Abstract
Background
Postmenopausal osteoporosis is a chronic metabolic bone disease caused by excessive osteoclast formation and function. Targeting osteoclast differentiation and activity can modulate bone resorption and alleviate osteoporosis. Cirsilineol, an active constituent of Vestita Wall, has shown numerous biological activities and has been used to treat many metabolic diseases. However, whether cirsilineol inhibits osteoclast activity and prevents postmenopausal osteoporosis still remain unknown.
Materials and methods
Primary bone marrow macrophages (BMMs) and RAW264.7 cells were used. Osteoclast activity was measured by TRAP staining, F-actin staining, and bone resorption assay after BMMs were treated with cirsilineol at concentrations of 0, 1, 2.5 and 5 µM. RT-PCR and western blotting were performed to evaluate the expression of osteoclast-related genes. In addition, female C57BL/6 mice underwent OVX surgery and were treated with cirsilineol (20 mg/kg) to demonstrate the effect of cirsilineol on osteoporosis.
Results
Cirsilineol significantly inhibited receptor activator of nuclear factor-kappa B ligand (RANKL)-induced osteoclast differentiation in a concentration- and time-dependent manner, respectively. Additionally, cirsilineol inhibited F-actin ring formation, thus reducing the activation of bone resorption ability. Cirsilineol suppressed the expression of osteoclast-related genes and proteins via blocking nuclear factor (NF)-κb, ERK, and p38 signaling cascades. More importantly, cirsilineol treatment in mice with osteoporosis alleviated osteoclasts hyperactivation and bone mass loss caused by estrogen depletion.
Conclusion
In this study, the protective effect of cirsilineol on osteoporosis has been investigated for the first time. In conclusion, our findings prove the inhibitory effect of cirsilineol on osteoclast activity via NF-κb/ERK/p38 signaling pathways and strongapplication of cirsilineol can be proposed as a potential therapeutic strategy.
Similar content being viewed by others
Introduction
Postmenopausal osteoporosis is a fairly common chronic disease characterized by abnormal bone metabolism and ultimately leads to decreased bone volume [1]. The decoupling of bone formation and bone resorption, which means the overactivation of osteoclasts and the silencing of osteoblasts, is the most common culprit in osteoporosis [2]. Osteoporosis usually has a poor prognosis and is accompanied by many complications, including systemic pain, blood vessel damage, nerve inflammation and a more increased risk of fractures [3,4,5]. Pathological fractures combine osteoporosis influence the activity of daily living of patients and can be life-threatening in severe cases [6].
Osteoclasts are multinucleated giant cells formed by the fusion of monocytes or macrophages derived from myeloid progenitor cells in bone marrow and are considered to be the sole regulator in osteolysis. Osteoclast precursors promote the expression of RANK on the cell surface in response to macrophage colony-stimulating factor (M-CSF) stimulation [7], making it more sensitive to RANKL signals. Binding of RANKL and RANK initiates the downstream signaling cascadeS including protein kinases (MAPKs), and NF-κb and thus triggers the activation of downstream osteoclast-related genes, leading to osteoclast differentiation [8, 9].
Excessive bone resorption by osteoclasts has been implicated in osteoporosis-induced bone mass loss, and the formation of F-actin ring makes a very positive contribution in this process [10]. F-actin ring, also known as sealing zone, forms during osteoclasts polarization and morphological changes, surrounding the resorption cavity and supporting the degradation of ECM by promoting environmental acidification [11,12,13]. F-actin ring formation is crucial for osteolytic activity.
Sustained inflammatory microenvironment and elevated inflammatory mediators are highly correlated with decreased bone mass and bone density in many patients with chronic inflammatory diseases [14, 15]. As previously reported, increased levels of inflammatory markers trigger the RANKL secretion from stromal cells, activate the RANKL/RANK signaling pathways in osteoclasts and affect bone metabolism [16, 17]. For the moment, inhibition of inflammation is an effective method to retard bone loss [18].
Anti-osteoporosis drugs mainly include basic supplements, bone resorption inhibitors and bone formation promoters [19, 20]. Clinically, inhibition of osteoclastic bone resorption is the main therapeutic strategy [21]. However, some of these drugs neither improve symptoms nor prevent side effects. Most drugs even show severe side effects ranging from gastro-intestinal tract bad effect to hepatorenal damage [22, 23].
Cirsilineol (4ʹ,5-dihydroxy-3ʹ,6,7-trimethoxyflavone), a kind of flavonoids bioactive compound isolated from vestita Wall, has been previously reported to possess numerous biological activities including anti-inflammatory [24], anti-tumor [25, 26], anti-plasmodial [27] and gastroprotective effect [28]. For local inflammation caused by Allergic Rhinitis, cirsilineol has shown a protective effect on immune imbalance by reducing inflammatory factor levels [29]. Additionally, cirsilineol has been reported to show considerable antibacterial activity against Helicobacter pylori [30]. Besides, a previous study has also shown that cirsilineol displayed excellent antioxidant and antidiabetic potential [31]. Given the diverse biological activities of cirsilineol, it has merged as a promising and safe approach to treat various diseases.
Considering the further evidence for links between oxidative stress, inflammatory processes and osteoclast activation, we investigated for the first time whether cirsilineol exerted protective effect on osteoporosis. Our studies suggest that cirsilineol suppresses RANKL-induced osteoclast differentiation and ameliorates osteoporosis-induced bone mass loss in vivo.
Materials and methods
Reagents
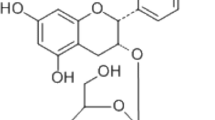
Cirsilineol (purity ≥ 97%; Fig. 1A) was purchased from MedChemExpress (MCE) Co. (New Jersey, America) and dissolved in dimethyl sulfoxide (DMSO) to make a solution of various concentrations. α-MEM and fetal bovine serum were purchased from Thermo Fisher Scientific (Scoresby, Vic, Australia). Recombinant human RANKL and M-CSF were purchased from Novoprotein Scientific Inc. (Shanghai, China). Rabbit-derived primary antibodies against p-ERK, ERK, p-JNK, JNK, p-p38, p38, p-p65, p65, p-ikβα, ikβα, p-akt, akt, NFATc1/NFAT2, c-Fos, RANK and GAPDH were all purchased form Cell Signaling Technology (Danvers, MA, USA). Second antibodies and CCK8 kits were acquired from Beyotime Biotechnology Co. (Shanghai, China). Tartrate-resistant acid phosphatase (TRAP) staining components and all other reagents were obtained from Sigma-Aldrich (St. Louis, MO, USA). All animals used for experiments were purchased from SLAC Laboratory Animal Co. (Shanghai, China).
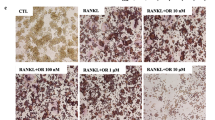
Inhibition of osteoclast differentiation by cirsilineol in vitro. A Chemical structure of cirsilineol. B Cirsilineol showed no toxicity to BMMs, until the concentration reaches 20 μM (n = 4). C–G Cirsilineol inhibited RANKL-induced osteoclast differentiation in a concentration- and time-dependent manner (n = 3). H–J The number and size of TRAP-positive osteoclasts both reduced in the early and late stage, and the inhibitory effect was more profound in the early stage (n = 3). Scale bar = 500 μm, *P < 0.05, **P < 0.01 vs. the control group
Cell isolation and cell viability assay
Long bones of both extremities were separated from 8-week-old C57BL/6 mice. By flushing the bone medullary cavity, macrophage precursors were obtained and then differentiated into macrophages with 50 ng/ml M-CSF stimulation for 5 days. All adherent cells were obtained for CCK8 assay and osteoclast differentiation.
To verify the cytotoxicity of cirsilineol, BMMs were seeded into 96-well plates and then cultured with different dosages of cirsilineol (0, 0.01, 0.1, 1, 2.5, 5, 10, 20 µM) for 1 day, 2 days and 4 days, respectively. In addition, MC3T3-E1 cells were also seeded into 96-well plates and cultured with different concentrations of cirsilineol (0, 0.5, 1, 2.5, 5, 10, 20, 50, 100 μM) for another 4 days. 10 μL CCK8 solution was added into each well and the change of cell viability was observed with CCK8 colorimeter using the ELX808 absorbance microplate reader (BioTek, Winooski, VT, USA).
TRAP staining assay
To determine the inhibition of cirsilineol on osteoclast formation, BMMs were seeded into 24-well plates at a density of 2 × 104 cells/well and cultured with osteoclastic medium containing 30 ng/ml M-CSF and 100 ng/ml RANKL for 4 days. During osteoclast differentiation, cells were also treated with different dosages of cirsilineol (0, 1, 2.5, 5 µM). After that, all cells were fixed with 4% paraformaldehyde and performed with TRAP staining assay. Mature osteoclasts meet the criteria of containing three or more nuclei. After the TRAP staining images were taken, the number and size of osteoclasts were quantitated compared to control.
F-actin ring staining and bone resorption assay
Mature osteoclasts were incubated with indicated dosages of cirsilineol during RANKL stimulation. Then cells were washed with PBS and fixed in 4% paraformaldehyde for 30 min. After cultured with rhodamine-phalloidin for 1 h, all cells were gently washed for three times, stained with DAPI for 5 min, and photographed by confocal microscopy.
To investigate the inhibitory effect of cirsilineol on bone resorption ability, BMMs were plated into 12-well plates at a density of 2 × 105 cells/well, and cultured with complete α-MEM containing 50 ng/ml M-CSF overnight. The cells were then stimulated with 100 ng/ml RANKL for 5 days until mature osteoclasts formed. Afterwards, all cells were thoroughly transplanted onto bone discs and treated with 5 μM cirsilineol for another 4 days. The resorption pits were photographed by the Hitachi (Chiyoda, Tokyo, Japan) S-3700N scanning electron microscope.
Quantitative real-time PCR
In order to identify the inhibitory effect of cirsilineol on osteoclast formation and function in gene levels, BMMs were seeded into 12-well plates and cultured with or without indicated dosages of cirsilineol (0, 1, 2.5, 5 μM). Besides, MC3T3-E1 cells were also seeded into 12-well plates and then cultured with differnet concentrations of cirsilineol (0, 5, 10 μM). Total RNA was collected using Trizol reagent (Takara, Dalian, China) and transcribed back into cDNA, which used for quantitative real-time PCR (RT-PCR). The expression of osteoclast-specific gene was normalized to the level of GAPDH, and the experiments were repeated for three times. The primer sequences for rt-PCR are as follows: GAPDH (Forward: 5′-ACCACAGTCCATGCCATCAC-3′; Reverse: 3′-TCCACCACCCTGTTGCTGTA-5′), NFATc1 (forward: 5′-CCGTTGCTTCCAGAAAATAACA-3′; reverse: 3′-TGTGGGATGTGAACTCGGAA-5′), TRAP (forward: 5′-CACTCCCACCCTGAGATTTGT-3′; reverse: 3′-CCCCAGAGACATGATGAAGTCA-5′); V-ATPase-a3 (forward: 5′-GCCTCAGGGGAAGGCCAGATCG-3; reverse: 3′-GGCCACCTCTTCACTCCGGAA-5′), cathepsin K, (forward: 5′-CTTCCAATACGTGCAGCAGA-3′; reverse: 3′-TCTTCAGGGCTTTCTCGTTC-5′), DC-STAMP (forward: 5′-AAAACCCTTGGGCTGTTCTT-3′; reverse: 3′-AATCATGGACGACTCCTTGG-5′), MMP-9 (forward: 5′-CAAAGACCTGAAAACCTCCAA-3′; reverse: 3′-GGTACAAGTATGCCTCTGCCA-5′), Tnf-α (forward: 5′-AAGCCTGTAGCCCACGTCGTA-3′; 3′-AGGTACAACCCATCGGCTGG-5′), IL-1β (forward: 5′-AAAAAAGCCTCGTGCTGTCG-3’; reverse: 3’-GTCGTTGCTTGGTTCTCCTTG-5’), IL-6 (forward: 5′-TCCATCCAGTTG CCTTCTTG-3’; reverse: 3’-TTCCACGATTTCCCAGAGAAC-5’), Collagen I (forward: 5′-AGAGCATGACCGATGGATTC-3′; reverse: 3′-CCTTCTTGAGGTTGCCAGTC-5′), Runx2 (forward: 5′-AAGTGCGGTGCAAACTTTCT-3′; reverse: 3’-AAGTGCGGTGCAAACTTTCT-5′).
Western blotting
Total cell lysate was collected and performed with high-speed centrifugation at 14,000 rpm for 15 min. All proteins were harvested from the supernatant of cell lysate. The obtained proteins were separated via SDS-PAGE electrophoresis and then transferred onto PVDF membranes for 2 h. The PVDF membranes were then blocked with 10% milk and incubated with primary antibodies overnight at 4 ℃. After washed with Tris-buffered saline three times, all samples were cultured with second antibodies for another 2 h at 4 ℃. Relative protein expression levels were detected using the Bio-Rad XRS chemiluminescence detection system (Hercules, CA, USA) and quantitated with the ImageJ software.
Alizarin red S (ARS) and Alkaline phosphatase (ALP) activity assay
To investigate whether cirsilineol affected bone formation and osteoblasts differentiation, MC3T3-E1 cells were used for further examination. Specifically, MC3T3-E1 cells (2 × 105 cells/well) were seeded into 6-well plates and then treated with different concentration of cirsilineol (0, 10, 20 μM) in osteogenic medium. Three days after osteogenic induction, MC3T3-E1 cells were fixed with 10% formaldehyde and then cultured with ALP staining kits. Another two weeks after osteogenic induction, MC3T3-E1 cells were incubated with 0.1% alizarin red dye for 30 min. After that, all plates were photographed using a high‐resolution microscope (Leica, Germany).
Establishment of osteoporosis mice model and bone histologic analysis
To verify whether cirsilineol exerted protective effect on osteoporosis-induced bone mass loss, ovariectomy mice models were established. Thirty 8-week-old female C57BL/6 mice were purchased and then randomly divided into six different groups: sham-surgery group, OVX group, OVX + cirsilineol (10 mg/kg) group, OVX + cirsilineol (20 mg/kg) group, OVX + cirsilineol (30 mg/kg) group and OVX + Zoledronate (0.1 mg/kg). One week after surgery, the cirsilineol-treated group mice were intraperitoneally injected different dosage of cirsilineol every 2 d. Equal volume of placebo was injected into each mouse in the sham-surgery group and OVX group. Four weeks later, all mice were euthanized by an overdose of anesthetic. Bilateral femurs were separated, fixed with 4% paraformaldehyde solution for 1 day, and then evaluated by microcomputed tomography to determine the appropriate dosage of cirsilineol. After decalcified with 10% EDTA for a month, long bones were cut into 4-μm sections and all sections were performed with histomorphology staining.
Statistical analysis
All experimental results are displayed as mean ± SD. Statistical difference was presented with Student's t-test and One-way ANOVA. P < 0.05 proved to be statistically significant.
Results
Cirsilineol inhibits RANKL-induced osteoclast formation, but shows no effect on osteoblast differentiation
Cell viability assay was performed to verify the toxic effect of cirsilineol on BMMs and MC3T3-E1 cells. After cirsilineol-treated BMMs and MC3T3-E1 cells were mixed with CCK-8 solution for 4 h, signal intensity was measured. The results demonstrated that the indicated concentrations of cirsilineol (0, 0.01, 0.1, 1, 2.5, 5 μM) had no obvious toxic effect after co-cultured with BMMs for 1 day, 2 days and 4 days, respectively (Fig. 1B). Besides, the results also showed that cirsilineol had no significant toxic effect on the cell viability of MC3T3-E1 cells, until cirsilineol was administrated at a concentration of 50 μM (Additional file 1: Fig. S1B).
Cirsilineol suppressed RANKL-induced osteoclast differentiation in concentration-dependent manner and time-dependent manner (Fig. 1C–G). Meanwhile, which stage did cirsilineol affect osteoclast formation was also been explored, including early stage (0–2 d), late stage (2–4 d) and all stage (0–4 d). The results showed that the number and size of TRAP staining positive cells both reduced in the early and late stage, and the early stage was more obviously inhibited (Fig. 1H–J). All data demonstrated that cirsilineol remarkably suppressed RANKL-induced osteoclast differentiation.
To better understand the effects of cirsilineol on bone formation, MC3T3-E1 cells were cultured with indicated concentrations of cirsilineol (0, 10, 20 μM) under osteogenesis-induced condition. Three days or fourth days after osteogenic induction, all cells were performed with ARS and ALP staining. Both imaging results and statistical results proved that cirsilineol had no obvious effects on osteoblasts differentiation (Additional file 1: Fig. S2A–C). Beyond that, there were also no significant differences in the expression of osteogenesis-related genes (Collagen I and Runx2) after cirsilineol treatment, either at mRNA or protein levels (Additional file 1: Fig. S2D, E).
Cirsilineol inhibits F-actin ring formation and bone resorption ability
In order to determine the inhibitory effect of cirsilineol on F-actin ring formation, BMMs were differentiated into mature osteoclasts with RANKL stimulation. Then mature osteoclasts were transplanted onto slides and incubated with different dosages of cirsilineol (0, 1, 2.5, 5 μM). After performed with rhodamine-phalloidin staining, the number and area of F-actin ring were photographed and analyzed. The results showed that the actin ring formation was most obviously inhibited with 5 μM cirsilineol treatment (Fig. 2A–C).
Cirsilineol inhibits bone resorption ability of mature osteoclast. A–C BMMs were differentiated into mature osteoclast for 4 days, and differentiated cells were treated with different concentrations of cirsilineol for another 2 days. The number of osteoclasts and the size of F-actin rings significantly reduced upon cirsilineol stimulation (n = 3). Scale bar = 50 μm. D, E Mature osteoclasts were seeded into bone slices and cultured with or without cirsilineol for 4 days. The osteolytic ability of osteoclasts was potently inhibited (n = 3). Scale bar = 50 μm.*P < 0.05, **P < 0.01 vs. the control group
The degradation and absorption of bone matrix is the most important ability of mature osteoclasts and also reflects osteoclast vitality. Hence, we tested whether cirsilineol suppressed osteolytic ability of osteoclasts. And the results verified that the bone resorption area remarkably decreased after treated with cirsilineol (Fig. 2D, E).
Cirsilineol inhibits osteoclast-specific genes expression and impairs NFATc1/c-Fos production
To investigate whether cirsilineol inhibits osteoclast formation and function in mRNA levels, numerous osteoclast-specific genes expression including NFATc1, TRAP, V-ATPase-a3, Cathepsink, DC-STAMP and MMP9 was detected with RT-PCR. The results confirmed that cirsilineol significantly suppressed the expression of osteoclast-related genes in a dosage- and time-dependent manner (Fig. 3A, B).
Cirsilineol inhibits osteoclast-specific genes expression at the transcriptional level. A BMMs were cultured with different concentrations of cirsilineol for 4 days, and the expression of osteoclast-specific genes including TRAP, DC-STAMP, CTSK, V-ATPase a3 and MMP-9 was significantly suppressed (n = 3). B BMMs were cultured with or without cirsilineol for 4 days, and the results showed that cirsilineol inhibited osteoclast-specific genes expression in a time-dependent manner (n = 3). C Cirsilineol inhibited the expression of inflammatory cytokines including IL-6, IL-1β and Tnf-α in a dose-dependent manner. *P < 0.05, **P < 0.01 vs. the control group
Postmenopausal sex steroid deficiency alters the production of several inflammatory cytokines, and in particular the cytokines IL-6, IL-1β and Tnf-α are regarded as the potent cytokines that promote bone resorption via activation of RANKL/RANK axis, thereby enhancing osteoclast activity [32,33,34]. In our study, we treated BMMs with different concentrations of cirsilineol (0, 1, 2.5, 5 μM) during osteoclastic induction. All results verified that cirsilineol inhibited the expression of IL-6, IL-1β and Tnf-α in a dose-dependent manner (Fig. 3C).
M-CSF acts as a crucial regulator in promoting RANK expression during osteoclastogenesis [7]. Activation of RANKL/RANK signal initiates the downstream signaling pathways and regulates NFATc1/c-Fos inductions, which is involved in mediating actin re-organization and cell differentiation of osteoclast precursors [35]. In this study, we also found that the inhibitory effect of cirsilineol on the protein expression of RANK, NFATc1 and c-Fos becomes more pronounced as the concentration increases (Fig. 4A–D). Meanwhile, cirsilineol also inhibited the above protein expression in a time-dependent manner (Fig. 4E–H).
Cirsilineol inhibits osteoclast-specific genes expression at the translational level. A–D With the increase of cirsilineol’s concentration, the expression levels of RANK, NFATc1 and c-Fos were on a downward trajectory (n = 3). E–H BMMs were seeded into 12-well plates and incubated with or without 5 μM cirsilineol for 4 days. The expression levels of RANK, NFATc1 and c-Fos increased over time and were suppressed with cirsilineol treatment (n = 3). *P < 0.05, **P < 0.01 vs. the control group
Cirsilineol blocks RANKL-activated NF-κb/ERK/p38 signaling pathways
In order to elucidate the exact molecular mechanism by which cirsilineol affected the activation of downstream signaling pathways, RAW264.7 cells were seeded into 6-well plates at a density of 8 × 105 cells/well. After pretreated with or without 5 μM cirsilineol for 6 h, all cells were stimulated with 100 ng/ml RANKL for 0, 5, 10, 20, 30, 60 min, respectively. Then total proteins were isolated and used for detecting the phosphorylation levels of MAPKs, AKT and NF-κb signaling cascades. The protein levels of p-ERK and p38 in cirsilineol -treated group obviously decreased while compared to control group. Nevertheless, the activation of JNK, which are also known to be part of the MAPK family, seem to make no significant difference (Fig. 5A, B).
Cirsilineol blocks RANKL-induced NF-κb/ERK/p38 signaling pathways activation. A, B To investigate the mechanisms in which cirsilineol inhibits osteoclast formation and activation, RAW264.7 cells were seeded into 6-well plates and cultured with or without cirsilineol for 6 h. Then all cells were stimulated with 100 ng/ml RANKL for 0, 5, 10, 20, 30, 60 min, respectively. The results demonstrated that cirsilineol inhibited the activation of NF-κb/ERK/p38 signaling cascades (n = 3). *P < 0.05, **P < 0.01 vs. the control group
NF-κb signaling cascade also plays an essential role in osteoclast differentiation and function [9]. However, the phosphorylation levels of p65 significantly reduced in the presence of 5 μM cirsilineol. In the meantime, the activation of the upstream IκBα pathway was also inhibited after cirsilineol treatment, which resulted in the decrease of p-p65 protein expression. Akt has been regarded as a master regulator in osteoclast differentiation and survival. In our study, we detected the phosphorylation of AKT, and the result proved that cirsilineol had no effect on p-AKT level (Fig. 5A, B).
Cirsilineol prevents OVX-induced bone mass loss and inhibits osteoclast activity in mice
In order to verify the possible therapeutic effect of cirsilineol in vivo, our study finally tested whether cirsilineol prevented bone volume loss and inhibited osteoclast action in OVX-induced osteoporosis mice. Thirty C57BL/6 female mice were randomly divided into six groups and treated with different dosage of cirsilineol as previously described. Zoledronate-treated group was served as a positive control group. After euthanized by overusing anesthetics, bilateral femurs were isolated and scanned by microcomputed tomography. An obvious bone loss occurred in the distal femurs of the OVX group mice, which indicated that successful osteoporosis mice models were established. Compared with the OVX group, cirsilineol treatment significantly increased bone volume in the femurs of osteoporosis animals. The therapeutic effect of cirsilineol at a concentration of 20 mg/kg is significantly better than that of 10 mg/kg, and there were no obvious differences between OVX + cirsilineol (20 mg/kg) group, OVX + cirsilineol (30 mg/kg) group and OVX + Zoledronate (0.1 mg/kg). All bone quality statistical results such as BV/TV, Conn.D, Tb.N, Tb.Th and TB.Sp amply proved the protective effect of cirsilineol (Additional file 1: Fig. S3A, B, Fig. 6A–F). Thus, we chose a dosage of 20 mg/kg for further study.
Cirsilineol ameliorates OVX-induced bone mass loss and osteoclast activation in vivo. A–F After the establishment of osteoporosis mice model, cirsilineol (20 mg/kg) was intraperitoneally injected into OVX mice for 1 month. Bilateral femurs were separated and photographed by micro-CT. The result verified that cirsilineol attenuated OVX-induced bone mass loss. The micromorphological quantification including BV/TV, Comn.D., Tb.N*, Tb.Th* and Tb.Sp* came to the same conclusion (n = 3). G–I The HE staining and TRAP staining results demonstrated that cirsilineol ameliorated bone mass loss and inhibited osteoclast activation at the histological level (n = 3). Scale bar (HE staining) = 200 μm; Scale bar (TRAP staining) = 100 μm. *P < 0.05, **P < 0.01 vs. the control group
Afterwards, we investigated whether cirsilineol protected against bone loss in histological level. All specimens were decalcified for two weeks and then performed with histological staining, including H&E staining and TARP staining. The results indicated that cirsilineol suppressed bone mass loss in OVX mice. Furthermore, the number and area of osteoclasts in vivo were measured after performed with TRAP staining. And the results suggested that osteoclast formation and activity were enhanced in the OVX group and significantly decreased in the cirsilineol-treated group (Fig. 6G, H).
All these data confirmed that cirsilineol prevented OVX-induced bone mass loss and inhibited osteoclast activity in osteoporosis mice.
Discussion
Osteoporosis, characterized by metabolic disorders of bone tissue, is a homeostasis disruption between bone formation and bone resorption [36]. Osteoporosis is often accompanied by bone deformities, pain and brittle fractures, resulting in a decline in life quality and a huge burden on public healthcare system. Considering the crucial role of osteoclast in osteolysis, regulation of hyperactive osteoclast activity seems to be a potential approach for treating disorders associated with abnormal bone metabolism.
In common pathological bone state such as rheumatoid arthritis and osteoporosis, the over-activated RANKL/RANK signaling cascade is the critical event resulting in osteoclast formation, survival and activation [37]. With M-CSF stimulation, RANK expression increases, and the binding of RANKL and RANK enhances the recruitment of TNF receptor-associated factor 6 (TRAF6). Subsequently, the TGF-b-activated kinase 1 (TAK1), which belongs to the downstream signal transduction factor of TRAF6, is phosphorylated and thus leads to the activation of the downstream signaling pathways, including the IKKα/β/Ikβα. Since Ikβα acts as an inhibitor to inactivate the downstream cascade, the phosphorylation of IKKα/β enhances the degradation of IκB, thus resulting in the activation of NF-κb and activated protein 1 (AP-1) [38]. Activated AP-1 and NF-κB facilitate the generation of NFATc1 and then increase osteoclast-related genes expression, thereby promoting osteoclast differentiation [39]. There are a plenty of receptors and transcriptional factors participating in osteoclast activation, and these molecules are considered to be mediated by NFATc1. Our research suggested that cirsilineol inhibited RANKL-induced NF-κb signaling pathways.
Significant promotion of osteoclast differentiation is previously reported when p38-MAPK axis is initiated [40]. RANKL-induced p38-MAPK activation regulates the expression of osteoclast-specific genes, which is involved in osteoclast formation. As for ERK, another subunit of MAPK super family, is activated with RANKL stimulation and regulates osteoclast formation and function [41]. In our study, we demonstrated that cirsilineol attenuated the phosphorylation of ERK and p38 upon RANKL stimulation.
Numerous drugs have been approved for treating osteoporosis. But in fact, the poor efficacy and severe side effects of these drugs limit the application for the clinical administration. Cirsilineol is one of the main high active components of vestita Wall and is a typical non-glycosylated flavonoid with anti-oxidant and anti-inflammatory activities. The chinese herb extract cirsilineol has been used to treat rheumatoid arthritis and dermatitis in traditional Chinese medicine for a long time. Cirsilineol significantly decreases the expression of proinflammatory mediators of LPS-stimulated macrophages at transcriptional and translational levels, thus improving the local inflammatory microenvironment [29]. Endogenously produced inflammatory factors generate strong oxydic free radicals that cause osteoclast activation and bone mass loss in osteoporosis [42]. Inhibition of various inflammatory factors levels may be one of the mechanisms in which cirsilineol exerted anti-osteoporosis effects. In our study, we confirmed that an anti-inflammatory nature compound cirsilineol reduced osteoclast formation in vitro and inhibited estrogen deficiency-induced bone loss by suppressing osteoclast activity in vivo.
Several limitations of our study still remain unsettled. Firstly, the exact mechanism of how cirsilineol inhibits osteoclast activation remains to be explored. Secondly, the intervention of cirsilineol on osteoblast differentiation and bone formation is still unknown. Lastly, the current delivery of cirsilineol is not suitable for the treatment of osteoporosis and still needs to be improved.
Conclusion
In conclusion, our study suggested that cirsilineol attenuated osteoclast differentiation by impairing NFATc1 induction, mainly through the NF-κB/ ERK/p38 signaling pathways. Meanwhile, cirsilineol improved osteoporosis-induced bone mass loss via inhibiting osteoclast formation and function.
Availability of data and materials
All the data and materials that support the findings of this study are included in the manuscript.
References
Lane JM, Russell L, Khan SN. Osteoporosis. Clin Orthop Relat Res. 2000;425:139–50.
Rachner TD, Khosla S, Hofbauer LC. Osteoporosis: now and the future. Lancet. 2011;377:1276–87.
Woolf AD, Pfleger B. Burden of major musculoskeletal conditions. Bull World Health Organ. 2003;81:646–56.
Sternberg Z. Cardiovascular autonomic dysfunction: link between multiple sclerosis osteoporosis and neurodegeneration. Neuromolecular Med. 2018;20:37–53.
Logan S, Thu WPP, Lay WK, Wang LY, Cauley JA, Yong EL. Chronic joint pain and handgrip strength correlates with osteoporosis in mid-life women: a Singaporean cohort. Osteoporos Int. 2017;28:2633–43.
Palacios S, Neyro JL, Fernandez de Cabo S, Chaves J, Rejas J. Impact of osteoporosis and bone fracture on health-related quality of life in postmenopausal women. Climacteric. 2014;17:60–70.
Boyce BF. Advances in the regulation of osteoclasts and osteoclast functions. J Dent Res. 2013;92:860–7.
Nagy V, Penninger JM. The RANKL-RANK story. Gerontology. 2015;61:534–42.
Park JH, Lee NK, Lee SY. Current understanding of RANK signaling in osteoclast differentiation and maturation. Mol Cells. 2017;40:706–13.
Okumura S, Mizoguchi T, Sato N, Yamaki M, Kobayashi Y, Yamauchi H, et al. Coordination of microtubules and the actin cytoskeleton is important in osteoclast function, but calcitonin disrupts sealing zones without affecting microtubule networks. Bone. 2006;39:684–93.
Teitelbaum SL. The osteoclast and its unique cytoskeleton. Ann N Y Acad Sci. 2011;1240:14–7.
Georgess D, Machuca-Gayet I, Blangy A, Jurdic P. Podosome organization drives osteoclast-mediated bone resorption. Cell Adh Migr. 2014;8:191–204.
Matsubara T, Kokabu S, Nakatomi C, Kinbara M, Maeda T, Yoshizawa M, et al. The actin-binding protein PPP1r18 regulates maturation, actin organization, and bone resorption activity of osteoclasts. Mol Cell Biol. 2018;38:e00425-e517.
Locantore P, Del Gatto V, Gelli S, Paragliola RM, Pontecorvi A. The interplay between immune system and microbiota in osteoporosis. Mediators Inflamm. 2020;2020:3686749.
Fischer V, Haffner-Luntzer M. Interaction between bone and immune cells: implications for postmenopausal osteoporosis. Semin Cell Dev Biol. 2022;123:14–21.
Kovacs B, Vajda E, Nagy EE. Regulatory effects and interactions of the Wnt and OPG-RANKL-RANK signaling at the bone-cartilage interface in osteoarthritis. Int J Mol Sci. 2019;20:4653.
Neumann E, Muller-Ladner U. Frommer KW [Inflammation and bone metabolism]. Z Rheumatol. 2014;73:342–8.
Park-Min KH, Lim E, Lee MJ, Park SH, Giannopoulou E, Yarilina A, et al. Inhibition of osteoclastogenesis and inflammatory bone resorption by targeting BET proteins and epigenetic regulation. Nat Commun. 2014;5:5418.
Tella SH, Gallagher JC. Prevention and treatment of postmenopausal osteoporosis. J Steroid Biochem Mol Biol. 2014;142:155–70.
Roush K. Prevention and treatment of osteoporosis in postmenopausal women: a review. Am J Nurs. 2011;111(26–35):quiz 6-7.
Black DM, Rosen CJ. Clinical practice. Postmenopausal Osteoporosis N Engl J Med. 2016;374:254–62.
Khosla S, Hofbauer LC. Osteoporosis treatment: recent developments and ongoing challenges. Lancet Diabetes Endocrinol. 2017;5:898–907.
Han P, Cui Q, Yang S, Wang H, Gao P, Li Z. Tumor necrosis factor-alpha and transforming growth factor-beta1 facilitate differentiation and proliferation of tendon-derived stem cells in vitro. Biotechnol Lett. 2017;39:711–9.
Ai M, Lin S, Zhang M, Wu T, Yang N, Li Y, et al. Cirsilineol attenuates LPS-induced inflammation in both in vivo and in vitro models via inhibiting TLR-4/NFkB/IKK signaling pathway. J Biochem Mol Toxicol. 2021;35: e22799.
Pathak G, Singh S, Kumari P, Hussain Y, Raza W, Luqman S, et al. Cirsilineol inhibits proliferation of lung squamous cell carcinoma by inducing ROS mediated apoptosis. Food Chem Toxicol. 2020;143: 111550.
Sheng X, Sun Y, Yin Y, Chen T, Xu Q. Cirsilineol inhibits proliferation of cancer cells by inducing apoptosis via mitochondrial pathway. J Pharm Pharmacol. 2008;60:1523–9.
Tasdemir D, Tierney M, Sen R, Bergonzi MC, Demirci B, Bilia AR, et al. Antiprotozoal effect of Artemisia indica extracts and essential oil. Planta Med. 2015;81:1029–37.
Gong G, Zhao R, Zhu Y, Yu J, Wei B, Xu Y, et al. Gastroprotective effect of cirsilineol against hydrochloric acid/ethanol-induced gastric ulcer in rats. Korean J Physiol Pharmacol. 2021;25:403–11.
Li E, Wang D, Xue Y, Yan J, Wang J. The Protective role of cirsilineol against ovalbumin-induced allergic rhinitis in mice by suppression of inflammation and oxidative stress. J Environ Pathol Toxicol Oncol. 2021;40:63–73.
Isobe T, Doe M, Morimoto Y, Nagata K, Ohsaki A. The anti-Helicobacter pylori flavones in a Brazilian plant, Hyptis fasciculata, and the activity of methoxyflavones. Biol Pharm Bull. 2006;29:1039–41.
Dawe A, Mbiantcha M, Yakai F, Jabeen A, Ali MS, Lateef M, et al. Flavonoids and triterpenes from Combretum fragrans with anti-inflammatory, antioxidant and antidiabetic potential. Z Naturforsch C J Biosci. 2018;73:211–9.
Yokota K, Sato K, Miyazaki T, Aizaki Y, Tanaka S, Sekikawa M, et al. Characterization and function of tumor necrosis factor and interleukin-6-induced osteoclasts in rheumatoid arthritis. Arthritis Rheumatol. 2021;73:1145–54.
Armas LA, Recker RR. Pathophysiology of osteoporosis: new mechanistic insights. Endocrinol Metab Clin North Am. 2012;41:475–86.
Tao H, Li W, Zhang W, Yang C, Zhang C, Liang X, et al. Urolithin A suppresses RANKL-induced osteoclastogenesis and postmenopausal osteoporosis by, suppresses inflammation and downstream NF-kappaB activated pyroptosis pathways. Pharmacol Res. 2021;174: 105967.
Miyamoto T. Regulators of osteoclast differentiation and cell-cell fusion. Keio J Med. 2011;60:101–5.
Appelman-Dijkstra NM, Papapoulos SE. Modulating bone resorption and bone formation in opposite directions in the treatment of postmenopausal osteoporosis. Drugs. 2015;75:1049–58.
Ono T, Nakashima T. Recent advances in osteoclast biology. Histochem Cell Biol. 2018;149:325–41.
Shifera AS, Friedman JM, Horwitz MS. IKK gamma (NEMO) is involved in the coordination of the AP-1 and NF-kappa B pathways. Mol Cell Biochem. 2008;310:181–90.
Iezaki T, Fukasawa K, Park G, Horie T, Kanayama T, Ozaki K, et al. Transcriptional modulator Ifrd1 regulates osteoclast differentiation through enhancing the NF-kappaB/NFATc1 pathway. Mol Cell Biol. 2016;36:2451–63.
Kim K, Kim JH, Kim I, Seong S, Kim N. Rev-erbalpha negatively regulates osteoclast and osteoblast differentiation through p38 MAPK signaling pathway. Mol Cells. 2020;43:34–47.
Agidigbi TS, Kang IS, Kim C. Inhibition of MEK/ERK upregulates GSH production and increases RANKL-induced osteoclast differentiation in RAW 264.7 cells. Free Radic Res. 2020;54:894–905.
Tao H, Ge G, Liang X, Zhang W, Sun H, Li M, et al. ROS signaling cascades: dual regulations for osteoclast and osteoblast. Acta Biochim Biophys Sin. 2020;52:1055–62.
Acknowledgements
Not applicable.
Funding
This work was supported by grants from the National Natural Science Foundation of China (No. 81572124, No. 82101649, No. 82102597), Natural Science Foundation of Zhejiang Province (LY19H060006, LQ20H060008, LQ18H070002, LQ21H060007).
Author information
Authors and Affiliations
Contributions
CW and RH: conception and design, intellectual input and supervision, article writing with contributions from other authors; CW, RZ and YL: experiments and/or data analysis; RH: fund support.
Corresponding author
Ethics declarations
Ethical approval and consent to participate
This work has been approved for animal ethics by the Second Affiliated Hospital, School of Medicine, Zhejiang University.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Additional file 1: Figure. S1
Cytotoxicity assay of cirsilineol to BMMs and MC3T3-E1 cells. Figure. S2 Cirsilineol has no effect on osteoblast differentiation. Figure. S3 Micro-CT assay.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Wang, C., Zeng, R., Li, Y. et al. Cirsilineol inhibits RANKL-induced osteoclast activity and ovariectomy-induced bone loss via NF-κb/ERK/p38 signaling pathways. Chin Med 19, 69 (2024). https://doi.org/10.1186/s13020-024-00938-6
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s13020-024-00938-6