Abstract
The role of gut microbiota and its products in human health and disease is profoundly investigated. The communication between gut microbiota and the host involves a complicated network of signaling pathways via biologically active molecules generated by intestinal microbiota. Some of these molecules could be assembled within nanoparticles known as outer membrane vesicles (OMVs). Recent studies propose that OMVs play a critical role in shaping immune responses, including homeostasis and acute inflammatory responses. Moreover, these OMVs have an immense capacity to be applied in medical research, such as OMV-based vaccines and drug delivery. This review presents a comprehensive overview of emerging knowledge about biogenesis, the role, and application of these bacterial-derived OMVs, including OMV-based vaccines, OMV adjuvants characteristics, OMV vehicles (in conjugated vaccines), cancer immunotherapy, and drug carriers and delivery systems. Moreover, we also highlight the significance of the potential role of these OMVs in diagnosis and therapy.
Similar content being viewed by others
Key points
-
OMVs are nanosized proteoliposomes derived from the outer membrane of Gram-negative bacteria.
-
Based on the physiological characteristics of OMVs, The delivery of therapeutic cargos, such as miRNAs and proteins to tissues, has now been identified.
-
Also, Designing powerful nanocarriers has administered bioengineering to target particular delivery of therapeutics for OMVs
Introduction
Gut microbiota plays a crucial role in the absorption of minerals and nutrients, synthesizing enzymes, vitamins, amino acids, and modulating the immune system [1,2,3]. Besides, a growing body of evidence shows that bacterial dysbiosis contributes to the development of some disorders, such as inflammatory bowel disease (IBD), obesity, irritable bowel syndrome (IBS), diabetes, cancer, multiple sclerosis (MS), and neurological diseases [4, 5]. On the other hand, the interplay between gut microbiota and immune cells is involved in the homeostasis of the gastrointestinal (GI) tract, health maintenance, and infection prevention in the host [6,7,8].
The shedding process of membrane vesicles (MVs) has been characterized as an evolutionarily conserved mechanism across eukaryotes and prokaryotes for intercellular communications [9]. These nano-sized, spherical, and bilayer proteolipid extracellular MVs harbor subsets of lipids, proteins, nucleic acids, as well as metabolites [9]. According to the hosts that extracellular vesicles (EVs) are derived from, these molecules are differently named, such as outer MVs (OMVs) for Gram-negative microorganisms; MVs for Gram-positive microorganisms; and microvesicles or exosomes for mammalian cells [10,11,12,13,14]. In this regard, microbiota-derived EVs have been identified as a carrier in host-bacteria interplays that, in terms of immune receptors, cause immune reactions [15]. It has been documented that non-pathogenic and pathogenic Gram-negative bacteria can generate vesicles [16]. The analysis and characterization of OMVs indicate that bacterial pathogens generate these secretory components to translocate virulent ingredients such as toxins, adhesins, and immunomodulatory factors, leading to cytotoxicity and modulation of immune response [16].
The ability of microbiota-derived OMVs to attach, enter, and deliver the cargos into host cells is based on the fusion capability of these vesicles to various membranes [17]. Based on the physiological characteristics of OMVs, the delivery of therapeutic cargos, such as microRNAs and proteins to tissues, has now been identified [18,19,20,21]. Also, bioengineering to target particular delivery of therapeutics has been administered by designing powerful nanocarriers [17, 18, 22]. The encapsulation, amphipathic nature, and bilayer topology of OMVs result in increased life span, enhanced stability, diminished side effects of these modules [22, 23]. Studies demonstrated that loading chemotherapeutic agents on OMVs, such as doxorubicin, can lead to increased accumulation of drugs in tumors and diminished toxicity compared to free doxorubicin [24, 25]. Besides, since MVs can easily transport molecules in the biological systems, they could be used to manufacture vaccines for effective antigen delivery [26]. For instance, it has been found that OMVs have powerful potential for adjuvants and are currently used in some vaccine platforms [27]. The essential activity of bacterial OMVs is to transfer biomolecules to particular targets [28]. Accordingly, they could be served as a new drug delivery tool because of various advantages, such as targeted delivery without causing toxicity on surrounding cells/tissue [28]. Bacteria OMVs can be loaded with many ligands using genetically handling their bacterial producers. These targeting ligands induce the deposition of drugs in target sites [28]. Besides, the OMV size is another advantage that allows the passively delivery of drugs to tumors via enhanced permeability and retention (EPR) inducing local immunity [28]. Targeted delivery to specific cells is another advantage of OMVs in drug delivery. OMVs originate from microorganisms and contain various pathogen-associated molecular patterns (PAMPs) that target cells as neutrophils and macrophages to quickly recognize and internalize [28]. Adjuvants can be highly beneficial in incorporated into OMVs, as they render full immunity and show low toxicity; hence, these molecules could also be employed as a novel mucosal delivery tool in vaccines [27]. In this review, we will discuss current updates on microbiota-derived OMVs in bacteria and their role in the host communication. We will also provide an overview of the current application and future perspective of OMVs for diagnostic and therapeutic purposes (Table 1).
Extracellular vesicles
EVs are lipid-based vesicles containing lipids, proteins, and nucleic acids that are generated by various cells released into the surrounding milieu [29,30,31]. These vesicles are lipid packages and include exosomes, microvesicles, ectosomes, oncosomes, and apoptotic bodies [32]. EVs have different sizes (< 50 nm to several μm), chemical ingredients, and activities [33]. Besides, both commensal and pathogenic bacteria generate EVs categorized as OMVs produced by Gram negative bacteria or as MVs synthesized by Gram-positive bacteria [34]. Bacteria-derived EVs could influence host immunity, resulting in pro-inflammatory reactions [34]. On the other hand, probiotic-derived EVs usually cause immune modulation [34]. In this section, we will discuss and provide an overview of the latest information on EVs derived from the host and bacteria.
Host-derived extracellular vesicles
In the host, micro-vesicles (MVs), exosomes, and apoptotic-derived bodies are listed to characterize host-derived EVs based on their biogenesis profile through membrane shedding, multicellular bodies, and apoptosis [35]. MVs are plasma membrane-derived vesicles with a size range of 100–1000 nm and are generated by vesiculation from eukaryotic cells. In this process, the asymmetry of phospholipid membrane mediated by cytoskeletal remodeling and enhanced cytosolic calcium play a vital role in shaping MVs [36]. MVs differ from other EVs in terms of the contents of phospholipids and proteins on their surface [37]. The importance of MVs in the propagation of coagulation and platelet aggregation due to the activity of membrane phospholipids has been addressed [38, 39]. Also, a growing body of evidence exhibits a connection between the overproduction of MVs and inflammatory reactions due to enhanced MV formation following the induction of tumor necrosis factor (TNF) [40].
Exosomes, another type of EVs, are sphingolipid- and cholesterol-rich membranes with a size range between 30 and 150 nm generated in all host cells [41]. It has been noted that exosomes are synthesized through inward budding of the endosomal compartments, followed by the fusion of multi-vesicular bodies to the cell membrane and the generation of intraluminal vesicles into the extracellular milieu [36]. It is known that the cargo of exosomes includes proteins, metabolites, lipids, as well as nucleic acids (mRNA, miRNA, and DNA) [36]. Exosomes can interact or be generated and internalized by recipient cells through various mechanisms such as fusion to the plasma membrane and/or adhesion to receptors mediating endocytosis [42, 43]. Finally, apoptotic bodies, another type of host EV, are larger than exosomes and contain cellular organelles, nuclear materials, and membrane/cytosolic contents. They are produced during the late phase of apoptosis [44]. Also, apoptotic bodies expose phosphatidylserine in their outer leaflet [44].
Microbiota-derived extracellular vesicles
Like other organisms, bacteria generate EVs with a size < 300 nm as a communication tool [45]. Bacteria-derived EVs could cause a particular advantage via the horizontal transfer of resistance genes to other bacteria [46]. Also, these vesicles are a detoxification system that facilitates the depletion of toxic materials from mother bacteria [46]. Besides, bacteria-derived EVs prompt their adaptation to a new condition, as seen in commensal bacteria in which their EVs are involved in the colonization of the gastrointestinal tract [47]. Most Gram-negative-derived EVs are categorized as OMVs, a bleb form of OM that contains lipids, lipoproteins, and OM proteins [48]. Also, several Gram-negative bacteria could produce another type of EV, outer-inner-MVs, containing cytoplasmic and periplasmic components such as adenosine triphosphate (ATP), DNA cytoplasmic proteins [49].
Some conditions are necessary for vesiculating and synthesizing bacteria-derived vesicles [50]. Studies performed on vesiculation mutants have found that vesiculation does not stem from lysis or disintegration of the bacterial envelope [51]. In summary, it has been found that survival is the main advantage of vesicle formation in bacteria, causing the liberation of toxic material and misfolded proteins and/or eliminating the surface-attacking factors involved in micro-nutrient acquisition [51].
Outer membrane vesicles characterization and biogenesis
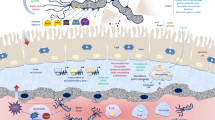
Gram-negative bacteria-derived OMV, a bilayer spherical nanostructure (100–300 nm) with an internal cavity created into the extracellular milieu, made of the phospholipid bilayer, lipopolysaccharide (LPS), membrane protein, cell wall components, peptidoglycan, ion metabolites, signaling molecules, and nucleic acids (Fig. 1) [52,53,54]. Bacterial pathogen-derived OMVs are enriched with proteins involved in an invasive activity that causes efficient internalization of these vesicles into host cells [18]. Invasins, and type III secretion system-dependent integration of the hydrophobic proteins IpaD, IpaB, and IpaC (key virulence factors) of Shigella flexneri and the Ail protein of Escherichia coli is considered exemplary proteins, facilitating the process of internalization [55]. Gram-negative species include E. coli, Shigella sp., Pseudomonas aeruginosa, Campylobacter jejuni, Salmonella sp., Helicobacter pylori, Vibrio sp., Neisseria sp., and Borrelia burgdorferi, have been found to generate OMVs [50, 53, 56,57,58,59,60,61,62,63,64,65,66,67]. Besides their communication activity, Gram-negative-derived OMVs can transfer bacterial virulence factors as cargos to OMVs, leading to increased bacterial survival [68, 69].
Biogenesis and cargo of outer membrane vesicles. It has been found that some components impact the OMV biogenesis including (1) Peptidoglycan endopeptidases, (2) cross-links of Meso-diaminopimelic acid– Meso-diaminopimelic acid within the peptidoglycan, (3) LPS or peptidoglycan fragments, (4) LPS-associated molecules, (5) insertion of PQS into the outer leaflet of the outer membrane, and (6) envelope components. OMV, outer membrane vesicle; LPS, lipopolysaccharide, PQS, Pseudomonas quinolone signal
To produce OMVs, OM must be released from the underlying peptidoglycan and swell outwards so that the vesicle membrane can detach [50]. Besides, the biophysical attribute of the OM-lipids and their interplays with proteins or other components that impact membrane bending has crucial activity in the biogenesis of OMVs [50]. Multiple models of OMV biogenesis have been proposed [18]. Studies found that reducing the cross-linking bond between OM and peptidoglycan induces the formation of OMVs [70, 71]. Vfgl, a different bacterial lipoprotein that iscontribute to the peptidoglycan production and degradation and mediates OMV biogenesis in Adherent-invasive E. coli (AIEC) and E. coli K12 strains [72]. These properties are presumably mediated by enhancing the synthesis of peptidoglycan or downregulation of lytic transglycosylases, leading to the maintenance of turgor pressure on the membrane [72, 73]. An increase in the number of OMVs produced as blebs to OM relieves the cells from the turgor pressure caused by peptidoglycan and muramic acid during cell wall synthesis [18].
In a study conducted by Mashburn and Whiteley [74], they found that enrichment of OM with phospholipids and LPS leads to the production of OMV. Besides, it has been shown that membrane curvature transformations via the membrane insertion of PQS (2-heptyl-3-hydroxy-4-quinolone), a quorum-sensing (QS) molecule, cause the formation of OMV in P. aeruginosa [75, 76]. Also, sequestration of positively-charged components and destabilization of calcium (Ca2+) and magnesium (Mg2+) by PQS can enhance the anionic repulsion of LPS, resulting in OMV formation [74]. Increased generation of OMVs has been detected by adding chelating agents, such as ethylenediaminetetraacetic acid (EDTA) [74, 77]. Also, OM proteins such as TolA/B (Tol-Pal), outer membrane protein A (OmpA), YbgF, and LppAB (all stabilize OM by increasing protein–protein or protein-peptidoglycan interplays) participate in the biogenesis of OMVs [78]. Some stress factors, such as high temperature and antibiotics also promote the production of OMVs [17, 79].
Role of outer membrane vesicles in bacteria
The pathogenic role of Gram-negative bacteria OMVs in infection has been well documented; nevertheless, the advantages of OMVs for non-pathogenic microorganisms are still under investigation [50]. The formation of OMVs gives bacteria advantages, although the energy cost needed to produce these large macromolecules would be high [50]. OMVs mediate the transfer of DNA fragments, cytotoxins, autolysins, and virulence factors [80,81,82]. The generation of OMVs helps bacteria communicate and interact with host cells [18]. OMV, among its unique activity in diverse physiological and pathological functions, has been found to play a pivotal role in acquiring micro-nutrients, stress reactions, and translocation of adhesion, toxins, and virulence components to evade the host immune reactions [18].
Interestingly, the diversity in peptidoglycan structure makes bacteria prone to death by OMVs, and the cytotoxicity of OMVs would be outstanding for those bacteria possessing the same peptidoglycan structure [18, 83]. The neutralization of some bacteria; activity is compromised because of the same degradative enzymes in bacteria and OMVs, resulting in less susceptibility to degradation [18]. The fusion of OMVs to a non-self-strain enhances the susceptibility of bacteria to degradative enzyme systems [84]. The enzyme cargo of OMVs enables bacteria to distinguish between self and non-self-cells, resulting in the target-specific eradication of non-similar cells [85]. For instance, OMVs derived from this system are operational in a Gram-negative bacterium, Lysobacter sp., that generates endopeptidase L5, resulting in degrading other competing Gram-negative bacteria [85]. Also, the same system for peptidoglycan hydrolase and OMVs containing peptidoglycan hydrolase produces destruction effects after making a clear distinction for non-self-microorganisms [79, 86, 87].
The packaging of enzymes, such as glycosidases and proteases, as cargo for bacteria-derived OMVs, shows an outstanding activity in acquiring micro-nutrients for microbial communities [18]. Myxococcus Xanthus-derived OMVs carry alkaline phosphatase that influences competitive bacteria, resulting in phosphate release that enhances the expansion of the multicellular community [88, 89]. Phosphoenolpyruvate (2-phosphoenolpyruvate, PEP), a catalytic cargo of OMVs carrying enolase, converts plasminogen into plasmin [18]. Also, PEP causes colonization of bacteria in the host to the degradation of matrix metalloproteins [90].
Additionally, it has been found that the limitation of metal ions in bacterial environments leads to competition between inter- and intra-species bacteria [17]. In this regard, loading trace elements on OMVs and serving them as a reservoir in interspecies competition result in the availability of metal ions for easy disposal through bacterial utilization [18]. Besides, the mutation in the stress-reactive genes enhances the formation of OMVs; also, the exposure of bacteria to antibacterial components has enormously evolved the production of these molecules, either by efflux pumps and/or the catalyzing the degradability of OMV cargo using the sequestration of antibacterial components from the extracellular environment [18, 91, 92]. It has been shown that the increased formation of surface receptors and ATP-binding cassette (ABC) transporters in OMVs, which act as sensors for micro-nutrients and transporters, can enhance bacterial survival [18].
Besides, it has been found that releasing exopolysaccharides via OMVs enhances the co-accumulation of bacterial cells in the biofilm mode of growth [93]. Biofilm is a surface adhering community of bacteria in response to stress that contains lipids, polysaccharides, proteins, nucleic acids, and appendages such as pili, flagella, as well as OMVs [5, 93,94,95,96,97]. The conversion from a planktonic growth mode into a biofilm mode of growth protects bacteria from numerous stress situations, such as starvation, desiccation, and anti-bacterial drugs [98]. In a biofilm, OMVs give a survival advantage to bacteria because it renders drug resistance with the help of biofilms that protect the embedded bacterial cells from anti-bacterial agents [99]. The connection of OMVs to the P. aeruginosa biofilm has intimidated the relation between stress and the increase of OMV formation during stress conditions [98, 99].
The interplay between bacteria with their host cells stimulates the generation of OMVs carrying different cargos, such as outer surface protein (Osp) A and OspB in B. burgdorferi, and BabA, SabA, and VacA in H. pylori, and UspA1 in Moraxella catarrhalis and aminopeptidase in P. aeruginosa [79]. GN-derived OMVs act as a bridge to enhance the bacterial adhesion to the host tissues and are also employed to increase bacterial adherence to the epithelial linings of the intestine and respiratory tract, leading to failure in bacterial elimination [18].
Role of outer membrane vesicles in host
Despite the unraveled mechanism underlying OMV biogenesis, the effect of bacterial OMVs, particularly on host cells, is a matter of numerous studies. OMVs can bypass the epithelial cell barrier and enter host cells [100]. Subsequently, OMVs will be presented by immune cells, such as macrophages (MQ), neutrophils, and dendritic cells (DCs) in the submucosa and mediate inflammatory reactions against OMVs [48, 100, 101]. Besides, adaptive immune cells, including T and B lymphocyte cells, will be triggered by signal molecules produced in response to antigen-presenting cells [65, 66, 100].
OMVs, in combination with PAMPs, such as porins and LPS, induces powerful immune reactions in endothelial cells and stimulate the pattern-recognizing receptors (PRRs) on MQ cells [68, 102]. It has been found that OMVs mediated by toxins, such as cytolysin A (ClyA), leukotoxin, and LPS, are more potent than their soluble forms [16, 103]. For instance, the release of stx1 and stx2 of Shigella dysenteriae and Shiga toxin of enterohemorrhagic E. coli (EHEC) O157:H7 as cargo for OMVs efficiently suppress the process of protein synthesis in the host [104, 105]. GN-OMVs harbor many virulence components, including LPS, cystic fibrosis transmembrane conductance regulator (CFTR) inhibitory factor (Cif), hemolytic phospholipase C (plcH), and alkaline phosphatase, and they remarkably influence the host cells [106]. Toxins and virulence factors formation help bacterial cells invade the host, hijack host machinery to acquire micronutrients, and suppress host immune reactions that are fundamental for survival in the host [18].
Some studies showed that OMVs could cause phenotypic alterations in host cells [107, 108] along with inflammatory reactions when exposed to the host cells [100]. For example, OMVs of Stenotrophomonas maltophili stimulate powerful inflammatory responses in A549 cells (lung epithelial cells) [109]. OMVs of V. cholerae trigger inflammatory mediators by synthesizing active proteases [110].
Additionally, OMVs belonging to P. aeruginosa stimulate inflammasome formation via caspase-5 in THP-1 monocyte cells [111]. It has been shown that OMVs isolated from E. coli incite immune reactions and induce the expression of interleukin-8 (IL-8) in intestinal epithelial cells [112, 113]. Nevertheless, such interplays would be different between various bacterial OMVs. In this regard, OMVs of Acinetobacter baumannii have been indicated to possess hemolytic, phospholipase, and leucotoxic effects on blood cells [114]. Besides, OMVs derived from H. pylori exhibit a crucial activity on the degranulation of eosinophil cells [115]. OMVs of Aggregatibacter actinomycetemcomitans can be internalized in embryonic kidney cells and induce innate immune reactions [116]. OMVs of Porphyromonas gingivalis stimulates calcification of vascular smooth muscles via Extracellular Signal-regulated Kinase 1 and 2 (ERK1/2)–Runt-related transcription factor 2 (RUNX2) and induce innate immune reactions by endothelial cells [117, 118]. OMVs of probiotic E. coli reinforce the epithelial barrier via the modulation of tight-junctions (TJ) expression in intestinal cells [119]. OMVs are capable of enhancing the expression of cell adhesions, as employed by E. coli to increase the binding of the bacterium to endothelial cells [120]. Finally, OMVs derived from Campylobacter jejuni play proteolytic effects on the cleavage of E-cadherin and Occludin proteins expressing on intestinal epithelial cells [121].
Of note, it has been found that bacteria-derived OMVs affect the activity of host immune cells [100]. For instance, OMVs can stimulate the production of inflammatory cytokines by neutrophils [100]. OMVs isolated from Neisseria meningitides can activate neutrophils to release pro-inflammatory cytokines and chemokines, such as IL-8, interleukin1-β (IL1-β), TNF-alpha (TNF-α), macrophage inflammatory protein 1α and 1β (MIP-1α and MIP-1β) [122]. Also, it has been found that interferon-gamma (IFN-γ) could alter the level of these cytokines to preserve the chronic inflammation situations [122]. It shows that OMVs could involve in protective immunity toward infection and these reactions to OMVs are similar to those exerted by bacterial infection [100]. Additionally, some virulence factors transferred by OMVs could oppress the antibacterial activity of neutrophils and hence involve in the attenuation of cytokine generation [100]. OMVs belonging to Uropathogenic E. coli (UPEC) can transfer cytotoxic necrotizing factor type 1 (CNF1), a bacterial toxin, which diminishes the membrane fluidity and causes functional impairment in neutrophils, resulting in decreased activity of cytokines and chemokines [100, 123]. Despite the impact of OMVs on neutrophils, recent findings demonstrate that OMVs isolated from N. meningitides could be neutralized by plasma and bactericidal/permeability-increasing protein (BPI), which is an essential protein found in the azurophilic granules of neutrophils [124]. It has been found that when neutrophils prevent bacterial invasion, in some cases, these innate immune cells degrade themselves to induce a defense mechanism toward bacteria [100]. Neutrophil extracellular trap (NET) is a killing factor that enables neutrophil cells to stop bacterial pathogens [125]. Most importantly, it has been noted that bacteria-derived OMVs can activate the formation of NETs [126]. Nevertheless, in terms of N. meningitides, this pathogen could escape NETs, enhancing the OMVs levels and the progression of infection [126].
Bacteria-derived OMVs could stimulate DCs by co-stimulatory molecules and cytokine expression [127]. N. meningitides-derived OMVs activate DCs by the expression of accessory molecules (CD40, CD83, CD80, and CD86), human leukocyte antigen (HLA)-DR, and programmed death-ligand 1(PD-L1) [100]. Besides, DCs activated by N. meningitides-derived OMVs generate cytokines, such as IL-1β and Interleukin 6 (IL-6) [128]. OMVs derived from H. pylori activate DCs to produce hemeoxygenase-1 (HO-1) through activating protein kinase B (PKB) (also known as Akt)- Nuclear factor erythroid 2-related factor 2 (Nrf2) and mammalian target of rapamycin (mTOR)-κB Kinase- Nuclear factor-κB (NF-κB) pathways [129]. In summary, the exposure of DCs to bacterial OMVs can stimulate innate immune reactions toward infection [100].
Macrophages could elicit powerful immune reactions when exposed to microbiota-derived OMVs [100]. OMVs stimulate macrophages to generate pro-inflammatory cytokines [100]. The pretreatment of macrophages with OMVs leads to evoked inflammatory responses [80, 130, 131]. It has been documented that bacterial OMVs phagocytosed by macrophages can induce the formation of IL-1β, TNF-α, and IL-8 via the activation of NF-κB [132]. Macrophages activated by OMVs derived from P. gingivalis produce IL-6, TNFα, Interleukin 10 (IL-10), Interleukin-12, p70 (IL-12 p70), IFN-β, and nitric oxide (NO) [133]. Also, OMVs of Legionella pneumophila initiate pro-inflammatory reactions in macrophages via toll-like receptor-2 and -4 (TLR2 and TLR4) pathways [134]. Meanwhile, OMVs enhance the replication of L. pneumophila inside macrophages, and it may characterize how OMVs increase the dissemination of L. pneumophila in the host cells [134, 135]. Guanylate-binding proteins are found as regulators of inflammation caused by OMVs derived from E. coli that could infect bone marrow-derived macrophages [136]. In addition, it has been shown that macrophages activated by OMVs can cause adaptive immune reactions [100]. In this regard, OMVs isolated from N. meningitidis and K. pneumoniae trigger the expression of CD80, CD86, major histocompatibility complex-II (MHC-II), HLA-DR, and intercellular adhesion molecules-1(ICAM-1) molecules that support antigen presentation on the surface of macrophages [80, 137, 138]. Macrophages, antigen-presenting cells, activate T lymphocytes to detect antigens of OMVs and subsequently enhance adaptive reactions [139]. Notably, naive macrophages exposed to OMV of Shigella boydii can induce the polarization of CD4+T cells to T helper type 1 (Th1) [140]. Several studies show that microbiota-derived OMVs can change the metabolic remodeling of macrophages and stimulate apoptosis and pyroptosis [133, 141]. These phenomena can result in diminished levels and dysfunction of protective immune cells, which can be considered significant in disorder progression.
On the other hand, bacteria-derived OMVs play anti-inflammatory roles in infected host cells [100]. It has been found that macrophages exposed to OMVs can synthesize IL-10 [133, 140]. For example, OMVs belonging to H. pylori promote the formation of IL-10, an immunosuppressive cytokine, in peripheral blood mononuclear cells (PBMCs) and inhibit apoptosis in Jurkat T cells (JTCs) [142]. Therefore, it seems that these vesicles are a double-edged sword, as they exert immunostimulatory activity against infection and also, at the same time, facilitate bacterial production by limiting immune cells to attack bacteria.
When bacteria-derived OMVs enter the host cells, antigen-presenting cells present their cargo antigen toward CD4+T lymphocytes and induce differentiation of T-helper cells toward Th1, Th2, and Th17 cells involved in cellular and humoral immune reactions [100]. OMVs have powerful adjuvant influences on cross-priming and contribute to developing CD4+ and CD8+T cells [143]. Nevertheless, it has been demonstrated that OMVs can inhibit T response and growth [143]. N. meningitides-derived OMVs transfer opacity-associated protein (Opa) that can influence the proliferation of T lymphocytes by changing receptor binding [144]. OMVs of H. pylori are reported to suppress the proliferation of T lymphocytes by stimulating Cyclooxygenase-2 (COX-2) in monocyte cells [145]. Besides, transferring of Porin B (PorB) by OMVs of Neisseria gonorrhoeae could inhibit the proliferation of CD4+T lymphocytes, while PorB proteosomes alter immunosuppressive reactions [146].
B-lymphocytes participate in humoral immunity through antibody synthesis to defend the host against microbial pathogens, and these cells need T lymphocytes to react to microbial antigens [100]. OMVs of Salmonella Typhimurium stimulate priming of B and T lymphocytes, and specific Immunoglobulin G could be recognized in in-vivo models immunized with OMVs [80]. It has been detected that OMVs can directly activate B lymphocytes [147]. In order to characterize OMVs-B cell interaction, a novel mechanism can explain the stimulation of B lymphocytes by OMVs.
Current applications of microbiota-derived outer membrane vesicles
Microbiota-derived OMVs possess different properties that make them attractive for various applications, such as drug delivery vehicles, microbial vaccines, cancer immunotherapy agents, adjuvants, and anti-bacterial adhesion components (Fig. 2) (Table 2) [28].
OMV as a drug delivery system
As previously noted, the essential activity of bacterial OMVs is to transfer biomolecules to particular targets [28]. Accordingly, they could be served as a new drug delivery tool because of various advantages, such as targeted delivery without causing toxicity on surrounding cells/tissue [28]. Bacteria OMVs can be loaded with many ligands using genetically handling their bacterial producers. These targeting ligands induce the deposition of drugs in target sites [28]. Besides, the OMV size is another advantage that allows the passively delivery of drugs to tumors via EPR [28]. Targeted delivery to specific cells is another advantage of OMVs in drug delivery. OMVs originate from microorganisms and contain various PAMPs that target cells to recognize and internalize [27] quickly.
The loading of drugs on bacteria-derived OMVs can protect these drugs from denaturation and degradation before reaching the targets [28]. Most importantly, in the case of cancer therapy, OMVs stimulate immune reactions that can be useful for the better elimination of tumors [28]. Nevertheless, if the immune reactions are not correctly controlled, they can damage the host. This implies why detoxified OMVs with lower inflammatory response capability are warranted. Taken together, the administration of microbiota-derived OMVs as a delivery tool would be promising for drug delivery systems.
OMVs as bacterial vaccines
Various models of vaccines are applied to protect the host from associated microbial infections [28]. As a result of possessing the pathogen components, vaccines can stimulate long-lasting pathogen-specific immune reactions [28]. Of note, microbiota-derived OMVs are currently noted to be used for this goal because OMVs contain some PAMPs, and also, they could enter the lymph nodes via lymphatic drainage after phagocytosis by antigen-presenting cells [28, 148]. The detection and uptake of bacteria-derived OMVs by antigen-presenting cells enhance their antigen presentation, co-stimulatory molecules formation, as well as pro-inflammatory cytokines formation [148].
One study showed a potential bacteria-derived OMV-based vaccine that was derived from N. meningitides. This type of OMV could be employed as an adjuvant to increase the immune response against meningitis type B [28]. OMV-derived vaccines have been used clinically for meningitis outbreaks in some countries, such as Norway and Cuba (efficacy up to 70%) [149,150,151,152,153]. This type of vaccine contains some antigens, such as PorA [154, 155]. The PorA protein is a crucial immunogenic factor of OMVs derived from N. meningitides and found in various strains [153]. Therefore, the immune reaction stimulated by OMV-based vaccines, similar to other types of vaccines, is specific to strain. Accordingly, a novel multivalent PorA vaccine has been administered from bioengineered OMVs containing various PorAs in the Netherlands [156, 157]. This OMV-based vaccine stimulated a four-fold enhancement in humoral immunity in a phase I trial [156, 157]. Other proteins in bacteria-derived OMVs also induce host reactions [153]. The FDA and European Medicines Agency approved the MenB vaccine. This vaccine contains OMV ingredients, such as minor proteins and PorA, to induce anti-pathogen reactions [28, 153]. Bacteria-derived OMV-based based vaccines have been extensively studied against bacterial pathogens, including S. flexneri, H. pylori, V. cholera, and S. Typhimurium [28, 158, 159]. It should be noted that these OMVs are generated from their parent bacteria that have been found to induce cellular and humoral immune reactions [28]. The generation of antibodies such as different Immunoglobulin G (IgG) and Immunoglobulin M (IgM) can be specific to pathogenic proteins as well as LPS [28]. In summary, different OMV vaccines with low toxicity and higher efficiency will be examined and entered the clinic.
OMVs as adjuvants
It has been well-documented that immunization with classical vaccines containing proteins or other antigens stimulates a medium immune reaction, particularly for cellular reactions [160]. Hence, currently, adjuvants were further evaluated to increase and shape immune reactions toward a particular antigen. In this regard, adjuvants act via producing depot, enhancing antigen presentation and uptake to lymph, and directly stimulating immune responses [161]. Thus, adjuvants can diminish the number of antigens and doses to achieve therapeutic and prophylactic goals, reducing the cost of treatment. Some properties of OMVs include non-replicating ability when isolated from their bacterial origin, size of < 300 nm, and containing PAMPs [162]. These properties made them an ideal candidate to be utilized as adjuvants [48]. The non-replicating ability of OMVs, in contrast to their bacterial origin, can solve safety problems existing in the application of a completed form of bacteria. Also, the size of bacteria-derived OMVs facilitates their entry into different sites, such as lymph nodes via lymphatic drainage and also phagocytosis by antigen-presenting cells [162]. Also, the pathogen-like property of OMVs triggers their uptake by antigen-presenting cells [163, 164]. Various types of PAMPs present on OMVs can interact with PRRs expressing on antigen-presenting cells and induce their full activation, leading to powerful adaptive immune reactions [163, 164]. It has been reported that lipoproteins and LPS present on the membrane of OMVs interplay with TLR2 and TLR4 on the surface of antigen-presenting cells, enhancing the uptake and recognition of OMVs by these cells [165]. RNA and DNA cargo of OMVs can interact with TLR3 and TLR9 in endosomes, stimulating the proliferation of antigen-presenting cells [165]. The administration of adjuvants can stimulate the synergistic formation of cytokines by antigen-presenting cells, resulting in enhanced T lymphocyte and antibody formation [166, 167].
It should be noted that vesicular compositions of bacteria-derived OMVs facilitate the inclusion of various antigens [168]. Hence, the entry of these OMVs into antigen-presenting cells can also mimic these antigens and contribute to the presentation and processing of antigens [28]. Most importantly, OMVs can be engineered to produce antigens by genetic manipulation of their bacterial origin [28]. A novel OMV-based vaccine was recently designed by loading Poly-β-1,6-N-acetyl-D-glucosamine (PNAG), an immunogen generated by bacterial pathogens, on OMVs to cause a robust immune response against PNAG- bacteria [169]. It has been indicated that the treatment of mice with OMVs protected them against the lethal effect of various PNAG-forming bacteria [28]. Taken together, the potential of microbiota-derived OMVs as an adjuvant in developing novel vaccines would be of note.
OMVs as cancer immunotherapy agents
The use of bacteria-derived OMVs for human cancer therapy is currently performed in multiple clinical trials [28]. The application of OMVs was relatively safer than live bacterial cells, as they are non-replicating particles [28]. OMVs contain different immunostimulatory components that help detect and uptake bacteria-derived OMVs and lead to the activation of immune reactions [28]. Due to the size of OMVs, they can enter or bind to tumor sites and stimulate local immunity via EPR effects [28]. In a study conducted by Kim et al. [170], they exhibited the remarkable anti-tumor activity of OMVs. They found that following the intravenous injection of OMVs are stored in tumor sites and stimulate anti-tumor immune reactions to eliminate tumors [170]. It has been shown that some OMV-derived bacteria can suppress tumor growth, and benefit cancer therapy [170]. Interestingly, the anti-tumor immune response stimulated by OMVs causes immunological memory in mice [170]. Notably, this anti-tumor influences the function of IFN-γ- and trypsin-sensitive proteins and has a crucial role in the formation of IFN-γ [170].
Bacteria-derived OMVs induce effective anti-tumor activity that can completely eliminate tumor sites and suppress tumor metastasis and recurrence [28]. Accordingly, a study by Chen et al. found that co-administration of bacteria-derived OMVs and chemotherapeutic drugs led to a better anti-tumor response. They loaded polyethylene glycol and the Arg-Gly-Asp peptide, a tumor-targeting ligand, on OMVs to enhance their blood circulation and enhance tumor-targeting properties [171]. In the next step, they coated OMVs with Tegafur, which made cancer cells sensitive to T lymphocytes and diminished the immunosuppressive cells such as myeloid-derived suppressor cells. These OMV-coated nanoparticles provided an anti-tumor activity that resulted in stimulating the host immune cells. The systemic injection of these OVMs increased the accumulation of particles in tumors via the EPR effect and active targeting through the Arg-Gly-Asp peptide [171].
OMVs as diagnostic and therapeutic biomarker
A key function of bio-imaging methods is to aid in the early detection and management of diseases. OMVs can have exogenous bio-imaging probes created and fixed onto them to deliver a visual signal by optical, magnetic, or nuclear means [172]. Due to this property, research into the processes by which OMVs mediate bacterial-host communication can be conducted. According to this principle, OMVs could be detected in body fluids, and their molecular compositions reflect their origin; hence, OMVs can be considered novel prognostic and diagnostic biomarkers for many infectious diseases. OMVs possess some distinct advantages, such as the ability to act as noninvasive biomarkers generated by almost all pathogens, reflect the progress of the infection, show treatment response, protect their cargos during long-term storage, as well as the biodegradability in all body fluids [173].
DiR iodide, a lipophilic fluorescent dye, labels membranes. By identifying OMVs with DiR, Liu et al. [174] showed that Akkermansia muciniphila OMVs can infiltrate and aggregate in bone tissues to enhance osteogenic activity and prevent osteoclast formation. Non-covalently bound lipophilic fluorescent dyes are unstable and lose fluorescence quickly.
As previously mentioned, OMVs carry various bacterial components such as LPS, proteins, DNA, and RNA [48, 175]. Ghosal et al. [176] evaluated the extracellular component of E. coli and found that OMVs derived from the E. coli MG1655 strain contain small non-coding RNAs. Besides, Sjöström et al. [177] revealed that OMVs belonging to V. cholerae contain sRNAs. Also, Resch et al. [178] reported non-coding RNAs enriched in OMVs belonging to group A Streptococcus. Koeppen et al. [54] revealed an inter-kingdom regulation by sRNAs through bacterial OMVs in which sRNA52320 from OMV of P. aeruginosa could be transferred into epithelial cells in the lung and diminish the immune reactions induced by LPS via targeting IL-8 mRNA. These findings have promisingly noted secretory sRNAs' pathological and biological significance in OMVs.
Moreover, optoacoustic imaging can be done using bacterial vesicles. Melanin's extensive optical absorption makes it excellent for optoacoustic imaging[179]. Melanin can be spontaneously packed into OMVs by overexpressing tyrosinase in E. coli, a crucial enzyme in melanin formation. OMVs create an improved multi-spectral optoacoustic tomography signal and induce local warmth when irradiated [180]. Engineered OMVs can aggregate in mouse tumor tissue for imaging and photothermal treatment after systemic delivery. Polydopamine nanoparticles produced by oxidative polymerization of dopamine are melanin-like and can be incorporated into the OMV–cancer cell hybrid membrane for tumor-targeted photoacoustic imaging and photothermal treatment [9].
Several studies showed that Gram-negative periodontal pathogens, including Treponema denticola, Tannerella forsythia, P. gingivalis, Fusobacterium nucleatum, Campylobacter rectus, Prevotella intermedia, Eikenella corrodens, and Peptostreptococcus anaerobius that are mediated periodontal attachment and disorder progression can generate OMVs [181, 182]. It has been demonstrated that OMVs of P. gingivalis trigger bacterial co-aggregation and impact the bacterial structure in periodontal plaque via sub-gingival biofilm formation.[181, 182]. Hence, characterization and detection of saliva-specific bacteria-derived OMVs are crucial to many definitions of the microbiome–host interplays in periodontal disorders. Accordingly, Han et al. [182] evaluated the specific periodontal pathogen-derived OMVs in salivary from periodontitis patients. They found that 5mC hypermethylation in salivary OMVs could distinguish periodontitis individuals from healthy individuals [182]. This result shows that OMV methylation can be a promising biomarker for human periodontitis.
By interacting with intestinal epithelia and the mucosal immune system, commensal OMVs maintain intestinal homeostasis. B. fragilis OMVs prevent intestinal inflammation and colitis in mice [183]. Bacteroides thetaiotaomicron OMVs induce IL-10 expression in healthy colonic DCs but not in IBD patients [184]. B. thetaiotaomicron-derived OMVs modulate immunological responses, making them potential IBD therapies. OMVs can be combined with innate immunogenicity to improve immunotherapy effectiveness. OMVs can penetrate through the stratum corneum, making them suitable for melanoma treatment. Peng and Wang [185] developed E. coli producing TNF-related apoptosis inducing ligand (TRAIL) protein and modified OMVs with v3 integrin peptide, targeting ligand, and indocyanine green for melanoma treatment. Multifunctional OMVs can boost antitumor performance in cutaneous melanoma with transdermal photo-TRAIL therapy.
OMVs and their promising application as biomarkers are useful candidates for therapeutic approaches. Despite the challenges in the clinical administration of OMVs, their physiological and biological characteristics have great power as diagnostic and therapeutic tools. In summary, further research can help introduce potential biomarkers and facilitate the clinical application of bacteria-derived OMVs.
Limitation of OMV application
Currently, considerable investigations have been carried out to evaluate the role of OMVs in bacterial communication and infection development [186, 187]. Besides, many groups have examined OMVs for their potential as delivery vehicles, bacterial vaccines, adjuvants cancer immunotherapy agents [22, 28, 188,189,190]. Nevertheless, there are some limitations, such as a lack of inadequate terminology, standardized methodology for the purification and/or isolation of different OMVs, and technical challenges in quantification and characterization [34, 191].
The difficult separation and purification processes necessary to get significant amounts of these microscopic vesicular structures are one of the primary challenges of investigating OMVs. The majority of investigations identify ultracentrifugation and ultrafiltration as techniques [192]. Notably, the isolation process can impact the shape and yield of OMVs, increase OMV aggregation, and/or collect lipoproteins and other undesirable cell debris. Therefore, the optimal OMV separation approach should deliver high OMV yields without compromising vesicles for further experimental investigations or biotechnology applications.
The generation of next-generation vaccinations has a lot of potential with OMV-based vaccines. There are still a lot of difficulties, including yields of OMVs after isolation and the composition, which affects immunogenicity and toxicity. OMVs are naturally advantageous to the bacterium, but they are not created in significant amounts during bacterial growth. However, there can be a very easy way to improve OMV yields [191]. According to research, OMV release rises in response to stress. Environmental stress, such as pressure, temperature, or nutrient depletion stress, is the least serious type of stress that bacteria can endure.
Along with the toxicity of wild-type LPS, bacteria-derived OMVs with several TLR antagonists occurring in OMVs such as lipoproteins, flagellin, and other OMPs can cause uncontrolled reactions such as excess inflammation [27]. Hence, OMV endotoxin components must be eliminated after isolation; for example, in Neisseria, the Factor H binding protein must be isolated from OMPs due to its cytotoxic nature [27]. Another challenge is that LPS-deficient OMVs usually show less immunogenicity than wild-type bacteria-derived OMVs. Hence, an optimal balance in the effective changes in LPS, such as low toxicity and high immunogenicity, is warranted.
Most importantly, if microbiota-derived OMVs are commercialized for the abovementioned applications, mass production should be considered [193, 194]. The mechanism underlying the production of OMVs is not fully understood, and hence consistent formation may be complex [193]. In this regard, during the Upstream Process of pre-culture of bacteria, another antifoam was needed for many scales up in the fermentation process. In contrast, a significant number of antifoams are not compatible with the generation processes of OMVs. Their surfactants may influence OMV function or even interfere with the integrity and purification of OMV [195, 196]. However, the use of antifoam is still considered a standard approach to inhibit excessive foaming due to required aeration at different densities [193]. Alternative approaches for mechanical foam breaking have been evaluated as part of the scale-up during the fermentation process [27].
Additionally, external components such as temperature and in rare cases, the absorption of phages, also influence OMV generation. Also, oxidative stress due to cysteine depletion in N. meningitides and/or sodium carbonate in V. cholera can affect the yield volume of the recombinant OMVs [27]. Hence, it is required to enhance mediated production technology and environmental situations.
The poor yield of OMVs, which are released spontaneously by bacteria but in very small numbers, together with the possibility of low levels of important protective antigens on their surface, are further barriers to their use as vaccines [197]. OMVs also contain endotoxins and deoxycholate extraction followed by differential centrifugation from the homogenized bacterial bulk can increase yield and decrease endotoxin levels; these are typically referred to as OMVs made using this technique detergent-extracted OMVs.
Lastly, several studies noted that LPS derivatives have a similar impact when compared with WT-LPS in vivo. These species-specific reactions can cause differences in the signaling and induction of TLRs [198]. Thus, this reaction highlighted the difficulty of in vivo analysis of the safety of microbiota-derived OMVs in humans. Hence, to improve the challenge of OMV applications, many human trials are needed to examine their biological effects.
Concluding remarks and future perspective
All in all, the current evidence implies that the gut microbiota and its metabolites have a crucial role in human health and disease. The disruption of the gut microbiota (which is called dysbiosis) balance can disturb the host's energy metabolism and immunity, significantly impacting the development of numerous human disorders. Recent investigations propose that OMVs could perform a critical role in shaping immune responses, including homeostasis and acute inflammatory responses. Following dysbiosis of the gut microbiota during infection, the number and type of these OMVs may change so that these molecules can be employed as targets for diagnosis. Also, we can apply OMVs as antibacterial agents. In this review, the application of OMVs for medical purposes, such as cancer immunotherapy, OVM-based vaccines, and drug delivery, were broadly addressed.
It should be noted that several obstacles exist in the application of these molecules, such as low yield volume and toxic effects owing to possessing some cytotoxic components (e.g., LPS). In this regard, some approaches have been proposed, such as genetic manipulation to reduce endotoxicity. One solution that seems to be optimal for increasing yields of OMVs would be heat induction [191]. In conclusion, future studies should focus on using OMVs and solving these challenges to pave the way for applying these molecules in the clinic.
Availability of data and materials
Not applicable.
Change history
02 February 2023
A Correction to this paper has been published: https://doi.org/10.1186/s13027-023-00484-0
Abbreviations
- IBD:
-
Inflammatory bowel disease
- IBS:
-
Irritable bowel syndrome
- MS:
-
Multiple sclerosis
- GI:
-
Gastrointestinal
- MVs:
-
Membrane vesicles
- EVs:
-
Extracellular vesicles
- OMVs:
-
Outer MVs
- MVs:
-
Micro-vesicles
- TNF:
-
Tumor necrosis factor
- AIEC:
-
Adherent-invasive E. coli
- QS:
-
Quorum-sensing
- Ca2+ :
-
Calcium
- Mg2+ :
-
Magnesium
- EDTA:
-
Ethylenediaminetetraacetic acid
- OmpA:
-
Outer membrane protein A
- MQ:
-
Macrophages
- DCs:
-
Dendritic cells
- PNAG:
-
Poly-β-1,6-N-acetyl-d-glucosamine
- PAMPs:
-
Pathogen-associated molecular patterns
- PRRs:
-
Pattern-recognizing receptors
- EHEC:
-
Enterohemorrhagic E. coli
- IL-8:
-
Interleukin-8
- ERK1:
-
Extracellular signal-regulated kinase
- RUNX2:
-
Runt related transcription factor 2
- TJ:
-
Tight-junctions
- IL1-β:
-
Interleukin1-β
- IFN-γ:
-
Interferon-gamma
- UPEC:
-
Uropathogenic E. coli
- CNF1:
-
Cytotoxic necrotizing factor type 1
- NET:
-
Neutrophil extracellular trap
- HLA:
-
Human leukocyte antigen
- IL-6:
-
Interleukin 6
- HO-1:
-
Hemeoxygenase-1
- PKB:
-
Protein kinase B
- MTOR:
-
Mammalian target of rapamycin
- IL-10:
-
Interleukin 10
- NO:
-
Nitric oxide
References
Mirzaei R, Afaghi A, Babakhani S, Sohrabi MR, Hosseini-Fard SR, Babolhavaeji K, Akbari SKA, Yousefimashouf R, Karampoor S. Role of microbiota-derived short-chain fatty acids in cancer development and prevention. Biomed Pharmacother. 2021;139:111619.
Mirzaei R, Bouzari B, Hosseini-Fard SR, Mazaheri M, Ahmadyousefi Y, Abdi M, Jalalifar S, Karimitabar Z, Teimoori A, Keyvani H. Role of microbiota-derived short-chain fatty acids in nervous system disorders. Biomed Pharmacother. 2021;139:111661.
Mirzaei R, Dehkhodaie E, Bouzari B, Rahimi M, Gholestani A, Hosseini-Fard SR, Keyvani H, Teimoori A, Karampoor S. Dual role of microbiota-derived short-chain fatty acids on host and pathogen. Biomed Pharmacother. 2021;145:112352.
Ahmadi Badi S, Moshiri A, Fateh A, Rahimi Jamnani F, Sarshar M, Vaziri F, Siadat SD. Microbiota-derived extracellular vesicles as new systemic regulators. Front Microbiol. 2017;8:1610.
Mirzaei R, Mirzaei H, Alikhani MY, Sholeh M, Arabestani MR, Saidijam M, Karampoor S, Ahmadyousefi Y, Moghadam MS, Irajian GR. Bacterial biofilm in colorectal cancer: What is the real mechanism of action? Microb Pathog. 2020;142:104052.
Jones EJ, Booth C, Fonseca S, Parker A, Cross K, Miquel-Clopés A, Hautefort I, Mayer U, Wileman T, Stentz R, Carding SR. The uptake, trafficking, and biodistribution of bacteroides thetaiotaomicron generated outer membrane vesicles. Front Microbiol. 2020;11:57.
Zeng MY, Inohara N, Nuñez G. Mechanisms of inflammation-driven bacterial dysbiosis in the gut. Mucosal Immunol. 2017;10:18–26.
Sommer F, Bäckhed F. The gut microbiota—masters of host development and physiology. Nat Rev Microbiol. 2013;11:227–38.
Yu Y-J, Wang X-H, Fan G-C. Versatile effects of bacterium-released membrane vesicles on mammalian cells and infectious/inflammatory diseases. Acta Pharmacol Sin. 2018;39:514–33.
Haurat MF, Elhenawy W, Feldman MF. Prokaryotic membrane vesicles: new insights on biogenesis and biological roles. Biol Chem. 2015;396:95–109.
Avila-Calderón ED, Araiza-Villanueva MG, Cancino-Diaz JC, López-Villegas EO, Sriranganathan N, Boyle SM, Contreras-Rodríguez A. Roles of bacterial membrane vesicles. Arch Microbiol. 2015;197:1–10.
Gould SB, Garg SG, Martin WF. Bacterial vesicle secretion and the evolutionary origin of the eukaryotic endomembrane system. Trends Microbiol. 2016;24:525–34.
Ailawadi S, Wang X, Gu H, Fan GC. Pathologic function and therapeutic potential of exosomes in cardiovascular disease. Biochim Biophys Acta. 2015;1852:1–11.
Mirzaei R, Babakhani S, Ajorloo P, Ahmadi RH, Hosseini-Fard SR, Keyvani H, Ahmadyousefi Y, Teimoori A, Zamani F, Karampoor S. The emerging role of exosomal miRNAs as a diagnostic and therapeutic biomarker in Mycobacterium tuberculosis infection. Mol Med. 2021;27:1–31.
Kuipers ME, Hokke CH, Smits HH, Nolte-‘t Hoen ENM. Pathogen-derived extracellular vesicle-associated molecules that affect the host immune system: an overview. Front Microbiol. 2018;9:2182.
Kuehn MJ, Kesty NC. Bacterial outer membrane vesicles and the host-pathogen interaction. Genes Dev. 2005;19:2645–55.
Kulp A, Kuehn MJ. Biological functions and biogenesis of secreted bacterial outer membrane vesicles. Annu Rev Microbiol. 2010;64:163–84.
Jan AT. Outer membrane vesicles (OMVs) of gram-negative bacteria: a perspective update. Front Microbiol. 2017;8:1053.
Li R, Liu Q. Engineered bacterial outer membrane vesicles as multifunctional delivery platforms. Front Mater. 2020;7:202.
Raposo G, Stoorvogel W. Extracellular vesicles: exosomes, microvesicles, and friends. J Cell Biol. 2013;200:373–83.
Mirzaei R, Zamani F, Hajibaba M, Rasouli-Saravani A, Noroozbeygi M, Gorgani M, Hosseini-Fard SR, Jalalifar S, Ajdarkosh H, Abedi SH. The pathogenic, therapeutic and diagnostic role of exosomal microrna in the autoimmune diseases. J Neuroimmunol. 2021;358:577640.
Wang S, Gao J, Wang Z. Outer membrane vesicles for vaccination and targeted drug delivery. Wiley Interdiscip Rev Nanomed Nanobiotechnol. 2019;11:e1523.
Ozpolat B, Sood AK, Lopez-Berestein G. Liposomal siRNA nanocarriers for cancer therapy. Adv Drug Deliv Rev. 2014;66:110–6.
Petros RA, DeSimone JM. Strategies in the design of nanoparticles for therapeutic applications. Nat Rev Drug Discov. 2010;9:615–27.
Kuerban K, Gao X, Zhang H, Liu J, Dong M, Wu L, Ye R, Feng M, Ye L. Doxorubicin-loaded bacterial outer-membrane vesicles exert enhanced anti-tumor efficacy in non-small-cell lung cancer. Acta Pharm Sin B. 2020;10:1534–48.
van der Pol L, Stork M, van der Ley P. Outer membrane vesicles as platform vaccine technology. Biotechnol J. 2015;10:1689–706.
Tan K, Li R, Huang X, Liu Q. Outer membrane vesicles: current status and future direction of these novel vaccine adjuvants. Front Microbiol. 2018;9:783–783.
Li M, Zhou H, Yang C, Wu Y, Zhou X, Liu H, Wang Y. Bacterial outer membrane vesicles as a platform for biomedical applications: an update. J Control Release. 2020;323:253–68.
Zaborowski MP, Balaj L, Breakefield XO, Lai CP. Extracellular vesicles: composition, biological relevance, and methods of study. Bioscience. 2015;65:783–97.
Yáñez-Mó M, Siljander PR, Andreu Z, Zavec AB, Borràs FE, Buzas EI, Buzas K, Casal E, Cappello F, Carvalho J, et al. Biological properties of extracellular vesicles and their physiological functions. J Extracell Vesicles. 2015;4:27066.
Doyle LM, Wang MZ. Overview of extracellular vesicles, their origin, composition, purpose, and methods for exosome isolation and analysis. Cells. 2019;8:727.
Veziroglu EM, Mias GI. Characterizing extracellular vesicles and their diverse RNA contents. Front Genet. 2020;11:700.
Théry C, Witwer KW, Aikawa E, Alcaraz MJ, Anderson JD, Andriantsitohaina R, Antoniou A, Arab T, Archer F, Atkin-Smith GK. Minimal information for studies of extracellular vesicles 2018 (MISEV2018): a position statement of the International Society for Extracellular Vesicles and update of the MISEV2014 guidelines. J Extracell Vesicles. 2018;7:1535750.
Macia L, Nanan R, Hosseini-Beheshti E, Grau GE. Host- and microbiota-derived extracellular vesicles, immune function, and disease development. Int J Mol Sci. 2019;21:107.
Brakhage AA, Zimmermann A-K, Rivieccio F, Visser C, Blango MG. Host-derived extracellular vesicles for antimicrobial defense. microLife. 2021;2:uqab003.
Macia L, Nanan R, Hosseini-Beheshti E, Grau GE. Host-and microbiota-derived extracellular vesicles, immune function, and disease development. Int J Mol Sci. 2020;21:107.
Latham SL, Tiberti N, Gokoolparsadh N, Holdaway K, Couraud PO, Grau GE, Combes V. Immuno-analysis of microparticles: probing at the limits of detection. Sci Rep. 2015;5:16314.
Zwaal RF, Schroit AJ. Pathophysiologic implications of membrane phospholipid asymmetry in blood cells. Blood. 1997;89:1121–32.
Nieuwland R, Berckmans RJ, McGregor S, Böing AN, Romijn FP, Westendorp RG, Hack CE, Sturk A. Cellular origin and procoagulant properties of microparticles in meningococcal sepsis. Blood. 2000;95:930–5.
Combes V, Coltel N, Alibert M, van Eck M, Raymond C, Juhan-Vague I, Grau GE, Chimini G. ABCA1 gene deletion protects against cerebral malaria: potential pathogenic role of microparticles in neuropathology. Am J Pathol. 2005;166:295–302.
Colombo M, Raposo G, Théry C. Biogenesis, secretion, and intercellular interactions of exosomes and other extracellular vesicles. Annu Rev Cell Dev Biol. 2014;30:255–89.
Lakkaraju A, Rodriguez-Boulan E. Itinerant exosomes: emerging roles in cell and tissue polarity. Trends Cell Biol. 2008;18:199–209.
French KC, Antonyak MA, Cerione RA. Extracellular vesicle docking at the cellular port: extracellular vesicle binding and uptake. Semin Cell Dev Biol. 2017;67:48–55.
Turturici G, Tinnirello R, Sconzo G, Geraci F. Extracellular membrane vesicles as a mechanism of cell-to-cell communication: advantages and disadvantages. Am J Physiol Cell Physiol. 2014;306:C621–33.
Brown L, Wolf JM, Prados-Rosales R, Casadevall A. Through the wall: extracellular vesicles in Gram-positive bacteria, mycobacteria and fungi. Nat Rev Microbiol. 2015;13:620–30.
Emamalipour M, Seidi K, Zununi Vahed S, Jahanban-Esfahlan A, Jaymand M, Majdi H, Amoozgar Z, Chitkushev LT, Javaheri T, Jahanban-Esfahlan R, Zare P. Horizontal gene transfer: from evolutionary flexibility to disease progression. Front Cell Dev Biol. 2020;8:229–229.
Bryant WA, Stentz R, Le Gall G, Sternberg MJE, Carding SR, Wilhelm T. In silico analysis of the small molecule content of outer membrane vesicles produced by bacteroides thetaiotaomicron indicates an extensive metabolic link between microbe and host. Front Microbiol. 2017;8:2440.
Kaparakis-Liaskos M, Ferrero RL. Immune modulation by bacterial outer membrane vesicles. Nat Rev Immunol. 2015;15:375–87.
Pérez-Cruz C, Delgado L, López-Iglesias C, Mercade E. Outer-inner membrane vesicles naturally secreted by gram-negative pathogenic bacteria. PLoS ONE. 2015;10:e0116896.
Schwechheimer C, Kuehn MJ. Outer-membrane vesicles from Gram-negative bacteria: biogenesis and functions. Nat Rev Microbiol. 2015;13:605–19.
Cuesta CM, Guerri C, Ureña J, Pascual M. Role of microbiota-derived extracellular vesicles in gut-brain communication. Int J Mol Sci. 2021;22:4235.
Qing G, Gong N, Chen X, Chen J, Zhang H, Wang Y, Wang R, Zhang S, Zhang Z, Zhao X, et al. Natural and engineered bacterial outer membrane vesicles. Biophys Rep. 2019;5:184–98.
Lindmark B, Rompikuntal PK, Vaitkevicius K, Song T, Mizunoe Y, Uhlin BE, Guerry P, Wai SN. Outer membrane vesicle-mediated release of cytolethal distending toxin (CDT) from Campylobacter jejuni. BMC Microbiol. 2009;9:1–10.
Koeppen K, Hampton TH, Jarek M, Scharfe M, Gerber SA, Mielcarz DW, Demers EG, Dolben EL, Hammond JH, Hogan DA. A novel mechanism of host-pathogen interaction through sRNA in bacterial outer membrane vesicles. PLoS Pathog. 2016;12:e1005672.
Kuehn MJ, Kesty NC. Bacterial outer membrane vesicles and the host–pathogen interaction. Genes Dev. 2005;19:2645–55.
McBroom AJ, Kuehn MJ. Release of outer membrane vesicles by Gram-negative bacteria is a novel envelope stress response. Mol Microbiol. 2007;63:545–58.
Bauman SJ, Kuehn MJ. Purification of outer membrane vesicles from Pseudomonas aeruginosa and their activation of an IL-8 response. Microbes Infect. 2006;8:2400–8.
Kadurugamuwa JL, Beveridge TJ. Membrane vesicles derived from Pseudomonas aeruginosa and Shigella flexneri can be integrated into the surfaces of other gram-negative bacteria. Microbiology. 1999;145:2051–60.
Elhenawy W, Bording-Jorgensen M, Valguarnera E, Haurat MF, Wine E, Feldman MF. LPS remodeling triggers formation of outer membrane vesicles in Salmonella. MBio. 2016;7:e00940-16.
Fiocca R, Necchi V, Sommi P, Ricci V, Telford J, Cover TL, Solcia E. Release of Helicobacter pylori vacuolating cytotoxin by both a specific secretion pathway and budding of outer membrane vesicles. Uptake of released toxin and vesicles by gastric epithelium. J Pathol. 1999;188:220–6.
Turner L, Praszkier J, Hutton ML, Steer D, Ramm G, Kaparakis-Liaskos M, Ferrero RL. Increased outer membrane vesicle formation in a Helicobacter pylori tolB mutant. Helicobacter. 2015;20:269–83.
Dorward DW, Schwan T, Garon CF. Immune capture and detection of Borrelia burgdorferi antigens in urine, blood, or tissues from infected ticks, mice, dogs, and humans. J Clin Microbiol. 1991;29:1162–70.
Chatterjee D, Chaudhuri K. Association of cholera toxin with Vibrio cholerae outer membrane vesicles which are internalized by human intestinal epithelial cells. FEBS Lett. 2011;585:1357–62.
Devoe I, Gilchrist J. Release of endotoxin in the form of cell wall blebs during in vitro growth of Neisseria meningitidis. J Exp Med. 1973;138:1156–67.
Goodarzi P, Mahdavi F, Mirzaei R, Hasanvand H, Sholeh M, Zamani F, Sohrabi M, Tabibzadeh A, JedaNiya ASMHK. Coronavirus disease 2019 (COVID-19): immunological approaches and emerging pharmacologic treatments. Int Immunopharmacol. 2020;88:106885.
Mirzaei R, Mohammadzadeh R, Mirzaei H, Sholeh M, Karampoor S, Abdi M, Alikhani MY, Kazemi S, Ahmadyousefi Y, Jalalifar S. Role of microRNAs in Staphylococcus aureus infection: potential biomarkers and mechanism. IUBMB Life. 2020;72:1856–69.
Mahdiun F, Mansouri S, Khazaeli P, Mirzaei R. The effect of tobramycin incorporated with bismuth-ethanedithiol loaded on niosomes on the quorum sensing and biofilm formation of Pseudomonas aeruginosa. Microb Pathog. 2017;107:129–35.
Ellis TN, Kuehn MJ. Virulence and immunomodulatory roles of bacterial outer membrane vesicles. Microbiol Mol Biol Rev. 2010;74:81–94.
Chattopadhyay MK, Jagannadham MV. Vesicles-mediated resistance to antibiotics in bacteria. Front Microbiol. 2015;6:758.
Burdett I, Murray R. Electron microscope study of septum formation in Escherichia coli strains B and B/r during synchronous growth. J Bacteriol. 1974;119:1039–56.
Hoekstra D, van der Laan JW, de Leij L, Witholt B. Release of outer membrane fragments from normally growing Escherichia coli. Biochim Biophys Acta (BBA) Biomembr. 1976;455:889–99.
Rolhion N, Barnich N, Claret L, Darfeuille-Michaud A. Strong decrease in invasive ability and outer membrane vesicle release in Crohn’s disease-associated adherent-invasive Escherichia coli strain LF82 with the yfgL gene deleted. J Bacteriol. 2005;187:2286–96.
Eggert US, Ruiz N, Falcone BV, Branstrom AA, Goldman RC, Silhavy TJ, Kahne D. Genetic basis for activity differences between vancomycin and glycolipid derivatives of vancomycin. Science. 2001;294:361–4.
Mashburn LM, Whiteley M. Membrane vesicles traffic signals and facilitate group activities in a prokaryote. Nature. 2005;437:422–5.
Mashburn-Warren L, Howe J, Brandenburg K, Whiteley M. Structural requirements of the Pseudomonas quinolone signal for membrane vesicle stimulation. J Bacteriol. 2009;191:3411–4.
Schertzer JW, Whiteley M. A bilayer-couple model of bacterial outer membrane vesicle biogenesis. MBio. 2012;3:e00297-11.
Lee J, Kim OY, Gho YS. Proteomic profiling of Gram-negative bacterial outer membrane vesicles: current perspectives. PROTEOMICS–Clin Appl. 2016;10:897–909.
Schwechheimer C, Sullivan CJ, Kuehn MJ. Envelope control of outer membrane vesicle production in Gram-negative bacteria. Biochemistry. 2013;52:3031–40.
MacDonald IA, Kuehn MJ. Offense and defense: microbial membrane vesicles play both ways. Res Microbiol. 2012;163:607–18.
Alaniz RC, Deatherage BL, Lara JC, Cookson BT. Membrane vesicles are immunogenic facsimiles of Salmonella typhimurium that potently activate dendritic cells, prime B and T cell responses, and stimulate protective immunity in vivo. J Immunol. 2007;179:7692–701.
Furuta N, Takeuchi H, Amano A. Entry of Porphyromonas gingivalis outer membrane vesicles into epithelial cells causes cellular functional impairment. Infect Immun. 2009;77:4761–70.
Biller SJ, Schubotz F, Roggensack SE, Thompson AW, Summons RE, Chisholm SW. Bacterial vesicles in marine ecosystems. Science. 2014;343:183–6.
Kulkarni HM, Jagannadham MV. Biogenesis and multifaceted roles of outer membrane vesicles from Gram-negative bacteria. Microbiology (Reading). 2014;160:2109–21.
Beveridge TJ. Structures of gram-negative cell walls and their derived membrane vesicles. J Bacteriol. 1999;181:4725–33.
Vasilyeva NV, Tsfasman IM, Suzina NE, Stepnaya OA, Kulaev IS. Secretion of bacteriolytic endopeptidase L5 of Lysobacter sp. XL1 into the medium by means of outer membrane vesicles. Febs J. 2008;275:3827–35.
Kadurugamuwa JL, Beveridge TJ. Bacteriolytic effect of membrane vesicles from Pseudomonas aeruginosa on other bacteria including pathogens: conceptually new antibiotics. J Bacteriol. 1996;178:2767–74.
Rasoul M, Rokhsareh M, Mohammad SM, Sajad K, Ahmadreza M. The human immune system against Staphylococcus epidermidis. Crit Rev™ Immunol. 2019;39:151–63.
Evans AGL, Davey HM, Cookson A, Currinn H, Cooke-Fox G, Stanczyk PJ, Whitworth DE. Predatory activity of Myxococcus xanthus outer-membrane vesicles and properties of their hydrolase cargo. Microbiology (Reading). 2012;158:2742–52.
Berleman JE, Allen S, Danielewicz MA, Remis JP, Gorur A, Cunha J, Hadi MZ, Zusman DR, Northen TR, Witkowska HE, Auer M. The lethal cargo of Myxococcus xanthus outer membrane vesicles. Front Microbiol. 2014;5:474.
Toledo A, Coleman JL, Kuhlow CJ, Crowley JT, Benach JL. The enolase of Borrelia burgdorferi is a plasminogen receptor released in outer membrane vesicles. Infect Immun. 2012;80:359–68.
Ciofu O, Beveridge TJ, Kadurugamuwa J, Walther-Rasmussen J, Høiby N. Chromosomal beta-lactamase is packaged into membrane vesicles and secreted from Pseudomonas aeruginosa. J Antimicrob Chemother. 2000;45:9–13.
Manning AJ, Kuehn MJ. Contribution of bacterial outer membrane vesicles to innate bacterial defense. BMC Microbiol. 2011;11:258.
Schooling SR, Beveridge TJ. Membrane vesicles: an overlooked component of the matrices of biofilms. J Bacteriol. 2006;188:5945–57.
Mirzaei R, Mohammadzadeh R, Alikhani MY, Shokri Moghadam M, Karampoor S, Kazemi S, Barfipoursalar A, Yousefimashouf R. The biofilm-associated bacterial infections unrelated to indwelling devices. IUBMB Life. 2020;72:1271–85.
Mirzaei R, Mohammadzadeh R, Sholeh M, Karampoor S, Abdi M, Dogan E, Moghadam MS, Kazemi S, Jalalifar S, Dalir A. The importance of intracellular bacterial biofilm in infectious diseases. Microb Pathog. 2020;147:104393.
Campoccia D, Mirzaei R, Montanaro L, Arciola CR. Hijacking of immune defences by biofilms: a multifront strategy. Biofouling. 2019;35:1055–74.
Mirzaei R, Abdi M, Gholami H. The host metabolism following bacterial biofilm: What is the mechanism of action? Rev Med Microbiol. 2020;31:175–82.
Klimentová J, Stulík J. Methods of isolation and purification of outer membrane vesicles from Gram-negative bacteria. Microbiol Res. 2015;170:1–9.
Beveridge TJ, Makin SA, Kadurugamuwa JL, Li Z. Interactions between biofilms and the environment. FEMS Microbiol Rev. 1997;20:291–303.
Cai W, Kesavan DK, Wan J, Abdelaziz MH, Su Z, Xu H. Bacterial outer membrane vesicles, a potential vaccine candidate in interactions with host cells based. Diagn Pathol. 2018;13:95.
Chatterjee D, Chaudhuri K. Vibrio cholerae O395 outer membrane vesicles modulate intestinal epithelial cells in a NOD1 protein-dependent manner and induce dendritic cell-mediated Th2/Th17 cell responses. J Biol Chem. 2013;288:4299–309.
Kim YS, Choi EJ, Lee WH, Choi SJ, Roh TY, Park J, Jee YK, Zhu Z, Koh YY, Gho YS, Kim YK. Extracellular vesicles, especially derived from Gram-negative bacteria, in indoor dust induce neutrophilic pulmonary inflammation associated with both Th1 and Th17 cell responses. Clin Exp Allergy. 2013;43:443–54.
Wai SN, Lindmark B, Söderblom T, Takade A, Westermark M, Oscarsson J, Jass J, Richter-Dahlfors A, Mizunoe Y, Uhlin BE. Vesicle-mediated export and assembly of pore-forming oligomers of the enterobacterial ClyA cytotoxin. Cell. 2003;115:25–35.
Yokoyama K, Horii T, Yamashino T, Hashikawa S, Barua S, Hasegawa T, Watanabe H, Ohta M. Production of shiga toxin by Escherichia coli measured with reference to the membrane vesicle-associated toxins. FEMS Microbiol Lett. 2000;192:139–44.
Dutta S, Iida K, Takade A, Meno Y, Nair GB, Yoshida S. Release of Shiga toxin by membrane vesicles in Shigella dysenteriae serotype 1 strains and in vitro effects of antimicrobials on toxin production and release. Microbiol Immunol. 2004;48:965–9.
Bomberger JM, Maceachran DP, Coutermarsh BA, Ye S, O’Toole GA, Stanton BA. Long-distance delivery of bacterial virulence factors by Pseudomonas aeruginosa outer membrane vesicles. PLoS Pathog. 2009;5:e1000382.
Rasti ES, Schappert ML, Brown AC. Association of Vibrio cholerae 569B outer membrane vesicles with host cells occurs in a GM1-independent manner. Cell Microbiol. 2018;20:e12828.
Aschtgen MS, Wetzel K, Goldman W, McFall-Ngai M, Ruby E. Vibrio fischeri-derived outer membrane vesicles trigger host development. Cell Microbiol. 2016;18:488–99.
Kim YJ, Jeon H, Na SH, Kwon HI, Selasi GN, Nicholas A, Park TI, Lee SH, Lee JC. Stenotrophomonas maltophilia outer membrane vesicles elicit a potent inflammatory response in vitro and in vivo. Pathog Dis. 2016;74:ftw104.
Mondal A, Tapader R, Chatterjee NS, Ghosh A, Sinha R, Koley H, Saha DR, Chakrabarti MK, Wai SN, Pal A. Cytotoxic and inflammatory responses induced by outer membrane vesicle-associated biologically active proteases from vibrio cholerae. Infect Immun. 2016;84:1478–90.
Bitto NJ, Baker PJ, Dowling JK, Wray-McCann G, De Paoli A, Tran LS, Leung PL, Stacey KJ, Mansell A, Masters SL, Ferrero RL. Membrane vesicles from Pseudomonas aeruginosa activate the noncanonical inflammasome through caspase-5 in human monocytes. Immunol Cell Biol. 2018;96:1120–30.
Cañas MA, Fábrega MJ, Giménez R, Badia J, Baldomà L. Outer membrane vesicles from probiotic and commensal Escherichia coli activate NOD1-mediated immune responses in intestinal epithelial cells. Front Microbiol. 2018;9:498.
Bielaszewska M, Marejková M, Bauwens A, Kunsmann-Prokscha L, Mellmann A, Karch H. Enterohemorrhagic Escherichia coli O157 outer membrane vesicles induce interleukin 8 production in human intestinal epithelial cells by signaling via Toll-like receptors TLR4 and TLR5 and activation of the nuclear factor NF-κB. Int J Med Microbiol. 2018;308:882–9.
Jha C, Ghosh S, Gautam V, Malhotra P, Ray P. In vitro study of virulence potential of Acinetobacter baumannii outer membrane vesicles. Microb Pathog. 2017;111:218–24.
Ko SH, Jeon JI, Kim YJ, Yoon HJ, Kim H, Kim N, Kim JS, Kim JM. Helicobacter pylori outer membrane vesicle proteins induce human eosinophil degranulation via a β2 Integrin CD11/CD18- and ICAM-1-dependent mechanism. Mediators Inflamm. 2015;2015:301716.
Thay B, Damm A, Kufer TA, Wai SN, Oscarsson J. Aggregatibacter actinomycetemcomitans outer membrane vesicles are internalized in human host cells and trigger NOD1- and NOD2-dependent NF-κB activation. Infect Immun. 2014;82:4034–46.
Yang WW, Guo B, Jia WY, Jia Y. Porphyromonas gingivalis-derived outer membrane vesicles promote calcification of vascular smooth muscle cells through ERK1/2-RUNX2. FEBS Open Bio. 2016;6:1310–9.
Ho MH, Guo ZM, Chunga J, Goodwin JS, Xie H. Characterization of innate immune responses of human endothelial cells induced by Porphyromonas gingivalis and their derived outer membrane vesicles. Front Cell Infect Microbiol. 2016;6:139.
Alvarez CS, Badia J, Bosch M, Giménez R, Baldomà L. Outer membrane vesicles and soluble factors released by probiotic Escherichia coli Nissle 1917 and commensal ECOR63 enhance barrier function by regulating expression of tight junction proteins in intestinal epithelial cells. Front Microbiol. 1981;2016:7.
Kim JH, Yoon YJ, Lee J, Choi EJ, Yi N, Park KS, Park J, Lötvall J, Kim YK, Gho YS. Outer membrane vesicles derived from Escherichia coli up-regulate expression of endothelial cell adhesion molecules in vitro and in vivo. PLoS ONE. 2013;8:e59276.
Elmi A, Nasher F, Jagatia H, Gundogdu O, Bajaj-Elliott M, Wren B, Dorrell N. Campylobacter jejuni outer membrane vesicle-associated proteolytic activity promotes bacterial invasion by mediating cleavage of intestinal epithelial cell E-cadherin and occludin. Cell Microbiol. 2016;18:561–72.
Lapinet JA, Scapini P, Calzetti F, Pérez O, Cassatella MA. Gene expression and production of tumor necrosis factor alpha, interleukin-1beta (IL-1beta), IL-8, macrophage inflammatory protein 1alpha (MIP-1alpha), MIP-1beta, and gamma interferon-inducible protein 10 by human neutrophils stimulated with group B meningococcal outer membrane vesicles. Infect Immun. 2000;68:6917–23.
Davis JM, Carvalho HM, Rasmussen SB, O’Brien AD. Cytotoxic necrotizing factor type 1 delivered by outer membrane vesicles of uropathogenic Escherichia coli attenuates polymorphonuclear leukocyte antimicrobial activity and chemotaxis. Infect Immun. 2006;74:4401–8.
Vida A, Troelstra A, Antal-Szalmás P, van Bommel TJ, Verheul AF, Verhoef J, van Kessel KP, van Strijp JA. Neutralization of Neisseria meningitidis outer membrane vesicles. Inflamm Res. 2011;60:801–5.
Pilsczek FH, Salina D, Poon KK, Fahey C, Yipp BG, Sibley CD, Robbins SM, Green FH, Surette MG, Sugai M, et al. A novel mechanism of rapid nuclear neutrophil extracellular trap formation in response to Staphylococcus aureus. J Immunol. 2010;185:7413–25.
Lappann M, Danhof S, Guenther F, Olivares-Florez S, Mordhorst IL, Vogel U. In vitro resistance mechanisms of Neisseria meningitidis against neutrophil extracellular traps. Mol Microbiol. 2013;89:433–49.
Laughlin RC, Mickum M, Rowin K, Adams LG, Alaniz RC. Altered host immune responses to membrane vesicles from Salmonella and Gram-negative pathogens. Vaccine. 2015;33:5012–9.
Zariri A, Beskers J, van de Waterbeemd B, Hamstra HJ, Bindels TH, van Riet E, van Putten JP, van der Ley P. Meningococcal outer membrane vesicle composition-dependent activation of the innate immune response. Infect Immun. 2016;84:3024–33.
Ko SH, Rho DJ, Jeon JI, Kim YJ, Woo HA, Kim N, Kim JM. Crude preparations of helicobacter pylori outer membrane vesicles induce upregulation of heme oxygenase-1 via activating Akt-Nrf2 and mTOR-IκB kinase-NF-κB pathways in dendritic cells. Infect Immun. 2016;84:2162–74.
McCaig WD, Loving CL, Hughes HR, Brockmeier SL. Characterization and vaccine potential of outer membrane vesicles produced by Haemophilus parasuis. PLoS ONE. 2016;11:e0149132.
Gao XJ, Li T, Wei B, Yan ZX, Hu N, Huang YJ, Han BL, Wai TS, Yang W, Yan R. Bacterial outer membrane vesicles from dextran sulfate sodium-induced colitis differentially regulate intestinal UDP-glucuronosyltransferase 1A1 partially through toll-like receptor 4/mitogen-activated protein kinase/phosphatidylinositol 3-kinase pathway. Drug Metab Dispos. 2018;46:292–302.
Cecil JD, O’Brien-Simpson NM, Lenzo JC, Holden JA, Singleton W, Perez-Gonzalez A, Mansell A, Reynolds EC. Outer membrane vesicles prime and activate macrophage inflammasomes and cytokine secretion in vitro and in vivo. Front Immunol. 2017;8:1017.
Fleetwood AJ, Lee MKS, Singleton W, Achuthan A, Lee MC, O’Brien-Simpson NM, Cook AD, Murphy AJ, Dashper SG, Reynolds EC, Hamilton JA. Metabolic remodeling, inflammasome activation, and pyroptosis in macrophages stimulated by Porphyromonas gingivalis and its outer membrane vesicles. Front Cell Infect Microbiol. 2017;7:351.
Jung AL, Hoffmann K, Herkt CE, Schulz C, Bertrams W, Schmeck B. Legionella pneumophila Outer membrane vesicles: isolation and analysis of their pro-inflammatory potential on macrophages. J Vis Exp. 2017;120:e55146.
Jung AL, Stoiber C, Herkt CE, Schulz C, Bertrams W, Schmeck B. Legionella pneumophila-derived outer membrane vesicles promote bacterial replication in macrophages. PLoS Pathog. 2016;12:e1005592.
Finethy R, Luoma S, Orench-Rivera N, Feeley EM, Haldar AK, Yamamoto M, Kanneganti TD, Kuehn MJ, Coers J. Inflammasome activation by bacterial outer membrane vesicles requires guanylate binding proteins. MBio. 2017;8:e01188-17.
Tavano R, Franzoso S, Cecchini P, Cartocci E, Oriente F, Aricò B, Papini E. The membrane expression of Neisseria meningitidis adhesin A (NadA) increases the proimmune effects of MenB OMVs on human macrophages, compared with NadA-OMVs, without further stimulating their proinflammatory activity on circulating monocytes. J Leukoc Biol. 2009;86:143–53.
Lee WH, Choi HI, Hong SW, Kim KS, Gho YS, Jeon SG. Vaccination with Klebsiella pneumoniae-derived extracellular vesicles protects against bacteria-induced lethality via both humoral and cellular immunity. Exp Mol Med. 2015;47:e183.
Gaudino SJ, Kumar P. Cross-talk between antigen presenting cells and T cells impacts intestinal homeostasis, bacterial infections, and tumorigenesis. Front Immunol. 2019;10:360–360.
Mitra S, Sinha R, Mitobe J, Koley H. Development of a cost-effective vaccine candidate with outer membrane vesicles of a tolA-disrupted Shigella boydii strain. Vaccine. 2016;34:1839–46.
Deo P, Chow SH. Outer membrane vesicles from Neisseria gonorrhoeae target PorB to mitochondria and induce apoptosis. PLoS Pathog. 2018;14:e1006945.
Winter J, Letley D, Rhead J, Atherton J, Robinson K. Helicobacter pylori membrane vesicles stimulate innate pro- and anti-inflammatory responses and induce apoptosis in Jurkat T cells. Infect Immun. 2014;82:1372–81.
Lee DH, Kim SH, Kang W, Choi YS, Lee SH, Lee SR, You S, Lee HK, Chang KT, Shin EC. Adjuvant effect of bacterial outer membrane vesicles with penta-acylated lipopolysaccharide on antigen-specific T cell priming. Vaccine. 2011;29:8293–301.
Youssef AR, van der Flier M, Estevão S, Hartwig NG, van der Ley P, Virji M. Opa+ and Opa- isolates of Neisseria meningitidis and Neisseria gonorrhoeae induce sustained proliferative responses in human CD4+ T cells. Infect Immun. 2009;77:5170–80.
Hock BD, McKenzie JL, Keenan JI. Helicobacter pylori outer membrane vesicles inhibit human T cell responses via induction of monocyte COX-2 expression. Pathog Dis. 2017;75:ftx034.
Zhu W, Tomberg J, Knilans KJ, Anderson JE, McKinnon KP, Sempowski GD, Nicholas RA, Duncan JA. Properly folded and functional PorB from Neisseria gonorrhoeae inhibits dendritic cell stimulation of CD4(+) T cell proliferation. J Biol Chem. 2018;293:11218–29.
Vaughan AT, Brackenbury LS, Massari P, Davenport V, Gorringe A, Heyderman RS, Williams NA. Neisseria lactamica selectively induces mitogenic proliferation of the naive B cell pool via cell surface Ig. J Immunol. 2010;185:3652–60.
Bachmann MF, Jennings GT. Vaccine delivery: a matter of size, geometry, kinetics and molecular patterns. Nat Rev Immunol. 2010;10:787–96.
Holst J, Oster P, Arnold R, Tatley M, Næss L, Aaberge I, Galloway Y, McNicholas A, O’Hallahan J, Rosenqvist E. Vaccines against meningococcal serogroup B disease containing outer membrane vesicles (OMV): lessons from past programs and implications for the future. Hum Vaccin Immunother. 2013;9:1241–53.
Bjune G, Høiby E, Grønnesby J, Arnesen Ø, Fredriksen JH, Lindbak A, Nøkleby H, Rosenqvist E, Solberg L, Closs O. Effect of outer membrane vesicle vaccine against group B meningococcal disease in Norway. The Lancet. 1991;338:1093–6.
Sierra G, Campa H, Varcacel N, Garcia I, Izquierdo P, Sotolongo P, Casanueva G, Rico C, Rodriguez C, Terry M. Vaccine against group B Neisseria meningitidis: protection trial and mass vaccination results in Cuba. NIPH Ann. 1991;14:195–207 (discussion 208).
Arnold R, Galloway Y, McNicholas A, O’Hallahan J. Effectiveness of a vaccination programme for an epidemic of meningococcal B in New Zealand. Vaccine. 2011;29:7100–6.
Holst J, Martin D, Arnold R, Huergo CC, Oster P, O’Hallahan J, Rosenqvist E. Properties and clinical performance of vaccines containing outer membrane vesicles from Neisseria meningitidis. Vaccine. 2009;27:B3–12.
Koeberling O, Delany I, Granoff DM. A critical threshold of meningococcal factor H binding protein expression is required for increased breadth of protective antibodies elicited by native outer membrane vesicle vaccines. Clin Vaccine Immunol. 2011;18:736–42.
van de Waterbeemd B, Mommen GP, Pennings JL, Eppink MH, Wijffels RH, van der Pol LA, de Jong AP. Quantitative proteomics reveals distinct differences in the protein content of outer membrane vesicle vaccines. J Proteome Res. 2013;12:1898–908.
Peeters C, Rümke H, Sundermann L, van der Voort ER, Meulenbelt J, Schuller M, Kuipers A, Van der Ley P, Poolman J. Phase I clinical trial with a hexavalent PorA containing meningococcal outer membrane vesicle vaccine. Vaccine. 1996;14:1009–15.
Claassen I, Meylis J, van der Ley P, Peeters C, Brons H, Robert J, Borsboom D, van der Ark A, van Straaten I, Roholl P. Production, characterization and control of a Neisseria meningitidis hexavalent class 1 outer membrane protein containing vesicle vaccine. Vaccine. 1996;14:1001–8.
Camacho A, De Souza J, Sánchez-Gómez S, Pardo-Ros M, Irache JM, Gamazo C. Mucosal immunization with Shigella flexneri outer membrane vesicles induced protection in mice. Vaccine. 2011;29:8222–9.
Acevedo R, Callicó A, Aranguren Y, Zayas C, Valdés Y, Pérez O, García L, Ferro VA, Pérez JL. Immune adjuvant effect of V. cholerae O1 derived Proteoliposome coadministered by intranasal route with Vi polysaccharide from Salmonella Typhi. BMC Immunol. 2013;14:1–4.
Reed SG, Orr MT, Fox CB. Key roles of adjuvants in modern vaccines. Nat Med. 2013;19:1597–608.
Awate S, Babiuk LAB, Mutwiri G. Mechanisms of action of adjuvants. Front Immunol. 2013;4:114.
Gerritzen MJH, Martens DE, Wijffels RH, van der Pol L, Stork M. Bioengineering bacterial outer membrane vesicles as vaccine platform. Biotechnol Adv. 2017;35:565–74.
Doyle SE, O’Connell RM, Miranda GA, Vaidya SA, Chow EK, Liu PT, Suzuki S, Suzuki N, Modlin RL, Yeh W-C. Toll-like receptors induce a phagocytic gene program through p38. J Exp Med. 2004;199:81–90.
Blander JM, Medzhitov R. Regulation of phagosome maturation by signals from toll-like receptors. Science. 2004;304:1014–8.
Cao X. Self-regulation and cross-regulation of pattern-recognition receptor signalling in health and disease. Nat Rev Immunol. 2016;16:35.
Napolitani G, Rinaldi A, Bertoni F, Sallusto F, Lanzavecchia A. Selected Toll-like receptor agonist combinations synergistically trigger a T helper type 1–polarizing program in dendritic cells. Nat Immunol. 2005;6:769–76.
Kasturi SP, Skountzou I, Albrecht RA, Koutsonanos D, Hua T, Nakaya HI, Ravindran R, Stewart S, Alam M, Kwissa M. Programming the magnitude and persistence of antibody responses with innate immunity. Nature. 2011;470:543–7.
Schetters STT, Jong WSP, Horrevorts SK, Kruijssen LJW, Engels S, Stolk D, Daleke-Schermerhorn MH, Garcia-Vallejo J, Houben D, Unger WWJ, et al. Outer membrane vesicles engineered to express membrane-bound antigen program dendritic cells for cross-presentation to CD8+ T cells. Acta Biomater. 2019;91:248–57.
Stevenson TC, Cywes-Bentley C, Moeller TD, Weyant KB, Putnam D, Chang Y-F, Jones BD, Pier GB, DeLisa MP. Immunization with outer membrane vesicles displaying conserved surface polysaccharide antigen elicits broadly antimicrobial antibodies. Proc Natl Acad Sci USA. 2018;115:E3106–15.
Kim OY, Park HT, Dinh NTH, Choi SJ, Lee J, Kim JH, Lee S-W, Gho YS. Bacterial outer membrane vesicles suppress tumor by interferon-γ-mediated antitumor response. Nat Commun. 2017;8:1–9.
Chen Q, Bai H, Wu W, Huang G, Li Y, Wu M, Tang G, Ping Y. Bioengineering bacterial vesicle-coated polymeric nanomedicine for enhanced cancer immunotherapy and metastasis prevention. Nano Lett. 2019;20:11–21.
Kai Xue K, Wang L, Liu J. Bacterial outer membrane vesicles and their functionalization as vehicles for bioimaging, diagnosis and therapy. Mater Adv. 2022;3:7185–97.
Xu K, Liu Q, Wu K, Liu L, Zhao M, Yang H, Wang X, Wang W. Extracellular vesicles as potential biomarkers and therapeutic approaches in autoimmune diseases. J Transl Med. 2020;18:1–8.
Liu JH, Chen CY, Liu ZZ, Luo ZW, Rao SS, Jin L, Wan TF, Yue T, Tan YJ, Yin H, et al. Extracellular vesicles from child gut microbiota enter into bone to preserve bone mass and strength. Adv Sci. 2021;8:2004831.
Pathirana RD, Kaparakis-Liaskos M. Bacterial membrane vesicles: biogenesis, immune regulation and pathogenesis. Cell Microbiol. 2016;18:1518–24.
Ghosal A, Upadhyaya BB, Fritz JV, Heintz-Buschart A, Desai MS, Yusuf D, Huang D, Baumuratov A, Wang K, Galas D. The extracellular RNA complement of Escherichia coli. Microbiologyopen. 2015;4:252–66.
Sjostrom A, Sandblad L, Uhlin B, Wai S. Membrane vesiclemediated release of bacterial RNA. Sci Rep. 2015;5:15329.
Resch U, Tsatsaronis JA, Le Rhun A, Stübiger G, Rohde M, Kasvandik S, Holzmeister S, Tinnefeld P, Wai SN, Charpentier E. A two-component regulatory system impacts extracellular membrane-derived vesicle production in group A Streptococcus. MBio. 2016;7:e00207-16.
Longo DL, Stefania R, Aime S, Oraevsky A. Melanin-based contrast agents for biomedical optoacoustic imaging and theranostic applications. Int J Mol Sci. 2017;18:1719.
Gujrati V, Prakash J, Malekzadeh-Najafabadi J, Stiel A. Bioengineered bacterial vesicles as biological nano-heaters for optoacoustic imaging. Nat Commun. 2019;10:1114.
Settem RP, El-Hassan AT, Honma K, Stafford GP, Sharma A. Fusobacterium nucleatum and Tannerella forsythia induce synergistic alveolar bone loss in a mouse periodontitis model. Infect Immun. 2012;80:2436–43.
Han P, Bartold PM, Salomon C, Ivanovski S. Salivary outer membrane vesicles and DNA methylation of small extracellular vesicles as biomarkers for periodontal status: a pilot study. Int J Mol Sci. 2021;22:2423.
Ahmadi Badi S, Khatami SH, Irani SH, Siadat SD. Induction effects of bacteroides fragilis derived outer membrane vesicles on toll like receptor 2, toll like receptor 4 genes expression and cytokines concentration in human intestinal epithelial cells. Cell J. 2019;21:57–61.
Durant L, Stentz R, Noble A, Brooks J, Gicheva N, Reddi D, O’Connor MJ, Hoyles L, McCartney AL, Man R, et al. Bacteroides thetaiotaomicron-derived outer membrane vesicles promote regulatory dendritic cell responses in health but not in inflammatory bowel disease. Microbiome. 2020;8:88.
Peng LH, Wang MZ. Engineering bacterial outer membrane vesicles as transdermal nanoplatforms for photo-TRAIL-programmed therapy against melanoma. Sci Adv. 2020;6:eaba2735.
Gao L, van der Veen S. Role of outer membrane vesicles in bacterial physiology and host cell interactions. Infect Microbes Dis. 2020;2:3–9.
Collins SM, Brown AC. Bacterial outer membrane vesicles as antibiotic delivery vehicles. Front Immunol. 2021;12:3773.
Zingl FG, Leitner DR, Thapa HB, Schild S. Outer membrane vesicles as versatile tools for therapeutic approaches. microLife. 2021;2:uqab006.
Kim OY, Park HT, Dinh NTH, Choi SJ, Lee J, Kim JH, Lee S-W, Gho YS. Bacterial outer membrane vesicles suppress tumor by interferon-γ-mediated antitumor response. Nat Commun. 2017;8:626.
Cheng K, Zhao R, Li Y, Qi Y, Wang Y, Zhang Y, Qin H, Qin Y, Chen L, Li C, et al. Bioengineered bacteria-derived outer membrane vesicles as a versatile antigen display platform for tumor vaccination via Plug-and-Display technology. Nat Commun. 2021;12:2041.
Balhuizen MD, Veldhuizen EJA, Haagsman HP. Outer membrane vesicle induction and isolation for vaccine development. Front Microbiol. 2021;12:629090–629090.
Reimer SL, Beniac DR, Hiebert SL, Booth TF, Chong PM, Westmacott GR, Zhanel GG, Bay DC. Comparative analysis of outer membrane vesicle isolation methods with an Escherichia coli tolA mutant reveals a hypervesiculating phenotype with outer-inner membrane vesicle content. Front Microbiol. 2021;12:628801.
Vipond C, Suker J, Jones C, Tang C, Feavers IM, Wheeler JX. Proteomic analysis of a meningococcal outer membrane vesicle vaccine prepared from the group B strain NZ98/254. Proteomics. 2006;6:3400–13.
Ahmadi Badi S, Moshiri A, Ettehad Marvasti F, Mojtahedzadeh M, Kazemi V, Siadat SD. Extraction and evaluation of outer membrane vesicles from two important gut microbiota members, Bacteroides Fragilis and Bacteroides Thetaiotaomicron. Cell J. 2020;22:344–9.
Fateh A, Vaziri F, Rahimi Janani F, Ahmadi Badi S, Ghazanfari M, Davari M, Arsang A, Siadat S. New insight into the application of outer membrane vesicles of Gram-negative bacteria. Vaccine Res. 2016;3:1–4.
Fazal S, Lee R. Biomimetic bacterial membrane vesicles for drug delivery applications. Pharmaceutics. 2021;13:1430.
Rossi O, Citiulo F, Mancini F. Outer membrane vesicles: moving within the intricate labyrinth of assays that can predict risks of reactogenicity in humans. Hum Vaccin Immunother. 2021;17:601–13.
Steeghs L, Keestra AM, van Mourik A, Uronen-Hansson H, van der Ley P, Callard R, Klein N, van Putten JP. Differential activation of human and mouse Toll-like receptor 4 by the adjuvant candidate LpxL1 of Neisseria meningitidis. Infect Immun. 2008;76:3801–7.
Gujrati V, Kim S, Kim S-H, Min JJ, Choy HE, Kim SC, Jon S. Bioengineered bacterial outer membrane vesicles as cell-specific drug-delivery vehicles for cancer therapy. ACS Nano. 2014;8:1525–37.
Wu J, An M, Zhu J, Tan Z, Chen GY, Stidham RW, Lubman DM. A Method for isolation and proteomic analysis of outer membrane vesicles from fecal samples by LC-MS/MS. J Proteomics Bioinformat. 2019;12:38.
Zhang H, Zhang Y, Song Z, Li R, Ruan H, Liu Q, Huang X. sncRNAs packaged by Helicobacter pylori outer membrane vesicles attenuate IL-8 secretion in human cells. Int J Med Microbiol. 2020;310:151356.
Sandbu S, Feiring B, Oster P, Helland OS, Bakke HS, Naess LM, Aase A, Aaberge IS, Kristoffersen AC, Rydland KM, et al. Immunogenicity and safety of a combination of two serogroup B meningococcal outer membrane vesicle vaccines. Clin Vaccine Immunol. 2007;14:1062–9.
Sharif E, Eftekhari Z, Mohit E. The Effect of growth stage and isolation method on properties of ClearColi™ outer membrane vesicles (OMVs). Curr Microbiol. 2021;78:1602–14.
Francis IP, Lui X, Wetzler LM. Isolation of naturally released gonococcal outer membrane vesicles as vaccine antigens. Methods Mol Biol. 2019;1997:121–41.
Marsay L, Dold C, Green CA, Rollier CS, Norheim G, Sadarangani M, Shanyinde M, Brehony C, Thompson AJ, Sanders H, et al. A novel meningococcal outer membrane vesicle vaccine with constitutive expression of FetA: a phase I clinical trial. J Infect. 2015;71:326–37.
Petousis-Harris H, Paynter J, Morgan J, Saxton P, McArdle B, Goodyear-Smith F, Black S. Effectiveness of a group B outer membrane vesicle meningococcal vaccine against gonorrhoea in New Zealand: a retrospective case-control study. Lancet. 2017;390:1603–10.
Gorringe AR, Taylor S, Brookes C, Matheson M, Finney M, Kerr M, Hudson M, Findlow J, Borrow R, Andrews N, et al. Phase I safety and immunogenicity study of a candidate meningococcal disease vaccine based on Neisseria lactamica outer membrane vesicles. Clin Vaccine Immunol. 2009;16:1113–20.
Findlow J, Lowe A, Deane S, Balmer P, van den Dobbelsteen G, Dawson M, Andrews N, Borrow R. Effect of sequence variation in meningococcal PorA outer membrane protein on the effectiveness of a hexavalent PorA outer membrane vesicle vaccine in toddlers and school children. Vaccine. 2005;23:2623–7.
Keiser PB, Gibbs BT, Coster TS, Moran EE, Stoddard MB, Labrie JE 3rd, Schmiel DH, Pinto V, Chen P, Zollinger WD. A phase 1 study of a group B meningococcal native outer membrane vesicle vaccine made from a strain with deleted lpxL2 and synX and stable expression of opcA. Vaccine. 2010;28:6970–6.
Sardiñas G, Reddin K, Pajon R, Gorringe A. Outer membrane vesicles of Neisseria lactamica as a potential mucosal adjuvant. Vaccine. 2006;24:206–14.
Liu Q, Tan K, Yuan J, Song K, Li R, Huang X, Liu Q. Flagellin-deficient outer membrane vesicles as adjuvant induce cross-protection of Salmonella Typhimurium outer membrane proteins against infection by heterologous Salmonella serotypes. Int J Med Microbiol. 2018;308:796–802.
Gu TW, Wang MZ, Niu J, Chu Y, Guo KR, Peng LH. Outer membrane vesicles derived from E. coli as novel vehicles for transdermal and tumor targeting delivery. Nanoscale. 2020;12:18965–77.
Huang W, Shu C, Hua L, Zhao Y, Xie H, Qi J, Gao F, Gao R, Chen Y, Zhang Q, et al. Modified bacterial outer membrane vesicles induce autoantibodies for tumor therapy. Acta Biomater. 2020;108:300–12.
Shi J, Ma Z, Pan H, Liu Y, Chu Y, Wang J, Chen L. Biofilm-encapsulated nano drug delivery system for the treatment of colon cancer. J Microencapsul. 2020;37:481–91.
Acknowledgements
The authors would like to thank Microbial Biotechnology Research Center, Iran University of Medical Sciences for funding this study (grant number: 1401-2-73-23786).
Author information
Authors and Affiliations
Contributions
SJ, RM, SRH, and BB participated in the study design, wrote the draft, and collected the documentation materials. GI, HMK and SK participated in the study design and helped revise the draft. All authors read and approved the manuscript.
Corresponding authors
Ethics declarations
Ethical approval and consent to participate
This study was approved by the Microbial Biotechnology Research Center, Iran University of Medical Sciences, Tehran, Iran, with code number: IR.IUMS.REC.1401.598.
Competing interests
The authors declare that they have no known competing financial interests or personal relationships that could have influenced the work reported in this paper.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
The original version of this article was revised: errors in the affiliations were corrected.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Jalalifar, S., Morovati Khamsi, H., Hosseini-Fard, S.R. et al. Emerging role of microbiota derived outer membrane vesicles to preventive, therapeutic and diagnostic proposes. Infect Agents Cancer 18, 3 (2023). https://doi.org/10.1186/s13027-023-00480-4
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s13027-023-00480-4