Abstract
Background
Spinocerebellar ataxia type 3 (SCA3) is an inherited, autosomal, and rare neurodegenerative disease. Serum/plasma biomarkers or functional magnetic resonance imaging used to assess progression, except for neurological examinations, is either inconvenient or expensive. Handgrip strength (HGS) may be considered as a biomarker to predict the progress of SCA3 and align with the alteration of plasma neurofilament light chain (NfL) and Scale for the Assessment and Rating of Ataxia (SARA).
Methods
Patients with SCA3 and healthy subjects were recruited from Changhua Christian Hospital. SARA, body mass index (BMI), and NfL were obtained for both groups. HGS was measured using a Jamar Plus + hand dynamometer.
Results
This study recruited 31 patients and 36 controls. HGS in the SCA3 group revealed a profound decrease (P < 0.001) compared with normal subjects. HGS also had a negative correlation with SARA (r = − 0.548, P = 0.001), NfL (r = − 0.359, P = 0.048), and a positive correlation with BMI (r = 0.680, P < 0.001). Moreover, HGS/BMI ratio correlated with SARA (r = − 0.441, P = 0.013). Controlling for gender and age, HGS still correlated with the above clinical items. The initial hypothesis was also proved in SCA3 84Q transgenic mice, showing grip strength weakness compared to normal mice.
Conclusions
HGS can be an alternative tool to assess the clinical severity of SCA3. Further research is needed to investigate the underlying mechanisms.
Similar content being viewed by others
Background
Spinocerebellar ataxia (SCA) is an autosomal inherited disease and one of the rare neurodegenerative diseases. SCA type 3 (SCA3), also known as Machado-Joseph disease, caused by abnormal CAG trinucleotide expansion greater than 62 at the gene of ataxin-3, is the most frequent SCA in most countries [1]. Common symptoms include imbalance, incoordination, muscle rigidity or spasticity, dysarthria, diplopia, and depression [2]. Scientists have been searching for disease-modifying therapies in recent years, such as varenicline, valproic acid, or Trehalose, because SCA3 is challenging to treat [3]. SCA3 is a slowly progressive neurodegenerative disease; however, monitoring the change of clinical status by neurological scales in a short period would be challenging [4].
Physicians are on the lookout for assessment tools that are both accessible and efficient when it comes to neurological status assessment for ataxia. The Scale for the Assessment and Rating of Ataxia (SARA) is a clinical scale dedicated to detecting disease progression of cerebellar symptoms [5]. Morphometric magnetic resonance imaging (MRI) offers vital insights into structural changes, such as volumetric reduction in cerebellum, basal ganglia, brain stem, and diencephalon. These are instrumental in confirming and enhancing our understanding of this disease [6]. Functional MRI has revealed cerebellar/cortical dissociation pattern specific to SCA3 patients, as opposed to healthy controls. This finding shed light on the evaluation of potential functional connectivity within cerebral-cerebellar motor networks [7]. Neurofilament light chain (NfL) is an emerging blood biomarker to evaluate neuroaxonal damage such as Alzheimer’s disease, multiple sclerosis, postoperative delirium, multiple system atrophy, and amyotrophic lateral sclerosis [8,9,10]. NfL also correlates with the disease severity of SCA3 and predicts its pre-ataxic and ataxic stages [11, 12]. Urinal polyglutamine ataxin-3 protein is elevated in patients with SCA3 [13]. while polyglutamine ataxin-3 in plasma or peripheral blood mononuclear cells correlates with disease severity [14, 15]. However, fMRI and the above biomarkers, except for SARA, are either expensive or inconvenient. Developing an easy and affordable assessment tool is highly anticipated and much needed for clinical use.
Handgrip strength (HGS), which is measured using a portable hand-held dynamometer, has been a practical tool to assess the well-being of the elderly. HGS could predict longitudinal declines in cognition, functional status, and mortality in older community populations [16]. The United Kingdom Biobank data reported that higher dementia incidence and mortality were independently associated with lower grip strength [17]. Grip strength could also assess muscle activity, which predicts functional loss in patients with Parkinson’s disease, where striatal dopamine and impaired motor unit degeneration are recruited [18]. Proper grip strength cutoff points are good predictors of functional independence and wheelchair skills for males with spinal cord injuries [19]. HGS might relate functional capacity or motor performance to cerebellar disorders in a small pilot study, including three patients with degenerative cerebellar ataxia, without mentioning the genetic background [20]. For animal models related to neurodegeneration diseases, HGS can be identified as a physiologic biomarker to forecast the disease course [21,22,23]. In a transgenic marmoset model of SCA3 diseases, age-associated decrease of HGS were significantly more prominent in transgenic symptomatic marmosets compared to either wide-type or asymptomatic marmosets [24]. However, the relationship between HGS and the clinical status of patients with SCA3 remains unknown.
Herein, we aim to establish the validity of HGS inspection as a predictor of the disease progress in patients and mice with SCA3, and its application in ataxia clinic for the disease following.
Result
Demographic data
This study enrolled 31 participants with SCA3 and 36 healthy controls. No significant difference was found in age or gender between the two groups (age: P = 0.517, gender: P = 0.988), as shown in detail in Table 1. The BMI of participants in the SCA3 group was lower than that of the control group (21.5 kg/m2 [19.5–25.5] vs. 23.6 kg/m2 [21.8–25.6], P = 0.043), consistent with the previous studies [25, 26]. The SCA3 group exhibited weaker HGS (24.3 kg [19.2–38.2] vs. 40.7 kg [27.1–49.3], P < 0.001, Fig. 1A) and lower HGS/BMI ratio compared to the control group (1.19 kg/(kg/m2) [0.95–1.55] vs. 1.38 kg/(kg/m2) [1.11–1.79], P < 0.001). A decrease in HGS was observed in either SCA3 female (20.10 kg [18.60–25.10] vs. 26.80 kg [25.45–30.4], P = 0.007) or SCA3 male (35.10 kg [23.30–38.33] vs. 47.20 kg [42.35–55.25], P < 0.001) patients compared with normal subjects in Fig. 1B. Additionally, a notable difference was observed in the significantly elevated plasma level of NfL in patients with SCA3 as compared to that in normal subjects (28.01 pg/mL [20.65–32.75] vs. 6.35 pg/mL [4.22–7.63], P < 0.001, Table 1). An elevated NfL level was found to predict the severity of SARA within the SCA3 group (r = 0.436, P = 0.014; see Additional file 1), consistent with previous reports [11, 12].
Correlation between HGS and clinical items
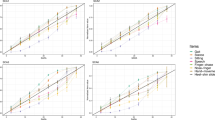
The high strength of HGS can predict low scoring on SARA (r = − 0.548, P = 0.001, Fig. 2A) and NfL (r = − 0.359, P = 0.048, Fig. 2B) in SCA3 patients. Additionally, greater HGS was associated with lower scores in each subscale of SARA, including gait (r = − 0.525, P = 0.002), stance ( r = − 0.487, P = 0.005), sitting (r = − 0.636, P < 0.001), speech disturbance (r = − 0.522, P = 0.003), finger chase (r = − 0.510, P = 0.003), finger-nose test (r = − 0.355, P = 0.050), fast alternating hand movements (r = − 0.505, P = 0.004), and heel-shin slide (r = − 0.403, P = 0.025), as shown in Table 2.
In the SCA3 patient group, HGS was effectively indicative of BMI (r = 0.680, P < 0.001, Fig. 2C). BMI has been demonstrated as a disease progression predictor in SCA3 [25, 26], and our study revealed a significant negative correlation with SARA (r = − 0.420, P = 0.019; see Additional file 1). BMI has been correlated with HGS in most studies [27, 28]; thus, the HGS/BMI ratio was calculated as a new variable after adjusting HGS for BMI. The HGS/BMI ratio still exhibited a negative correlation with SARA scores (r = − 0.441, P = 0.013, Fig. 2D). Even after considering gender and age as controlling factors, HGS continued to illustrate a consistent relationship with SARA, NfL, and BMI. Moreover, lower HGS can predict SCA3 patients with a higher CAG repeat number under adjusting for age (r = − 0.396, P = 0.030; see Additional file 2).
Grip strength in SCA3 mice
The grip strength of SCA3 84Q transgenic mice was significantly decreased at the age of 18 months than that of WT mice (P = 0.003, Fig. 3A). After correcting for body weight, the grip strength of SCA3 mice remained lower when compared to that of the WT mice(P = 0.007, Fig. 3B).
Discussion
The current assessment of disease progression in SCA3 involves neurological examinations, such as SARA, for evaluating ataxia. NfL [12] and BMI [26] have established themselves as reliable predictors of SCA3, and our research uncovered a robust relationship between HGS and SARA, even after adjusting for gender and age. The increased strength of HGS can predict higher BMI and lower plasma levels of NfL. Additionally, a higher HGS/BMI ratio indicated lower scores in the SARA evaluation. Moreover, our study demonstrated that patients with SCA3 exhibited significantly lower HGS compared to healthy controls. This study represents the first instance where HGS or the HGS/BMI ratio has independently predicted the disease severity of SCA3, including all eight subscores of SARA. HGS can serve as an easily accessible tool to estimate the disease status of SCA3 in a clinical setting, even when considering the gene loading of SCA3-CAG repeat expansion.
HGS is a widely used measure of muscular strength, but various factors can influence its accuracy. HGS in patients with SCA3 may relate to myopathy, neuropathy or other comorbidity. Our study revealed a significant inverse correlation between HGS and disease severity, even after controlling for common factors. Various conditions, including arthritis, tendinitis, and major vascular or neurological disorders, can affect grip strength. Other factors, such as sex, age, handedness, and nutritional status, also impact HGS [29]. Participants with severe comorbidities, such as stroke, cancer, heart failure, or kidney failure, were excluded, and the absence of arthritis or tendinitis was screened to ensure the accuracy of our study. Handedness was determined according to the standard protocol for using a hand dynamometer [30], while sex and age were considered during statistical analysis.
Accordingly, recent literature revealed a report investigating grip strength in transgenic SCA3 135Q mice without mentioning the pathogenesis that contributes to the reduction of grip strength [31]. In the present study, we found a similar result in SCA3 84Q mice with weak grip strength compared with WT mice during the late disease stage. To account for the potential confounding effect of decreased body weight in SCA3 84Q mice at 18 months, we adjusted for body weight and still detected weaker grip strength in SCA3 mice compared to WT mice. Our previous studies have also demonstrated a decrease in the ratios of muscle mass and body weight in the quadriceps, gastrocnemius, tibialis, extensor digitorum longus, and soleus muscles compared to WT mice. The cross-sectional area of muscle fibers was found to be reduced in SCA3 84Q mice [32]. Atrophic signaling involving Akt/Forkhead box-O and myosin heavy chain (MyHC) expressions were implicated in these findings, indicating the existence of sarcopenia or muscle disease [33, 34]. Specifically, our study revealed a significant decrease in phosphorylated AKT and the muscle cell differentiation marker, MyHC, in SCA3 84Q mice compared to WT mice which indicated the possible muscular pathogenesis involved in the disease of SCA3 [32].
Muscle weakness is a common feature in patients with SCA3, and recent studies indicate that myopathic origin may contribute to distal muscle weakness in these individuals [35]. Further, evidence indicates a potential association between SCA3 and sarcopenia, as patients with SCA3 display lower muscle strength and lean mass than healthy controls [36]. Therefore, the muscle atrophy pathway may be related to the lower HGS observed in patients with SCA3 who have sarcopenia. Our study revealed that sarcopenia-related BMI and HGS could provide valuable insights into the clinical progression of patients with SCA3. A declining BMI signifies deterioration in the condition of patients with SCA3 and may indicate the possible occurrence of comorbidity with sarcopenia. Another report suggests that hyperkinesia may lead to increased energy expenditure, while dysphagia results in decreased nutrition intake, both potentially contributing to a body composition resembling sarcopenia [36]. The Foundation for the National Institutes of Health Biomarkers Consortium Sarcopenia Project also recommends grip strength measurements, such as HGS and HGS/BMI ratio, as non-Dual-energy X-ray absorptiometry approaches to identify sarcopenic patients among the elderly [37]. Our study revealed that HGS not only helps assess the clinical severity of SCA3 but is also significantly related to BMI. HGS can be regarded as a valuable tool for evaluating the progression of SCA3 patients with sarcopenia.
Therefore, further investigation into the possible mechanism of decreased HGS, including peripheral neuropathy, such as electromyography, nerve conduction studies, or even muscle biopsy, should be considered as the next step. In the future, we will conduct long-term longitudinal observations to examine the relationship between HGS and disease progression and expand our sample size to include more SCA3 patients and normal subjects.
Conclusions
This is the first report on HGS in patients with SCA3. HGS exhibited an inverse correlation with the neurological status, namely SARA and NfL, and demonstrated a strong correlation with BMI, which is another predictor of disease progression. The increased HGS/BMI ratio consistently showed a relationship with SARA, providing valuable insights into disease severity. Combined with the grip strength information obtained from SCA3 84Q mice in the present study, HGS can serve as a practical and accessible tool to evaluate the clinical status of SCA3.
Methods
Patient recruitment
Subjects diagnosed with SCA3 by genetic mutational screening were recruited at Changhua Christian Hospital (CCH), Taiwan. The inclusion criteria for patients were a SARA score of ≥ 3 points [38]. Informed consent was collected from May 2021 to January 2022 (CCH-IRB approval No:200703). Participants were aged from 20 to 80 years. Exclusion criteria were pregnancy, comorbidity with stroke, cancer, heart failure, or renal failure. Patients with < 3 Points on the SARA scale were excluded. Demographic data such as age, age of disease onset, disease duration, CAG trinucleotide repeat number, and body mass index (BMI) were recorded. Age- and sex-matched individuals with negative genetic screening for mutant ataxin-3 were recruited as healthy controls from the normal population of staff in CCH (CCH-IRB approval No:200730).
HGS
A single physician measured the HGS with a Jamar Plus + hand dynamometer (Sammons Preston Rolyan, Bolingbrook, IL, USA). Participants were asked to sit on a straight back chair, keep their elbow flexed at 90 degrees, and hold the dynamometer with handle position 2, which is the distance of 4.8 cm from the handle to the fixed part of the dynamometer [30]. Participants had to apply maximal power for 3 s by the dominant hand. Grip strength was measured three times, with rest for 15 s between each effort [39]. The handgrip strength was the maximal value of the three measurements.
SARA
A single experienced neurologist measured SARA for the SCA3 group. SARA is a universal scale to assess the severity of ataxia. SARA has eight subscores, including gait, stance, sitting, speech disturbance, finger chase, nose-finger test, fast alternating hand movements, and heel-shin slide [40]. The total scores ranged from 0 to 40, with higher points indicating worse cerebellar conditions.
Plasma NfL
Blood was collected in BD EDTA tubes and then centrifuged at 2500 × g for 10 min at 4 °C to obtain plasma for each participant. Plasma samples were diluted at a ratio of 1:4. The single-molecule (Simoa) array technology by an ultra-high sensitivity protein molecular detection instrument (Simoa HD-X, Quanterix, MA, USA) and the Simoa NfL Advantage kit (Quanterix, MA, USA) measured the plasma NfL level [41]. All NfL values were within the linear ranges of the assays. The average intraassay coefficient of variation was 4.94%.
Animal model and grip strength
C57BL/6 wild-type (WT) mice were obtained from the National Laboratory Animal Center (Taipei, Taiwan), and SCA3 84Q transgenic mice (C57BL/6 background) have been previously described [42]. The Institutional Animal Care and Use Committee of Changhua Christian Hospital approved all animal experiments (approval number: CCH-AE-108-021). The genotype of SCA3 84Q mice was confirmed through the polymerase chain reaction of a DNA sample obtained from the mouse tail (primer sequences for forward: 5′-TGGCCTTTCACATGGATGTGAA-3′, reverse: 5′-CCAGTGACTACTTTGATTCG-3′). The 430-bp molecule refers to the positive of SCA3 84Q. Mice received standard diets and were housed under a 12-h light/dark cycle in a temperature-controlled room. The body weights of the mice were recorded weekly to monitor their health status. Grip strength tests were performed only on 18-month-old mice. A Digital Force Gauge (Model DPS-5R: range of 0–5 kgf, Imada, Japan) was used to measure the mice’s four-limb grip strength. The mouse was placed on a metal grid, and its tail was gently pulled back in parallel, and the apparatus automatically recorded the peak force when the mouse released its grip. The maximal force (grams) was represented as four-limb grip strength, while relative grip strength was normalized to the weight of the mouse. Each mouse underwent a grip strength test three times at 1-min intervals [43].
Statistics
Continuous variables were presented as the medians and interquartile ranges (25th–75th percentile), whereas the categorical variables were presented as numbers and percentages because most of the continuous variables did not follow a normal distribution. The Mann–Whitney U test was used to assess the difference in continuous variables between the healthy and diseased populations, while the chi-square test was employed for categorical variables. Additionally, the Spearman rank test was used to determine the strength of the relationship between the two variables. The partial correlation was used to adjust for age and gender. All data were analyzed using IBM Statistical Package for the Social Sciences for Windows, Version 22.0 (IBM Corp., Armonk, NY). A P value of < 0.05 was considered statistically significant.
Availability of data and materials
We understand the importance of providing a data availability statement as part of our research manuscript. While we acknowledge the significance of transparency and reproducibility, due to privacy and confidentiality concerns, we are unable to publicly disclose the data used in this study. We assure you that the data supporting our findings will be made available to interested researchers upon reasonable request, subject to ethical and legal considerations. For inquiries regarding access to the data, please contact Chin-San Liu (liu48111@gmail.com) and Chung-Min Chiu (shongdiah@gmail.com). We are committed to promoting scientific integrity and collaboration while maintaining the confidentiality of sensitive information.
Abbreviations
- SCA:
-
Spinocerebellar ataxia
- SCA3:
-
SCA type 3
- SARA:
-
Scale for the assessment and rating of ataxia
- MRI:
-
Magnetic resonance imaging
- NfL:
-
Neurofilament light chain
- HGS:
-
Handgrip strength
- CCH:
-
Changhua Christian Hospital
- BMI:
-
Body mass index
- WT:
-
Wild-type
- MyHC:
-
Myosin heavy chain
References
Schols L, et al. Autosomal dominant cerebellar ataxias: clinical features, genetics, and pathogenesis. Lancet Neurol. 2004;3(5):291–304.
Paulson H. Machado-Joseph disease/spinocerebellar ataxia type 3. Handb Clin Neurol. 2012;103:437–49.
Ghanekar SD, et al. Current and emerging treatment modalities for spinocerebellar ataxias. Expert Rev Neurother. 2022;22(2):101–14.
Lee YC, et al. Comparison of cerebellar ataxias: a three-year prospective longitudinal assessment. Mov Disord. 2011;26(11):2081–7.
Yabe I, et al. Usefulness of the scale for assessment and rating of ataxia (SARA). J Neurol Sci. 2008;266(1–2):164–6.
Arruda WO, et al. Volumetric MRI changes in spinocerebellar ataxia (SCA3 and SCA10) patients. The Cerebellum. 2020;19:536–43.
Yap KH, et al. Magnetic resonance imaging and its clinical correlation in spinocerebellar ataxia type 3: a systematic review. Front Neurosci. 2022;16:859651.
Narayanan S, et al. Neurofilament light: a narrative review on biomarker utility. Fac Rev. 2021;10:46.
Zhang L, et al. Neurofilament light chain predicts disease severity and progression in multiple system atrophy. Mov Disord. 2022;37(2):421–6.
Verde F, Otto M, Silani V. Neurofilament light chain as biomarker for amyotrophic lateral sclerosis and frontotemporal dementia. Front Neurosci. 2021;15:679199.
Wilke C, et al. Neurofilaments in spinocerebellar ataxia type 3: blood biomarkers at the preataxic and ataxic stage in humans and mice. EMBO Mol Med. 2020;12(7):e11803.
Li QF, et al. Neurofilament light chain is a promising serum biomarker in spinocerebellar ataxia type 3. Mol Neurodegener. 2019;14(1):39.
Koike Y, et al. Urine levels of the polyglutamine ataxin-3 protein are elevated in patients with spinocerebellar ataxia type 3. Parkinsonism Relat Disord. 2021;89:151–4.
Hubener-Schmid J, et al. Polyglutamine-expanded ataxin-3: a target engagement marker for spinocerebellar ataxia type 3 in peripheral blood. Mov Disord. 2021;36(11):2675–81.
Gonsior K, et al. PolyQ-expanded ataxin-3 protein levels in peripheral blood mononuclear cells correlate with clinical parameters in SCA3: a pilot study. J Neurol. 2021;268(4):1304–15.
Rijk JM, et al. Prognostic value of handgrip strength in people aged 60 years and older: a systematic review and meta-analysis. Geriatr Gerontol Int. 2016;16(1):5–20.
Esteban-Cornejo I, et al. Handgrip strength and all-cause dementia incidence and mortality: findings from the UK Biobank prospective cohort study. J Cachexia Sarcopenia Muscle. 2022;13(3):1514–25.
Jones GR, et al. Handgrip strength related to long-term electromyography: application for assessing functional decline in parkinson disease. Arch Phys Med Rehabil. 2017;98(2):347–52.
Neto FR, et al. Handgrip strength cutoff points for functional independence and wheelchair ability in men with spinal cord injury. Top Spinal Cord Inj Rehabil. 2021;27(3):60–9.
Giangiardi VF, et al. Functional capacity and motor performance of upper limbs in individuals with cerebellar disorders: a pilot study. Behav Neurol. 2017;2017:8980103.
Flis DJ, et al. Swim training modulates mouse skeletal muscle energy metabolism and ameliorates reduction in grip strength in a mouse model of amyotrophic lateral sclerosis. Int J Mol Sci. 2019;20(2):233.
Cho IK, et al. Combination of stem cell and gene therapy ameliorates symptoms in Huntington’s disease mice. NPJ Regenerat Med. 2019;4(1):7.
Tomczyk M, et al. Rosiglitazone ameliorates cardiac and skeletal muscle dysfunction by correction of energetics in huntington’s disease. Cells. 2022;11(17):2662.
Tomioka I, et al. Transgenic monkey model of the polyglutamine diseases recapitulating progressive neurological symptoms. Eneuro. 2017. https://doi.org/10.1523/ENEURO.0250-16.2017.
Diallo A, et al. Body mass index decline is related to spinocerebellar ataxia disease progression. Mov Disord Clin Pract. 2017;4(5):689–97.
Yang JS, et al. association between body mass index and disease severity in chinese spinocerebellar ataxia type 3 patients. Cerebellum. 2018;17(4):494–8.
Krakauer NY, Krakauer JC. Association of Body Shape Index (ABSI) with Hand Grip Strength. Int J Environ Res Public Health. 2020;17(18):6797.
Schlussel MM, et al. Reference values of handgrip dynamometry of healthy adults: a population-based study. Clin Nutr. 2008;27(4):601–7.
Amo-Setien FJ, et al. Factors associated with grip strength among adolescents: an observational study. J Hand Ther. 2020;33(1):96–102.
Trampisch US, et al. Optimal Jamar dynamometer handle position to assess maximal isometric hand grip strength in epidemiological studies. J Hand Surg Am. 2012;37(11):2368–73.
Gamage H, et al. Machado Joseph disease severity is linked with gut microbiota alterations in transgenic mice. Neurobiol Dis. 2023;179:106051.
Wu YL, et al. Coenzyme Q10 supplementation increases removal of the ATXN3 polyglutamine repeat, reducing cerebellar degeneration and improving motor dysfunction in murine spinocerebellar ataxia type 3. Nutrients. 2022;14:17.
Umeki D, et al. Protective effects of clenbuterol against dexamethasone-induced masseter muscle atrophy and myosin heavy chain transition. PLoS ONE. 2015;10(6):e0128263.
Samant SA, et al. The histone deacetylase SIRT6 blocks myostatin expression and development of muscle atrophy. Sci Rep. 2017;7(1):11877.
Li J, et al. Autophagic vacuolar myopathy involving the phenotype of spinocerebellar ataxia type 3. Neuropathology. 2023;43(2):135–42.
Leite C, et al. Body composition in spinocerebellar ataxia type 3 and 10 patients: comparative study with control group. Nutr Neurosci. 2020;23(1):49–54.
Studenski SA, et al. The FNIH sarcopenia project: rationale, study description, conference recommendations, and final estimates. J Gerontol A Biol Sci Med Sci. 2014;69(5):547–58.
Maas RP, et al. The preclinical stage of spinocerebellar ataxias. Neurology. 2015;85(1):96–103.
Roberts HC, et al. A review of the measurement of grip strength in clinical and epidemiological studies: towards a standardised approach. Age Ageing. 2011;40(4):423–9.
Schmitz-Hubsch T, et al. Scale for the assessment and rating of ataxia: development of a new clinical scale. Neurology. 2006;66(11):1717–20.
Khalil M, et al. Serum neurofilament light levels in normal aging and their association with morphologic brain changes. Nat Commun. 2020;11(1):812.
Lin YS, et al. IGF-1 as a potential therapy for spinocerebellar ataxia type 3. Biomedicines. 2022;10(2):505.
Takeshita H, et al. Modified forelimb grip strength test detects aging-associated physiological decline in skeletal muscle function in male mice. Sci Rep. 2017;7:42323.
Acknowledgements
We thank the physician Tsung-Han Lee for helping submit the documents to IRB. We appreciate the assistance provided by the Health Examination and Manage Center of Changhua Christian Hospital in collecting blood samples from the participants.
Funding
The research was funded by research Grants 109-CCH-IRP-055 and 110-CCH-MST-123 from Changhua Christian Hospital, Taiwan. It was also financially supported by the "Chinese Medicine Research Center, China Medical University" from The Featured Areas Research Center Program within the framework of the Higher Education Sprout Project by the Ministry of Education (MOE) in Taiwan (MOST 110-2320-B-039-070).
Author information
Authors and Affiliations
Contributions
CMC, CSL and HHC conceptualised and contributed to the study design. WLC, TTL and HJC execution for patient recruitment and performed the experiments. CMC, WLC and YSL contributed to the data analysis, interpretation and wrote the manuscript. CMC and YJC contributed to the data analysis of statistics. CMC and CJL verified the underlying data. All authors contributed to the article and editing of the final version of the manuscript. All authors read and approved by the final manuscript.
Corresponding authors
Ethics declarations
Ethics approval and consent to participate
All procedures of this study were approved by the Independent Ethics Committee of the Changhua Christian Hospital (CCH-IRB approval No:200703 and No:200730). In addition, written informed consent was obtained from all participants prior to study inclusion.
Consent for publication
All the patients included signed the consent for publication.
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Additional file 1: Fig. S1.
C orrelation between NfL and SARA in the SCA3 group. Plasma NfL positively correlated with SARA. Fig. S2. Correlation between BMI and SARA in the SCA3 group. BMI negatively correlated with SARA.
Additional file2 : Table 1.
Correlation between HGS and A ge, Gender, SARA, NfL, BMI and CAG repeat count.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Chiu, C., Cheng, W., Lin, Y. et al. A pilot study: handgrip as a predictor in the disease progression of SCA3. Orphanet J Rare Dis 18, 317 (2023). https://doi.org/10.1186/s13023-023-02948-3
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s13023-023-02948-3