Abstract
Background
Glycemic control is an important issue in the treatment of diabetic patients. However, traditional methods, such as medication (the usual treatment), have limitations. Cognitive behavioral therapy (CBT) might be a useful option to help control the glycemic condition. The effects can be revealed by systemic review or meta-analysis of randomized clinical trials (RCT).
Methods
A systematic search and a meta-analysis for the RCT were done of the short- and long-term effects of CBT on the glycemic control of diabetic patients in a comparison with the usual treatment. Nineteen RCT studies and 3,885 diabetic patients were enrolled in this meta-analysis. Subgroup analyses of types 1 and 2 diabetes and individual and group CBT were also performed.
Results
Patients treated with CBT showed no significant difference in HbA1c when compared to the usual treatment within six months. However, CBT was more effective in reducing HbA1c when compared to usual treatment with at least six months of treatment duration [standardized mean difference: -0.44 (95% confidence interval (CI): -0.63 ~ -0.25), Z = 4.49]. Subgroup analysis of type 1 and 2 diabetic patients supported a long-term effect of CBT on glycemic control [standardized mean difference: -0.85 (95% CI: -1.19 ~ -0.10), Z = 2.23, standardized mean difference: -0.33 (95% CI:-0.47 ~ -0.19), Z = 4.52, respectively].
Conclusions
CBT would be a useful option for improving the glycemic control of diabetic patients undergoing long-term treatment. The advantages of the long-term effects of CBT should be considered by clinicians and staff.
Similar content being viewed by others
Introductions
Diabetes mellitus (DM) is an important, chronic physical illness commonly seen in clinical practice. It is associated with physical and mental health problems, which contribute to comorbidity and increase the difficulty of treatment [1]. Glycated hemoglobin (HbA1c) is an important index for glycemic control. The maintenance of an ideal HbA1c is helpful in decreasing the complications of DM, such as peripheral neuropathy or nephropathy. In recent years, even with the improvement of diabetes medications, the glycemic control rate has not reached an ideal standard, which contributes to the microvascular and macrovascular complications of DM [2]. Because a third of type 2 DM patients do not achieve their target HbA1c, [3] methods to improve glycemic control are needed in addition to the medication treatment. Up to 50% of people with DM have poor mental health, which should not be neglected as a crucial comorbidity [4]. According to the American Diabetes Association, the standard care for people with DM should not focus only on the standard medication treatment. Self-management and psychological interventions might also play a useful role in improving glycemic control [1]. Self-regulation and self-integration are important for people with DM to achieve better psychological adjustment to their disease by fostering self-growth and resilience [5] Even with the differences in the underlying psychological mechanisms of type 1 and 2 DM, the importance of establishing better self-regulation and self-integration is crucial for both subtypes. A randomized trial of people with type 2 DM supported nurse-led motivational interventions that improve self-management, self–efficacy, quality of life, and glycemic control, which indicates that psychological intervention improves glycemic control through a bidirectional interaction between mind and body [6]. Diabetes-related stress contributes to the psychological burden and emotional responses of people with DM, influencing them via psychological domains, such as cognition and behavior [7].
From the above literature, the dysfunctional cognitive and behavioral responses of people with DM are crucial issues for improving their standard care. Cognitive-behavioral therapy (CBT) is commonly used in the treatment of dysfunctional cognitive beliefs and behaviors. It improves the management of DM through the replacement of dysfunctional cognition with a self-helping and realistic cognition [8]. The results of a recent network meta-analysis of adult patients with type 1 DM showed that CBT has potential treatment effectiveness, even without significant treatment effects of psychological interventions [9]. For type 2 DM, the latest network meta-analysis showed CBT to have a high treatment effect, even though there has been an impression of minimal clinical benefits for psychological interventions [3]. However, other meta-analyses showed that CBT might be beneficial for reducing the HbA1c and fasting blood sugar of people with DM, [10, 11] especially for people with DM comorbid with depression [12]. Another meta-analysis showed that CBT-based interventions improve glycemic control and depression symptoms with medium to large effects in people with type 1 and 2 DM. [13]. A previous meta-analysis reported that CBT might have short to medium-term treatment effectiveness for reducing HbA1c. The lack of long-term treatment effectiveness might be a disadvantage of CBT for the treatment of people with DM [14]. However, there may be overlap in the primary studies, and the heterogeneity of results could be due to differences in the methods used in these systematic reviews. For example, several meta-analytic studies enrolled studies of DM patients with comorbid depression [11, 12]. One meta-analysis focused only on patients with type 1 DM [9]. Several meta-analytic studies surveyed both type 1 and 2 DM [10, 13, 14]. One mixed pure DM patients and DM patients with comorbid depression [13]. CBT content, duration and frequency, concurrent medication treatment, and other variables were difficult to standardize in the previous meta-analytic studies. Therefore, it will be important to conduct meta-analyses with less bias than the enrolled studies and a more standardized group of DM patients.
From the above literature, we found heterogeneity of the meta-analysis results based on previous meta-analytic studies of people with DM. This study was done to clarify and confirm the treatment duration-related treatment effects of CBT on the glycemic control of people with DM. In this systematic review and meta-analysis we enroll only the randomized clinical trials of pure CBT for people with DM. In addition, we clarify the short- and long-term treatment effects of CBT for people with DM. Finally, we perform separate subgroup analyses of type 1 and 2 DM to confirm the short- and long-term treatment effects on glycemic control.
Methods
Inclusion criteria
To find all articles related to the effects of CBT for patients with DM, we searched and collected prospective RCT articles from the following databases: PubMed, ScienceDirect, EmBase, Web of Science, Scopus databases, Cumulative Index for Nursing and Allied Health Literature (CINAHL), and the Cochrane Central Register of Controlled Trials (CENTRAL). The keywords included “cognitive behavioral therapy”, “cognitive”, “behavioral”, “diabetes”, “diabetic”, “randomized”, “clinical”, “controlled”, “trial”, “HbA1c”, “glycemic control” or “outcome”, “comparison”, “versus”, “treatment”, “usual treatment”. The article search was limited to those published or e-published online before July 2022. The date of our last search of PubMed, ScienceDirect, EmBase, and Web of Science was June 30, 2022. The date of our last search of Scopus databases, CINAHL, and CENTRAL was June 29, 2022.
The inclusion criteria were (1) Comparisons between CBT and usual treatment for diabetic patients. (2) Studies with an outcome of glycemic control and specific for HbA1c. (3) Studies with detailed data on the outcome of glycemic control and with data specific for HbA1c. (4) Clinical trials with a randomized design. (5) Articles written in English that were published in the journals of the science citation index database. All authors (DN, WX, and YL) participated the study and DN and WX performed the article selection process according to the inclusion criteria.
Risk of bias assessment
The Cochrane Handbook for Systematic Reviews and Interventions gave us the direction to conduct the current meta-analysis [15]. The risk of bias was evaluated by the following dimensions: bias arising from the randomization process, bias due to deviations from intended interventions, bias due to missing outcome data, bias in measurement of the outcome, and bias in selection of the reported result [16]. The results are reported according to the preferred reporting items for systematic reviews and meta-analyses (PRISMA) guidelines [17].
Extraction and critical appraisal of data
The data from the enrolled articles was collected by DN and WX. First, the baseline HbA1c of diabetic patients in the CBT and usual treatment groups. Second, the short-term (less than 6 months) endpoint HbA1c after CBT or usual treatment. Third, the long-term (more than 6 months) endpoint HbA1c after CBT or usual treatment. The rationale for our classification of short- and long-term was that we aimed to find the treatment duration-related effects of CBT on the HbA1c of people with DM. Fourth, the short- and long-term endpoint HbA1c of the CBT and usual treatment groups of patients with different subtypes of diabetes (types 1 and 2 diabetes). DN and WX assessed the abstracts to screen the articles and independently evaluated the full text version of the selected citations. The clinical outcome data was independently collected from text, tables, and figures of the enrolled articles by the two reviewers. The enrolled articles basically had data on the short-term endpoint HbA1c, long-term endpoint HbA1c, or subtypes of diabetes in the full text content. All authors (DN, WX, and YL) participated a collaborative review to resolve any discrepancies and to achieve agreement (kappa = 0.8) and participated in the review of the final results. The GRADE (grading of recommendations, assessment, development, and evaluation) approach was used by DX and YL to assess the certainty of evidence.
Meta-a and statistical analysis
The current meta-analysis was analyzed using the Cochrane Collaboration Review Manager Software Package (Rev Man Version 5.4). The CBT and usual treatment groups were compared to find which treatment would be superior for the glycemic control of diabetic patients. The overall effect size of the short- and long-term HbA1c endpoints were calculated as the weighted average of the inverse variance for the study-specific estimates. The weighted standardized mean difference was used to estimate numerical variables for the continuous variables. Heterogeneity was estimated using the χ2 distribution test, Higgins I2 index, Cochran's Q, and Tau square test. The synthesized results were conducted by pooling the data and using a random effects model meta-analysis. Subgroup analyses of the subtypes of diabetes were performed to confirm the treatment effects of CBT or usual treatment on glycemic control. In addition, forest plotting was done to demonstrate if the meta-analysis would favor CBT or usual treatment for improving the glycemic control of diabetes. Finally, a test for overall effect was done to calculate the Z and p-values for significance. The above meta-analysis steps were performed by DN and YL.
Results
Characteristics of the studies enrolled
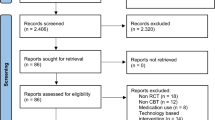
The article selection process is presented as a PRISMA flow diagram in Fig. 1. The eligibility of the 46 screened articles was assessed according to the content of the full text after the article selection process: 27 were eliminated (Fig. 1), leaving the data of 19 available for the qualitative analysis [18,19,20,21,22,23,24,25,26,27,28,29,30,31,32,33,34,35,36]. The characteristics of the enrolled 19 studies are summarized in Table 1. In addition, the risk of bias assessment for the short-term studies is presented in Fig. 2 and the risk of bias assessment for the long-term studies is presented in Fig. 3.
Search and selection flowchart. The current meta-analysis followed the PRISMA guidelines and used abstract and title selection to identify the potentially relevant literature and then to screen it. The full text of the screened literature was assessed to find studies eligible for inclusion in the final meta-analysis
Meta-analysis results for the effect of CBT vs usual treatment on HbA1c over the short-term
Of the 19 articles enrolled, 13 had outcome data for short-term HbA1c. The mean difference in the short-term HbA1c of the CBT and usual treatment groups in the random effects model was -0.05 [95% confidence interval (CI): -0.16 ~ 0.06], without significance (test for overall effect Z = 0.92, p = 0.36). Low heterogeneity was noted [Tau2 = 0.00, Chi2 = 8.90, df = 12 (p = 0.71), I2 = 0%].
Meta-analysis results for the long-term HbA1c of CBT vs usual treatment
Of the 19 articles, 13 had outcome data of the long-term endpoint HbA1c. The mean difference in the long-term HbA1c of the CBT group and usual treatment groups in the random effects model was -0.43 (95% CI: -0.58 ~ -0.27). Long-term HbA1c was significantly lower in the CBT group than in the usual treatment group (test for overall effect Z = 5.42, p < 0.00001). High heterogeneity was noted [Tau2 = 0.04, Chi2 = 43.67, df = 13 (p < 0.0001), I2 = 70%] (Fig. 4). The statistical heterogeneity might be a consequence of clinical heterogeneity (variability in the participants, interventions, and/or outcomes) or methodological heterogeneity (variability in study design, outcome measurement tools, and/or risk of bias) among the selected RCTs (https://training.cochrane.org/handbook/current/chapter-10#section-10-10). Due to the lack of patient-level data, we performed the following subgroup analyses of types 1 and 2 DM to survey for potential clinical heterogeneity.
Analysis of the effect on HbA1c of long-term CBT vs usual treatment for the type 1 DM subgroup
The mean long-term difference in the HbA1c of the CBT and usual treatment groups was -0.49 (95% CI: -0.80 ~ -0.17) for the type 1 DM subgroup, which indicates a significantly lower long-term endpoint HbA1c for the CBT group than was found for the usual treatment group (test for overall effect Z = 3.02, p = 0.003). High heterogeneity was noted [Tau2 = 0.07, Chi2 = 13.19, df = 4 (p = 0.01), I2 = 70%] (Fig. 5).
Analysis of the effect on HbA1c of long-term CBT vs usual treatment for the type 2 DM subgroup
The mean long-term difference in the HbA1c of the CBT and usual treatment groups was -0.46 (95% CI: -0.68 ~ -0.25) for the type 2 DM subgroup, which indicates significantly lower long-term HbA1c in the CBT group than was found in the usual treatment group (test for overall effect Z = 4.22, p < 0.0001). High heterogeneity was noted [Tau2 = 0.05, Chi2 = 20.65, df = 7 (p = 0.004), I2 = 66%] (Fig. 6).
Assessments of certainty (or confidence) in the body of evidence
Our evaluation found moderate confidence in the effect estimate.
Discussion
In the current meta-analysis, the treatment effect of CBT demonstrates a long-term effect on HbA1c. The significantly lower HbA1c after at least six months of study duration indicates that CBT expresses its potential and augmentation role in improving the glycemic control of diabetic patients. The subgroup analysis of people with type 1 and 2 DM over the long-term supports the potential and augmentation role of CBT for relieving the hyperglycemic status; however, the short-term effect was not significant for CBT on the glycemic control of diabetic patients. The subgroup analyses of types 1 and 2 DM individually also supported a lack of significant treatment effect over the short-term on endpoint HbA1c. Our meta-analysis results indicate that the augmentation and potential effects of CBT on the glycemic control of diabetic patients might be significant only over the long-term (more than six months).
The current meta-analysis failed to find a significant short-term effect of CBT on HbA1c, possibly for the following reasons. First, the definition of short-term effect in current meta-analyses is within six months, which might be different from the definition of previous meta-analyses. Second, the RCTs enrolled in the current meta-analysis might be more up-to-date than previous meta-analyses. However, future comprehensive review and meta-analysis will be needed to confirm the short-term effect of CBT on the HbA1c of people with DM.
Our results also corresponded to those of a previous meta-analysis that showed that CBT is effective in reducing fasting blood glucose, with a long-term effect [12]. Another meta-analysis of psychological interventions showed only that CBT has the potential to be effective for adult patients with type 1 DM [9]. In addition, a meta-analysis of people with type 2 DM showed that psychological interventions can effectively reduce HbA1c and that CBT has the highest probability of intervention effectiveness [3]. A recent meta-analysis of CBT-based intervention (not pure CBT) found improved glycemic control, with a moderate to large effect size, however, the heterogeneity of CBT may have limited the interpretations [13]. Another meta-analysis of diabetic patients with depression reported that CBT might be most effective for glycemic control within six months [11]. A meta-analysis focused on the content and style of CBT showed that, in group-based and face-to-face methods, psycho-education, behavioral, cognitive, goal-setting, and homework assignment strategies all significantly reduced HbA1c, which indicates that CBT of different content and styles have a similar effect on glycemic control [10]. These meta-analyses supported the importance of the augmentation therapy role of CBT for glycemic control and psychological symptoms [37]. The effect of CBT on glycemic control might be related to the reduction of negative thoughts, attitudes, and beliefs regarding diabetes and might lead to a change in dysfunctional self-care behaviors and improve subsequent glycemic control [14].
However, all these meta-analyses demonstrated only that the effectiveness of CBT on glycemic control might be limited to medium- or short-term duration [14]. Uchendu et al. stated that the lack of long-term effects might be due to not so many studies at that time having focused on the long-term treatment duration (2017). The long-term effects might appear if more long-term treatment studies of CBT are enrolled, [14] which might explain the reason for the long-term effects of CBT on people with DM in the current meta-analysis. These previous meta-analyses did not find a significant reduction of HbA1c over the long-term. Our meta-analysis enrolled more long-term studies (nine done since 2017) and showed a significant long-term effect on HbA1c, which corresponds to Uchendu et al.’s conclusion about the lack of significant long-term effects in previous meta-analyses. In the current meta-analysis, a significant short-term effect on glycemic control was not found: only a long-term effect was significant. Our subgroup analysis of people with type 1 and 2 DM also supports the significant role of long-term CBT in reducing HbA1c. Long-term CBT may be associated with gray matter changes of brain structures, such as the amygdala. It might reduce self-referential criticisms and might be helpful in reducing dysfunctional belief and behaviors [38]. The connectivity between the prefrontal cortex and amygdala might also be modulated by CBT, which might also reduce dysfunctional belief and behaviors [39]. Therefore, future study to clarify the short and long-term effects of CBT on glycemic control will be necessary.
There are several limitations to the current meta-analysis. First, even though we enrolled 19 studies with a relatively large sample size, only 13 with 3,296 total participants reported short-term effects and 14 with 3,562 participants reported long-term effects. The sample size is somewhat small, which might limit the generalization of our results. The enrollment of more randomized studies with more participants will be necessary in the future. Second, the variable content of CBT limits the results. This variation includes the frequency, intensity, duration, session content, total number of sessions, group or individual, real face-to-face or web-delivered, training of the therapists, and directions of CBT. These variables might interfere the solidness of evidence for CBT. Future studies with a more consistent structure and duration of CBT will be helpful in reducing such confounding factors. Third, the types of DM and the subject’s demographic data limit the interpretations of the current meta-analysis. Our meta-analysis enrolled studies of people with type 1 and 2 DM. Even the subgroup analysis of type 1 and 2 DM separately showed consistent, significant results for the long-term effects of CBT on glycemic control. However, the pathophysiology and epidemiology of type 1 and 2 DM are different, which might have contributed to different responses to CBT. In addition, many adolescents have type 1 DM, as was reported in several enrolled studies. The variability of the age of the diabetic patients in the current meta-analysis means that our results must be interpreted with caution. In addition, the predominance of female patients enrolled in the studies may have led to a gender-specific influence on the current results. Fourth, the lack of patient-level data may also limit the interpretation of our results due to the lack of a full evaluation of patient-level covariates in cross comparisons. It is impossible to confirm a possible subgroup effect related to patient age or to explore the impact of variation in the disease condition of the patients in the enrolled studies. Fifth, due to the lack of such data in most studies, the current meta-analysis compares only the difference of the HbA1c endpoints, not the change of HbA1c. Future studies of changes in HbA1c by CBT for diabetic patients are warranted. Sixth, our method of assignment to intervention may have led to bias in our results. For interventions over a long period, only subjects who did not drop out (good adherence patients) are included in the analysis. The intention-to-treat effect was not properly evaluated as a result, which might influence the interpretation. Seventh, the variations of the interventions and significant statistical heterogeneity might influence the validity of conducting a meta-analysis under such conditions. However, because we used the random effects model, it should be appropriate due to its ability to capture uncertainty resulting from heterogeneity among studies [40].
Availability of data and materials
Can be obtained from the corresponding author under a reasonable request.
Abbreviations
- CBT:
-
Cognitive behavioral therapy
- DM:
-
Diabetes mellitus
- HbA1c:
-
Hemoglobin A1c
- RCT:
-
Randomized clinical trial
References
American DA. Standards of medical care in diabetes–2013. Diabetes Care. 2013;36(Suppl 1):S11-66.
Koro CE, Bowlin SJ, Bourgeois N, Fedder DO. Glycemic control from 1988 to 2000 among U.S. adults diagnosed with type 2 diabetes: a preliminary report. Diabetes Care. 2004;27(1):17–20.
Winkley K, Upsher R, Stahl D, et al. Psychological interventions to improve glycemic control in adults with type 2 diabetes: a systematic review and meta-analysis. BMJ Open Diabetes Res Care. 2020;8(1):e001150.
The Lancet Diabetes E. Poor mental health in diabetes: still a neglected comorbidity. Lancet Diabetes Endocrinol. 2015;3(6):393.
Gois CJ, Ferro AC, Santos AL, et al. Psychological adjustment to diabetes mellitus: highlighting self-integration and self-regulation. Acta Diabetol. 2012;49(Suppl 1):S33-40.
Chen SM, Creedy D, Lin HS, Wollin J. Effects of motivational interviewing intervention on self-management, psychological and glycemic outcomes in type 2 diabetes: a randomized controlled trial. Int J Nurs Stud. 2012;49(6):637–44.
Chew BH, Shariff-Ghazali S, Fernandez A. Psychological aspects of diabetes care: Effecting behavioral change in patients. World J Diabetes. 2014;5(6):796–808.
Tolin DF. Is cognitive-behavioral therapy more effective than other therapies? A meta-analytic review Clin Psychol Rev. 2010;30(6):710–20.
Winkley K, Upsher R, Stahl D, et al. Systematic review and meta-analysis of randomized controlled trials of psychological interventions to improve glycaemic control in children and adults with type 1 diabetes. Diabetic Med. 2020;37(5):735–46.
Li Y, Storch EA, Ferguson S, Li L, Buys N, Sun J. The efficacy of cognitive behavioral therapy-based intervention on patients with diabetes: A meta-analysis. Diabetes Res Clin Pract. 2022;189: 109965.
Lu X, Yang D, Liang J, et al. Effectiveness of intervention program on the change of glycaemic control in diabetes with depression patients: A meta-analysis of randomized controlled studies. Prim Care Diabetes. 2021;15(3):428–34.
Li C, Xu D, Hu M, et al. A systematic review and meta-analysis of randomized controlled trials of cognitive behavior therapy for patients with diabetes and depression. J Psychosom Res. 2017;95:44–54.
Yang X, Li Z, Sun J. Effects of cognitive behavioral therapy-based intervention on improving glycaemic, psychological, and physiological outcomes in adult patients with diabetes mellitus: a meta-analysis of randomized controlled trials. Front Psychiatry. 2020;11:711.
Uchendu C, Blake H. Effectiveness of cognitive-behavioural therapy on glycaemic control and psychological outcomes in adults with diabetes mellitus: a systematic review and meta-analysis of randomized controlled trials. Diabetic Med. 2017;34(3):328–39.
Cumpston M, Li T, Page MJ, et al. Updated guidance for trusted systematic reviews: a new edition of the Cochrane Handbook for Systematic Reviews of Interventions. Cochrane Database Syst Rev. 2019;10:ED000142.
Sterne JAC, Savovic J, Page MJ, et al. RoB 2: a revised tool for assessing risk of bias in randomised trials. BMJ. 2019;366:l4898.
Knobloch K, Yoon U, Vogt PM. Preferred reporting items for systematic reviews and meta-analyses (PRISMA) statement and publication bias. J Cranio-Maxillo-Facial Surgery. 2011;39(2):91–2.
Henry JL, Wilson PH, Bruce DG, Chisholm DJ, Rawling PJ. Cognitive-behavioural stress management for patients with non-insulin dependent diabetes mellitus. Psychol Health Med. 1997;2(2):109–18.
Lustman PJ, Griffith LS, Freedland KE, Kissel SS, Clouse RE. Cognitive behavior therapy for depression in type 2 diabetes mellitus. A randomized, controlled trial. Ann Internal Med. 1998;129(8):613–621.
van der Ven NC, Hogenelst MH, Tromp-Wever AM, et al. Short-term effects of cognitive behavioural group training (CBGT) in adult Type 1 diabetes patients in prolonged poor glycaemic control. A randomized controlled trial Diabetic Med. 2005;22(11):1619–23.
Snoek FJ, van der Ven NC, Twisk JW, et al. Cognitive behavioural therapy (CBT) compared with blood glucose awareness training (BGAT) in poorly controlled Type 1 diabetic patients: long-term effects on HbA moderated by depression. A randomized controlled trial Diabetic Med. 2008;25(11):1337–42.
Amsberg S, Anderbro T, Wredling R, et al. A cognitive behavior therapy-based intervention among poorly controlled adult type 1 diabetes patients—A randomized controlled trial. Patient Educ Counsel. 2009;77(1):72–80.
Welschen LMC, van Oppen P, Bot SDM, Kostense PJ, Dekker JM, Nijpels G. Effects of a cognitive behavioural treatment in patients with type 2 diabetes when added to managed care; a randomised controlled trial. J Behav Med. 2013;36(6):556–66.
Safren SA, Gonzalez JS, Wexler DJ, et al. A randomized controlled trial of cognitive behavioral therapy for adherence and depression (CBT-AD) in patients with uncontrolled type 2 diabetes. Diabetes Care. 2014;37(3):625–33.
Menting J, Tack CJ, van Bon AC, et al. Web-based cognitive behavioural therapy blended with face-to-face sessions for chronic fatigue in type 1 diabetes: a multicentre randomised controlled trial. Lancet Diabetes Endocrinol. 2017;5(6):448–56.
Newby J, Robins L, Wilhelm K, et al. Web-based cognitive behavior therapy for depression in people with diabetes mellitus: a randomized controlled trial. J Medical Internet Res. 2017;19(5): e157.
Whitehead LC, Crowe MT, Carter JD, et al. A nurse-led education and cognitive behaviour therapy-based intervention among adults with uncontrolled type 2 diabetes: A randomised controlled trial. J Eval Clin Pract. 2017;23(4):821–9.
Fisher L, Hessler D, Polonsky WH, et al. T1-REDEEM: a randomized controlled trial to reduce diabetes distress among adults with type 1 diabetes. Diabetes Care. 2018;41(9):1862–9.
Stanger C, Lansing AH, Scherer E, Budney A, Christiano AS, Casella SJ. A web-delivered multicomponent intervention for adolescents with poorly controlled type 1 diabetes: a pilot randomized controlled trial. Ann Beh Med. 2018;52(12):1010–22.
Wei C, Allen RJ, Tallis PM, et al. Cognitive behavioural therapy stabilises glycaemic control in adolescents with type 1 diabetes-outcomes from a randomised control trial. Pediatr Diabetes. 2018;19(1):106–13.
Cummings DM, Lutes LD, Littlewood K, et al. Randomized trial of a tailored cognitive behavioral intervention in type 2 diabetes with comorbid depressive and/or regimen-related distress symptoms: 12-month outcomes from COMRADE. Diabetes Care. 2019;42(5):841–8.
Alshehri MM, Alothman SA, Alenazi AM, et al. The effects of cognitive behavioral therapy for insomnia in people with type 2 diabetes mellitus, pilot RCT part II: diabetes health outcomes. BMC Endocr Disord. 2020;20(1):136.
Zuo X, Dong Z, Zhang P, et al. Effects of cognitive behavioral therapy on sleep disturbances and quality of life among adults with type 2 diabetes mellitus: A randomized controlled trial. Nutr Metab Cardiovasc Dis. 2020;30(11):1980–8.
Xu C, Dong Z, Zhang P, et al. Effect of group cognitive behavioural therapy on psychological stress and blood glucose in people with type 2 diabetes mellitus: A community-based cluster randomized controlled trial in China. Diabetic Med. 2021;38(2): e14491.
Zhang HZ, Zhang P, Chang GQ, et al. Effectiveness of cognitive behavior therapy for sleep disturbance and glycemic control in persons with type 2 diabetes mellitus: A community-based randomized controlled trial in China. World J Diabetes. 2021;12(3):292–305.
Schmitt A, Kulzer B, Reimer A, et al. Evaluation of a stepped care approach to manage depression and diabetes distress in patients with type 1 diabetes and type 2 diabetes: results of a randomized controlled trial (ECCE HOMO Study). Psychotherapy Psychosom. 2022;91(2):107–22.
Vlachou E, Ntikoudi A, Owens DA, Nikolakopoulou M, Chalimourdas T, Cauli O. Effectiveness of cognitive behavioral therapy-based interventions on psychological symptoms in adults with type 2 diabetes mellitus: An update review of randomized controlled trials. J Diabetes Complications. 2022;36(5): 108185.
Mansson KNT, Salami A, Carlbring P, Boraxbekk CJ, Andersson G, Furmark T. Structural but not functional neuroplasticity one year after effective cognitive behaviour therapy for social anxiety disorder. Behav Brain Res. 2017;318:45–51.
Sandman CF, Young KS, Burklund LJ, Saxbe DE, Lieberman MD, Craske MG. Changes in functional connectivity with cognitive behavioral therapy for social anxiety disorder predict outcomes at follow-up. Behav Res Ther. 2020;129: 103612.
Dettori JR, Norvell DC, Chapman JR. Fixed-effect vs random-effects models for meta-analysis: 3 points to consider. Global Spine J. 2022;12(7):1624–6.
Acknowledgements
None.
Funding
None.
Author information
Authors and Affiliations
Contributions
Research idea and study design: DN and WX; data acquisition: DN and WX; data analysis/interpretation: DN and LY; statistical analysis: LY; supervision or mentorship: JM. DN, WX and LY takes responsibility that this study has been reported honestly, accurately and transparently, and accepts accountability for the overall work by ensuring that questions pertaining to the accuracy or integrity of any portion of the work are appropriately investigated and resolved. The author(s) read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
We consent for the publication of our manuscript if accepted by the journal.
Competing interests
None.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Dong, N., Wang, X. & Yang, L. The short- and long-term effects of cognitive behavioral therapy on the glycemic control of diabetic patients: a systematic review and meta-analysis. BioPsychoSocial Med 17, 18 (2023). https://doi.org/10.1186/s13030-023-00274-5
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s13030-023-00274-5