Abstract
Background
Individuals with Friedreich ataxia (FRDA) can find it difficult to access specialized clinical care. To facilitate best practice in delivering healthcare for FRDA, clinical management guidelines (CMGs) were developed in 2014. However, the lack of high-certainty evidence and the inadequacy of accepted metrics to measure health status continues to present challenges in FRDA and other rare diseases. To overcome these challenges, the Grading of Recommendations Assessment and Evaluation (GRADE) framework for rare diseases developed by the RARE-Bestpractices Working Group was adopted to update the clinical guidelines for FRDA. This approach incorporates additional strategies to the GRADE framework to support the strength of recommendations, such as review of literature in similar conditions, the systematic collection of expert opinion and patient perceptions, and use of natural history data.
Methods
A panel representing international clinical experts, stakeholders and consumer groups provided oversight to guideline development within the GRADE framework. Invited expert authors generated the Patient, Intervention, Comparison, Outcome (PICO) questions to guide the literature search (2014 to June 2020). Evidence profiles in tandem with feedback from individuals living with FRDA, natural history registry data and expert clinical observations contributed to the final recommendations. Authors also developed best practice statements for clinical care points that were considered self-evident or were not amenable to the GRADE process.
Results
Seventy clinical experts contributed to fifteen topic-specific chapters with clinical recommendations and/or best practice statements. New topics since 2014 include emergency medicine, digital and assistive technologies and a stand-alone section on mental health. Evidence was evaluated according to GRADE criteria and 130 new recommendations and 95 best practice statements were generated.
Discussion and conclusion
Evidence-based CMGs are required to ensure the best clinical care for people with FRDA. Adopting the GRADE rare-disease framework enabled the development of higher quality CMGs for FRDA and allows individual topics to be updated as new evidence emerges. While the primary goal of these guidelines is better outcomes for people living with FRDA, the process of developing the guidelines may also help inform the development of clinical guidelines in other rare diseases.
Similar content being viewed by others
Introduction
Friedreich ataxia (FRDA) is a multisystem autosomal recessive disease affecting approximately 1 in 29,000 individuals with Caucasian ancestry [1]. Neurological features of FRDA include progressive appendicular and axial ataxia, spasticity, absent lower limb reflexes, dysarthria, visual and hearing dysfunction and impaired vibration sense and proprioception [2]. In addition, non-neurologic features including scoliosis, foot deformity, cardiomyopathy, diabetes mellitus and mental health issues add to the complexity of the disease [3]. The onset of symptoms is on average at 10 years of age but can range from as young as two years to beyond 40 years (in the case of Very Late Onset FRDA) [4]. Individuals with FRDA with an onset of symptoms younger than 15 years lose the capacity to ambulate on average 11.5 years after disease onset [5]. Despite a historical mean age of death reported to be 36 years [6], clinical experience indicates that with targeted care, individuals with FRDA can live for many decades beyond loss of ambulation [7].
Despite significant progress in the search for disease modifying agents, the chronic, progressive course of FRDA cannot yet be significantly slowed. Disease progression continues to have a profound impact on the health and well-being of people with FRDA. Given the rarity of the condition, individuals with FRDA often find it difficult to access experienced specialist multi-disciplinary care for long-term, evidence-based management. Rare diseases present a unique set of challenges in evidence based clinical care. In particular, the lack of adequately powered studies means that many management recommendations are based on low-certainty evidence. Specifically, the developmental and degenerative issues related to FRDA and the need for clinical care across childhood and adulthood requires a diverse approach to clinical management [8]. Recommendations developed without a strong evidence base do not provide clear clinical guidance and run the risk of contributing to sub-optimal care for individuals with rare diseases such as FRDA [9].
In 2014, identifying the requirement for FRDA-specific clinical management guidelines, 39 international expert clinicians wrote the first iteration of the Consensus Clinical Management Guidelines for Friedreich ataxia [10]. In this first iteration, the evidence underscoring the guidelines was evaluated according to the recommendations of the Guidelines International Network (http://www.g-i-n.net/). Recommendations were established according to the criteria developed by the National Health and Medical Research Council (NHMRC) Australia. In the absence of robust evidence, most (62%) of the 142 recommendations in these guidelines were based on the collective expertise of the clinicians and were thus presented as good practice points.
Given the time that had elapsed since 2014, an update of the guidelines was required; however, the challenge of developing guidelines for rare diseases remained. Paucity of available evidence, the predominance of low-quality studies and the inconsistency in metrics used to measure outcomes in FRDA make it challenging to apply standard comparative effectiveness strategies to this and other rare diseases [9]. In particular, the first iteration of the CMGs exemplified the difficulty in generating strong recommendations for diagnosis and/or treatment strategies due to the lack of high-certainty evidence [9].
The time between the first and second iteration of the guidelines provided an opportunity to consider alternative methods of grading the evidence and establishing the strength of the recommendations. The RARE-Bestpractices Working Group has provided a framework for developing guidelines using the Grading of Recommendations Assessment and Evaluation (GRADE) system [11]. Pai and colleagues [9] proposed supplementing the GRADE approach with three strategies specific to rare diseases: (a) reviewing and including literature in like conditions, thus providing “Indirect evidence”, (b) systematic collection of expert observations and/or patient perceptions via “Structured observation forms” and, (c) use of available clinical registry data. This paper describes the application of this approach in developing the updated iteration of clinical management guidelines for FRDA.
Methods
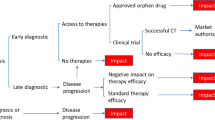
Figure 1 summarizes the overall process for guidelines development, with each phase described below.
Flow chart of the stages of the guidelines development and stakeholder involvement. Red arrows indicate process for obtaining indirect evidence. CMG = Clinical Management Guidelines; PICO = Patient, Intervention, Comparator, Outcome; FACOMS = Friedreich’s Ataxia Clinical Outcomes Measures; EFACTS = European Friedreich’s Ataxia Consortium for Translational Studies; FRDA = Friedreich ataxia
Stakeholders
The key stakeholders in the development of the guidelines were:
Guideline panel. An executive guideline panel comprising seven international clinicians provided oversight of the guideline development process and endorsed the final recommendations.
Project coordinator. The panel was supported by a project coordinator and administrative support.
Expert authors. International expert clinicians and researchers were invited by the guideline panel to author chapters and develop recommendations for their designated topic.
Methods experts. Expert authors were assisted by two methodological experts who completed the literature searches and summarized and synthesized the evidence using the GRADE structure.
Individuals with FRDA and/or their families. In contrast to the preceding iteration of the guidelines, individuals with FRDA and/or their families were included throughout the development of the guidelines to provide a lived perspective of FRDA on the development of topics and the final recommendations, in particular the lay summaries of recommendations.
Figure 1 shows how and when these stakeholders contributed to the development of the guidelines.
Topic list
The guideline panel reviewed the topic list from the previous CMG iteration, identified gaps in the available topics and suggested new topics for inclusion.
Developing the Patient, Intervention, Comparator, Outcome (PICO) questions
Expert authors were allocated topics relevant to their expertise and were asked to define the scope of their designated topic. Authors were instructed that each recommendation should answer a focused and sensible healthcare question designed according to the Patient, Intervention, Comparator, Outcome (PICO) framework. Individuals with FRDA and/or their families provided feedback on the PICO questions. The PICO questions guided the literature review and generation of other evidence if required.
Grading of Recommendations Assessment and Evaluation (GRADE)
The GRADE approach is a system for rating the quality of a body of evidence for clinical practice guidelines and grading recommendations in health care. GRADE offers a transparent and structured process for developing and presenting evidence summaries and for carrying out the steps involved in developing recommendations [12]. The GRADEpro Guideline Development Tool (GRADEpro GDT: GRADEpro Guideline Development Tool [Software]; McMaster University and Evidence Prime, 2021; available from https://www.gradepro.org), including the evidence table, summary of findings table and evidence to recommendation table, was used to generate the guidelines. The bespoke structured observation forms used to develop these guidelines were based on those presented in the GRADE tool (see Additional file 1 for an example of a structured observation form).
Literature search
A literature search for each PICO was completed using a comprehensive search of three electronic databases (Cochrane Library, CINAHL and MEDLINE) for publications from January 2014 to June 2020, including only English-language publications. Reference lists and the authors’ personal libraries were searched for further publications. Randomized, non-randomized controlled and observational studies were included. Papers identified in the 2014 guidelines (published prior to 2014) were included in the review if not superseded by more recent, higher quality literature.
The populations included adults and/or children with FRDA. However, when there was minimal published evidence, populations with like conditions (such as spinocerebellar ataxia and multiple sclerosis) were included in the search.
For each PICO, the review of the literature was summarized and presented to expert authors via the GRADE evidence table and summary of findings table.
Registry data
In instances where there was limited published evidence on the PICO question, authors had the opportunity to interrogate registry data from two international natural history registries of clinical information from individuals with FRDA: the Friedreich’s Ataxia Clinical Outcomes Measures (FACOMS) and the European Friedreich’s Ataxia Consortium for Translational Studies (EFACTS). The information provided was descriptive only; for example, frequency of use of a medication in individuals with a specific impairment related to FRDA.
Structured observation forms
If the expert authors anticipated there was limited or no published evidence on the PICO question, structured observation forms were used to systematically document clinical observations by experienced clinicians that could contribute to the strength of the recommendation. In the first instance, the expert authors (clinicians) working on that topic completed the form/s independently. If responses showed large discrepancies, all expert authors were invited to complete the structured observation forms. The questions were targeted at clinicians known to provide healthcare to individuals with FRDA.
Generation of recommendations
GRADE Evidence to Recommendation tables were used to provide the structure for expert authors to decide the strength of the recommendation and the level of evidence. The evidence base related to each question was evaluated according to the criteria of: problem (is the issue a significant problem?); desirable effects (of the intervention); undesirable effects (of the intervention); certainty of evidence; values; balance of effects; and acceptability (of the intervention). Based on the responses to these criteria, the type (for or against the intervention, or neither) and strength of the recommendation (strong or conditional) were generated. For the rating of the strength of the recommendation, in addition to evidence from studies in FRDA, evidence from like conditions, clinical experience and expert consensus were taken into account when published evidence was not available. In this situation, the level of evidence was rated as “low” or “very low”. Table 1 provides an explanation of the symbols used to grade recommendations.
For strong recommendations, consistent with the GRADE working group we suggested authors should use terminology such as "we recommend…" or "clinicians should…", “clinicians should not…” or “do…”, “don’t…” For conditional recommendations, we suggested less definitive wording, such as "we suggest…" or "clinicians might…" or “we conditionally recommend…” or “we make a qualified recommendation that…”.
Along with each recommendation, authors provided clinical and research justification for the recommendation and a description of subgroups that might need particular consideration in implementing the recommendation, if any.
Best practice statements
Best practice statements were included when application of the GRADE framework was not appropriate. This was in circumstances where the reverse would not be credible; for example, ambulant individuals with FRDA who are falling frequently should be offered a detailed assessment of balance and gait. In addition if recommendations from the 2014 guidelines were deemed still relevant, yet not incorporated into a new PICO thus enabling scrutiny from a GRADE framework, they were included as best practice statements.
Chapter content
For each topic, expert authors were asked to write a chapter elaborating on the evidence and providing background and context to the best practice statements and recommendations. For chapters that did not include recommendations or best practice statements, expert authors provided an evidence-based overview of the topic.
Lay summaries
Expert authors wrote a lay summary of the recommendations related to the topic. Given the target audience for this section is individuals living with FRDA, review of the lay summaries by individuals with FRDA and their families was a crucial component of the guideline development.
Achieving consensus
Each of the expert authors needed to agree on the grading of recommendations. In the case of not achieving consensus we proposed the authors use a Delphi survey to achieve consensus.
Process of endorsement
The executive panel reviewed the chapter content and recommendations and responded to a set of questions via an online survey (see Additional file 2). If the panel had any reservations about the wording of a recommendation, this was discussed with the relevant authors and agreement was reached. Endorsement was then established after resolution of these issues with the authors.
Results
Seventy expert clinicians contributed to writing the guidelines. In addition, seven individuals with FRDA and four individuals who care for someone with FRDA provided input from the perspective of the lived experience of FRDA.
Based on the contents of the previous iteration of the guidelines and identified gaps, the guidelines panel defined 17 overall topics for inclusion in the new guidelines, resulting in 17 separate chapters. As shown in Table 2, within the main topics there are specific sub-topics included. For example, there are 11 separate health issues within Neurological Components of FRDA (Sects. 3.1 to 3.11). New topics (since 2014) include emergency medicine, digital and assistive technologies and a separate topic (with 3 sub-topics) dedicated to mental health (this was previously included as part of the Quality of Life topic). Three of the chapters (1, 2 and 12) provide overviews of topics that did not generate PICOs, and consequently do not contain recommendations, although chapter 12 contains best practice statements. For the other 14 main topics, expert authors used the GRADE rare-diseases process to assess the evidence for 130 PICOs, generating 130 recommendations. Authors were able to achieve consensus on all recommendations without the use of a Delphi survey. In addition to the 130 new recommendations, there are 95 best practice statements included across the 17 chapters.
Table 3 lists all best practice statements and recommendations under each topic and sub-topic heading, along with the symbols denoting the strength of the recommendation and the level of evidence for each recommendation (the symbols denoting the strength of the recommendation and level of evidence are explained in Table 1). The full tables of recommendations, each with accompanying justification (based on evidence and clinical expertise) and subgroup considerations, as well as chapter contents and lay summaries are available online. “Evidence to Recommendation” tables showing how each recommendation was developed with summaries of the evidence can also be viewed on the website (https://frdaguidelines.org/).
Table 3 shows that the majority of recommendations are supported by a very low or low level of evidence, reflecting the lack of high-quality clinical studies of many aspects of FRDA. However, in many cases, a conditional or strong recommendation was made based on the criteria specified in the GRADE rare-disease process. For 23 of the 130 PICO questions, the authors were not able to generate a recommendation either for or against the intervention. In these cases, the authors considered there was not enough data in FRDA or other conditions, or enough solid clinical experience to make any judgement about whether these particular interventions may be useful or not in FRDA. Sixteen recommendations were either conditional or strong recommendations against the intervention, while the rest (n = 91) were conditional or strong recommendations for the intervention.
Discussion
FRDA presents with a unique, heterogeneous phenotype that spans both childhood and adulthood. As such, CMGs for FRDA need to provide expedient clinical guidance for clinicians, particularly those less familiar with the condition. The diverse manifestation and complexity of FRDA mandates appropriate CMGs developed in a transparent manner, via a process able to synthesize available evidence and delivered in a clear and concise manner [9]. Consistent with the approach taken by Pai and colleagues [9], we demonstrated that by adopting the RARE-Bestpractices Working Group structure, developing robust CMGs according to the GRADE framework is feasible for FRDA. Adopting the RARE-Bestpractices Working Group framework made it possible to utilize evidence in like conditions, clinical expertise, registry data and feedback from the structured observation forms to make useful recommendations even where evidence in FRDA was low. This process has seen the genesis of 130 new recommendations and 95 best practice statements across 17 broad topic areas.
The addition of new topics was crucial to this iteration. For example, a consistent theme in the development of these guidelines was the frustration of both affected individuals and clinicians alike regarding the experience of someone with FRDA presenting to the emergency room. By most accounts, many emergency room physicians have scarce clinical experience with FRDA and as such may be unsure how to manage issues such as chest pain, trauma or urinary tract infection in the context of FRDA. These CMGs now specifically address this knowledge gap by providing an additional resource to the clinician. Inclusion of such topics reflects the importance of incorporating those who have a lived experience of FRDA and the challenges of navigating the health system, into each step of the development of these guidelines. The inclusion of the lived experience of FRDA provided an important differentiation from the 2014 guidelines and was pivotal in identifying the gaps in those guidelines that required attention. In addition, the inclusion of lay summaries of the recommendations was a significant new initiative. Whilst the target audience of the guidelines is healthcare professionals, provision of a lay summary of the clinical recommendations will assist individuals with FRDA and their families in understanding how best to manage their health and well-being and support shared decision-making with healthcare providers. Lay summaries underwent comprehensive scrutiny by individuals living with FRDA to ensure they address this requirement.
Ensuring the contemporaneous nature and sustainability of recommendations is an important component of guideline development [13]. As new evidence comes to light it is important that recommendations can be modified in real time. The use of the GRADE framework provides not only a robust, transparent structure to the development of the guidelines, but ensures a process of continual updating of the recommendations as new evidence emerges. Moreover, it is anticipated that the guidelines will not only identify the gaps in evidence but also facilitate further studies to address these gaps. Indeed, by the end of this iteration of guideline development, two papers have already arisen from this important process [14, 15].
A further critical component to developing guidelines is dissemination and ensuring the guidelines are openly available and easily accessed by clinicians and others in need of the information. Again, the opinions of clinicians and individuals with a lived experience of FRDA was sought in deciding the best repository and process of dissemination of the CMGs. An important component of dissemination is ensuring transparency, access to the evidence to recommendation tables that underscore the recommendations and the opportunity for future work such as translation into languages other than English. Incorporating suggestions from stakeholders, a dedicated website has been designed to house the CMGs, including chapter content, lay summaries, recommendations, best practice statements and the evidence to recommendation tables. Ongoing review of this process of dissemination will occur via the website.
Finally, it is important to reiterate these CMGs are intended to assist healthcare professionals to make informed treatment decisions about the care of individuals with FRDA. They are not intended as a sole source of guidance in managing issues related to FRDA. Rather, they were designed to assist clinicians by providing an evidence-based framework for decision-making. Ultimately, healthcare professionals must make their own treatment decisions about care on a case-by-case basis, after consultation with individuals living with FRDA, consideration of local or geographical issues including fiscal issues, and using their clinical judgement, knowledge and expertise. Developing guidelines for rare diseases such as FRDA is challenging; however, we believe the blueprint we have provided for this process will be applicable to other clinicians striving, as we are, to provide the best possible clinical care to people who live with rare conditions.
Availability of data and materials
The datasets used and/or analysed during the current study are available from the corresponding author on reasonable request until such time this manuscript is published. Thereafter the datasets used in this study will be publicly available on a website to be made publically available after publication of this manuscript.
Abbreviations
- EKG:
-
Electrocardiogram
- FRDA:
-
Friedreich Ataxia
- FXN :
-
Frataxin
- GAA:
-
Guanine–Adenine–Adenine
- LVEF:
-
Left Ventricular Ejection Fraction
- NHMRC:
-
National Health and Medical Research Council
- NYHA:
-
New York Heart Association
- OSA:
-
Obstructive Sleep Apnea
- PICO:
-
Patient, Intervention, Comparator, Outcome
- RLS:
-
Restless Leg Syndrome
References
Cossee M, Schmitt M, Campuzano V, Reutenauer L, Moutou C, Mandel JL, et al. Evolution of the Friedreich’s ataxia trinucleotide repeat expansion: founder effect and premutations. Proc Natl Acad Sci USA. 1997;94(14):7452–7.
Delatycki MB, Bidichandani SI. Friedreich ataxia-pathogenesis and implications for therapies. Neurobiol Dis. 2019;132:104606.
Reetz K, Dogan I, Hohenfeld C, Didszun C, Giunti P, Mariotti C, et al. Nonataxia symptoms in Friedreich Ataxia: report from the Registry of the European Friedreich’s Ataxia Consortium for Translational Studies (EFACTS). Neurology. 2018;91(10):e917–30.
Dürr A, Cossee M, Agid Y, Campuzano V, Mignard C, Penet C, et al. Clinical and genetic abnormalities in patients with Friedreich’s ataxia. N Engl J Med. 1996;335(16):1169–75.
Rummey C, Farmer JM, Lynch DR. Predictors of loss of ambulation in Friedreich’s ataxia. EClinicalMedicine. 2020;18:100213.
Tsou AY, Paulsen EK, Lagedrost SJ, Perlman SL, Mathews KD, Wilmot GR, et al. Mortality in Friedreich ataxia. J Neurol Sci. 2011;307:46–9.
Lynch DR, Farmer JM, Wilson RB. Mortality in Friedreich ataxia. Texan Heart Inst J. 2007;34(4):502–3.
Lynch DR, Schadt K, Kichula E, McCormack S, Lin KY. Friedreich ataxia: multidisciplinary clinical care. J Multidiscip Healthc. 2021;14:1645–58.
Pai M, Yeung CHT, Akl EA, Darzi A, Hillis C, Legault K, et al. Strategies for eliciting and synthesizing evidence for guidelines in rare diseases. BMC Med Res Methodol. 2019;19(1):67.
Corben LA, Lynch D, Pandolfo M, Schulz JB, Delatycki MB, Clinical Management Guidelines Writing G. Consensus clinical management guidelines for Friedreich ataxia. Orphanet J Rare Dis. 2014;9:184.
Pai M, Iorio A, Meerpohl J, Taruscio D, Laricchiuta P, Mincarone P, et al. Developing methodology for the creation of clinical practice guidelines for rare diseases: a report from RARE-Bestpractices. Rare Dis. 2015;3(1):e1058463.
Schünemann H, Brożek J, Guyatt G, Oxman A, editors. GRADE handbook for grading quality of evidence and strength of recommendations. Updated October 2013: The GRADE Working Group 2013. Available from: guidelinedevelopment.org/handbook.
Vernooij RW, Sanabria AJ, Sola I, Alonso-Coello P, Martinez GL. Guidance for updating clinical practice guidelines: a systematic review of methodological handbooks. Implement Sci. 2014;9:3.
Naeije G, Schulz JB, Corben LA. The cognitive profile of Friedreich ataxia: a systematic review and meta-analysis. BMC Neurol. 2022;22(1):97.
Tamaroff J, DeDio A, Wade K, Wells M, Park C, Leavens K, et al. Friedreich’s ataxia related diabetes: epidemiology and management practices. Diabetes Res Clin Pract. 2022;186:109828.
Milne S, Campagna E, Delatycki MB, Corben LA. Rehabilitation in Friedreich ataxia. In: Iansek R, Morris ME, editors. Rehabilitation in movement disorders. Cambridge: Cambridge University Press; 2013. p. 185–202.
Ilg W, Brotz D, Burkard S, Giese MA, Schols L, Synofzik M. Long-term effects of coordinative training in degenerative cerebellar disease. Mov Disord. 2010;25(13):2239–46.
Ilg W, Schatton C, Schicks J, Giese MA, Schols L, Synofzik M. Video game-based coordinative training improves ataxia in children with degenerative ataxia. Neurology. 2012;79(20):2056–60.
Synofzik M, Ilg W. Motor training in degenerative spinocerebellar disease: ataxia-specific improvements by intensive physiotherapy and exergames. Biomed Res Int. 2014;2014:583507.
Ilg W, Synofzik M, Brotz D, Burkard S, Giese MA, Schols L. Intensive coordinative training improves motor performance in degenerative cerebellar disease. Neurology. 2009;73(22):1823–30.
Synofzik M, Schatton C, Giese M, Wolf J, Schols L, Ilg W. Videogame-based coordinative training can improve advanced, multisystemic early-onset ataxia. J Neurol. 2013;260(10):2656–8.
Perlman SL. Symptomatic and disease-modifying therapy for the progressive ataxias. Neurologist. 2004;10(5):275–89.
Delatycki MB, Holian A, Corben L, Rawicki HB, Blackburn C, Hoare B, et al. Surgery for equinovarus deformity in Friedreich’s ataxia improves mobility and independence. Clin Orthop Relat Res. 2005;430:138–41.
Akuthota V, Ferreiro A, Moore T, Fredericson M. Core stability exercise principles. Curr Sports Med Rep. 2008;7(1):39–44.
Stokes IA, Gardner-Morse M, Henry SM, Badger GJ. Decrease in trunk muscular response to perturbation with preactivation of lumbar spinal musculature. Spine (Phila Pa 1976). 2000;25(15):1957–64.
Beauchamp M, Labelle H, Duhaime M, Joncas J. Natural history of muscle weakness in Friedreich’s ataxia and its relation to loss of ambulation. Clin Orthop Relat Res. 1995;311:270–5.
Cabanas-Valdes R, Bagur-Calafat C, Girabent-Farres M, Caballero-Gomez FM, du-Port-de-Pontcharra-Serra H, German-Romero A, et al. Long-term follow-up of a randomized controlled trial on additional core stability exercises training for improving dynamic sitting balance and trunk control in stroke patients. Clin Rehabil. 2017;31(11):1492–9.
Cabanas-Valdes R, Cuchi GU, Bagur-Calafat C. Trunk training exercises approaches for improving trunk performance and functional sitting balance in patients with stroke: a systematic review. NeuroRehabilitation. 2013;33(4):575–92.
Coyne KS, Kaplan SA, Chapple CR, Sexton CC, Kopp ZS, Bush EN, et al. Risk factors and comorbid conditions associated with lower urinary tract symptoms: EpiLUTS. BJU Int. 2009;103(Suppl 3):24–32.
Fowler CJ, Panicker JN, Drake M, Harris C, Harrison SC, Kirby M, et al. A UK consensus on the management of the bladder in multiple sclerosis. J Neurol Neurosurg Psychiatry. 2009;80(5):470–7.
Larson RD. Psychometric properties of the modified fatigue impact scale. Int J MS Care. 2013;15(1):15–20.
Galindo-Zavala R, Bou-Torrent R, Magallares-Lopez B, Mir-Perello C, Palmou-Fontana N, Sevilla-Perez B, et al. Expert panel consensus recommendations for diagnosis and treatment of secondary osteoporosis in children. Pediatr Rheumatol Online J. 2020;18(1):20.
Miyasaki JM, Aldakheel A. Movement disorders in pregnancy. Continuum (Minneap Minn). 2014;20(1):148–61.
American Diabetes Association. 2. Classification and diagnosis of diabetes: standards of Medical Care in Diabetes-2021. Diabetes Care. 2021;44:S15–33.
Armstrong BA, Howat PW. Pregnancy in a woman with Friedreich’s ataxia complicated by pulmonary embolism. Aust N Z J Obstet Gynaecol. 2002;42(1):88–90.
Friedman LS, Paulsen EK, Schadt KA, Brigatti KW, Driscoll DA, Farmer JM, et al. Pregnancy with Friedreich ataxia: a retrospective review of medical risks and psychosocial implications. Am J Obstet Gynecol. 2010;203(3):224.e1-5.
The Royal Australian and New Zealand College of Obstetricians and Gynaecologists. Intrapartum fetal surveillance. Clinical guidelines—fourth edition. www.ranzcog.edu.au: RANZCOG; 2019.
Kubal K, Pasricha SK, Bhargava M. Spinal anesthesia in a patient with Friedreich’s ataxia. Anesth Analg. 1991;72(2):257–8.
MacKenzie WE. Pregnancy in women with Friedreich’s ataxia. Br Med J Clin Res Ed. 1986;293(6542):308.
Acknowledgements
Our grateful thanks are extended to the Friedreich's Ataxia Research Alliance (FARA, USA) for funding this project. We are also grateful to the group of individuals living with Friedreich ataxia and their families for their welcome, insightful and critical feedback during the development of these guidelines.
In addition we wish to acknowledge the Clinical Management Guidelines Writing Group (in alphabetical order): Hamed Akhlaghi, Sanjay I. Bidichandani, Sylvia Boesch, Miriam Cnop, Louise Corben, Manuela Corti, Martin B. Delatycki, Antoine Duquette, Alexandra Durr, Andreas Eigentler, Anton Emmanuel, Jennifer Farmer, John M. (Jack) Flynn, Noushin Chini Foroush, Anne Fournier, Marcondes C. França Jr, Paola Giunti, Ellen W. Goh, Lisa Graf, Marios Hadjivassiliou, Maggie-Lee Huckabee, Mary G. Kearney, Arnulf H. Koeppen, Yenni Lie, Kimberly Y. Lin, Anja Lowit, David Lynch, Caterina Mariotti, Katherine Mathews, Shana E. McCormack, Sarah C. Milne, Lisa Montenegro, Thierry Morlet, Gilles Naeije, Massimo Pandolfo, Jalesh N. Panicker, Michael H. Parkinson, Aarti Patel, Ronald Mark Payne, Susan Perlman, Roger E. Peverill, Francoise Pousset, Hélène Puccio, Myriam Rai, Gary Rance, Kathrin Reetz, Tennille J. Rowland, Phoebe Sansom, Konstantinos Savvatis, Ellika T. Schalling, Ludger Schöls, Jörg B. Schulz, Barbara Smith, Elisabetta Soragni, Caroline Spencer, Sub H. Subramony, Matthis Synofzik, David J. Szmulewicz, Geneieve Tai, Jaclyn Tamaroff, Lauren Treat, Ariane Veilleux Carpentier, Adam P. Vogel, Susan E. Walther, David R. Weber, Neal J. Weisbrod, George Wilmot, Robert B. Wilson, Grace Yoon, Theresa Zesiewicz.
Funding
This project was funded by the Friedreich’s Ataxia Research Alliance (USA).
Author information
Authors and Affiliations
Consortia
Contributions
LAC provided project leadership, participated in expert writing groups, oversaw the guideline development process and guideline panel and drafted the manuscript. VC assisted expert authors with editing chapter contents and was involved with drafting the manuscript. SM completed the literature reviews, supported expert authors through the guideline development process, participated in expert writing groups and revised the manuscript. JF was a member of the guideline panel, provided project leadership, review and feedback on the guideline development process, review and endorsement of the recommendations and chapter content and reviewed the manuscript. KL, MP, MBD, SS, JBS, DL were members of the guideline panel, participated in expert writing groups and provided critical edits to the manuscript. AM provided administrative support including development of online questionnaires and webpage design and development. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
VC, SM, KL, JBS, SS, DL and LAC have no competing interests to report. MBD has research funding from the Friedreich's Ataxia Research Alliance, National Health and Medical Research Council (Australia) and Medical Research Future Fund (Australia). MP has research funding from the Friedreich's Ataxia Research Alliance and is a consultant with Design Therapeutics, Aavantibio, Larimar, Minoryx for the development of FRDA therapeutics. JF and AM are employees of the Friedreich’s Ataxia Research Alliance.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Additional file 1.
Example of a structured observation form.
Additional file 2.
Recommendations review survey.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Corben, L.A., Collins, V., Milne, S. et al. Clinical management guidelines for Friedreich ataxia: best practice in rare diseases. Orphanet J Rare Dis 17, 415 (2022). https://doi.org/10.1186/s13023-022-02568-3
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s13023-022-02568-3