limited global clinical data [11, 25]. A more recent meta-analysis of published data from large cohorts from across the world reported that hypertension, diabetes, and cancer significantly exacerbate the severity of COVID-19 in patients resulting in death, however, chronic kidney diseases contributed the most to death [11]. Analyzing the association between chronic comorbidity and COVID-19 severity/fatality by country of residence, Zhou et al. found that such odds were highly and significantly increased for obesity in France (compared to that in USA, UK and China), for hypertension/ cardiovascular diseases in China and for diabetes in the USA [26]. HIV-infection and/or tuberculosis independently predicted the hazard of death in South Africa [12] and of severity in Ethiopia [27], but not in studies from the USA [15, 28]. As such, the call by The African Forum for Research and Education in Health [6, 7] to pool COVID-19 data from African countries very necessary to enhance COVID-19 epidemiology in Africa to ensure an adaptive response at both hospital and community levels.
COVID-19 typical symptoms include fever, cough and shortness of breath [29,30,31,32]. The most frequent symptoms reported in our cohort were cough, fever/chills and shortness of breath with half of patients who died exhibiting fever/chills and/ or shortness of breath at presentation. Furthermore, shortness of breath was also a predictor of death. Latest evidence indicate that a number of patients with serious COVID-19 might have the cytokine storm syndrome [23]. The cytokine storm syndrome might explain COVID-19-related clinical symptoms and signs such as fever, cough/sputum, shortness of breath, faster respiratory rates, stuffy nose and generalized malaise, as these are common to other diseases-associated cytokine storm syndrome [33]. However, shortness of breath (regardless of degree of severity) has been identified as critically important core outcomes by more than 9,300 patients, health professionals, and the public from 111 countries in the global Coronavirus Disease 2019 Core Outcome Set Initiative [34]. This is important since it will help to capture the dynamic nature of COVID-19, illustrate the debilitating severity, and measure the progress in the resumption of daily activities. The congestion of the intensive care unit can be correlated with the fatality of COVID-19 [35]. Prioritizing patients in need of intensive treatment is required to reduce the death rate during the pandemic [36]. Therefore, knowing the predictive potential of baseline symptoms in a deadly pandemic is particularly valuable in settings with resource shortages, such as rural Africa. In agreement with many prognosis scores simply excluding laboratory/imagery data (machine learning model or not), shortness of breath/ dyspnea significantly predicted COVID-19 severity (hospitalization, stay in intensive care and or death) [8, 35, 36]. Moreover, in one model, laboratory data only predicted clinical deterioration while baseline clinical data such age and shortness of breath/ dyspnea significantly predicted the hazards of death among critically ill patients [37].
Although informative, our research highlights the need to design prospective studies to address the identified shortfalls. The use of registry-based data designed at the onset of the pandemic (at risk of missing data) is one of the main shortcomings of our research. This did not allow us to provide a full understanding of comorbidity (e.g., very low prevalence of reported obesity and HIV) and, as a result, we did not include each comorbidity in the updated models to prevent misleading interpretation. Similarly, roughly 2% of patients hospitalized for COVID-19 missed final clinical outcome but no difference was observed for other baseline characteristics compared to included patients. Moreover, COVID-19 clinical severity, laboratory and imaging results were not routinely recorded in the national public health record and were consequently not included in this first study. Although one might assume that hospitalized patients were more likely to present with moderate to severe COVID-19 stage we also acknowledge that not considering this information might bias our findings either away, or towards the true effect. However, our research included a comprehensive data from all eight provinces in Niger and thus offered a thorough real-world description of COVID-19 epidemiology in this humanitarian setting. Moreover, while this is the first report from Niger, we are also the first to compare the variation in clinical features among all hospitalized patients quarantined for COVID-19 infection, considering this region has the highest burden of other respiratory diseases in Africa.
Conclusion
In this retrospective study, respiratory distress upon admission, as measured by shortness of breath, high-risk age groups, and comorbidities significantly predicted mortality among COVID-19 patients hospitalized in Niger. In addition, by following the inpatient route during COVID-19 pandemic, we have characterized the clinical profile of patients needing extensive care in resource-constrained settings to assist risk-adapted choices on the allocation of medical resources. Further research may concentrate on converting existing data into a meaningful prognostic score for physicians working in conflict zones.
Availability of data and materials
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Abbreviations
- ACE-2:
-
Angiotensin-converting enzyme 2
- aHR:
-
Adjusted hazards ratio
- aOR:
-
Adjusted odds ratio
- CD4:
-
Cluster of differentiation 4
- COPD:
-
Chronic obstructive pulmonary diseases
- COVID-19:
-
Novel coronavirus disease 2019
- DHIS2:
-
District health information software 2
- DRSP:
-
Directions générales de la santé publique
- ECD:
-
Equipes cadres du district
- HIV:
-
Human immunodéficience virus
- RT-PCR:
-
Real time polynuclear chain reaction
- SARS-CoV-2:
-
Severe acute respiratory syndrome coronavirus 2
- UK:
-
United Kingdom
- USA:
-
United State of America
References
WHO Director-General’s opening remarks at the media briefing on COVID-19 - 11 March 2020. https://www.who.int/director-general/speeches/detail/who-director-general-s-opening-remarks-at-the-media-briefing-on-covid-19---11-march-2020. Accessed 25 Nov 2020.
Coronavirus (COVID-19). In: WHO | Regional Office for Africa. https://www.afro.who.int/health-topics/coronavirus-covid-19. Accessed 25 Nov 2020.
Niger: WHO Coronavirus Disease (COVID-19) Dashboard. https://covid19.who.int. Accessed 25 Nov 2020.
Happi C. Violent extremism in the Lake Chad Basin region: evolution and impact of Boko Haram. In: Africa Portal. 2020. https://www.africaportal.org/publications/violent-extremism-lake-chad-basin-region-evolution-and-impact-boko-haram/. Accessed 23 Dec 2020.
UNHCR Update Diffa Region: June 2020-Looking beyond the emergency toward development-Niger. In: ReliefWeb. https://reliefweb.int/report/niger/unhcr-update-diffa-region-june-2020-looking-beyond-emergency-toward-development. Accessed 23 Dec 2020.
Sam-Agudu NA, Rabie H, Pipo MT, et al. The critical need for pooled data on COVID-19 in African children: an AFREhealth call for action through multi-country research collaboration. Clin Infect Dis. 2021. https://doi.org/10.1093/cid/ciab142.
Nachega JB, Sam-Agudu NA, Budhram S, et al. Effect of SARS-CoV-2 infection in pregnancy on maternal and neonatal outcomes in Africa: an AFREhealth call for evidence through multicountry research collaboration. Am J Trop Med Hyg. 2020. https://doi.org/10.4269/ajtmh.20-1553.
Cho S-Y, Park S-S, Song M-K, Bae YY, Lee D-G, Kim D-W. Prognosis score system to predict survival for COVID-19 cases: a Korean Nationwide Cohort Study. J Med Internet Res. 2021;23:e26257.
Niger Population. Worldometer. 2021. https://www.worldometers.info/world-population/niger-population/. Accessed 24 Feb 2021.
Overview. In: World Bank. https://www.worldbank.org/en/country/niger/overview. Accessed 24 Feb 2021.
Ng WH, Tipih T, Makoah NA, Vermeulen J-G, Goedhals D, Sempa JB, Burt FJ, Taylor A, Mahalingam S. Comorbidities in SARS-CoV-2 patients: a systematic review and meta-analysis. MBio. 2021. https://doi.org/10.1128/mBio.03647-20.
Boulle A, Davies M-A, Hussey H, et al. Risk factors for COVID-19 death in a population cohort study from the Western Cape Province, South Africa. Clin Infect Dis. 2020. https://doi.org/10.1093/cid/ciaa1198.
Ejaz H, Alsrhani A, Zafar A, Javed H, Junaid K, Abdalla AE, Abosalif KOA, Ahmed Z, Younas S. COVID-19 and comorbidities: deleterious impact on infected patients. J Infect Public Health. 2020;13:1833–9.
Wu Z, McGoogan JM. Characteristics of and important lessons from the Coronavirus disease 2019 (COVID-19) outbreak in China: summary of a report of 72 314 cases from the Chinese Center for Disease Control and Prevention. JAMA. 2020;323:1239–42.
Kalyanaraman Marcello R, Dolle J, Grami S, et al. Characteristics and outcomes of COVID-19 patients in New York City’s public hospital system. PLoS ONE. 2020;15:e0243027.
CDC. Cases, data, and surveillance. Centers for Disease Control and Prevention. 2020. https://www.cdc.gov/coronavirus/2019-ncov/covid-data/investigations-discovery/hospitalization-death-by-age.html. Accessed 22 Feb 2021.
Gesesew HA, Koye DN, Fetene DM, et al. Risk factors for COVID-19 infection, disease severity and related deaths in Africa: a systematic review. BMJ Open. 2021;11:e044618.
Simon AK, Hollander GA, McMichael A. Evolution of the immune system in humans from infancy to old age. Proc Biol Sci. 2015;282:20143085.
Liu PP, Blet A, Smyth D, Li H. The science underlying COVID-19: implications for the Cardiovascular system. Circulation. 2020;142:68–78.
Zhou T, Su TT, Mudianto T, Wang J. Immune asynchrony in COVID-19 pathogenesis and potential immunotherapies. J Exp Med. 2020. https://doi.org/10.1084/jem.20200674.
Mohammed M, Muhammad S, Mohammed FZ, et al. Risk factors associated with mortality among patients with novel Coronavirus disease (COVID-19) in Africa. J Racial Ethnic Health Disparities. 2021;8:1267–72.
Reindl-Schwaighofer R, Hödlmoser S, Eskandary F, Poglitsch M, Bonderman D, Strassl R, Aberle JH, Oberbauer R, Zoufaly A, Hecking M. Angiotensin-converting enzyme 2 (ACE2) elevation in severe COVID-19. Am J Respir Crit Care Med. 2021. https://doi.org/10.1164/rccm.202101-0142LE.
Mehta P, McAuley DF, Brown M, Sanchez E, Tattersall RS, Manson JJ, HLH Across Speciality Collaboration, UK. COVID-19: consider cytokine storm syndromes and immunosuppression. Lancet. 2020;395:1033–1034
Mahumud RA, Kamara JK, Renzaho AMN. The epidemiological burden and overall distribution of chronic comorbidities in coronavirus disease-2019 among 202,005 infected patients: evidence from a systematic review and meta-analysis. Infection. 2020;48:813–33.
Oikonomidi T, Boutron I, Pierre O, Cabanac G, Ravaud P, the COVID-19 NMA Consortium. Changes in evidence for studies assessing interventions for COVID-19 reported in preprints: meta-research study. BMC Med. 2020;18:402
Zhou Y, Yang Q, Chi J, Dong B, Lv W, Shen L, Wang Y. Comorbidities and the risk of severe or fatal outcomes associated with coronavirus disease 2019: a systematic review and meta-analysis. Int J Infect Dis. 2020;99:47–56.
Abdela SG, Abegaz SH, Demsiss W, Tamirat KS, van Henten S, van Griensven J. Clinical profile and treatment of COVID-19 patients: experiences from an ethiopian treatment center. Am J Trop Med Hyg. 2020. https://doi.org/10.4269/ajtmh.20-1356.
Karmen-Tuohy S, Carlucci PM, Zervou FN, Zacharioudakis IM, Rebick G, Klein E, Reich J, Jones S, Rahimian J. Outcomes among HIV-positive patients hospitalized with COVID-19. J Acquir Immune Defic Syndr. 2020;85:6–10.
Azer SA. COVID-19: pathophysiology, diagnosis, complications and investigational therapeutics. New Microb New Infect. 2020;37:100738.
Nachega JB, Ishoso DK, Otokoye JO, et al. Clinical characteristics and outcomes of patients hospitalized for COVID-19 in Africa: early insights from the democratic Republic of the Congo. Am J Trop Med Hyg. 2020;103:2419–28.
Ruíz-Quiñonez JA, Guzmán-Priego CG, Nolasco-Rosales GA, et al. Features of patients that died for COVID-19 in a hospital in the south of Mexico: a observational cohort study. PLoS ONE. 2021;16:e0245394.
Zhong Z-F, Huang J, Yang X, et al. Epidemiological and clinical characteristics of COVID-19 patients in Hengyang, Hunan Province, China. World J Clin Cases. 2020;8:2554–65.
Yongzhi X. COVID-19-associated cytokine storm syndrome and diagnostic principles: an old and new Issue. Emerg Microb Infect. 2021;10:266–76.
Tong A, Baumgart A, Evangelidis N, et al. Core outcome measures for trials in people with Coronavirus disease 2019: respiratory failure, multiorgan failure, shortness of breath, and recovery. Crit Care Med. 2021;49:503–16.
Heo J, Han D, Kim H-J, Kim D, Lee Y-K, Lim D, Hong SO, Park M-J, Ha B, Seog W. Prediction of patients requiring intensive care for COVID-19: development and validation of an integer-based score using data from Centers for Disease Control and Prevention of South Korea. J Intensive Care. 2021;9:16.
Kim H-J, Han D, Kim J-H, et al. An easy-to-use machine learning model to predict the prognosis of patients with COVID-19: retrospective cohort study. J Med Internet Res. 2020. https://doi.org/10.2196/24225.
Qin W, Bai W, Liu K, Liu Y, Meng X, Zhang K, Zhang M. Clinical course and risk factors of disease deterioration in critically Ill Patients with COVID-19. Hum Gene Ther. 2021. https://doi.org/10.1089/hum.2020.255.
Acknowledgements
We thank clinics and all health workers across Niger involved in the response against COVID-19 pandemics. PDMCK is supported by the US National Institutes of Health (NIH)-Fogarty Postdoctoral Fellowship (University of Pittsburgh and Stellenbosch University): Grant No. 1D43TW010937-01A1.
Funding
The implementation of this activity was supported by the State of Niger and its partners.
Author information
Authors and Affiliations
Contributions
PDMCK, IA, BO, BPMA and CSW were responsible for the design; PDMCK, BO, EA, AM, AM, EKI, GAD, BJSM and TD acquired the data, PDMCK, IA and CSW analyzed the data; PDMCK wrote the first draft, and all the authors were responsible for the interpretation of data, writing the manuscript, approval of the version to be published, and agreement to be accountable for all aspects of the work. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
This study used routine program data retrospectively collected with no individual data identifiers revealed or used. The study was approved by the Niger, Ministry of Public health, and the Niger National COVID-19 Multisectoral Response Committee.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Katoto, P.D.M.C., Aboubacar, I., Oumarou, B. et al. Clinical features and predictors of mortality among hospitalized patients with COVID-19 in Niger. Confl Health 15, 89 (2021). https://doi.org/10.1186/s13031-021-00426-w
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s13031-021-00426-w