Abstract
Feline hyperthyroidism is a rather new disease, first reported from the North American east coast in 1979. The prevalence is increasing, especially in older cats, and hyperthyroidism is now reported worldwide as the most common feline endocrinopathy. Several studies have been performed trying to identify important etiological factors such as exposure to persistent organic pollutants, and especially brominated flame retardants, have been suggested to be of importance for the development of the disease. Recent studies have shown higher concentrations of these contaminants in serum of hyperthyroid cats in comparison to cats with normal thyroid status. However, other still unknown factors are most probably of importance for the development of this disease.
Similar content being viewed by others
Introduction
Before the end of the 1970s feline hyperthyroidism (FHT) was a rare disease generally caused by carcinomas of the thyroid gland resulting in an overproduction of thyroid hormones. However, in 1979 Peterson et al. [1] published a report on a new type of FHT with a still unknown etiology. Typically, FHT is more commonly seen in elderly cats (> 8 years), and rarely in younger cats (less than 5% of the cases).
Histologically the thyroid gland may demonstrate follicular adenoma or a multinodular hyperplasia in cases with FHT. Adenoma and hyperplasia may occur simultaneously within the same thyroid gland [2]. Since the first report from the North American east coast, this new disease has now been reported from all continents and it is showing an increasing prevalence [3,4,5,6]. In North America, FHT has increased from 0.3% in 1979 to 4.5% in 1985, whilst another study reported an increase from 0.1% in 1978–1982 to 2% in the period 1993–1997 [5, 7]. In an English study (n = 2276 cases) the overall prevalence of FHT was 2.4%, but in older cats (> 10 years) the prevalence was 8.7% [8].
At the same time, the severity of clinical signs, typically weight loss, polyphagia, polydipsia, hyper-activity, aggression, diarrhea, vomiting and tachycardia [9] has decreased, because of an increased awareness of FHT in the veterinary profession [10]. The ever-higher age of the cat population due to an increase in social status and better care in Europe, North America, Australia and Japan may account for some of the increasing number of FHT cases. However, this reasoning does not solely explain the increasing prevalence of FHT seen worldwide [7].
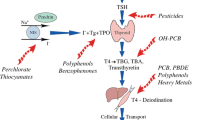
The physiological effects of the thyroid hormones T4 (thyroxine, 3,3′,5,5′-tetraiodo-l-thyrone) and T3 (triiodothyronine, 3,3′,5-tetraiodo-l-thyronine) on the mammalian metabolism are similar in all mammalian species studied. However, cats differ from other vertebrate species in respect of the plasma proteins transporting thyroid hormones. Cats appear to lack the protein thyroxin binding globulin (TBG), which is an important transport protein in other mammalians, therefore transthyretin (TTR) becomes the main thyroid transporting plasma protein [11].
In 2009, Dye et al. [12] introduced the hypothesis that anthropogenic chemicals could explain the increased numbers of reported FHT cases since the occurrence of FHT started in parallel with the introduction of flame retardants in household products, e.g. the polybrominated diphenyl ethers (PBDEs). The question was raised if these compounds have an etiological role and consequently, several studies started to analyze for anthropogenic chemicals in cat serum to search for a cause-effect relationship [12,13,14,15,16,17,18,19,20,21,22,23,24,25].
Search strategy
This critical review is summarizing the existing studies on persistent organic pollutants analyzed in cat samples, and is putting the gathered information in perspective to FHT, metabolism and other etiological studies. The search for relevant literature in PubMed (http://www.ncbi.nlm.nih.gov/PubMed) was using the terms “cat” and “feline” in combination with terms such as; “persistent organic pollutant, organohalogen compound, pesticide (HCH, PCP, DDT etc.), PCB, flame retardant (incl. subgroups such as brominated FR, organophosphorus FR, PDBE, HBCD, TBBPA, TBP etc.), hydroxylated metabolites, phenolic compound, poly- and perflouroalkyl substances (PFAS, incl. PFOA and PFOS), etc.” For the general discussion regarding feline hyperthyroidism, thyroid functionality and metabolism no general search strategy was applied. The authors’ gathered expertise in the field was used to find literature where knowledge gaps demanded it.
Feline metabolism of xenobiotics
Cats are hyper carnivores and have lower activity of certain cytochrome P450-enzymes involved in both phase I and II reactions, hence limiting their ability to metabolize certain xenobiotics [26]. In particular, the conjugation enzymes glucuronyl transferase and sulphotransferases in phase II have lower activities in cats compared to e.g. dogs and humans [27]. Sulfation of T4 appear to be insignificant in cats according to a study on thyroid hormone transport across the feline gut wall, which is an important route of elimination of T4 [28]. This will affect the metabolism and elimination of most xenobiotic compounds in the cat, including natural plant substances and toxins, substances easily metabolized by other animals. Glucuronidation accounts for detoxification of a number of phenolic substances in other species but clearance of these substances are slower in cats. A well-known example of this is the toxicity in cats of acetaminophen which is not toxic to dogs or humans in therapeutic doses. Glucuronides are also the main bile excretion form of T4 in cats [29], but if this has any etiological importance in FHT is not known. In euthyroid cats with normal thyroid function, excretion of glucoronidated T4 is obviously sufficient but if this mechanism is overloaded in FHT and if this further aggravate the condition is to our knowledge not known.
Epidemiology
Several epidemiological studies have been performed trying to identify important etiological factors explaining FHT. Even though several hypotheses have been tested, no single factor has yet been proved in these studies explaining the outbreak of this new disease.
In an early North American study, it was found that cats with FHT more often used litter boxes, had been treated for ectoparasites and were fed canned food [4]. The more frequent use of litter boxes was also observed by Wakeling et al. [6] as a risk factor for FHT, although this is probably more likely an indicator of a cat with an indoor life, as stated by the authors. In the study by Kass et al. [4] it was found that Siamese and Himalayan breeds were less prone to be affected by FHT, indicating that genetics are important for the development of FHT. Martin et al. [30] and Stephens et al. [8] also found that the Siamese breed had less risk of developing FHT. In the latter and more extensive study also Burmese, Persian and purebred cats were found to have an overall lower prevalence of FHT. In a New Zealand study, purebred cats also showed a lower risk than domestic short- and long-haired cats [31]. Further, these authors also suggested that females were at higher risk of developing FHT, which is not shown in other studies where no differences have been seen between genders (e.g. [7, 8]). In humans, females are at much higher risk developing toxic adenomatous goiter, which is the human equivalent of FHT [9].
Canned food was identified as a risk factor in several studies [6, 7, 30, 31]. The studies by Martin et al. [30] and Wakeling et al. [6] identified especially the fish flavored canned food to be associated to a higher incidence of FHT. On the other hand, De Wet et al. [3] found no correlation to the type of food eaten by the cats and FHT. However, more than 90% of the ingredients in cat food from the same producer is identical for all the different flavors [32].
Commonly, affected cats live a predominantly indoor life and have a high intake of canned food. A possible risk factor in the canned food is bisphenol A (BPA) a known endocrine disrupting compound (EDC) [33]. The content of BPA was analyzed in 15 canned Japanese cat food samples and found to be 60- to 70-fold below the limits established for human food by EU and Japanese authorities [34]. However, BPA has not been shown to bind to the transport protein TTR, which makes BPA less probable as an important etiological factor for FHT [47]. In addition, other possibly goitrogenic factors associated with the food have been identified in epidemiological studies performed around the world. Different vegetable substances like isoflavones and phthalates coming from soy and corn are identified as possible goitrogenic compounds in cat food [4, 26, 34, 35]. Isoflavones were determined in New Zealand cat food by Bell et al. [26] and found in concentrations known to have endocrine disrupting effects in other species. Several of these vegetable substances are eliminated via the glucuronidation pathway, a process known to be slow in cats due to their low activity of the enzyme glucuronyl transferase [27].
In commercial cat foods highly variable iodine content was found, an element essential for the synthesis of thyroid hormones [7]. In some cases the iodine content was clearly deviating from the recommendations given by the National Research Council (NRC) and Association of American Food Control Officials (AAFCO) for cat food.
In a review from South Africa it was suggested that there is a geographical difference in the incidence of FHT based on the different figures found in the open international literature [36]. However, Stephens et al. [8] found no significant differences between different regions of England. It is possible that the different figures from different countries presented in the literature are due to differences in data collection and classification of cases by the reporting clinicians.
The disparity of results obtained in epidemiological studies are difficult to evaluate due to e.g. different methods used to define study populations and other factors associated with the design of the studies. Furthermore, the recollection of the owner’s memory of living conditions, feeding habits of their cat several years ago is of crucial importance. However, a prudent evaluation of the different etiological factors found in published studies is that FHT is a multifactorial disease.
Endocrine disrupting chemicals in cats
After the report by Dye et al. [12], domestic cats have been used as sentinels for human exposure to indoor pollutants like persistent organic pollutants (POPs), and especially the PBDEs and polychlorinated biphenyls (PCBs). Hydroxylated metabolites of these halogenated compounds have structural similarities of the natural thyroid hormones T4 and T3 [33, 37]. Analyses of cat blood serum from different parts of the world have shown high levels of PBDEs, higher than found in humans from the same area [12, 15, 18, 19, 22, 24]. The usage of PBDEs were introduced to the market just prior to the first cases of FHT were recorded [38]. These pollutants are emitted from electrical and electronic equipment (computers, TV-sets etc.), furniture and textiles. The reason for cats being especially exposed to these environmental pollutants is their natural behavior to regularly groom their fur coat, thereby removing and ingesting dust particles [14, 19, 20, 39]. Dust is a matrix collecting volatile and semi-volatile chemicals released from the indoor products, flooring, textiles, furniture and any human activity (skin, hair) [40]. Toddlers will be exposed to these compounds to a higher degree than adults in the same household due to the dust ingestion associated to their hand-to-mouth behavior. Studies in California [14] and Sweden [41] have shown that cats have approximately 50 times higher concentrations of PBDE in their blood than human adults from the same geographical area.
The structural similarities between OH-metabolites of PBDEs and PCBs and thyroid hormones have initiated studies trying to find correlations between the concentrations of these compounds in cat blood and FHT [14, 16, 17, 20, 42]. Two studies have been able to statistically show a significant correlation between cats with FHT and the blood concentration of some PBDEs and PCBs. The congeners associated with FHT differed between the studies. Norrgran et al. [20] found association to BDE-99, BDE-153, BDE-183, and CB153, whereas Walter et al. [25] found associations to BDE17, BDE100, BDE47, BDE49 and CB131, CB153, CB174, CB180 and CB196. The latter study analyzed serum from cats living in the US and reported total median levels eight times higher than the first study analyzing serum from cats living in Sweden. The pattern of PBDE congeners found in cats and humans differ, suggesting different exposure sources of the pollutant [14, 19, 43] and/or different metabolic capacity. The pattern of PBDEs found in cat serum is similar to the pattern found in dust suggesting dust to be the exposure source. In the studies by Chow et al. [19, 44] and Guo et al. [14] no correlation was found between house dust levels, serum levels and the thyroid status of matched cats, possibly because the serum concentrations were not age adjusted for.
In a later study by Norrgran Engdahl et al. [39] paired samples of cat serum, feed and house dust were used, a significant correlation between serum levels and dust concentrations were found. This was the first time that dust could be confirmed as being a relevant exposure pathway of these compounds, although it has been hypothesized earlier as the major contamination source for POP in cats [14, 44].
In addition, the Swedish study analysed 28 different cat food brands (dry and wet cat food), and found a significant correlation between cat serum and cat food concentrations for the flame retardant decabromobiphenyl (BB209) and two phenolic compounds 2,4,6-tribromophenol (2,4,6-TBP) and 6-hydroxy-2,2′,4,4′tetrabromo-diphenylether (6-OH-BDE47), suggesting cat food to be an important exposure pathway for these compounds [39]. Both 2,4,6-TBP and 6-OH-BDE47 are natural compounds formed by marine plants and may enter the food chain via sea food [45, 46]. Further, it was shown that cat liver microsomes were unable to form the 2,4,6-TBP metabolite from the parent PBDEs by incubation, indicating that cat food may be the source of the 2,4,6-TBP found in serum [24]. These phenolic compounds have a high binding affinity to the transport protein TTR and can competitively replace the natural ligand to be transported to its target tissue [47]. Also, these compounds could be found at higher concentration and more frequently in wet food compared to dry food. This is in accordance with previous epidemiological observations that cats eating wet/canned food had a higher prevalence for FHT [6, 7, 30, 31]. In fact, serum levels of 6-OH-BDE47 were 2–50 times higher in Japanese stray cats compared to cats living in UK and Sweden, which could support this association as their food is likely more influenced by fish from the ocean [17, 18, 20, 39].
Other compounds with potency to bind to TTR are the per- and polyfluoroalkyl substances (PFASs), which are ubiquitously found in our indoor environment, coming from products with functions such as water and grease repellents (food packaging, kitchen utensils and outdoor clothing) or low surface tension (sprays in cleaning products, floor polishing etc.) [47]. Cats from the US [21, 48] had 2–4 times higher serum levels than cats from Sweden [49]. The US study could show that serum from hyperthyroid cats showed higher PFAS level (9.50 ng/mL) compared to nonhyperthyroid cats (7.24 ng/mL), due to the levels of perfluorooctanoic acid (PFOA, P < 0.05). The Swedish study could demonstrate an association between serum levels of PFOA with the dust concentrations in their homes, confirming dust to be a relevant exposure pathway [49].
Recently, the non-halogenated organophosphate ester flame retardants (OPFR) was analyzed in cat serum [23]. It has been suggested that OPFR can enhance the T4 binding to human TTR, a mechanism not totally understood yet [50]. If this is also an effect in cats is not known to our knowledge. Many more chemicals, with TH disrupting potency and analyzed in dust have not yet been searched for in cat serum, e.g. phthalates, BPA, musk compounds, and parabens, commonly found in personal care products [39]. Considering the growing evidence of the endocrine effects of these compounds, and the increasing number of monitoring data in dust and serum (both for cats and humans) it is reasonable to attribute chemical exposure as contributing to the observed increased incidence of endocrine disorders in cats and humans, such as FHT.
Conclusions
The correlation between pet cats spending the majority of time indoor, hence having a considerable dust intake, and the increase in reported incidence of FTH could possibly be associated to the vast number of chemicals analyzed in house dust, several of them showing thyroid hormone disrupting potency. The correlation to cats eating wet/canned food and FHT may be associated to the naturally occurring 6-OH-BDE-47 and 2,4,6-TBP derived from the marine environment, or the presence of other possible phenolic compounds not yet identified in the wet cat food. Although there are indications that anthropogenic chemicals, such as PBDEs and other POPs, could have etiological importance for the development of FHT a plausible time course for development of the disease, and more detailed biochemical mechanisms are needed to fully elucidate the mode of action behind this rather new disease. A thorough understanding of their role in the etiopathogenesis of FHT would need longitudinal studies. To protect our animals, and our children it is of outermost importance to identify and regulate the mixture of EDCs in our indoor environment.
Availability of data and materials
Not applicable.
Abbreviations
- AAFCO:
-
Association of American food control officials
- BDE:
-
brominated diphenyl ethers
- BPA:
-
bisphenol A
- EDC:
-
endocrine disrupting compound
- FHT:
-
feline hyperthyroidism
- NRC:
-
National Research Council
- OPFR:
-
organophosphate ester flame retardants
- PBDE:
-
polybrominated diphenyl ethers
- PCB:
-
polychlorinated biphenyl
- POP:
-
persistent organic pollutants
- T3 :
-
triiodothyronine
- T4 :
-
thyroxine
- TTR:
-
transthyretin
References
Peterson ME, Johnson GF, Andrews LK. Spontaneous hyperthyroidism in the cat. In: Proceedings of the ACVIM, vol. 108; 1979.
Maxie MG. Neoplasma of the thyroid gland. In: Maxie MG, editor. Jubb, Kennedy, and Palmer’s pathology of domestic animals. 5th ed. St Louis: Saunders/Elsevier; 2007. p. 96.
De Wet CS, Mooney CT, Thompson PN, Schoolman JP. Prevalence and risk factors for feline hyperthroidism in Hong Kong. J Feline Med Surg. 2009;11:315–21.
Kass PH, Peterson ME, Levy J, James K, Baker DV, Cowgill LD. Evaluation of environmental, nutritional, and host factors in cats with hyperthyroidism. J Vet Intern Med. 1999;13:323–9.
Scarlett JM, Moise JN, Rayl J. Feline hyperthyroidism: a descriptive case–control study. Prev Vet Med. 1988;6:295–309.
Wakeling J, Everard A, Broadbelt D, Elliot J, Syme H. Risk factors for feline hyperthyroidism in the UK. J Small Anim Pract. 2009;50:406–14.
Edinboro CH, Scott-Moncriefff JC, Glickman LT. Potential relationship with iodine supplement requirements of commercial cat foods. J Feline Med Surg. 2010;12:672–9.
Stephens MJ, O’Neill DG, Church DB, McGreevy PD, Thomson PC, Brodbelt DC. Feline hyperthyroidism reported in primary-care veterinary practices in England: prevalence, associated factors and spatial distribution. Vet Rec. 2014. https://doi.org/10.1136/vr.102431.
Peterson ME, Ward CR. Etiopathological findings of hyperthyroidism in cats. Vet Clin Small Anim Pract. 2007;37:633–45.
Broussard JD. Changes in clinical and laboratory findings in cats with hyperthyroidism from 1983 to 1993. J Am Vet Med Assoc. 1995;206:302–5.
Larsson M, Pettersson T, Carlström A. Thyroid hormone binding in serum of 15 vertebrate species: isolation of thyroxine-binding globulin and prealbumin analogs. Gen Comp Endocrinol. 1985;58:360–75.
Dye J, Venier M, Zhu L, Ward CR, Hites RA, Birnbaum LS. Elevated PBDE levels in pet cats: sentinels for humans? Environ Sci Technol. 2007;41:6350–6.
Kunisue T, Tanabe S. Hydroxylated polychlorinated biphenyls (OD-PCBs) in the blood of mammals and birds from Japan: lower chlorinated OD-PCBs and profiles. Chemosphere. 2009;74:950–61.
Guo W, Park J-S, Wang Y, Gardner S, Baek C, Petreas M, et al. High brominated diphenyl ether levels in Californian house cats: house dust a primary source. Environ Toxicol Chem. 2012;31:301–6.
Mensching DA, Slater M, Scott JW, Ferguson DC, Beasly VR. The feline thyroid gland: a model for endocrine disruption by polybrominated diphenyl ethers (PBDEs)? J Toxicol Environ Health A. 2012;75:201–12.
Ali N, Malik RN, Mehdi T, Equani SAMAS, Javeed A, Neels H, et al. Organohalogenated contaminants (OHCs) in the serum and hair of pet cats and dogs: biosentinels of indoor pollution. Sci Total Environ. 2013;449:29–36.
Dirtu AC, Niessen SJM, Jorens PG, Covaci A. Organohalogenated contaminants in domestic cats’ plasma in relation to spontaneous acromegaly and type 2 diabetes mellitus: a clue for endocrine disruption in humans. Environ Int. 2013;57:60–7.
Mizukawa H, Nomiyama K, Nakasuto S, Yashimoro S, Hayashi T, Tashiro Y, et al. Species-specific differences in the accumulation features of organohalogen contaminants and their metabolites in the blood of Japanese terrestrial animals. Environ Pollut. 2013;174:28–37.
Chow K, Hearn LK, Zuber M, Beatty JA, Mueller JF, Barrs VR. Evaluation of polybrominated diphenyl ethers (PBDEs) in matched cat sera and house dust samples: investigation of a potential link between PBDEs and spontaneous feline hyperthyroidism. Environ Res. 2015;136:173–9.
Norrgran J, Jones B, Bignert A, Athanassiadis I, Bergman Å. Higher PBDE serum concentrations may be associated with feline hyperthyroidism in Swedish cats. Environ Sci Technol. 2015;49:5107–14.
Bost PC, Strynar MJ, Reiner JL, Zweigenbaum JA, Secoura PL, Lindstrom AB, et al. U.S. domestic cats as sentinels for perflouroalkyl substances: possible linkages with housing, obesity, and disease. Environ Res. 2016;151:145–53.
Mizukawa H, Nomiyama K, Nakasuto S, Iwata H, Yoo J, Kubota A, et al. Organohalogen compounds in pet dog and cat: do pets biotransform natural brominated products in food to harmful hydroxylated compounds? Environ Sci Technol. 2016;50:444–52.
Henriquez-Hernández LA, Carretón E, Camacho M, Montoya-Alonso JA, Boada LD, Bernal Martin V, et al. Potential role of cats as a sentinel species for human exposure to flame retardants. Front Vet Sci. 2017;4:79.
Mizukawa H, Nomiyama K, Nakasuto S, Yamamoto M, Ishizuka M, Ikenaka Y, et al. Anthropogenic and naturally produced brominated phenols in pet blood and pet food in Japan. Environ Sci Technol. 2017;51:11354–62.
Walter KM, Lin Y, Kass PH, Puschner B. Association of polybrominated diphenyl ethers (PBDEs) and polychlorinated biphenyls (PCBs) with hyperthyroidism in domestic felines, sentinels for thyroid hormone disruption. BMC Vet Res. 2017. https://doi.org/10.1186/s12917-017-1031-626.
Bell KM, Rutherfurd SM, Hendriks WH. The isoflavone content of commersially-available feline diets in New Zealand. N Z Vet J. 2006;54:103–8.
Hill K, Shaw I. Does exposure to thyroxin-mimics cause feline thyroid hyperplasia? Vet Rec. 2014. https://doi.org/10.1136/vr.g3754.
Hays MT, Hsu L, Kohatsu S. Transport of the thyroid hormones across the feline gut wall. Thyroid. 1992;2:45–56.
Myant NB. Excretion of the glucuronide of thyroxine in cat bile. Biochem J. 1966;99:341–6.
Martin KM, Rossing MA, Ryland LM, DiGiacomo RF, Freitag WA. Evaluation of dietary and environmental risk factors for hyperthyroidism in cats. JAVMA. 2000;217:853–6.
Olzak J, Jones BR, Pfeffer DU, Squires RA, Morris RS, Markwell PJ. Multivariate analysis of risk factors for feline hyperthyroidism in New Zealand. N Z Vet J. 2004;53:53–8.
Norrgran Engdahl J. Cats as biomarkers for exposure to POPs in home environment. Doctoral dissertation, Department of Environmental Science and Analytical Chemistry, Stockholm University; 2015. p. 30. ISBN 978-91-7649-190-4.
Zoeller RT. Environmental chemicals as thyroid hormone analogues: new studies indicate that the thyroid hormone receptors are targets of industrial chemicals? Mol Cell Endocrinol. 2005;242:10–5.
Kang J-H, Kondo F. Determination of bisphenol A in canned pet food. Res Vet Sci. 2002;73:177–82.
Court MH, Freeman LM. Identification and concentration of soy isoflavones in commercial cat foods. Am J Vet Res. 2002;63:181–5.
McLean JL, Lobetti RG, Schoeman JP. Worldwide prevalence and risk factors for feline hyperthyroidism: a review. JSAVA. 2014. https://doi.org/10.4102/jsava.v85i1.1097.
Peterson ME. Hyperthyroidism in cats: what’s causing this this epidemic of thyroid disease and how can we prevent it? J Feline Med Surg. 2012;14:804–18.
Holzworth J, Theran P, Carpenter JL, Harpster NK, Todoroff RJ. Hyperthyroidism in the cat: ten cases. JAVMA. 1980;176:345–53.
Norrgran Engdahl J, Bignert A, Jones A, Athanassiadis I, Bergman Å, Weiss JM. Cats’ internal exposure to selected BFRs and organochlorines correlated to house dust and cat food. Environ Sci Technol. 2017;51:3012–20.
Zhang J, Kamstra JH, Ghorbbanzadeh M, Weiss JM, Hamers T, Andersson PL. In silico approach to identify potential thyroid hormone disruptors among currently known dust contaminants and their metabolites. Environ Sci Technol. 2015;49:10099–107.
Kupryianchyk D, Hovander L, Jones B, Lindqvist NG, Eriksson S, Bergman Å. Hyperthyroidism, a new disease in cats - Is it caused by exposure to environmental organic pollutants? Organohalogen Compd. 2009;71:2720–5.
Meeker JD, Johnson PI, Camann D, Hauser R. Polybrominated diphenyl ether (PBDE) concentrations in house dust are related to hormone levels in man. Sci Total Environ. 2009;407:3425–9.
Norrgran J, Jones B, Lindquist N-G, Bergman Å. Decabromobiphenyl, polybrominated diphenyl ethers, and brominated phenolic compounds in serum from cats diagnosed with the endocrine disease feline hyperthyroidism. Arch Environ Contam Toxicol. 2012;63:161–8.
Chow K, Beatty JA, Barrs VR, Hearn LK, Zuber M. PBDEs and feline hyperthyroidism. Vet Rec. 2014;175:433–4.
Malmvärn A, Zebühr Y, Kautsky L, Bergman Å, Asplund L. Hydroxylated and methoxylated polybrominated diphenyl ethers and polybrominated dibenzo-p-dioxins in red alga and cyanobacteria living in the Baltic Sea. Chemosphere. 2008;2:910–6.
Haglund P, Löfstrand K, Malmvärn A, Bignert A, Asplund L. Temporal variations of polybrominated dibenzo-p-dioxin and methoxylated diphenyl ether concentrations in fish revealing large differences in exposure and metabolic stability. Environ Sci Technol. 2010;44:2466–73.
Weiss JM, Andersson PL, Zhang P, Simon E, Leonards PEG, Hamers T, et al. Tracing thyroid hormone disrupting compounds: database compilation and structure activity evaluation for an effect-directed analysis of sediment. Anal Bioanal Chem. 2015;407:5625–34.
Wang M, Guo W, Gardner S, Petreas M, Park J-S. Per- and polyfluoroalkyl substances in Northern California cats: temporal comparison and a possible link to cat hyperthyroidism. Environ Chem. 2018;37:2523–9.
Weiss JM, Jones B, Koekkoek J, Bignert A, Lamoree MH. Per- and polyfluoroalkyl substances (PFAS) in Swedish household dust and exposure to pet cats. Submitted to Environ Health; 2019.
Hill KL, Hamers T, Kamstra JK, Willmore WG, Letcher RJ. Organophosphate triesters and selected metabolites enhance binding of thyroxine to human transthyretin in vitro. Toxicol Lett. 2018;285:87–93.
Acknowledgements
The authors acknowledge Swedish Research Council FORMAS for financial support.
Funding
Drs. Jones and Weiss research is funded by the Swedish Research Council FORMAS (Grant No. 210-2012-131).
Author information
Authors and Affiliations
Contributions
JNE and JW searched the chemical literature and read the appropriate articles. BJ searched and read the veterinary literature. All three authors drafted the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated.
About this article
Cite this article
Jones, B., Engdahl, J.N. & Weiss, J. Are persistent organic pollutants important in the etiology of feline hyperthyroidism? A review. Acta Vet Scand 61, 45 (2019). https://doi.org/10.1186/s13028-019-0478-9
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s13028-019-0478-9