Abstract
Traditional Chinese medicine (TCM) has been widely used for several centuries for metabolic diseases, including non-alcoholic fatty liver disease (NAFLD). At present, NAFLD has become the most prevalent form of chronic liver disease worldwide and can progress to non-alcoholic steatohepatitis (NASH), cirrhosis, and even hepatocellular carcinoma. However, there is still a lack of effective treatment strategies in Western medicine. The development of NAFLD is driven by multiple mechanisms, including genetic factors, insulin resistance, lipotoxicity, mitochondrial dysfunction, endoplasmic reticulum stress, inflammation, gut microbiota dysbiosis, and adipose tissue dysfunction. Currently, certain drugs, including insulin sensitizers, statins, vitamin E, ursodeoxycholic acid and betaine, are proven to be beneficial for the clinical treatment of NAFLD. Due to its complex pathogenesis, personalized medicine that integrates various mechanisms may provide better benefits to patients with NAFLD. The holistic view and syndrome differentiation of TCM have advantages in treating NAFLD, which are similar to the principles of personalized medicine. In TCM, NAFLD is primarily classified into five types based on clinical experience. It is located in the liver and is closely related to spleen and kidney functions. However, due to the multi-component characteristics of traditional Chinese medicine, its application in the treatment of NAFLD has been considerably limited. In this review, we summarize the advances in the pathogenesis and treatment of NAFLD, drawn from both the Western medicine and TCM perspectives. We highlight that Chinese and Western medicine have complementary advantages and should receive increased attention in the prevention and treatment of NAFLD.
Similar content being viewed by others
Introduction
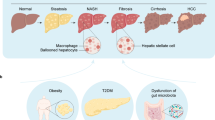
NAFLD is the most common chronic liver disease worldwide with a global prevalence of 32.4% [1]. This disease encompasses a spectrum of liver disorders ranging from non-alcoholic fatty liver (NAFL), characterized by simple steatosis, to non-alcoholic steatohepatitis (NASH), characterized by hepatocyte steatosis accompanied by inflammation, hepatocyte ballooning, progressive fibrosis, and even further progression to cirrhosis and hepatocellular carcinoma (HCC) [2]. The multiple-hits hypothesis suggests that insulin resistance, lipotoxicity, endoplasmic reticulum stress, inflammation, mitochondrial dysfunction, oxidative stress, gut microbiota, and genetics synergistically contribute to the development of NAFLD [3]. Furthermore, NAFLD frequently coexists with many metabolic diseases such as type 2 diabetes (T2D), which is associated with an increased risk of adverse outcomes in NAFLD patients [4]. Although many clinical and experimental studies have been performed, the detailed molecular mechanisms involved in the pathogenesis of NAFLD remain to be elucidated. Lifestyle modifications, including weight loss, a Mediterranean diet, and physical activity, are recognized as the most effective non-drug treatment strategies, while there are still no specific drugs available for NAFLD in clinical settings due to its complex pathogenesis [2]. However, increasing evidence indicates that incorporating drugs with different mechanisms and personalized medicine may improve treatment efficacy in NAFLD patients [5].
Traditional Chinese medicine (TCM) has been widely used in China and is increasingly recognized as a complementary and alternative form of modern medicine, including herbal medicine, acupuncture, and other physical therapies such as massage [6]. Holistic view and syndrome differentiation are the theoretical guidance of TCM and reflect system physiology approaches and personalized medicine in Western medicine [7], demonstrating their advantages in NAFLD treatment [8]. In TCM, NAFLD pathogenesis is primarily attributed to deficiency of the spleen (Pi) or kidney (Shen), stagnation of liver qi, and internal retention of phlegm, dampness, and blood stasis [9]. Consistently, the theoretical guidance of NAFLD treatment includes removing phlegm and blood stasis, nourishing the liver and kidneys, relaxing the liver and spleen, eliminating moisture, and clearing heat [10]. However, the diagnosis and treatment of NAFLD in TCM are subject to subjectivity and complexity. Therefore, it is necessary to establish standardization and objective quantitative analysis methods in combination with Western medicine to improve the clinical efficacy of NAFLD treatment [11]. This review presents the current progress in the pathogenesis and treatment of NAFLD in Chinese and Western medicine (Fig. 1).
The pathogenesis of NAFLD in Western medicine
The two-hit theory was proposed in 1998, which posits that the first hit, steatosis, increases liver sensitivity to the second hits that mediate liver injury. However, with the emergence of new findings, the traditional two-hit theory is no longer sufficient to explain the complex pathogenesis of NAFLD, and multiple parallel strikes are gradually replacing the second strike theory to explain the pathogenesis of NAFLD. Such hits include genetic factors, insulin resistance, lipotoxicity, mitochondrial dysfunction, endoplasmic reticulum stress, inflammation, gut microbiota dysbiosis, and adipose tissue dysfunction [12, 13] (Fig. 2). However, the interactions and synergies between these mechanisms and their changing patterns in NAFLD development require further clarification.
The pathogenesis of NAFLD in Western medicine. The risk of developing NAFLD is determined by environmental and genetic factors. High-fat diet-induced insulin resistance and disruptions in hepatic lipid metabolism, such as imbalances in lipid synthesis and catabolism or impaired lipid transport, result in the excessive accumulation of lipids in hepatocytes. Concurrently, factors such as lipotoxicity, endoplasmic reticulum stress and mitochondrial dysfunction contribute to the inflammation and apoptosis of overloaded hepatocytes. Inflammation can exacerbate hepatocyte injury and stimulate hepatic stellate cell activation, leading to hepatic fibrosis. Additionally, the interplay between the liver, adipose tissue and the gut contributes to metabolic dysregulation and inflammation in NAFLD
Genetics
Genetic and epigenetic changes interact with environmental factors to determine susceptibility to NAFLD [14]. A cross-sectional analysis indicated that the risk of severe liver fibrosis was 12-fold higher in first-degree relatives of patients with NAFLD-related cirrhosis than in the general population [15]. Moreover, the incidence of NAFLD in the United States has significant racial and ethnic disparities [16]. Genome-wide association studies in recent years have revealed the important role of genetic polymorphisms in the progression of NAFLD.
The I148M variant disrupts the phospholipase activity of patatin-like phospholipase domain-containing 3 (PNPLA3), a triglyceride lipase, thereby interfering with lipid catabolism and promoting hepatic fat accumulation to increase the risk of liver-related mortality in patients with NAFLD [17, 18]. The E167K variant of transmembrane 6 superfamily member 2 (TM6SF2) decreases the secretion of very low-density lipoprotein (VLDL) to favor hepatic fat accumulation in NAFLD patients but confers cardiovascular protection by reducing the amount of circulating lipids [19, 20]. The rs641738 variant downregulates the expression of membrane-bound O-acyltransferase domain-containing 7 (MBOAT7) and reduces the levels of phosphatidylinositol species with arachidonoyl side chains in hepatocytes, which might contribute to fibrosis independent of inflammation in NAFLD [21, 22]. The P446L variant of glucokinase regulatory protein (GCKR) increases hepatic glucose uptake and the production of malonyl-CoA, thereby promoting lipid accumulation in the liver by increasing lipogenesis substrates and blocking fatty acid oxidation [23, 24]. In addition, several other genetic determinants of NAFLD have been identified, including hydroxysteroid 17-beta dehydrogenase 13 (HSD17B13) [25], phosphatase 1 regulatory subunit 3B (PPP1R3B) [26], immunity-related GTPase M (IRGM) [27] and Lpin1 [28].
Insulin resistance
Insulin, a hormone, regulates cellular functions, stimulates sugar transport, and thus governs cell growth, energy balance and gene expression, and inhibits lipolysis in adipose tissue. It binds to its receptors, initiating the phosphorylation of various downstream substrates, such as insulin receptor substrate (IRS)-1, -2, -3, and -4, thereby activating the insulin signaling pathway [29]. IRS-1 and IRS-2 phosphorylate phosphoinositide 3-kinase (PI3K) and activate protein kinase B (AKT), resulting in the translocation of glucose transporter protein 4 (GLUT4). This process enhances glucose uptake and modulates forkhead transcription factors, increasing lipid gene expression and decreasing glucose xenobiotic gene expression [30]. Molecular substances associated with NAFLD interfere with insulin signaling connections and exacerbate insulin resistance, including non-esterified fatty acids, tumor necrosis factor, nuclear factor-κB (NF-κB), suppressor of cytokine signaling (SOCS), Jun amino-terminal kinase 1 (JNK1), and cytochrome P4502E1 [31]. Furthermore, substantial evidence suggests that IR may precede NAFLD. Reduced metabolic activity may protect against excessive transport of free fatty acids (FFAs) to the liver [32].
Lipotoxicity
The core mechanisms of NASH are endoplasmic reticulum stress, oxidative stress, and inflammatory responses caused by free cholesterol, FFAs, and their metabolites. The conventional view is that triglycerides in hepatocytes promote lipid peroxidation, oxidative stress, inflammation, and fibrosis and are drivers of NAFLD progression. However, this viewpoint is increasingly challenged because triglycerides may antagonize lipotoxicity. A study revealed that inhibiting triglyceride synthesis in the mouse liver reduced hepatic steatosis but exacerbated liver damage and fibrosis [33]. Triglyceride (TG) deposition in the mouse liver was insufficient to induce insulin resistance and liver inflammation [34]. These results suggest that triglycerides may not be the primary type of lipid that promotes NAFLD disease progression.
Studies have shown that palmitic acid (C16:0) and stearic acid (C18:0), which are abundant in animal fat and dairy products, can promote the progression of NAFLD. Furthermore, ceramides can increase mitochondrial membrane permeability to promote cell death by activating JNK activity [35]. The accumulation of lysophosphatidylcholine in hepatocytes can cause apoptosis by activating the death receptor DR5 while promoting macrophage activation through pro-inflammatory exosomes [36]. The increase in excess free cholesterol in cells can disrupt the fluidity of mitochondrial membranes, leading to mitochondrial malfunction and oxidative stress. The accumulation of cholesterol crystals in hepatocytes can activate inflammasome and promote the production of inflammatory factors such as IL-1β and TNF-α [37]. Therefore, in some animal models of NASH, high cholesterol and free fatty acid levels promote NASH phenotype development [38].
Endoplasmic reticulum stress
The endoplasmic reticulum (ER) is the major site of protein and lipid synthesis in hepatocytes [39]. The accumulation of misfolded or unfolded proteins leads to ER stress and the activation of the unfolded protein response (UPR), which mainly relies on three transmembrane ER stress sensors, inositol-requiring enzyme 1 (IRE1), PKR-like ER kinase (PERK) and activating transcription factor (ATF6), to restore homeostasis [40].
IRE1α in NAFLD demonstrates a dichotomous impact. IRE1α facilitates serine palmitoyltransferase gene transcription via XBP1s, leading to the release of extracellular vesicles (EVs) from hepatocytes, thereby recruiting macrophages to foster hepatic inflammation and injury in NASH mice [41]. Conversely, S-nitrosylation of IRE1α impedes its degradation of miR-34 and miR-200, which are pivotal microRNAs that govern hepatic lipid metabolism [42]. This finding was further corroborated by liver-specific IRE1α knockout, which exacerbates liver fibrosis and inflammation in high-fat diet-induced mice [43].
Activation of the PERK-eIF2α pathway leads to the up-regulation of UPR target genes, including the proapoptotic protein C/EBP homologous protein (CHOP). Interestingly, CHOP-deficient hepatocytes exhibit attenuated ER stress-induced cell death [44], while CHOP-dependent macrophage apoptosis protects against NAFLD [45]. Moreover, salubrinal, an eIF2α inhibitor, has been shown to have hepatoprotective effects on high-fat diet-induced obese mice [46].
Reduced endoplasmic reticulum (ER) membrane fluidity, which selectively activates ATF6α, is implicated in NASH [47]. ATF6p50, a cleaved form of ATF6α, may indirectly suppress lipogenic genes by inducing CHOP through dominant-negative inhibition of C/EBPα, leading to diminished fatty acid oxidation and lipoprotein secretion [48].
In addition, deletion of sterol regulatory element-binding protein (SREBP) cleavage-activating protein (SCAP) exacerbates ER stress and liver injury through impairing FA synthesis and discerning FA incorporation into phosphatidylcholine [49]. SID1 transmembrane family member 2 (SIDT2) deficiency induces ER stress and results in hepatic steatosis by upregulating SREBP1, sterol regulatory element binding transcription factor 1 (SREBF1) and fatty acid biosynthesis [50]. Forkhead box A3 (FOXA3) is selectively induced by XBP1s during endoplasmic reticulum stress and directly orchestrates Period1 (Per1) expression, thereby enhancing the expression of the lipogenic gene Srebp1c [51].
Mitochondrial dysfunction
Mitochondria, which are abundant in hepatocytes and reliant on oxygen for adenosine triphosphate (ATP) synthesis, play a pivotal role in the metabolic functions of the liver. During fatty acid catabolism, FFAs are initially converted to fatty acyl-CoA in the cytosol of hepatocytes and subsequently transported into mitochondria by carnitine palmitoyl-transferase 1 (CPT1). β-Oxidation efficiently breaks down FFAs into acetyl-CoA, which is subsequently metabolized to CO2 and H2O via the tricarboxylic acid (TCA) cycle [52]. However, an imbalance between saturated and unsaturated FAs leads to reduced levels of linoleic and arachidonic acids in mitochondrial phospholipids, resulting in decreased CPT1 activity [53]. The electron transport chain (ETC) is situated in the inner mitochondrial membrane and consists of five complexes, including complexes I–IV. High-fat diets increase calcium uptake to modulate the influx of sodium into the mitochondrial matrix via sodium/calcium exchangers, resulting in a reduction in inner mitochondrial membrane fluidity and negatively impacting mitochondrial ATP production [54]. Consistently, studies have shown increased mitochondrial mass and reduced efficiency of respiratory chain complexes, accompanied by elevated mitochondrial uncoupling and leakage activity in patients with NAFLD [55]. Furthermore, a recent study reported the presence of two distinct mitochondrial populations in the liver, which are named cytoplasmic mitochondria and lipid droplet-associated mitochondria. Lipid droplet-associated mitochondria exhibit increased fatty acid oxidation, as indicated by elevated levels of phosphorylated ACC2 (pACC2), Mitofusin 2 protein (MFN2) and CPT1 activity, but are damaged by fatty acid oxidation in an NAFLD rat model [56]. Furthermore, when mitochondrial β-oxidation is impaired, alternative peroxisomal and cytochrome oxidation pathways for FFAs are activated, resulting in the generation of substantial levels of reactive oxygen species (ROS) and oxidative stress [57].
Inflammation
A crucial pathological feature in the progression of NAFLD is chronic, persistent, low-grade non-infectious inflammation. Elevated levels of FFAs and their lipotoxicity, IR, ER stress and adipose tissue dysfunction activate and sustain the production and release of hepatic pro-inflammatory cytokines [58]. High caloric intake causes the expansion of adipose depots, which is associated with increased infiltration of macrophages [59]. IR is closely related to the inflammatory response and is now considered a chronic subclinical inflammatory process [60]. Under physiological conditions, the IRS undergoes tyrosine phosphorylation. Moreover, inflammatory factors activate a series of kinases to induce serine/threonine phosphorylation in the IRS, which prevents normal tyrosine phosphorylation and leads to the loosening of the binding of the IRS to the insulin receptor and the inability of the IRS to activate its downstream substrate phosphatidylinositol 3-kinase (PI3-K), thus interfering with the passage of IR/IRS/PI insulin signaling [60]. ER stress plays an essential role in the activation of the NLRP3 inflammasome, which results in the development of NAFLD. The expression of various inflammatory cytokines, such as TNF-α, IL-1β and MCP-1, is induced by the NLRP3 inflammasome through the JNK/AP-1 and IKK/NF-κB pathways [61]. Furthermore, overnutrition increases intestinal permeability and results in bacterial and lipopolysaccharide (LPS) migration via the portal vein to the liver, where they react with Toll-like receptors (TLRs) and activate NF-κB to induce the secretion of inflammatory cytokines, thereby maintaining and aggravating hepatic inflammation [62]. Inflammatory cytokines activate Kupffer cells and hepatic stellate cells (HSCs), which exacerbate the inflammatory response and lead to hepatic damage and fibrosis [58].
The gut microbiota and metabolites
Both the liver and intestine originate from the ventral foregut endoderm. They are intrinsically linked in anatomy and biological function [63]. The liver and the intestine are connected through the portal vein, through which 70–75% of the blood supply to the liver passes, while the portal vein originates from the intestinal capillaries. Moreover, a range of bacteria, bacterial metabolites, and environmental toxins produced in the intestine can reach the liver through the portal vein [64], making the liver the most accessible place for intestinal bacteria and their derivatives. The tight junctions of intestinal epithelial cells act as a natural barrier to bacteria and their metabolites [65]. Antigens from pathogenic microorganisms or foods are recognized by dendritic cells in the intestinal epithelium to activate adaptive T cells. Pathogen-associated molecular patterns (PAMPs), such as lipopolysaccharide (LPS), peptidoglycan, and flagellin, are recognized by Toll-like receptors (TLRs) and Nod-like receptors (NLRs). PAMPs activate HSCs involved in the fibrotic process, but Kupffer cells respond more strongly to LPS than HSCs [66].
Primary bile acids (BAs) are synthesized by the liver and secreted into the gallbladder. These compounds are released into the duodenum after feeding and are metabolized by intestinal bacteria to secondary bile acids and then reabsorbed into the portal vein, where most of the molecules are captured by the liver for recirculation [67]. The most crucial bile acid receptors in NAFLD are the farnesyl X nuclear receptor (FXR), which is activated mainly by primary bile acids, and transmembrane G-protein-coupled receptor 5 (TGR5), which is activated primarily by secondary bile acids [68]. FXRs are members of the nuclear receptor superfamily and play a role in hepatic bile acid uptake and transport. Hepatic TG and cholesterol levels are significantly increased in FXR-deficient mice [69]. However, intestinal bacteria interact with FXRs to reduce hepatic lipogenesis by reducing glucagon-like peptide 1 (GLP-1) expression in the gut and increasing hepatic gluconeogenesis [70]. TGR5 activation induces the transcription factor cAMP-responsive element binding protein (CREB) and enhances the production of GLP-1 to regulate glucose and energy metabolism [71].
Short-chain fatty acids (SCFAs) are volatile fatty acids produced mainly by the fermentation of soluble dietary fiber and indigestible carbohydrates by intestinal microorganisms [72]. SCFAs play various roles in body metabolism, such as regulating appetite and energy expenditure, stimulating insulin sensitivity, and activating adenosine monophosphate in the liver and skeletal muscle [73]. One of the mechanisms by which SCFAs affect hepatic adiposity and adipose tissue accumulation is the regulation of insulin sensitivity in adipose tissue through G-protein coupled receptor 43 (GPR43). In terms of insulin sensitivity, GRP43 in adipose tissue is activated by SCFAs to promote energy expenditure and inhibit adipose tissue accumulation [74]. Moreover, the recognition of SCFAs by the intestine can cause the release of insulin-sensitive peptide tyrosine (PYY) and GLP-1, which inhibits gastrointestinal motility and prolongs gastrointestinal transit time [75].
Adipose tissue dysfunction
Adipose tissue is an important site of energy metabolism and an essential endocrine organ of the body. Increasingly, studies have shown that fatty endocrine factors can regulate the function of other metabolic organs, thereby regulating metabolic homeostasis throughout the body. In obesity, insulin resistance, and metabolic syndrome, fatty tissue becomes chronically inflamed and secretes increased levels of inflammatory factors such as IL-1β, TNF-α, and IL-6, which stimulate liver inflammation and disrupt glycolipid metabolism through the body circulation [58]. Moreover, adiponectin from adipose tissue inhibits insulin resistance, reduces hepatic steatosis, and has anti-inflammatory, anti-apoptotic, and insulin-sensitizing effects in NAFLD [76].
Leptin may play a dual role in the pathogenesis of NAFLD. In animal models, leptin has been found to alleviate hepatic steatosis by inhibiting the de novo synthesis of hepatic lipids (DNL) in the early stages of NAFLD disease, but promoting inflammation and hepatic fibrosis in the progressive stages of NAFLD disease [77, 78]. A recent study demonstrated that leptin in the brain increases very-low-density lipoprotein triglyceride (VLDL-TG) levels and reduces hepatic lipid content through the vagus nerve [79]. Lipocalin-2 activates HSCs to secrete matrix metalloproteinase 9 (MMP9) in leptin-deficient obesity, thereby promoting the transition from simple steatosis to NASH [80]. Consistently, a study reported that a decrease in lipocalin levels may be associated with the development of NAFLD [81]. Neuregulin 4 (Nrg4), from brown adipose tissue, negatively regulates de novo lipogenesis mediated by liver X receptor (LXR) and SREBP1c [82]. In addition, Nrg4 restrains tumor-associated macrophage (TAM)-like macrophages and the exhaustion of cytotoxic CD8+ T cells to suppress NASH progression to liver cancer [83].
The pathogenesis of NAFLD in TCM
NAFLD is a term used in Western medicine, but there is no direct equivalent of this disease in TCM. However, TCM has recorded this disease for a long time. Su Wen registered, “Excessive diet will damage the gastrointestinal tract”. According to its symptoms and pathogenesis, NAFLD can be recognized as a hepatic syndrome such as distention and fullness, phlegm syndrome, turbidity, hypochondriac pain, a lump at the left hypochondrium and damp obstruction disease [8]. In TCM, the primary causes of NAFLD are identified as emotional disorders, irregular diet, work stress, chronic diseases and congenital deficiencies. It is located mainly in the liver and is closely related to spleen and kidney functions [8].
There are many theories on the treatment of NAFLD in TCM, primarily based on the theory of liver and spleen [9]. Spleen deficiency can result in impaired organ transformation and transportation functions, resulting in gastrointestinal disturbances. Correspondingly, disturbance in the gut microbiota can lead to functional impairments in the digestive system, resulting in spleen deficiency syndrome [84]. Physiologically, the transportation function of the spleen depends on the maintenance of liver qi, while the drainage and blood storage functions of the liver require vital qi produced by spleen metabolism. This interplay reflects the roles and relationships between the liver and spleen in transportation, blood production and blood storage.
Most related research emphasizes NAFLD treatment based on the liver and spleen theories, while the importance of the kidney is frequently overlooked. In Su Wen Yin Yang Xiang Da Lun, the theory of reciprocal generation of the essence and blood between the liver and kidney is discussed: “North generates cold, cold generates water, water generates salt, salt generates kidney, kidney generates bone marrow, marrow generates liver.” This elucidates the liver-kidney relationship, forming the basis of the “liver and kidney share the same origin” theory [85]. One of the fundamental theories of TCM is that the kidney collects essence, and the liver stores blood, then they continuously transform within the body along with qi. Sufficient blood can nourish and continuously enrich kidney essence, which is the physiological relationship between the liver and kidney.
Identification of NAFLD patterns in TCM
NAFLD can be classified into five main types based on clinical experience: spleen-deficiency and phlegm-turbid stagnation; stagnation of liver-qi; accumulated damp-heat; stasis blocking channels and deficiency of liver and kidney [86, 87].
Spleen-deficiency and phlegm-turbid stagnation
Primary symptoms include fullness in the right hypochondrium. Secondary symptoms include obesity, bodily heaviness, lethargy, chest tightness, dizziness and nausea.
Stagnation of liver-qi
Primary symptoms consist of congestion or wandering pain in the right hypochondrium, often accompanied by depression due to irritability. Secondary symptoms include abdominal distension, diarrhea, abdominal pain, fatigue, and chest tightness.
Accumulated damp-heat
The primary symptoms involve fullness in the right hypochondrium. Secondary symptoms include nausea, vomiting, jaundice, chest fullness, and body heaviness.
Stasis blocking channels
Primary symptoms include a lump or tingling pain in the right abdomen. Secondary symptoms include anorexia, chest tightness, and a dark complexion.
Deficiency of liver and kidney
Primary symptoms present as pain in the right hypochondrium. Secondary symptoms include physical weakness, lumbar and knee soreness, frequent nocturnal urination, and diarrhea.
Pharmacological Treatment of NAFLD in Western Medicine
In Western medicine, although there are currently no approved drugs for NAFLD, some drugs, such as insulin sensitizers, statins, vitamin E, ursodeoxycholic acid and betaine, are beneficial for the clinical treatment of NAFLD. Among these, insulin sensitizers, statins and vitamin E are primarily recommended in clinical practice guidelines for NAFLD [88, 89]. Here, we summarize the research progress related to these drugs in NAFLD treatment.
Insulin sensitizers
Insulin sensitizers are applicable in patients with both NAFLD/NASH and type 2 diabetes mellitus. Metformin is the first-line treatment for type 2 diabetes, which elevates hepatic glucose output while reducing insulin-stimulated glucose uptake in peripheral tissues and fatty acid oxidation in adipose tissue [90]. A study demonstrated that metformin can reverse transaminase abnormalities, steatosis, and inflammation in NAFLD and NASH patients [91]. Metformin alleviates hepatic steatosis by restoring SIRT1-mediated autophagy and inducing tristetraprolin to regulate lipophagy and necroptosis in hepatocytes [92, 93]. A recent study demonstrated that metformin can rescue the impairment of hepatic CD8+ T cell metabolism and the efficacy of anti-PD-1 therapy against NASH-related liver cancer [94]. Furthermore, combination of metformin, both liraglutide and sitagliptin can reduce body weight, intrahepatic lipids, and visceral adipose tissue and improve glycemic control in patients with T2DM and NAFLD under conditions of inadequate glycemic control caused by metformin [95]. However, long-term metformin treatment might promote NAFLD progression in leptin-insensitive individuals [96].
Thiazolidinediones (TZDs) are a class of oral antidiabetic drugs. They induce the nuclear transcription factor peroxisome proliferator-activated receptor-γ (PPAR-γ), which is predominantly expressed in adipose tissue and contributes to decreased hepatic fat content and enhanced glycemic control and insulin sensitivity [97]. Furthermore, TZDs elevate plasma adiponectin levels and activate adenosine monophosphate [98]. Studies have elucidated the impact of TZDs on liver enzymes and histological profiles. Research in rat models has shown that pioglitazone and rosiglitazone inhibit the activation of hepatic stellate cells in vitro and ameliorate hepatic steatosis and fibrosis in vivo [99]. The primary adverse effect of TZDs is weight gain, but they can be effectively combined with metformin [100, 101].
Liraglutide, a synthetic long-acting GLP-1 receptor agonist, is approved for the treatment of diabetes mellitus and obesity. Clinical studies have indicated that liraglutide can reduce metabolic dysfunction, insulin resistance and lipotoxicity in patients with NASH without improving fibrosis [102, 103]. However, a recent study demonstrated that combining bempedoic acid, an inhibitor of liver ATP citrate lyase (ACLY), with liraglutide, reduces liver steatosis, hepatocellular ballooning and hepatic fibrosis in a mouse model of NASH [104].
Statins
Statins have anti-inflammatory, antioxidant, and anti-fibrotic effects and reduce cholesterol biosynthesis by inhibiting HMG-CoA reductase [105]. Atorvastatin, lovastatin, simvastatin and fluvastatin are lipophilic drugs. Conversely, pravastatin and pitavastatin are hydrophilic, resulting in minimal metabolism in the liver. Studies indicate that hydrophobic statins, such as lovastatin and simvastatin, accumulate more in the liver than hydrophilic statins. However, the specific clinical implications of these differences remain unclear [105]. A comprehensive review of the safety and efficacy of statins in NAFLD patients revealed that these drugs effectively reduce liver enzyme levels and positively impact liver histology [106]. Moreover, high cumulative doses of certain statins, including rosuvastatin, pravastatin, atorvastatin, simvastatin, and fluvastatin, have been shown to reduce the risk of NAFLD-related decompensated liver cirrhosis in T2DM patients [107]. The combination of rosuvastatin and ezetimibe was found to more effectively reduce hepatic lipid accumulation than monotherapy in NAFLD patients [108]. Another primary benefit of statins is their association with decreased mortality from cardiovascular disease (CVD). Post hoc analysis of three extensive randomized clinical trials suggested that certain statins, particularly atorvastatin, not only ameliorate NAFLD/NASH but also reduce CVD events twice as in those with normal liver function [109]. Nonetheless, statins have been underprescribed in patients with NAFLD, primarily due to apprehensions about hepatotoxicity [110].
Vitamin E
Vitamins possessing antioxidant properties are widely recognized for their health benefits. This effectiveness is attributed to their ability to reduce reactive oxygen species levels in the body via diverse mechanisms, thereby preventing oxidative cellular damage, which can culminate in cellular senescence and apoptosis [111]. Elevated transport of fatty acids to the liver during metabolic synthesis escalates oxidative stress through fatty acid oxidation and oxidative phosphorylation, creating a milieu rich in reactive oxygen species that potentially results in hepatocellular damage and progressive liver injury [112]. Vitamin E enhances the activity of the antioxidant enzymes catalase and glutathione peroxidase [113]. Furthermore, genetic factors, including variations in haptoglobin and fatty acid desaturase 1/2 (FADS1/FADS2), may influence the therapeutic efficacy of Vitamin E in NAFLD [114]. Clinical studies have demonstrated Vitamin E ameliorates liver fibrosis in patients with NASH [115]. Additionally, a combination treatment of vitamin E and hydroxytyrosol has been shown to inhibit hepatic stellate cell proliferation by disrupting the nuclear translocation/activation of SMAD2/3 transcription factors, thereby improving NAFLD-related fibrosis in children [116].
Ursodeoxycholic acid
Ursodeoxycholic acid (UDCA) is well known for dissolving cholesterol stones, which can stabilize cell membranes, safeguard mitochondria, mitigate bile salt toxicity, and be employed in the treatment of primary biliary cholangitis and cholestatic liver disease. UDCA has demonstrated improvements in hepatic steatosis and inflammation in animal models [117, 118]. This improvement was partially attributed to the inhibition of the miR-34a/SIRT1/p53 pathway [119]. Additionally, UDCA has been shown to partially restore gut microbiota, repair gut barrier integrity, and reduce hepatic inflammation in NASH [120]. Moreover, UDCA significantly normalizes liver enzymes within the initial 3 months of treatment, enhances lipid profiles, and alleviates hepatic steatosis independently of weight loss. It also positively influences carotid intima-media thickness (CIMT) and reduces 10-year atherosclerotic cardiovascular disease risk in women following 6 months of treatment [121].
Betaine
Betaine, a compound synthesized in the liver via oxidation of dietary choline, is a naturally occurring dietary component [122]. Betaine deficiency commonly arises from malnutrition, notably in chronic alcoholism, and is implicated in both alcoholic steatohepatitis (ASH) and NASH [123]. As a key component of the methyl donor pathway, betaine is present in carbohydrate-rich foods. However, diets deficient in choline or betaine lead to the hypomethylation of homocysteine and phosphatidylethanolamine, which in turn causes hepatic steatosis and inflammation. Evidence from a case‒control study revealed significantly lower betaine levels in patients with NASH than those with NAFLD [124]. Additionally, betaine mitigates hepatic lipid accumulation, gluconeogenesis, and inflammation by restoring LXRα and PPARα expression and reducing ER stress in fructose-fed rats [125]. Recent research indicates that betaine counteracts HFD-induced disruptions in hepatic lipid and iron metabolism by decreasing CpG methylations on the promoters of lipogenic and iron-metabolic genes [126]. Furthermore, maternal betaine supplementation was found to ameliorate MHFD-induced NAFLD, potentially by modulating gut microbiota and SCFAs in offspring mice [127].
Non-pharmacological Treatment of NAFLD in Western Medicine
At present, there is no approved pharmacological treatment for NAFLD. Consequently, non-pharmacological interventions, including dietary modifications, exercise and bariatric surgical procedures, have emerged as crucial components in the treatment of NAFLD.
Mediterranean diet
The Mediterranean diet (MD) is a traditional dietary pattern prevalent in European countries, characterized by high consumption of whole grains, fruits, legumes, vegetables, and seafood, moderate dairy intake, and limited consumption of meat and poultry [128]. A 6-week cross-over study revealed that adherence to the MD significantly ameliorates hepatic steatosis and diminishes insulin resistance, correlating with weight loss [128]. Moreover, adherence to the MD not only positively impacts clinical parameters such as weight, waist circumference, hepatic fat accumulation, insulin resistance, alanine transaminase levels, gamma-glutamyl transferase, total cholesterol and triglycerides, but also beneficially influences inflammatory biomarkers, including adhesion molecules and cytokines [129]. Furthermore, the new suggested strategy of the use of a green-Mediterranean diet enriched with green plant-based proteins/polyphenols such as mankai, green tea, and walnuts and reduced in red/processed meat, has been shown to decrease NAFLD incidence by half [130].
Fitness exercise
Aerobic exercise has been demonstrated to facilitate fat breakdown in various tissues, enhance oxidative enzyme activities, activate γ receptors, and modify adipose factor levels, thereby contributing to the improvement of NAFLD [131]. A study examining the impact of different aerobic exercise intensities on 220 Chinese NAFLD patients over 6 and 12 months revealed that the experimental group exhibited enhanced liver adaptability to exercise and a decrease in hepatic fat content [132]. Additionally, the combination of intermittent fasting and exercise has proven more effective at reducing hepatic steatosis in NAFLD patients than either fasting or exercise alone [133]. Consequently, it is advisable for NAFLD patients to engage in moderate exercise when they have good nutritional status.
Surgical
Metabolic surgery currently represents a reliable method for treating NAFLD, and procedures such as gastric banding are utilized to diminish gastric capacity and limit food intake [134]. There is substantial evidence suggesting that metabolic surgery leads to optimal results in terms of weight reduction, improvement in dyslipidemia, decreased mortality from obesity-related diseases, and enhanced glucose and lipid metabolism, as well as hepatic fibrosis mitigation. Notably, it also significantly reduces the risk of liver cirrhosis by approximately 70% [135]. Furthermore, for obese patients with NAFLD, bariatric surgery is a viable alternative characterized by a relatively low rate of perioperative complications. In patients with severe obesity accompanied by complex conditions, this approach should be considered a therapeutic option [136].
Pharmacological treatment of NAFLD in TCM
The TCM treatment principles of NAFLD advocate phased approaches: initial treatment focuses on soothing the liver and qi, as well as fortifying the spleen and stomach, while intermediate and advanced stages emphasize enhancing the spleen and qi, resolving blood stasis, dissipating nodules, clearing heat, and detoxifying. TCM formulas are distinguished by their individualized treatment strategies, which exhibit multi-component and multi-pathway pharmacological effects in the treatment of NAFLD [137]. Given the varied syndrome types in NAFLD patients, specific prescription treatments are essential, which should be tailored to the primary symptoms while also considering secondary manifestations. Here, we introduce the classic formulas and summarize the progress of research on formulas for the treatment of different types of NAFLD (Table 1).
Removing dampness and turbidity
The primary formula for treating spleen-deficiency and phlegm-turbid stagnation is Modified Weiling Decoction from Dan Xi Xin Fa. It primarily comprises ingredients such as Atractylodes macrocephala, Tangerine peel, Magnolia officinalis, Glycyrrhiza glabra, Polygonum multiflorum, Poria cocos, Bupleurum falcatum and Cinnamon. Ingredient modifications of these formulas based on clinically specific symptoms: Gynostemma and Hawthorn are added for those who were obese and had apparent surrounding dampness. Modified Weiling Decoction has been found to effectively reduce the levels of TG, TC, ALT and AST in the serum of patients with NAFLD [138].
The Fu Fang Zhen Zhu Formula (FTZ) improves hepatic steatosis and fibrosis in NAFLD with type 2 diabetes by activating the AMPK signaling pathway [139]. In addition, FTZ ameliorates NASH by inhibiting gut inflammation, improving intestinal barrier function, and modulating the intestinal microbiota composition [140].
Liver-soothing and spleen-invigorating
The primary formula for treating stagnation of liver-qi is Xiao Yao Powder from the Tai Ping Hui Min He Ji Ju Fang. It primarily comprises ingredients such as Angelica sinensis, Paeonia alba, Bupleurum, Poria, Atractylodes macrocephala, Glycyrrhiza, Gentian and Peppermint. Ingredient modifications of these formulas were performed based on clinically specific symptoms: Citrus Medica and ginger peel are added for obvious abdominal distension; Mongolian milkvetch and Codonopsis pilosula are added for short-term fatigue. A meta-analysis based on 12 studies with 1012 participants suggested the potential therapeutic effect of Xiao Yao Powder for NAFLD [141].
Linggui Zhugan Decoction alleviates hepatic steatosis through cytokine signaling 2 modification by N6-methyladenosine in mice and improves insulin resistance in overweight/obese patients with NAFLD through regulating the DNA N6-methyladenine modification of phosphatase 1 regulatory subunit 3A and autophagy related 3 [142, 143]. Moreover, Linggui Zhugan Decoction ameliorates HFD-induced hepatic lipid deposition in mice by inhibiting STING-mediated inflammation in macrophages [144]. Danshao Shugan Granules had significant effects on total cholesterol, triglyceride, aspartate transaminase and γ-glutamyl transpeptidase in NAFLD patients [145]. Chaihu Shugan Powder inhibits the expression of 15 miRNAs, such as miR-34a-5p, miR-146a-5p, miR-20b-5p and miR-142-3p, and fatty acid biosynthesis related enzymes, thus reducing fatty acid biosynthesis in NAFLD [146]. Qinlian Hongqu Decoction inhibits the IRE1-α/IKKB-β/NF-κB signaling pathway in the liver, thereby attenuating inflammation and insulin resistance in NAFLD rats [147].
Clearing heat and removing dampness
The primary formula for treating accumulated damp-heat is Yinchen Wuling Powder from Jin Kui Yao Lue. It primarily comprises ingredients such as Capillaris Herba, Alismatis Rhizoma, Atractylodes Macrocephalae Rhizoma stir-fried with wheat bran, Poria and Cinnamomi Ramulus. Ingredient modifications of these formulas were performed based on clinically specific symptoms: Ginger pinellia and Bamboo shavings are added for Jaundice; Polygonum cuspidatum is added for those trapped by damp-evil. Yinchen Wuling Powder shows hepatoprotective effects by regulating the imbalance of the gut microbiota and increasing the abundance of butyrate-producing bacteria to regulate pro-inflammatory cytokines and immune function [148].
Gegen Qinlian Decoction inhibits the TLR4 signaling pathway to reduce ALT, AST, liver tissue IL-6, and TNF-α levels to reduce liver injury and improve NASH [149]. Huangqin Decoction ameliorates hepatic inflammation in NAFLD rats by blocking the TLR4/NF-κB/NLRP3 pathway [150]. Zexie Baizhu Decoction impedes lipogenesis and promotes fatty acid oxidation to alleviate lipid disorders and protect the liver in NAFLD [151]. Danshen Zexie Decoction suppresses the ROS/NLRP3/IL-1β signaling pathway by activating Nrf2 to reduce lipid accumulation and alleviate oxidative stress, inflammation and pyroptosis in NAFLD rats [152]. Qige Decoction inhibits hepatic steatosis by reducing hepatic insulin resistance and triglyceride biosynthesis via the PPP1R3C/SIK1 signaling axis [153]. Si Miao Formula attenuates NAFLD, which is linked to the modulation of the gut microbiota composition, especially that of Akkermansia muciniphila[154].
Removing blood stasis and dispersing knots
The primary formulas for treating stasis blocking channels are Gexia Zhuyu Decoction, sourced from Yi Lin Correction, and Er Chen Decoction, derived from Tai Ping Hui Min He Ji Prescription. These formulas primarily include ingredients such as Peach kernel, Peony bark, Radix paeoniae rubra, Solanum nigrum, Ligusticum wallichii, Angelica sinensis, Safflower, Coix lacryma, Poria and Ginger. Ingredient modifications of these formulas were performed based on clinically specific symptoms: Curcuma longa and Ginger are added for blood stasis, such as a dark face. Gexia Zhuyu Decoction has been found to modify miRNA-24 and transient receptor potential melastatin 4 (TRPM4) expression, thereby enhancing NAFLD treatment efficacy in mice [155]. Er Chen Decoction alleviates NAFLD in rats by preventing oxidative stress, diminishing inflammatory responses, and ameliorating gut microbiota dysbiosis, as well as modulating metabolites in serum, including taurine and hypotaurine, cysteine and methionine and vitamin B6 [156].
Qushi Huayu Decoction reduces liver injury in NASH by suppressing the MAPK pathway to protect the colonic tight junction and alters serum lipids by regulating the gut microbiota, including Bacteroides, Blautia, Lachnoclostridium and Turicibacter [157, 158]. Tianhuang Formula increases the abundance of Lactobacillus and the content of 5-MIAA in the intestinal contents and liver to reduce the expression of Nrf2 and oxidative stress, thereby improving NAFLD [159]. Sanhuang Tang alleviates insulin resistance by activating the INSR/IRS1/AKT/FoxO1 pathway, which contributes to NAFLD treatment [160].
Tonifying spleen and kidney
The primary formulas for treating spleen and kidney deficiency are Si Jun Zi Decoction, sourced from Tai Ping Hui Min He Ji Ju Fang, and Jin Kui Shenqi Pill, derived from Jin Kui Yao Lue. These formulas primarily comprise ingredients such as Ginseng, Poria, Atractylodes, Licorice, Rehmannia, Cornu Cervi pantotrichum, Yam, and Peony peel. Ingredient modifications of these formulas are performed based on specific clinical symptoms: Boswellia root and Eucommia are added for waist and knee soreness, dizziness and weakness; Radix pseudostellariae and Cinnamon are added for cold extremities; Radix chinensis and Cuttlebone are added for frequent nocturnal urination; Fried lentils and Fried coix seeds are added for diarrhea.
Yiqi BuShen Tiaozhi Formula primarily improves NASH by modulating the expression of various miRNAs, such as mmu-let-7a-5p, mmu-let-7b-5p, mmu-let-7 g-3p, and mmu-miR-106b-3p [161]. Yinchen Linggui Zhugan Decoction effectively inhibits the expression of the TNF signaling pathway and alleviates HFD-induced liver injury and inflammatory response in rats with NAFLD [162]. Sini San reduces lipid droplet accumulation and YAP1 expression in hepatocytes, and the knockout of hepatocellular YAP1 reduces the effect of Sini San on reducing lipid deposition [163]. Spleen-strengthening and liver-draining herbal Formula can improve liver function and glucolipid metabolism in patients with NAFLD by regulating the disturbance of intestinal flora, including Coprococcus, Lachnospiraceae and Ruminococcus genus [164].
Non-pharmacological Treatment of NAFLD in TCM
Acupuncture
Acupuncture, a widely-used adjunctive therapy in TCM, originates from China and boasts a history exceeding 4000 years. It is employed to promote health and treat diverse diseases via techniques such as manual needling, electroacupuncture, moxibustion, and acupressure at specific anatomical sites, termed acupoints [165]. Numerous studies have demonstrated the efficacy of acupuncture in treating NAFLD, notably in impacting liver fat, lipid metabolism, and insulin resistance [166]. Recent research underscores the beneficial role of traditional acupuncture therapy in treating NAFLD, as it provides convenient treatment with minimal adverse reactions and considerable efficacy [167].
Acupuncture can inhibit the progression of NAFLD by suppressing inflammation, mitigating oxidative stress, and enhancing lipid metabolism in liver cells [166]. A study revealed that electroacupuncture at the bilateral acupoints “Spleen Shu,” “Kidney Shu,” and “Diaphragm Shu” effectively decreased the expression of TNF-α in NAFLD rats, thereby ameliorating the inflammatory state of NAFLD and reducing liver damage [168]. Furthermore, acupuncture has been shown to significantly enhance lipid metabolism and the systemic inflammatory response in HFD-induced NAFLD rats, possibly through the regulation of the intestinal microbiota composition [169]. Numerous clinical studies have indicated that acupuncture is effective at lowering total cholesterol (TC), TG, and low-density lipoprotein cholesterol (LDL-C) levels and increasing high-density lipoprotein cholesterol (HDL-C) levels, thus improving lipid metabolism in patients with dyslipidemia [170].
Acupoint embedding
Acupoint embedding is an improved technique based on traditional acupuncture intervention. The core principle of this technique entails the placement of absorbable sutures, such as sheep gut sutures, within acupoints to extend the stimulation duration and augment efficacy, thus diminishing the necessity for frequent interventions. Additionally, acupoint embedding offers inherent practical convenience [171]. Extensive clinical research has been undertaken to establish the efficacy and safety of acupoint embedding therapy in the treatment of NAFLD [172]. Studies have shown its superior effectiveness in treating NAFLD with abnormal liver enzyme levels compared with traditional pharmacotherapy [173]. Significantly, a meta-analysis confirmed the lipid-lowering impact of acupoint embedding in NAFLD [174].
Martial arts
Traditional Chinese martial arts, such as Tai Chi, have demonstrated therapeutic efficacy as a form of physical exercise in the treatment of metabolic diseases [175]. In a recent study, Tai Chi was observed to significantly decrease waist circumference and elevate high-density lipoprotein (HDL) cholesterol levels in obese adults [176]. Furthermore, Tai Chi has been associated with reductions in waist circumference, body mass index (BMI), blood glucose levels, and insulin resistance in T2MD patients [177]. These studies suggest the potential of Tai Chi in the treatment of NAFLD. Interestingly, Tai Chi has been also found to be more effective than conventional exercise in reducing anxiety and depression, as well as in enhancing overall mental health [178]. This distinction highlights the unique aspects of Tai Chi, which combines physical movement with mindfulness and deep breathing techniques. The practice's holistic approach not only addresses physical well-being but also significantly impacts psychological health, offering a more comprehensive benefit compared to traditional forms of exercise that primarily focus on physical fitness.
Integration of TCM and Western medicine for the treatment of NAFLD
The current research on the integration of TCM and Western medicine for the treatment of NAFLD is still in early stages. However, there is already some evidence indicating that this integrative approach may offer superior treatment outcomes for NAFLD. A study indicates that combining Xiao Yao San with simvastatin enhances treatment efficacy compared to simvastatin alone, significantly improving liver function and lipid profiles in NAFLD patients [179]. Similarly, a combination of Compound Danshen Tablets and metformin for treating diabetes with NAFLD shows greater improvement in liver function, blood sugar, and lipid levels than using metformin alone [180]. Additionally, electroacupuncture combined with lifestyle modifications has been found to be more effective than lifestyle modifications alone in treating obesity-related NAFLD, improving liver fat status, glycemic control, and insulin resistance [181]. While the integration of TCM and Western medicine shows promise in treating NAFLD, more research is needed to fully understand its efficacy. Future research should focus on large-scale clinical trials to validate these findings and guide clinical practice in the holistic management of NAFLD.
Conclusions
In the foreseeable future, Chinese medicine is poised to become a significant source of potential therapeutics for the treatment of NAFLD, although realizing this vision entails overcoming considerable challenges. The first challenge involves translating traditional Chinese medicine terminology into a universally recognized scientific language and establishing a standard, repeatable method for identifying the contents of various Chinese herbs, facilitating improved recognition and communication between Chinese and Western medicine. The second challenge entails conducting rigorous clinical research on TCM for the treatment of NAFLD and elucidating the benefits of evidence-based approaches in this context. The third challenge involves conducting comprehensive research on the mechanisms of action of Chinese formula medicines in NAFLD and verifying whether their observed effects can be optimized through a scientifically robust, practical, and accredited validation platform. However, finding the precise target for improving NAFLD through molecular research is challenging due to the diverse compositions of Chinese herbal medicines. The current strategy is dissecting the molecular mechanisms of the prescription by analyzing individual herbs or active ingredients. However, this approach deviates from the principles of syndrome differentiation in TCM.
In the management of NAFLD, both TCM and Western medicine offer distinct approaches with respective advantages and limitations. Western medicine, grounded in clinical trials and scientific research, provides evidence-based treatments such as antidiabetic drugs (e.g., Metformin) and antioxidants (e.g., Vitamin E), which can improve liver function and reduce hepatic fat. However, these treatments may come with side effects like gastrointestinal symptoms and weight gain, and are often limited in reversing the progression of advanced fibrosis stage. Conversely, TCM focuses on holistic balance and regulation, utilizing herbal medicine and acupuncture to treat NAFLD. While generally associated with fewer side effects and suitable for long-term use, TCM's efficacy and mechanisms require further validation through rigorous clinical studies, and treatments may vary significantly among individuals. In summary, Western medicine is offering rapid symptom amelioration and TCM is enhancing overall bodily function, possibly reducing the side effects of conventional drugs and potentially improving overall therapeutic outcomes. Investigations into the integration of TCM and Western medicine for the treatment of NAFLD are nascent and require further investigation and dedication. This emerging field could potentially shape the future direction of NAFLD prevention and management. However, the effectiveness and optimization of such integrated therapeutic strategies necessitate rigorous scientific inquiry and clinical trials.
Availability of data and materials
Not applicable.
Abbreviations
- AMPK:
-
AMP-activated protein kinase
- AKT:
-
Protein Kinase B
- ATG3:
-
Autophagy-related proteins 3
- BA:
-
Bile acid
- CRTC2:
-
CREB-regulated transcription coactivator2
- FoxO1:
-
Forkhead box protein O1
- GCKR:
-
Glucokinase regulatory protein
- HFD:
-
High-fat diet
- INSR:
-
Insulin receptor
- IRE1-α:
-
Inositol requiring enzyme 1-α
- IRS1:
-
Insulin receptor substrate 1
- IKKB:
-
IkBa kinase B
- L-1β:
-
Interleukin-1β
- LPS:
-
Lipopolysaccharides
- MAPK:
-
Mitogen-activated protein kinases
- MBOAT7:
-
Membrane-bound O-acyltransferase domain-containing 7
- NAFL:
-
Non-alcoholic fatty liver
- NASH:
-
Non-alcoholic steatohepatitis
- NF‑κB:
-
Nuclear factor-κB
- NLRP3:
-
Nod-like receptor family pyrin domain-containing 3
- NRF2:
-
NF-E2-related factor 2
- PI3K:
-
Phosphoinositide 3-kinase
- PNPLA3:
-
Patatin-like phospholipase domain-containing 3
- PPP1R3A:
-
Protein phosphatase 1 regulatory subunit 3A
- PPP1R3C:
-
Protein phosphatase 1 regulatory subunit 3C
- ROS:
-
Oxygen species
- SCFA:
-
Short-chain fatty acid
- SIRT1:
-
Sirtuin 1
- SIK1:
-
Salt-inducible kinase 1
- SOCS2:
-
Cytokine signaling 2
- SREBP1:
-
Sterol regulatory element binding protein 1
- STING:
-
Stimulator of interferon genes
- STZ:
-
Streptozotocin
- T2DM:
-
Type 2 diabetes mellitus
- TM6SF2:
-
Transmembrane 6 superfamily member 2
- TNF:
-
Tumor necrosis factor
- TLR4:
-
Toll-like receptor 4
- TRPM4:
-
Transient receptor potential melastatin 4
- YAP1:
-
Yes-associated protein 1
References
Riazi K, Azhari H, Charette JH, Underwood FE, King JA, Afshar EE, et al. The prevalence and incidence of NAFLD worldwide: a systematic review and meta-analysis. Lancet Gastroenterol Hepatol. 2022;7(9):851–61.
Paternostro R, Trauner M. Current treatment of non-alcoholic fatty liver disease. J Intern Med. 2022;292(2):190–204.
Buzzetti E, Pinzani M, Tsochatzis EA. The multiple-hit pathogenesis of non-alcoholic fatty liver disease (NAFLD). Metabolism. 2016;65(8):1038–48.
Stefan N, Cusi K. A global view of the interplay between non-alcoholic fatty liver disease and diabetes. Lancet Diabetes Endocrinol. 2022;10(4):284–96.
Xu X, Poulsen KL, Wu L, Liu S, Miyata T, Song Q, et al. Targeted therapeutics and novel signaling pathways in non-alcohol-associated fatty liver/steatohepatitis (NAFL/NASH). Signal Transduct Target Ther. 2022;7(1):287.
Wang J, Wong YK, Liao F. What has traditional Chinese medicine delivered for modern medicine? Expert Rev Mol Med. 2018;20: e4.
Wang M, Liu W, Ge J, Liu S. The immunomodulatory mechanisms for acupuncture practice. Front Immunol. 2023;14:1147718.
Dai X, Feng J, Chen Y, Huang S, Shi X, Liu X, et al. Traditional Chinese Medicine in nonalcoholic fatty liver disease: molecular insights and therapeutic perspectives. Chin Med. 2021;16(1):68.
Dong H, Lu FE, Zhao L. Chinese herbal medicine in the treatment of nonalcoholic fatty liver disease. Chin J Integr Med. 2012;18(2):152–60.
Gong P, Long H, Guo Y, Wang Z, Yao W, Wang J, et al. Chinese herbal medicines: The modulator of nonalcoholic fatty liver disease targeting oxidative stress. J Ethnopharmacol. 2024;318(Pt B): 116927.
Zhang S, Wang W, Pi X, He Z, Liu H. Advances in the application of traditional chinese medicine using artificial intelligence: a review. Am J Chin Med. 2023;51(5):1067–83.
Tilg H, Adolph TE, Moschen AR. Multiple parallel hits hypothesis in nonalcoholic fatty liver disease: revisited after a decade. Hepatology. 2021;73(2):833–42.
Peng C, Stewart AG, Woodman OL, Ritchie RH, Qin CX. Non-alcoholic steatohepatitis: a review of its mechanism models and medical treatments. Front Pharmacol. 2020;11:603926. https://doi.org/10.3389/fphar.2020.603926.
Eslam M, Valenti L, Romeo S. Genetics and epigenetics of NAFLD and NASH: clinical impact. J Hepatol. 2018;68(2):268–79.
Caussy C, Soni M, Cui J, Bettencourt R, Schork N, Chen CH, et al. Nonalcoholic fatty liver disease with cirrhosis increases familial risk for advanced fibrosis. J Clin Invest. 2017;127(7):2697–704.
Rich NE, Oji S, Mufti AR, Browning JD, Parikh ND, Odewole M, et al. Racial and ethnic disparities in nonalcoholic fatty liver disease prevalence, severity, and outcomes in the United States: a systematic review and meta-analysis. Clin Gastroenterol Hepatol. 2018;16(2):198-210 e2.
Romeo S, Kozlitina J, Xing C, Pertsemlidis A, Cox D, Pennacchio LA, et al. Genetic variation in PNPLA3 confers susceptibility to nonalcoholic fatty liver disease. Nat Genet. 2008;40(12):1461–5.
Unalp-Arida A, Ruhl CE. Patatin-like phospholipase domain-containing protein 3 I148M and liver fat and fibrosis scores predict liver disease mortality in the U.s. Population. Hepatology. 2020;71(3):820–34.
Mahdessian H, Taxiarchis A, Popov S, Silveira A, Franco-Cereceda A, Hamsten A, et al. TM6SF2 is a regulator of liver fat metabolism influencing triglyceride secretion and hepatic lipid droplet content. Proc Natl Acad Sci U S A. 2014;111(24):8913–8.
Dongiovanni P, Petta S, Maglio C, Fracanzani AL, Pipitone R, Mozzi E, et al. Transmembrane 6 superfamily member 2 gene variant disentangles nonalcoholic steatohepatitis from cardiovascular disease. Hepatology. 2015;61(2):506–14.
Thangapandi VR, Knittelfelder O, Brosch M, Patsenker E, Vvedenskaya O, Buch S, et al. Loss of hepatic Mboat7 leads to liver fibrosis. Gut. 2021;70(5):940–50.
Luukkonen PK, Zhou Y, Hyotylainen T, Leivonen M, Arola J, Orho-Melander M, et al. The MBOAT7 variant rs641738 alters hepatic phosphatidylinositols and increases severity of non-alcoholic fatty liver disease in humans. J Hepatol. 2016;65(6):1263–5.
Beer NL, Tribble ND, McCulloch LJ, Roos C, Johnson PR, Orho-Melander M, et al. The P446L variant in GCKR associated with fasting plasma glucose and triglyceride levels exerts its effect through increased glucokinase activity in liver. Hum Mol Genet. 2009;18(21):4081–8.
Jonas W, Schurmann A. Genetic and epigenetic factors determining NAFLD risk. Mol Metab. 2021;50: 101111.
Pirola CJ, Garaycoechea M, Flichman D, Arrese M, San Martino J, Gazzi C, et al. Splice variant rs72613567 prevents worst histologic outcomes in patients with nonalcoholic fatty liver disease. J Lipid Res. 2019;60(1):176–85.
Seidelin AS, Nordestgaard BG, Tybjaerg-Hansen A, Stender S. Genetic variation at PPP1R3B increases hepatic CT attenuation and interacts with prandial status on plasma glucose. J Clin Endocrinol Metab. 2020;105(6):dgaa51. https://doi.org/10.1210/clinem/dgaa151.
Lin YC, Chang PF, Lin HF, Liu K, Chang MH, Ni YH. Variants in the autophagy-related gene IRGM confer susceptibility to non-alcoholic fatty liver disease by modulating lipophagy. J Hepatol. 2016;65(6):1209–16.
Valenti L, Motta BM, Alisi A, Sartorelli R, Buonaiuto G, Dongiovanni P, et al. LPIN1 rs13412852 polymorphism in pediatric nonalcoholic fatty liver disease. J Pediatr Gastroenterol Nutr. 2012;54(5):588–93.
Postic C, Girard J. Contribution of de novo fatty acid synthesis to hepatic steatosis and insulin resistance: lessons from genetically engineered mice. J Clin Invest. 2008;118(3):829–38.
Dowman JK, Tomlinson JW, Newsome PN. Pathogenesis of non-alcoholic fatty liver disease. QJM. 2010;103(2):71–83.
Taniguchi CM, Emanuelli B, Kahn CR. Critical nodes in signalling pathways: insights into insulin action. Nat Rev Mol Cell Biol. 2006;7(2):85–96.
Manco M. Insulin resistance and NAFLD: a dangerous liaison beyond the genetics. Children. 2017;4(8):74. https://doi.org/10.3390/children4080074.
Yamaguchi K, Yang L, McCall S, Huang J, Yu XX, Pandey SK, et al. Inhibiting triglyceride synthesis improves hepatic steatosis but exacerbates liver damage and fibrosis in obese mice with nonalcoholic steatohepatitis. Hepatology. 2007;45(6):1366–74.
Monetti M, Levin MC, Watt MJ, Sajan MP, Marmor S, Hubbard BK, et al. Dissociation of hepatic steatosis and insulin resistance in mice overexpressing DGAT in the liver. Cell Metab. 2007;6(1):69–78.
Summers SA. Ceramides in insulin resistance and lipotoxicity. Prog Lipid Res. 2006;45(1):42–72.
Hirsova P, Ibrahim SH, Krishnan A, Verma VK, Bronk SF, Werneburg NW, et al. Lipid-induced signaling causes release of inflammatory extracellular vesicles from hepatocytes. Gastroenterology. 2016;150(4):956–67.
Ioannou GN. The role of cholesterol in the pathogenesis of NASH. Trends Endocrinol Metab. 2016;27(2):84–95.
Guo L, Zhang P, Chen Z, Xia H, Li S, Zhang Y, et al. Hepatic neuregulin 4 signaling defines an endocrine checkpoint for steatosis-to-NASH progression. J Clin Invest. 2017;127(12):4449–61.
Lebeaupin C, Vallee D, Hazari Y, Hetz C, Chevet E, Bailly-Maitre B. Endoplasmic reticulum stress signalling and the pathogenesis of non-alcoholic fatty liver disease. J Hepatol. 2018;69(4):927–47.
Wang M, Kaufman RJ. Protein misfolding in the endoplasmic reticulum as a conduit to human disease. Nature. 2016;529(7586):326–35.
Lisbona F, Rojas-Rivera D, Thielen P, Zamorano S, Todd D, Martinon F, et al. BAX inhibitor-1 is a negative regulator of the ER stress sensor IRE1alpha. Mol Cell. 2009;33(6):679–91.
Wang JM, Qiu Y, Yang Z, Kim H, Qian Q, Sun Q, et al. IRE1alpha prevents hepatic steatosis by processing and promoting the degradation of select microRNAs. Sci Signal. 2018;11(530):eaao4617. https://doi.org/10.1126/scisignal.aao4617.
Maiers JL, Malhi H. Endoplasmic reticulum stress in metabolic liver diseases and hepatic fibrosis. Semin Liver Dis. 2019;39(2):235–48.
Lebeaupin C, Proics E, de Bieville CH, Rousseau D, Bonnafous S, Patouraux S, et al. ER stress induces NLRP3 inflammasome activation and hepatocyte death. Cell Death Dis. 2015;6(9): e1879.
Malhi H, Kropp EM, Clavo VF, Kobrossi CR, Han J, Mauer AS, et al. C/EBP homologous protein-induced macrophage apoptosis protects mice from steatohepatitis. J Biol Chem. 2013;288(26):18624–42.
Li J, Li X, Liu D, Zhang S, Tan N, Yokota H, et al. Phosphorylation of eIF2alpha signaling pathway attenuates obesity-induced non-alcoholic fatty liver disease in an ER stress and autophagy-dependent manner. Cell Death Dis. 2020;11(12):1069.
Lee S, Kim S, Hwang S, Cherrington NJ, Ryu DY. Dysregulated expression of proteins associated with ER stress, autophagy and apoptosis in tissues from nonalcoholic fatty liver disease. Oncotarget. 2017;8(38):63370–81.
Kammoun HL, Chabanon H, Hainault I, Luquet S, Magnan C, Koike T, et al. GRP78 expression inhibits insulin and ER stress-induced SREBP-1c activation and reduces hepatic steatosis in mice. J Clin Invest. 2009;119(5):1201–15.
Kawamura S, Matsushita Y, Kurosaki S, Tange M, Fujiwara N, Hayata Y, et al. Inhibiting SCAP/SREBP exacerbates liver injury and carcinogenesis in murine nonalcoholic steatohepatitis. J Clin Invest. 2022;132(11):e151895. https://doi.org/10.1172/JCI151895.
Gao J, Zhang Y, Yu C, Tan F, Wang L. Spontaneous nonalcoholic fatty liver disease and ER stress in Sidt2 deficiency mice. Biochem Biophys Res Commun. 2016;476(4):326–32.
Liu C, Zhou B, Meng M, Zhao W, Wang D, Yuan Y, et al. FOXA3 induction under endoplasmic reticulum stress contributes to non-alcoholic fatty liver disease. J Hepatol. 2021;75(1):150–62.
Mansouri A, Gattolliat CH, Asselah T. Mitochondrial dysfunction and signaling in chronic liver diseases. Gastroenterology. 2018;155(3):629–47.
Myint M, Oppedisano F, De Giorgi V, Kim BM, Marincola FM, Alter HJ, et al. Inflammatory signaling in NASH driven by hepatocyte mitochondrial dysfunctions. J Transl Med. 2023;21(1):757. https://doi.org/10.1186/s12967-023-04627-0.
Shum M, Ngo J, Shirihai OS, Liesa M. Mitochondrial oxidative function in NAFLD: friend or foe? Mol Metab. 2021;50: 101134.
Longo M, Meroni M, Paolini E, Macchi C, Dongiovanni P. Mitochondrial dynamics and nonalcoholic fatty liver disease (NAFLD): new perspectives for a fairy-tale ending? Metabolism. 2021;117: 154708.
Talari NK, Mattam U, Meher NK, Paripati AK, Mahadev K, Krishnamoorthy T, et al. Lipid-droplet associated mitochondria promote fatty-acid oxidation through a distinct bioenergetic pattern in male Wistar rats. Nat Commun. 2023;14(1):766.
Ramanathan R, Ali AH, Ibdah JA. Mitochondrial dysfunction plays central role in nonalcoholic fatty liver disease. Int J Mol Sci. 2022;23(13):7280. https://doi.org/10.3390/ijms23137280.
Schuster S, Cabrera D, Arrese M, Feldstein AE. Triggering and resolution of inflammation in NASH. Nat Rev Gastroenterol Hepatol. 2018;15(6):349–64.
Pafili K, Roden M. Nonalcoholic fatty liver disease (NAFLD) from pathogenesis to treatment concepts in humans. Mol Metab. 2021;50: 101122.
Rohm TV, Meier DT, Olefsky JM, Donath MY. Inflammation in obesity, diabetes, and related disorders. Immunity. 2022;55(1):31–55.
Li W, Cao T, Luo C, Cai J, Zhou X, Xiao X, et al. Crosstalk between ER stress, NLRP3 inflammasome, and inflammation. Appl Microbiol Biotechnol. 2020;104(14):6129–40.
Chen J, Vitetta L. Gut microbiota metabolites in NAFLD pathogenesis and therapeutic implications. Int J Mol Sci. 2020;21(15):5214. https://doi.org/10.3390/ijms21155214.
Macpherson AJ, Heikenwalder M, Ganal-Vonarburg SC. The liver at the nexus of host-microbial interactions. Cell Host Microbe. 2016;20(5):561–71.
Milosevic I, Vujovic A, Barac A, Djelic M, Korac M, Radovanovic Spurnic A, et al. Gut-liver axis, gut microbiota, and its modulation in the management of liver diseases: a review of the literature. Int J Mol Sci. 2019;20(2):395. https://doi.org/10.3390/ijms20020395.
Cui Y, Wang Q, Chang R, Zhou X, Xu C. Intestinal barrier function-non-alcoholic fatty liver disease interactions and possible role of gut microbiota. J Agric Food Chem. 2019;67(10):2754–62.
Yiu JH, Dorweiler B, Woo CW. Interaction between gut microbiota and toll-like receptor: from immunity to metabolism. J Mol Med (Berl). 2017;95(1):13–20.
Cai J, Rimal B, Jiang C, Chiang JYL, Patterson AD. Bile acid metabolism and signaling, the microbiota, and metabolic disease. Pharmacol Ther. 2022;237: 108238.
Chiang JYL, Ferrell JM. Bile acid receptors FXR and TGR5 signaling in fatty liver diseases and therapy. Am J Physiol Gastrointest Liver Physiol. 2020;318(3):G554–73.
Zhou SP, You HM, Qiu ST, Yu DW, Bai Y, He JC, et al. A new perspective on NAFLD: focusing on the crosstalk between peroxisome proliferator-activated receptor alpha (PPARα) and farnesoid X receptor (FXR). Biomed Pharmacother. 2022;154:113577. https://doi.org/10.1016/j.biopha.2022.113577.
Albaugh VL, Banan B, Antoun J, Xiong Y, Guo Y, Ping J, et al. Role of bile acids and GLP-1 in mediating the metabolic improvements of bariatric surgery. Gastroenterology. 2019;156(4):1041-51 e4.
Chavez-Talavera O, Tailleux A, Lefebvre P, Staels B. Bile acid control of metabolism and inflammation in obesity, Type 2 diabetes, dyslipidemia, and nonalcoholic fatty liver disease. Gastroenterology. 2017;152(7):1679-94 e3.
van der Hee B, Wells JM. Microbial regulation of host physiology by short-chain fatty acids. Trends Microbiol. 2021;29(8):700–12.
Morrison DJ, Preston T. Formation of short chain fatty acids by the gut microbiota and their impact on human metabolism. Gut Microbes. 2016;7(3):189–200.
Liu WX, Luo XL, Tang J, Mo QF, Zhong H, Zhang H, et al. A bridge for short-chain fatty acids to affect inflammatory bowel disease, type 1 diabetes, and non-alcoholic fatty liver disease positively: by changing gut barrier. Eur J Nutr. 2021;60(5):2317–30.
Christiansen CB, Gabe MBN, Svendsen B, Dragsted LO, Rosenkilde MM, Holst JJ. The impact of short-chain fatty acids on GLP-1 and PYY secretion from the isolated perfused rat colon. Am J Physiol Gastrointest Liver Physiol. 2018;315(1):G53–65.
Shabalala SC, Dludla PV, Mabasa L, Kappo AP, Basson AK, Pheiffer C, et al. The effect of adiponectin in the pathogenesis of non-alcoholic fatty liver disease (NAFLD) and the potential role of polyphenols in the modulation of adiponectin signaling. Biomed Pharmacother. 2020;131:110785. https://doi.org/10.1016/j.biopha.2020.110785.
Abu-Tair L, Doron S, Mahamid M, Amer J, Safadi R. Leptin modulates lymphocytes’ adherence to hepatic stellate cells is associated with oxidative status alterations. Mitochondrion. 2013;13(5):473–80.
Procaccini C, Galgani M, De Rosa V, Carbone F, La Rocca C, Ranucci G, et al. Leptin: the prototypic adipocytokine and its role in NAFLD. Curr Pharm Des. 2010;16(17):1902–12.
Metz M, Beghini M, Wolf P, Pfleger L, Hackl M, Bastian M, et al. Leptin increases hepatic triglyceride export via a vagal mechanism in humans. Cell Metab. 2022;34(11):1719-31 e5.
Kim KE, Lee J, Shin HJ, Jeong EA, Jang HM, Ahn YJ, et al. Lipocalin-2 activates hepatic stellate cells and promotes nonalcoholic steatohepatitis in high-fat diet-fed Ob/Ob mice. Hepatology. 2023;77(3):888–901.
Wang Y, Zhou M, Lam KS, Xu A. Protective roles of adiponectin in obesity-related fatty liver diseases: mechanisms and therapeutic implications. Arq Bras Endocrinol Metabol. 2009;53(2):201–12.
Wang GX, Zhao XY, Meng ZX, Kern M, Dietrich A, Chen Z, et al. The brown fat-enriched secreted factor Nrg4 preserves metabolic homeostasis through attenuation of hepatic lipogenesis. Nat Med. 2014;20(12):1436–43.
Zhang P, Chen ZM, Kuang HY, Liu TY, Zhu JQ, Zhou LK, et al. Neuregulin 4 suppresses NASH-HCC development by restraining tumor-prone liver microenvironment. Cell Metab. 2022;34(9):1359-1376.e7.
Merenstein DJ, Foster J, D’Amico F. A randomized clinical trial measuring the influence of kefir on antibiotic-associated diarrhea: the measuring the influence of Kefir (MILK) Study. Arch Pediatr Adolesc Med. 2009;163(8):750–4.
Li P, Zhang HJ, Zheng LT. The theory of homogeny of liver and kidney in the treatment of kidney and liver fibrosis. Chin J Integr Med. 2012;18(4):250–2.
Ji LS, Li Q, He Y, Zhang X, Zhou ZH, Gao YT, et al. Therapeutic potential of traditional Chinese medicine for the treatment of NAFLD: a promising drug Potentilla discolor Bunge. Acta Pharmaceutica Sinica B. 2022;12(9):3529–47.
Zhou Z, Zhang J, You L, Wang T, Wang K, Wang L, et al. Application of herbs and active ingredients ameliorate non-alcoholic fatty liver disease under the guidance of traditional Chinese medicine. Front Endocrinol. 2022;13:1000727.
Tokushige K, Ikejima K, Ono M, Eguchi Y, Kamada Y, Itoh Y, et al. Evidence-based clinical practice guidelines for nonalcoholic fatty liver disease/nonalcoholic steatohepatitis 2020. Hepatol Res. 2021;51(10):1013–25.
Kang SH, Lee HW, Yoo JJ, Cho Y, Kim SU, Lee TH, et al. KASL clinical practice guidelines: management of nonalcoholic fatty liver disease. Clin Mol Hepatol. 2021;27(3):363–401.
Stumvoll M, Nurjhan N, Perriello G, Dailey G, Gerich JE. Metabolic effects of metformin in non-insulin-dependent diabetes mellitus. N Engl J Med. 1995;333(9):550–4.
Lin HZ, Yang SQ, Chuckaree C, Kuhajda F, Ronnet G, Diehl AM. Metformin reverses fatty liver disease in obese, leptin-deficient mice. Nat Med. 2000;6(9):998–1003.
Park J, Rah SY, An HS, Lee JY, Roh GS, Ryter SW, et al. Metformin-induced TTP mediates communication between Kupffer cells and hepatocytes to alleviate hepatic steatosis by regulating lipophagy and necroptosis. Metabolism. 2023;141: 155516.
Song YM, Lee YH, Kim JW, Ham DS, Kang ES, Cha BS, et al. Metformin alleviates hepatosteatosis by restoring SIRT1-mediated autophagy induction via an AMP-activated protein kinase-independent pathway. Autophagy. 2015;11(1):46–59.
Wabitsch S, McCallen JD, Kamenyeva O, Ruf B, McVey JC, Kabat J, et al. Metformin treatment rescues CD8(+) T-cell response to immune checkpoint inhibitor therapy in mice with NAFLD. J Hepatol. 2022;77(3):748–60.
Yan JH, Yao B, Kuang HY, Yang XB, Huang Q, Hong TP, et al. Liraglutide, sitagliptin, and insulin glargine added to metformin: the effect on body weight and intrahepatic lipid in patients With Type 2 diabetes mellitus and nonalcoholic fatty liver disease. Hepatology. 2019;69(6):2414–26.
Liu BL, Xu JY, Lu LY, Gao LL, Zhu SJ, Sui Y, et al. Metformin induces pyroptosis in leptin receptor-defective hepatocytes via overactivation of the AMPK axis. Cell Death Dis. 2023;14(2):82. https://doi.org/10.1038/s41419-023-05623-4.
Yki-Jarvinen H. Thiazolidinediones. N Engl J Med. 2004;351(11):1106–18.
Ahmed MH, Byrne CD. Current treatment of non-alcoholic fatty liver disease. Diabetes Obes Metab. 2009;11(3):188–95.
Duvnjak M, Tomasic V, Gomercic M, Duvnjak LS, Barsic N, Lerotic I. Therapy of nonalcoholic fatty liver disease: current status. J Physiol Pharmacol. 2009;60:57–66.
Lavynenko O, Abdul-Ghani M, Alatrach M, Puckett C, Adams J, Abdelgani S, et al. Combination therapy with pioglitazone/exenatide/metformin reduces the prevalence of hepatic fibrosis and steatosis: The efficacy and durability of initial combination therapy for type 2 diabetes (EDICT). Diabetes Obes Metab. 2022;24(5):899–907.
Jianfang F, Wanxia X, Xiling G, Jing X, Wenjuan Y, Jianrong L, et al. Effect and Safety of Pioglitazone-Metformin Tablets in the Treatment of Newly Diagnosed Type 2 Diabetes Patients with Nonalcoholic Fatty Liver Disease in Shaanxi Province: a randomized, double-blinded, Double-Simulated Multicenter Study. J Diabetes Res. 2023;2023:2044090.
Armstrong MJ, Hull D, Guo K, Barton D, Hazlehurst JM, Gathercole LL, et al. Glucagon-like peptide 1 decreases lipotoxicity in non-alcoholic steatohepatitis. J Hepatol. 2016;64(2):399–408.
Armstrong MJ, Gaunt P, Aithal GP, Barton D, Hull D, Parker R, et al. Liraglutide safety and efficacy in patients with non-alcoholic steatohepatitis (LEAN): a multicentre, double-blind, randomised, placebo-controlled phase 2 study. Lancet. 2016;387(10019):679–90.
Desjardins EM, Wu J, Lavoie DCT, Ahmadi E, Townsend LK, Morrow MR, et al. Combination of an ACLY inhibitor with a GLP-1R agonist exerts additive benefits on nonalcoholic steatohepatitis and hepatic fibrosis in mice. Cell Rep Med. 2023;4(9): 101193.
Janicko M, Drazilova S, Pella D, Fedacko J, Jarcuska P. Pleiotropic effects of statins in the diseases of the liver. World J Gastroentero. 2016;22(27):6201–13.
Boutari C, Pappas PD, Anastasilakis D, Mantzoros CS. Statins’ efficacy in non-alcoholic fatty liver disease: s systematic review and meta-analysis. Clin Nutr. 2022;41(10):2195–206.
Wu SY, Chen WM, Chiang MF, Lo HC, Wu MS, Lee MC, et al. Protective effects of statins on the incidence of NAFLD-related decompensated cirrhosis in T2DM. Liver Int. 2023;43(10):2232–44.
Cho Y, Rhee H, Kim YE, Lee M, Lee BW, Kang ES, et al. Ezetimibe combination therapy with statin for non-alcoholic fatty liver disease: an open-label randomized controlled trial (ESSENTIAL study). BMC Med. 2022;20(1):93.
Doumas M, Imprialos K, Dimakopoulou A, Stavropoulos K, Binas A, Athyros VG. The role of statins in the management of nonalcoholic fatty liver disease. Curr Pharm Design. 2018;24(38):4587–92.
Shang Y, Hagström H. Statins are underused in women with NAFLD after cardiovascular events compared with matched control subjects. Clin Gastroenterol Hepatol. 2023;21(5):1359-1361.e2. https://doi.org/10.1016/j.cgh.2022.03.020.
Liu ZW, Ren ZP, Zhang J, Chuang CC, Kandaswamy E, Zhou TY, et al. Role of ROS and nutritional antioxidants in human diseases. Front Physiol. 2018;9:477. https://doi.org/10.3389/fphys.2018.00477.
Oseini AM, Sanyal AJ. Therapies in non-alcoholic steatohepatitis (NASH). Liver Int. 2017;37:97–103.
Tabei SMB, Fakher S, Djalali M, Javanbakht MH, Zarei M, Derakhshanian H, et al. Effect of vitamins A, E, C and omega-3 fatty acids supplementation on the level of catalase and superoxide dismutase activities in streptozotocin-induced diabetic rats. Bratisl Med J. 2015;116(2):115–8.
Civelek M, Podszun MC. Genetic factors associated with response to vitamin E treatment in NAFLD. Antioxidants-Basel. 2022;11(7):1284. https://doi.org/10.3390/antiox11071284.
Wang MY, Prabahar K, Gaman MA, Zhang JL. Vitamin E supplementation in the treatment on nonalcoholic fatty liver disease (NAFLD): evidence from an umbrella review of meta-analysis on randomized controlled trials. J Digest Dis. 2023;24(6–7):380–9.
Panera N, Braghini MR, Crudele A, Smeriglio A, Bianchi M, Condorelli AG, et al. Combination treatment with hydroxytyrosol and vitamin e improves NAFLD-related fibrosis. Nutrients. 2022;14(18):3791. https://doi.org/10.3390/nu14183791.
Pathil A, Mueller J, Warth A, Chamulitrat W, Stremmel W. Ursodeoxycholyl lysophosphatidylethanolamide improves steatosis and inflammation in murine models of nonalcoholic fatty liver disease. Hepatology. 2012;55(5):1369–78.
Buko VU, Kuzmitskaya-Nikolaeva IA, Naruta EE, Lukivskaya OY, Kirko SN, Tauschel HD. Ursodeoxycholic acid dose-dependently improves liver injury in rats fed a methionine- and choline-deficient diet. Hepatol Res. 2011;41(7):647–59.
Castro RE, Ferreira DMS, Afonso MB, Borralho PM, Machado MV, Cortez-Pinto H, et al. miR-34a/SIRT1/p53 is suppressed by ursodeoxycholic acid in the rat liver and activated by disease severity in human non-alcoholic fatty liver disease. J Hepatol. 2013;58(1):119–25.
Li H, Wang QL, Chen PZ, Zhou CH, Zhang XX, Chen L. Ursodeoxycholic acid treatment restores gut microbiota and alleviates liver inflammation in non-alcoholic steatohepatitic mouse model. Front Pharmacol. 2021;12:788558. https://doi.org/10.3389/fphar.2021.788558.
Nadinskaia M, Maevskaya M, Ivashkin V, Kodzoeva K, Pirogova I, Chesnokov E, et al. Ursodeoxycholic acid as a means of preventing atherosclerosis, steatosis and liver fibrosis in patients with nonalcoholic fatty liver disease. World J Gastroentero. 2021;27(10):959–75.
Chen WQ, Xu MJ, Xu MW, Wang YC, Zou QY, Xie SX, et al. Effects of betaine on non-alcoholic liver disease. Nutr Res Rev. 2022;35(1):28–38.
Wang C, Ma C, Gong LH, Dai S, Li YX. Preventive and therapeutic role of betaine in liver disease: A review on molecular mechanisms. Eur J Pharmacol. 2021;912:174604. https://doi.org/10.1016/j.ejphar.2021.174604.
Sookoian S, Puri P, Castaño GO, Scian R, Mirshahi F, Sanyal AJ, et al. Nonalcoholic steatohepatitis is associated with a state of betaine-insufficiency. Liver Int. 2017;37(4):611–9.
Ge CX, Yu R, Xu MX, Li PQ, Fan CY, Li JM, et al. Betaine prevented fructose-induced NAFLD by regulating LXRa/PPARcic pathway and alleviating ER stress in rats. Eur J Pharmacol. 2016;770:154–64.
Li YL, Jiang WD, Feng Y, Wu L, Jia YM, Zhao RQ. Betaine alleviates high-fat diet-induced disruption of hepatic lipid and iron homeostasis in Mice. Int J Mol Sci. 2022;23(11):6263. https://doi.org/10.3390/ijms23116263.
Sun LQ, Tan XY, Liang XP, Chen HJ, Ou Q, Wu QM, et al. Maternal betaine supplementation mitigates maternal high fat diet-induced NAFLD in offspring mice through gut microbiota. Nutrients. 2023;15(2):284. https://doi.org/10.3390/nu15020284.
Ryan MC, Itsiopoulos C, Thodis T, Ward G, Trost N, Hofferberth S, et al. The Mediterranean diet improves hepatic steatosis and insulin sensitivity in individuals with non-alcoholic fatty liver disease. J Hepatol. 2013;59(1):138–43.
Suárez M, Boqué N, del Bas JM, Mayneris-Perxachs J, Arola L, Caimari A. Mediterranean diet and multi-ingredient-based interventions for the management of non-alcoholic fatty liver disease. Nutrients. 2017;9(10):1052. https://doi.org/10.3390/nu9101052.
Meir AY, Rinott E, Tsaban G, Zelicha H, Kaplan A, Rosen P, et al. Effect of green-Mediterranean diet on intrahepatic fat: the DIRECT PLUS randomised controlled trial. Gut. 2021;70(11):2085–95.
Hashida R, Kawaguchi T, Bekki M, Omoto M, Matsuse H, Nago T, et al. Aerobic vs. resistance exercise in non-alcoholic fatty liver disease: a systematic review. J Hepatol. 2017;66(1):142–52.
Zhang HJ, He J, Pan LL, Ma ZM, Han CK, Chen CS, et al. Effects of moderate and vigorous exercise on nonalcoholic fatty liver disease a randomized clinical trial. Jama Intern Med. 2016;176(8):1074–82.
Ezpeleta M, Gabel K, Cienfuegos S, Kalam F, Lin SH, Pavlou V, et al. Effect of alternate day fasting combined with aerobic exercise on non-alcoholic fatty liver disease: a randomized controlled trial. Cell Metab. 2023;35(1):56-70.e3.
Lassailly G, Caiazzo R, Ntandja-Wandji LC, Gnemmi V, Baud G, Verkindt H, et al. Bariatric surgery provides long-term resolution of nonalcoholic steatohepatitis and regression of fibrosis. Gastroenterology. 2020;159(4):1290-1301.e5. https://doi.org/10.1053/j.gastro.2020.06.006.
Wirth KM, Sheka AC, Kizy S, Irey R, Benner A, Sieger G, et al. Bariatric surgery is associated with decreased progression of nonalcoholic fatty liver disease to cirrhosis a retrospective cohort analysis. Ann Surg. 2020;272(1):32–9.
Ghiassi S, Morton JM. Safety and efficacy of bariatric and metabolic surgery. Curr Obes Rep. 2020;9(2):159–64.
Liu C, Liao JZ, Li PY. Traditional Chinese herbal extracts inducing autophagy as a novel approach in therapy of nonalcoholic fatty liver disease. World J Gastroentero. 2017;23(11):1964–73.
Zhang Y. Modified weiling decoction in treating nonalcoholic fatty liver disease randomized controlled study. J Pract Tradit Chin Internal Med. 2012;27(02):10–1.
Wang H, Huang MY, Bei WJ, Yang YQ, Song LX, Zhang DX, et al. FTZ attenuates liver steatosis and fibrosis in the minipigs with type 2 diabetes by regulating the AMPK signaling pathway. Biomed Pharmacother. 2021;138:111532. https://doi.org/10.1016/j.biopha.2021.111532.
Lan T, Xu TH, Fu YF, Jiang S, Liang XL, Yu Z, et al. Fufang Zhenzhu Tiaozhi capsule prevents intestinal inflammation and barrier disruption in mice with non-alcoholic steatohepatitis. Front Endocrinol. 2022;13:864703. https://doi.org/10.3389/fendo.2022.864703.
Liu N, Yang JY, Ma W, Li CY, An L, Zhang X, et al. Xiaoyao Powder in the treatment of non-alcoholic fatty liver disease: a systematic review and meta-analysis. J Ethnopharmacol. 2022;288:114999. https://doi.org/10.1016/j.jep.2022.114999.
Dang YQ, Xu JJ, Yang Y, Li CL, Zhang Q, Zhou WJ, et al. Ling-gui-zhu-gan decoction alleviates hepatic steatosis through SOCS2 modification by N6-methyladenosine. Biomed Pharmacother. 2020;127:109976. https://doi.org/10.1016/j.biopha.2020.109976.
Dai L, Xu J, Liu B, Dang Y, Wang R, Zhuang L, et al. Lingguizhugan Decoction, a Chinese herbal formula, improves insulin resistance in overweight/obese subjects with non-alcoholic fatty liver disease: a translational approach. Front Med. 2022;16(5):745–59.
Cao L, Xu E, Zheng RD, Zhangchen ZL, Zhong RL, Huang F, et al. Traditional Chinese medicine Lingguizhugan decoction ameliorate HFD-induced hepatic-lipid deposition in mice by inhibiting STING-mediated inflammation in macrophages. Chin Med-UK. 2022;17(1):7. https://doi.org/10.1186/s13020-021-00559-3.
Wang H, Xu ZJ, Wang Q, Shu S. Danshao Shugan Granule therapy for non-alcoholic fatty liver disease. Lipids Health Dis. 2022;21(1):76.
Zheng CY, Nie H, Pan MX, Fan W, Pi DJ, Liang Z, et al. Chaihu Shugan powder influences nonalcoholic fatty liver disease in rats in remodeling microRNAome and decreasing fatty acid synthesis. J Ethnopharmacol. 2024;318:116967. https://doi.org/10.1016/j.jep.2023.116967.
Zhang Y, Guo Z, Wang J, Yue Y, Yang Y, Wen Y, et al. Qinlian hongqu decoction ameliorates hyperlipidemia via the IRE1-α/IKKB-β/NF-κb signaling pathway: Network pharmacology and experimental validation. J Ethnopharmacol. 2024;318(Pt A): 116856.
Zhang YM, Zhao M, Jiang X, Qiao QY, Liu TT, Zhao CJ, et al. Comprehensive analysis of fecal microbiome and metabolomics in hepatic fibrosis rats reveal hepatoprotective effects of yinchen wuling powder from the host-microbial metabolic axis. Front Pharmacol. 2021;12:713197. https://doi.org/10.3389/fphar.2021.713197.
Zhang CH, Xiao Q, Sheng JQ, Liu TT, Cao YQ, Xue YN, et al. Gegen Qinlian Decoction abates nonalcoholic steatohepatitis associated liver injuries via anti-oxidative stress and anti-inflammatory response involved inhibition of toll-like receptor 4 signaling pathways. Biomed Pharmacother. 2020;126:110076. https://doi.org/10.1016/j.biopha.2020.110076.
Yan BF, Wang Y, Wang WB, Ding XJ, Wei B, Liu SJ, et al. Huangqin decoction mitigates hepatic inflammation in high-fat diet-challenged rats by inhibiting TLR4/NF-?B/NLRP3 pathway. J Ethnopharmacol. 2023;303:115999. https://doi.org/10.1016/j.jep.2022.115999.
Cao YH, Shi JY, Song LY, Xu JJ, Lu HL, Sun JH, et al. Multi-omics integration analysis identifies lipid disorder of a non-alcoholic fatty liver disease (NAFLD) mouse model improved by Zexie-Baizhu decoction. Front Pharmacol. 2022;13:858795. https://doi.org/10.3389/fphar.2022.858795.
Biao Y, Chen J, Liu C, Wang R, Han X, Li L, et al. Protective effect of Danshen Zexie decoction against non-alcoholic fatty liver disease through inhibition of ROS/NLRP3/IL-1β pathway by Nrf2 signaling activation. Front Pharmacol. 2022;13: 877924.
Fan S, Zhou Z, Ye J, Li Y, Huang K, Ke X. Integration of lipidomics and transcriptomics reveals the efficacy and mechanism of Qige decoction on NAFLD. Evid Based Complement Alternat Med. 2022;2022:9739032.
Han RT, Qiu HH, Zhong J, Zheng NN, Li BB, Hong Y, et al. Si Miao Formula attenuates non-alcoholic fatty liver disease by modulating hepatic lipid metabolism and gut microbiota. Phytomedicine. 2021;85:153544. https://doi.org/10.1016/j.phymed.2021.153544.
Zhao YW, Yang J, Niu J, Wang T, Liang XD, Ren Y, et al. Pharmacodynamic evaluation of the Gexia Zhuyu decoction in the treatment of NAFLD and the molecular mechanism underlying the TRPM4 pathway regulation. Evid-Based Compl Alt. 2021;2021:3364579. https://doi.org/10.1155/2021/3364579.
Zhang L, Chen N, Zhan L, Bi T, Zhou W, Zhang L, et al. Erchen Decoction alleviates obesity-related hepatic steatosis via modulating gut microbiota-drived butyric acid contents and promoting fatty acid β-oxidation. J Ethnopharmacol. 2023;317: 116811.
Leng J, Huang F, Hai Y, Tian H, Liu W, Fang Y, et al. Amelioration of non-alcoholic steatohepatitis by Qushi Huayu decoction is associated with inhibition of the intestinal mitogen-activated protein kinase pathway. Phytomedicine. 2020;66: 153135.
Ni Y, Wang X, Wu Q, Yao Y, Xu Y, Li Y, et al. Qushi Huayu decoction ameliorates non-alcoholic fatty liver disease in rats by modulating gut microbiota and serum lipids. Front Endocrinol. 2023;14:1272214.
Luo DS, Yang L, Pang HT, Zhao YT, Li KP, Rong XL, et al. Tianhuang formula reduces the oxidative stress response of NAFLD by regulating the gut microbiome in mice. Front Microbiol. 2022;13(13):984019. https://doi.org/10.3389/fmicb.2022.984019.
Shi H, Qiao F, Huang K, Lu W, Zhang X, Ke Z, et al. Exploring therapeutic mechanisms of San-Huang-Tang in nonalcoholic fatty liver disease through network pharmacology and experimental validation. J Ethnopharmacol. 2022;296: 115477.
Hong W, Li SS, Cai YQ, Zhang TT, Yang QR, He BH, et al. The target microRNAs and potential underlying mechanisms of Yiqi-Bushen-Tiaozhi recipe against-non-alcoholic steatohepatitis. Front Pharmacol. 2020. https://doi.org/10.3389/fphar.2020.529553.
Jiang H, Mao TY, Liu YY, Tan X, Sun ZM, Cheng Y, et al. Protective effects and mechanisms of Yinchen Linggui Zhugan decoction in HFD-induced nonalcoholic fatty liver disease rats based on network pharmacology and experimental verification. Front Pharmacol. 2022. https://doi.org/10.3389/fphar.2022.90812.
Zheng KN, Zhou WH, Ji JM, Xue Y, Liu YW, Li CG, et al. Si-Ni-San reduces lipid droplet deposition associated with decreased YAP1 in metabolic dysfunction-associated fatty liver disease. J Ethnopharmacol. 2023;305:116081. https://doi.org/10.1016/j.jep.2022.116081.
Hui D, Liu L, Azami NLB, Song J, Huang Y, Xu W, et al. The spleen-strengthening and liver-draining herbal formula treatment of non-alcoholic fatty liver disease by regulation of intestinal flora in clinical trial. Front Endocrinol. 2022;13:1107071.
Zhao YD, Zhang ZC, Qin SR, Fan W, Li W, Liu JY, et al. Acupuncture for Parkinson’s disease: efficacy evaluation and mechanisms in the dopaminergic neural circuit. Neural Plast. 2021;2021:1–23. https://doi.org/10.1155/2021/9926445.
Li B, Fang L. Research progress on the mechanism of acupuncture treatment for nonalcoholic fatty liver disease. Gastroenterol Res Pract. 2022;2022:5259088.
Wang HY, Liang CM, Cui JW, Pan L, Hu H, Fang HJ. Acupuncture improves hepatic lipid metabolism by suppressing oxidative stress in obese nonalcoholic fatty liver disease rats. Zhen Ci Yan Jiu. 2019;44(3):189–94.
Zeng ZH, Zeng MH, Huang XK, Chen R, Yu H. Effect of electroacupuncture stimulation of back-shu points on expression of TNF-alpha and lipid peroxidation reaction in the liver tissue in non-alcoholic fatty liver disease rats. Zhen Ci Yan Jiu. 2014;39(4):288–92.
Wang HY, Wang Q, Liang CM, Pan L, Hu H, Fang HJ. Acupuncture improved hepatic steatosis in HFD-induced NAFLD rats by regulating intestinal microbiota. Front Microbiol. 2023. https://doi.org/10.3389/fmicb.2023.1131092.
Gao Y, Chen R, Liang F. Mechanisms of acupuncture for non-alcoholic fatty liver disease: researches progress and prospects. Zhongguo Zhen Jiu. 2018;38(1):109–13.
Guo T, Ren Y, Kou J, Shi J, Tianxiao S, Liang F. Acupoint catgut embedding for obesity: systematic review and meta-analysis. Evid Based Complement Alternat Med. 2015;2015: 401914.
Zhao JJ, Rong PJ, Shi L, Ben H, Zhu B. Somato stimulation and acupuncture therapy. Chin J Integr Med. 2016;22(5):394–400.
Huo J, Zhao J, Yuan Y, Wang J. Research status of the effect mechanism on catgut-point embedding therapy. Zhongguo Zhen Jiu. 2017;37(11):1251–4.
Dai L, Ooi VV, Zhou W, Ji G. Acupoint embedding therapy improves nonalcoholic fatty liver disease with abnormal transaminase: a PRISMA-compliant systematic review and meta-analysis. Medicine. 2020;99(3): e18775.
Yu X, Chau JPC, Huo L. The effectiveness of traditional Chinese medicine-based lifestyle interventions on biomedical, psychosocial, and behavioral outcomes in individuals with type 2 diabetes: a systematic review with meta-analysis. Int J Nurs Stud. 2018;80:165–80.
Siu PM, Yu AP, Chin EC, Yu DS, Hui SS, Woo J, et al. Effects of Tai Chi or conventional exercise on central obesity in middle-aged and older adults : a three-group randomized controlled trial. Ann Intern Med. 2021;174(8):1050–7.
Chau JPC, Leung LYL, Liu X, Lo SHS, Choi KC, Zhao J, et al. Effects of Tai Chi on health outcomes among community-dwelling adults with or at risk of metabolic syndrome: a systematic review. Complement Ther Clin Pract. 2021;44: 101445.
Yin J, Yue C, Song Z, Sun X, Wen X. The comparative effects of Tai chi versus non-mindful exercise on measures of anxiety, depression and general mental health: a systematic review and meta-analysis. J Affect Disord. 2023;337:202–14.
Li W. Clinical observation of Xiaoyao Powder combined with simvastatin in treatment of nonalcoholic fatty liver. Guangming J Chin Med. 2019;34:2213–5.
Yu Yang YH, Lihua W. Curative effect of compound Danshen tablet combined with metformin in the treatment of diabetic patients with nonalcoholic fatty liver disease. J Modern Clin Med. 2016;42:49–51.
Dong C, Zhang CR, Xue BY, Miu WF, Fang NY, Li K, et al. Electroacupuncture combined with lifestyle control on obese nonalcoholic fatty liver disease: a randomized controlled trial. Zhongguo Zhen Jiu. 2020;40(2):129–34.
Acknowledgements
Not applicable.
Funding
This work was supported by grants from the National Natural Science Foundation of China (82070590 and 82373930).
Author information
Authors and Affiliations
Contributions
XD and XH contributed equally to this work. XD, XH and BT wrote the manuscript. XD and XH generated the figures. TL developed the concept and edited the manuscript. All authors have read and agreed to the published version of the manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
All authors consent to the publication of this work in Chinese Medicine.
Competing interests
The authors declare that there are no conflicts of interest. Figure 1 is created using Figdraw (www.figdraw.com).
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Ding, X., He, X., Tang, B. et al. Integrated traditional Chinese and Western medicine in the prevention and treatment of non-alcoholic fatty liver disease: future directions and strategies. Chin Med 19, 21 (2024). https://doi.org/10.1186/s13020-024-00894-1
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s13020-024-00894-1