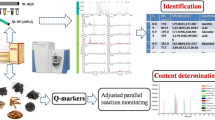
Abstract
Wushicha Granule, an over-the-counter-drug (OTC) prescription, consists of 19 traditional Chinese herbals medicines (CHMs), such as Chaihu, Hongcha, Chuanxiong, Houpo, and Gancao. The five however have not been effectively characterized by the quality-markers (Q-markers) system in current Pharmacopoeia. The study therefore established a novel database-aided ultra-high performance liquid chromatography-quadrupole-orbitrap mass spectrometry (UHPLC-Q-orbitrap MS/MS) strategy. The strategy has putatively identified 52 compounds from Wushicha Granule, mainly including flavonoids, saponins, alkaloid, lignins, and lactones. Especially, saponin “glycyrrhetinic acid” in the Granule was specifically identified as 18β-configuration (rather than 18α-configuration). Meanwhile, two pairs of isomers were fully discriminated, including vitexin vs isovitexin and daidzein vs 7,4'-dihydroxyflavone. 8β-Glycyrrhetinic acid, together with saponin saikosaponin A, alkaloid caffeine, lactone S-senkyunolide A, and lignin magnolol, were further studied using quantum chemical calculation, UV–vis spectra, and anti-counterfeiting validation experiment. In the validation experiment, they have successfully recognized 6 counterfeit Wushicha Granules, by means of a LC–MS equipped extraction software. Based on these results, 8β-glycyrrhetinic acid is recommended to replace the old Q-marker “glycyrrhetinic acid”; while saikosaponin A, caffeine, S-senkyunolide A, and magnolol are recommended as new Q-markers. These recommendations can not only recognize the counterfeits regarding Chaihu, Hongcha, Chuanxiong, Houpo, and Gancao, but also prevent the possible safety-incident. All these will greatly improve the efficiency and specificity of current Pharmacopoeia.
Similar content being viewed by others
Introduction
Wushicha (Wushi Tea, 午時茶, Fig. 1) Granule is a Chinese traditional health-care prescription medicine with an over 200-year history. It is documented to be related to Dragon Boat Festival (端午節) in China [1, 2]. From the angle of traditional Chinese medicine (TCM), Wushicha Granule however has multiple functions of dispelling wind and relieving exterior syndrome, as well as resolving damp and regulating stomach. Thus, it is usually used to treat several wind-cold-induced gastroenterology disorders, such as ulcerative colitis, nausea, vomiting, abdominal pain, and diarrhea [2, 3].
These functions have facilitated it wide consumption in China. Nowadays, Wushicha Granule, as an over-the-counter-drug (OTC) prescription, can be accessed via online and pharmacy sales. According to the data from National Medical Products Administration of China [4], at least 52 pharmaceutical manufacturers, including Guangzhou Wanglaoji Pharmaceutical Co., Ltd, have been approved to manufacture the Granule.
The manufacture of Wushicha Granule is fulfilled by mixing 19 Chinese herbal medicines (CHMs) (Table 1). The manufacturing techniques are expected to comply with the Pharmacopoeia (Chinese Pharmacopoeia, 2020 version). However, in 2021, two batches of Wushicha Granule have been identified as unqualified products [5, 6]. This has attracted public attention regarding its quality-markers (Q-markers) in Pharmacopoeia.
The current Pharmacopoeia only defines three Q-markers. One Q-marker hesperidin is for HPLC analysis; while another Q-markers phillyrin and “glycyrrhetinic acid” Q-markers however are for TLC (thinner layer chromatography) analysis [3]. In line with Table 1 and the accumulated literatures [3, 7,8,9], the current Q-markers system only involves four CHMs (i.e., Lianqiao, Chenpi, Zhishi, and Gancao). Some important CHMs, such as Hongcha, Chaihu, Chuanxiong, and Houpo, have not been involved [3]. As a result, the current system could not recognize the counterfeits concerning Hongcha, Chaihu, Chuanxiong, and Houpo. This is considered as the first limitation of current Pharmacopoeia Q-markers system.
In addition, the current Q-markers system has not yet discriminated two configurations of glycyrrhetinic acid, i.e., 18α- and 18β-. The former presents 18S-; while the latter shows 18R-, according to the updated nomenclature guideline. Thus, the two actually are a pair of stereo-isomers. Two stereo-isomers have been reported to possess different pharmacological effects. 18β-Glycyrrhetinic acid had hepatocyte protection effect; while 18α-glycyrrhetinic acid did not. On the other hand, 18α-glycyrrhetinic acid could selectively inhibit 11-hydroxysteroid dehydrogenase I, whilst 18β-glycyrrhetinic acid could not [10]. This situation is similar to two thalidomide stereo-isomers (i.e., R- and S-thalidomides). Therefore, the confusion of 18α-, and 18β-glycyrrhetinic acids, may cause a tragedy similar to “Thalidomide Disaster” in 1960s. This can be regarded as the second limitation of current Pharmacopoeia Q-markers system.
Two limitations urge pharmacists to update the current Q-markers system, by means of an appropriate method, such as addition of new Q-marker. The update of course includes a fundamental work to discriminate 18α-glycyrrhetinic acid and 18β-glycyrrhetinic acid. All these obviously require a systematical and reliable identification for the main compounds in Wushicha Granule. Thereby, the study attempted to use a novel database-aided cutting-edge ultra-high performance liquid chromatography-quadrupole-orbitrap mass spectrometry (UHPLC-Q-orbitrap MS/MS) strategy, to fulfill the identification. Some identified compounds would further be recommended as Q-marker candidates for consideration by the Pharmacopoeia Commission.
Materials and methods
Wushicha Granule and its counterfeits
Wushicha Granule was purchased from Hubei Wushi Pharmaceutical Co., LTD (Anlu, Hubei, China). Its Lot No. was Z42020134, and production date was Jan. 13, 2022.
Six counterfeit Wushicha Granules were prepared by our team through replacement method. Both Zhishi and Chenpi were replaced by wood powder, to prepared the first counterfeit Wushicha Granule, i.e., CWG 1. Similarly, Chaihu was replaced by wood powder, to obtain CWG 2. In addition, Hongcha, Chuanxiong, Houpo, and Gancao were by wood powder, to produce CWG 3, CWG 4, CWG 5, and CWG 6, respectively (Table 2).
Chemicals
Methyl gallate (C8H8O5, M.W. 192.16, Cas. 99–24-1, 98%), S-senkyunolide A (C12H16O2, M.W. 192.25, Cas. 63038-10-8, 98%), saikosaponin A (C42H68O13, M.W. 780.98, Cas. 20736-09-8, 98%), licoricesaponin H2 (C42H62O16, M.W. 822.9, Cas. 118441-85-3, 98%), quinic acid (C7H12O6, M.W. 192.16, Cas. 77–95-2, 98%), myricetin (Cas. 529–44-2, C15H10O8, M.W. 318.24, 97%), liquiritin (C21H22O9, M.W. 418.39, Cas. 551–15-5, 98%), scoparone (C11H10O4, M.W. 206.19, Cas. 120–08-1, 98%), platycodin D (C57H92O28, M.W. 1225.32, Cas. 58479-68-8, 98%), 18α-glycyrrhetinic acid (C30H46O4, M.W. 470.69, Cas. 1449–05-4, 98%), and 18β-glycyrrhetinic acid (C30H46O4, M.W. 470.69, Cas. 471–53-4, 98%) were obtained from Herbest Biotech Co., Ltd (Baoji, China). ( +)-4-Cholesten-3-one (Cas. 601–57-0, C27H44O, M.W. 384.64, 98%), ethyl stearate (Cas. 111–61-5, C20H40O2, M.W. 312.53, 98%), and 5-hydroxyflavone (Cas. 491–78-1, C15H10O3, M.W. 238.24, 97%) were from TCI Chemical Co. (Shanghai, China). D-gluconic acid (Cas. 526–95-4, C6H12O7, M.W. 196.155, 98%) was from Sigma-Aldrich Co., Ltd. (Shanghai, China). Randaiol (Cas. 87562-14-9, C15H14O3, M.W. 242.27, 97%), luteolin (Cas. 491–70-3, C15H10O6, M.W. 286.24, 97%), (-)-pinoresinol (Cas. 81446-29-9, C20H22O6, 358.39, 97%), isorhamnetin-3-O-β-D-glucoside (Cas. 5041–82-7, C22H22O12, M.W. 478.4, 97%), and acteoside(Cas. 61276-17-3, C29H36O15, M.W. 624.59, 97%) were from BioBioPha Co., Ltd. (Kunming, China). Hypericin (Cas. 548–04-9, C30H16O8, M.W. 504.45, 97%), S-hesperetin (Cas. 520–33-2, C16H14O6, M.W. 302.28, 97%), naringenin chalcone (Cas. 73692-50-9, M.W. C15H12O5, 272.25, 97%), S-naringenin (Cas. 480–41-1, C15H12O5, M.W. 272.253, 98%), 7,4’-dihydroxyflavone (Cas. 2196–14-7, C15H10O4, M.W. 254.238, 98%), astragalin (Cas. 480-10-4, C21H20O11, M.W. 448.38, 97%), isochlorogenic acid A (Cas. 2450–53-5, C25H24O12, M.W. 516.45, 97%), rosmarinic acid (Cas. 20283-92-5, C18H16O8, M.W. 360.31, 97%), naringin (Cas. 10236-47-2, C27H32O14, M.W. 580.53, 97%), rutin (Cas. 153–18-4, C27H30O16, M.W. 610.518, 98%), isoquercitrin (Cas. 21637-25-2, C21H20O12, 464.38, 97%), isovitexin (Cas. 38953-85-4, C21H20O10, M.W. 432.37, 98%), isoliquiritin (Cas. 5041–81-6, C21H22O9, M.W. 418.39, 97%), and puerarin (Cas. 3681-99-0, C21H20O10, M.W. 416.38, 97%) were from Chengdu Alfa Biostrategy Co., Ltd. (Chengdu, China). Formononetin (Cas. 485–72-3, C16H12O4, M.W. 268.264, 98%), isoliquiritigenin (Cas. 961-29-5, C15H12O4, M.W. 256.253, 98%), daidzein (Cas. 486-66-8, C15H10O4, M.W. 254.24, 97%), naringenin-7-O-β-D-glucoside (Cas. 529-55-5, C 21H22O10, M.W. 434.393, 98%), 5-caffeoylquinic acid (Cas. 906-33-2, C16H18O9, M.W. 354.31, 98%), and gallic acid (Cas. 149–91-7, C7H6O5, M.W. 170.1, 99%) were from Chengdu Biopurify Phytochemicals Ltd. (Chengdu, China).Magnolol (Cas. 528–43-8, C18H18O2, M.W. 266.32, 97%), 3,3',4',5,6,7,8-heptamethoxyflavone (Cas. 1178-24-1, C16H12O3, M.W. 252.26, 97%), quercetin(Cas. 117–39-5, C15H10O7, M.W. 302.23, 97%), vitexin (Cas. 3681-93-4, C21H10O10, M.W. 432.11, 97%), schaftoside (Cas. 51938-32-0, C26H28O14, M.W. 564.49, 98%), vicenin-2 (Cas. 23666-13-9, C27H30O15, M.W. 594.518, 98%), and protocatechuic acid (Cas. 99-50-3, C7H6O4, M.W. 154.12, 97%) were from Sichuan Weikeqi Biological TechnologyCo., Ltd. (Chengdu, China). Caffeine (Cas. 58-08-2, C8H10N4O2, M.W. 194.19, 98%) was prepared by our laboratory. Methanol, and water were of mass spectra purity grade. All other reagents used in this study were purchased as analytical grade from the Guangzhou Chemical Reagent Factory (Guangzhou, China).
The preparation of sample solution
The purchased Wushicha Granule was dissolved using distilled water under ultrasound treatment, to avoid the possible solvent effect [11]. The dissolution however brought about a turbid liquid. The turbid liquid was then filtered through a 0.45 μm membrane, to prepare a filtrate. The filtrate (at 30 mg/mL) was then kept in cell-bottle at 2–6 °C for analysis [12].
Furthermore, 6 premixed counterfeit Granules (i.e., CWG 1 ~ CWG 6) were prepared for their lyophilized aqueous extract powders, in line with Jiang’s method [13]. Then, 6 lyophilized powders were dissolved using distilled water under ultrasound treatment at 30 mg/mL and filtered through a 0.45 μm membrane to prepare a filtrate, respectively. All filtrates were then kept in cell-bottle at 2–6 °C for analysis [12].
Database establishment and UHPLC-Q-orbitrap MS analysis
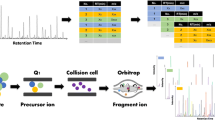
The database has been built up using the corresponding authentic standards, according to the previous study [14]. In brief, these authentic standards were dissolved in methanol at 30 μg/mL, respectively. The methanolic solution was then filtered through a 0.45 μm membrane, and kept in cell-bottle at 2–6 °C for analysis. The ultrahigh-performance liquid chromatography (UHPLC) was conducted, according to the previous method [15, 16]. In brief, the UHPLC separation was achieved by the mobile phase comprising 0.1% HCOOH (phase A) and methanol (phase B). The binary gradient was set as following: 0–5 min, 10% B; 5–14.5 min, 10 → 100% B; 14.5–16 min, 100% B; 16–16.1 min, 100 → 0% B. Then, the 10% B mobile phase was kept for 4 min to equilibrate the system. The mobile phase run at a flow rate of 0.4 mL min 1. The column temperature was maintained at 40 °C and injection volume was 3 μL.
The Q-orbitrap MS analysis was performed a high-resolution Q-orbitrap mass spectrometer (Thermo Fisher Scientific, Waltham, MA, USA) and consulted with our previous instrument settings [17, 18]. The operating parameters were detailed as follows: auxiliary gas, 10; sheath gas, 40; sweep gas, 0; spray voltage, 4.5 kV. The temperature of auxiliary gas heater and capillary were both set at 450 °C. The full MS resolution and dd-MS2 were 70,000 and 17,500, respectively, and their AGC target was 2 × 105. Nitrogen (N2) was applied for spray stabilization and the damping gas in the C- trap. The stepped normalized collision energy was set to 20, 50, and 90 V. The MS scanning scope was set as m/z 0–1500. The analyses were conducted under both negative and positive models. The positive model however was a supplement of the negative one. The UHPLC-Q-orbitrap MS analysis only focused on the authentic standards and Wushicha Granule (except for 6 counterfeits).
Putative identification using software and MS spectra elucidation
Xcalibur 4.1 software package and TraceFinder General Quan (Thermo Fisher Scientific Inc., Waltham, MA, USA) were used for data acquisition and analysis [14]. The acquired data included retention time (R.T.), molecular peak, MS/MS profile, and diagnostic MS/MS fragments of authentic standards. The acquisition was achieved based on the previous conditions [19]. Through the comparison with the database, 63 compounds were preliminarily identified from the Wushicha sample solution. After manual elucidation of MS spectra fragmenting, 52 compounds were further confirmed to finish putative identification.
Quantum chemical calculation details
Seven compounds were investigated for quantum chemical calculation, including caffeine, hesperidin, saikosaponin A, S-senkyunolide A, 18α-glycyrrhetinic acid, 18β-glycyrrhetinic acid, and magnolol. All calculations were accomplished using the Gaussian 16 in Linux system, including conformational optimization, dipole moment calculation, and highest occupied molecular orbital (HOMO)–lowest unoccupied molecular orbital (LUMO) energy gap. The basis set was at (U)B3LYP-D3(BJ)/ 6–31 + G(d,p) level [20,21,22]. The most stable conformation was optimized until no imaginary frequency; while the calculation results (including optimized conformation) were viewed via Gaussian View 6.1.1 [23]. The optimized conformation was further exported using Chem3D pro. 14.0. Gaussian 16, and Gaussian View 6.1.1 (Gaussian Inc., Wallingford, CT, USA).
UV–vis spectra scanning experiments
The UV–vis spectra scanning experiments were conducted based on the previous method [24]. In brief, 6 compounds were dissolved in methanol at appropriate concentrations, respectively. Their methanolic solutions were then scanned using a UV–vis spectrophotometer (Unico 2600A, Shanghai, China) from 200 to 800 nm, respectively. Six compounds referred to hesperidin, phillyrin, magnolol, caffeine, S-senkyunolide A, 18β-glycyrrhetinic acid, and saikosaponin A. All these compounds were at ~ 0.02 mg/mL concentration.
Anti-counterfeiting validation experiment based on 6 counterfeits
The anti-counterfeiting validation experiment was performed using ultra-performance liquid chromatography coupled with electrospray ionization quadrupole time-of-flight tandem mass spectrometry (UHPLC-ESI-Q-TOF–MS/MS), an analytic technology inferior to UHPLC-Q-orbitrap MS/MS. The chromatography analysis protocol was based on our previous studies [25, 26]; while the MS spectra monitoring was achieved using a Q − TOF − MS/MS apparatus (i.e., Triple TOF 5600plus mass spectrometer, AB SCIEX, Framingham, MA, U.S.A.) [27]. However, the analytes were 6 counterfeit Wushicha Granules, i.e., CWG 1–CWG 6. For comparison, the Wushicha sample solution was also prepared for this analysis, under the same conditions.
Results
Putative identification based on UHPLC-Q-orbitrap MS/MS
The Wushicha sample solution was firstly analyzed using UHPLC-Q-orbitrap MS/MS strategy. Through analysis, the total ion current (TIC) diagrams were obtained (Fig. 2); while the main ion peaks were further investigated for the R.T. values, molecular ion peak, and diagnostic MS/MS fragments (Table 3). These information was compared with that of authentic standards in the database; and thereafter the MS spectra of all compounds were fully elucidated, according to relevant principles (e.g., Retro-Diels–Alder fragmenting, Additional file 1: S1, Additional file 2: S2, Additional file 3: S3, Additional file 4: S4, Additional file 5: S5, Additional file 6: S6, Additional file 7: S7, Additional file 8: S8, Additional file 9: S9, Additional file 10: S10, Additional file 11: S11, Additional file 12: S12, Additional file 13: 13, Additional file 14: S14, Additional file 15: S15, Additional file 16: S16, Additional file 17: S17, Additional file 18: S18, Additional file 19: S19, Additional file 20: S20, Additional file 21: S21, Additional file 22: S22, Additional file 23: S23, Additional file 24: S24, Additional file 25: S25, Additional file 26: S26, Additional file 27: S27, Additional file 28: S28, Additional file 29: S29, Additional file 30: S30, Additional file 31: S31, Additional file 32: S32, Additional file 33: S33, Additional file 344: S34, Additional file 35: S35, Additional file 36: S36, Additional file 37: S37, Additional file 38: S38, Additional file 39: S39, Additional file 40: S40, Additional file 41: S41, Additional file 42: S42, Additional file 43: S43, Additional file 44: S44, Additional file 45: S45, Additional file 46: S46, Additional file 47: S47, Additional file 48: S48, Additional file 49: S49, Additional file 50: S50, Additional file 51: S51). In particular, 6 compounds, including saikosaponin A, platycodin D, vitexin, isovitexin, daidzein, and 7,4'-dihydroxyflavone, were also shown their MS spectra elucidation in Figs. 3, 4, 5, 6. Finally, the structures (and even configurations) of all identified compounds were detailed in Fig. 7, for the convenience of readers.
The putative identification of saikosaponin A (28) based on MS spectra under negative model. A MS/MS spectra and the relevant elucidation of standard saikosaponin A; B MS/MS spectra of the sample peak from Wushicha Granule. The m/z values in purple indicated the calculated ones. The detailed MS elucidation, and identification process were shown in in Additional file 27: S27
The putative identification of platycodin D (38) based on MS spectra under negative model. A MS/MS spectra and the relevant elucidation of standard platycodin D; B MS/MS spectra of the sample peak from Wushicha Granule. The m/z values in purple indicated the calculated ones. The detailed MS elucidation, and identification process were shown in Additional file 37: S37
The structures of 52 identified compounds from Wushicha Granule by database-aided UHPLC-Q-orbitrap MS/MS strategy. A 48 non-isomeric compounds; B two pairs of isomeric compounds. The red “√” indicates the old Pharmacopoeia Q-markers; while the purple “√” means the new Q-marker candidates. The wave lines in 18 and 21 indicate the uncertain stereo-configuration
Quantum chemical calculation
The main calculation results of 7 compounds were shown in Table 4. Seven compounds referred to hesperidin (23), 18α-glycyrrhetinic acid, 18β-glycyrrhetinic acid (45), caffeine (7), saikosaponin A (28), S-senkyunolide A (40), and magnolol (44).
UV–vis spectra scanning
Anti-counterfeiting validation experiment using 6 counterfeit Wushicha Granules
Discussion
The study established a novel strategy, i.e., database-aided UHPLC-Q-orbitrap MS/MS, to simultaneously identify 52 compounds from Wushicha Granule. Compared with the conventional HPLC–UV strategy which could simultaneously identify 2–10 compounds [66,67,68,69,70], our strategy was undoubtedly of high-efficiency. Besides high-efficiency, our strategy was of high-reliability as well. As seen in Figs. 3, 4, 5, 6 and Additional file 1: S1, Additional file 2: S2, Additional file 3: S3, Additional file 4: S4, Additional file 5: S5, Additional file 6: S6, Additional file 7: S7, Additional file 8: S8, Additional file 9: S9, Additional file 10: S10, Additional file 11: S11, Additional file 12: S12, Additional file 13: 13, Additional file 14: S14, Additional file 15: S15, Additional file 16: S16, Additional file 17: S17, Additional file 18: S18, Additional file 19: S19, Additional file 20: S20, Additional file 21: S21, Additional file 22: S22, Additional file 23: S23, Additional file 24: S24, Additional file 25: S25, Additional file 26: S26, Additional file 27: S27, Additional file 28: S28, Additional file 29: S29, Additional file 30: S30, Additional file 31: S31, Additional file 32: S32, Additional file 33: S33, Additional file 344: S34, Additional file 35: S35, Additional file 36: S36, Additional file 37: S37, Additional file 38: S38, Additional file 39: S39, Additional file 40: S40, Additional file 41: S41, Additional file 42: S42, Additional file 43: S43, Additional file 44: S44, Additional file 45: S45, Additional file 46: S46, Additional file 47: S47, Additional file 48: S48, Additional file 49: S49, Additional file 50: S50, Additional file 51: S51, our strategy was carried out via multiple comparisons including molecular ion peak comparison, diagnostic MS/MS peak comparison, MS/MS profile comparison, and R.T. value comparison. All these comparisons were based on authentic standards in the database. Therefore, the identification was highly-convincing and thus described as “putative identification” in the study.
One of putative identification instances was saikosaponin A (30). As seen in Fig. 3, the sample peak was highly similar to that of authentic standard, especially in molecular ion peak, diagnostic MS/MS peak, and MS/MS profile. Further MS elucidation suggested 10–5 ~ 10–6 relative standard deviation (RSD) values between the experimental and calculated m/z values. Such high-accuracy could also be observed in the identification of platycodin D (38), a non-isomeric compound (Fig. 4).
Our strategy however could also be used to discriminate isomers, e.g., two vitexin isomers (13 and 17). The two showed similar molecular ion peaks, diagnostic MS/MS fragments, and even MS/MS profiles. However, their R.T. values were different from each other (Fig. 5). The two were thus successfully discriminated using the strategy [18]. Similarly, two isomers (29 and 31) were also successfully discriminated in the study (Fig. 6).
Both non-isomeric compound identification and isomers discrimination have offered detailed MS elucidation in Additional file 1: S1, Additional file 2: S2, Additional file 3: S3, Additional file 4: S4, Additional file 5: S5, Additional file 6: S6, Additional file 7: S7, Additional file 8: S8, Additional file 9: S9, Additional file 10: S10, Additional file 11: S11, Additional file 12: S12, Additional file 13: 13, Additional file 14: S14, Additional file 15: S15, Additional file 16: S16, Additional file 17: S17, Additional file 18: S18, Additional file 19: S19, Additional file 20: S20, Additional file 21: S21, Additional file 22: S22, Additional file 23: S23, Additional file 24: S24, Additional file 25: S25, Additional file 26: S26, Additional file 27: S27, Additional file 28: S28, Additional file 29: S29, Additional file 30: S30, Additional file 31: S31, Additional file 32: S32, Additional file 33: S33, Additional file 344: S34, Additional file 35: S35, Additional file 36: S36, Additional file 37: S37, Additional file 38: S38, Additional file 39: S39, Additional file 40: S40, Additional file 41: S41, Additional file 42: S42, Additional file 43: S43, Additional file 44: S44, Additional file 45: S45, Additional file 46: S46, Additional file 47: S47, Additional file 48: S48, Additional file 49: S49, Additional file 50: S50, Additional file 51: S51. This has become a great contrast with the previous tentative identification works [71,72,73]. For example, Duan and colleagues used old document data (2015 and 2019) to “recognize” flavonoid vitexin in the newest experiment (2021). Obviously, there was no comparability between the old document data and new experiment data; Correspondingly, there was no detailed MS elucidation [73]. Some identification works might be arbitrarily, e.g., the identification of vitexin. This is because its highly similar isomer (isovitexin) has not been completely excluded. These tentative identification works may cause inappropriate (and even wrong) structure, to mislead the readers.
The present study however has detailed the structures of 52 compounds (1–52). Structurally, these putatively identified compounds covered 15 main structural types, that is, flavone-glycoside, flavone, lipid, saponin, phenolic acid, lignin, quinic acid derivative, isoflavone, chalcone, sugar, lkaloid, coumarin, naphthodianthrone, lactone, phenylpropanoid, and steroid. From the perspective of stereo-chemistry, most chiral atoms have been verified for the stereo-configuration. The putative identification, along with the isomers discrimination and stereo-configuration verification, have provided a foundation for Q-marker addition.
The Q-marker addition however is required to comply with five principles proposed by academician Chang-xiao Liu. These principles can be briefly described as traceability, testability, relevance to pharmacology, relevance to TCM theory, and specificity [74, 75]. Apparently, Liu’s principles have not completely excluded industrialization of Q-marker, and thus has not prohibited massive and illegal addition industrialized Q-marker into the Granule yet. This may lead to a tragedy similar to Sanlu Melamine Incident in China (2008), as predicted by our team [19].
To prevent a similar tragedy, a new principle named “non-industrialization” was recently proposed by our team [17]. According to the principle, one Q-marker candidate should not be easily obtained via industrialization. Therefore, 9 identified compounds were firstly excluded as Q-marker candidate, including D-gluconic acid (1), quinic acid (2), gallic acid (3), protocatechuic acid (4), methyl gallate (6), linoleic acid (46), palmitic acid (47), oleic acid (48), ethyl palmitate (49), and ethyl stearate (50). This is because these natural compounds could be industrially synthesized as well [76,77,78,79,80,81,82].
From other 43 compounds, four compounds were recommended as new Q-marker candidates, including caffeine (7), saikosaponin A (28), S-senkyunolide A (40), and magnolol (44), in accordance with the above principles (Table 5). In particular, four new Q-markers (7, 28, 40, and 44), together with one revised one (45) possessed higher HOMO–LUMO energy gaps than the old Q-marker (23, Table 3). This has implied that the former five (7, 28, 40, 44, and 45) would possess better traceability than the latter one (23). This is because that, high energy gap indicates high stability, from the angle of chemical thermodynamics. Thus, during the processes of manufacturing, handling, transportation, and metabolism, five Q-markers (7, 28, 40, 44, and 45) would not be destroyed by external stimulation (e.g., illumination, heat, or catalyst), to show excellent traceability. Of these, caffeine (7) is a government-controlled chemical in China and cannot be massively and industrially synthesized. In short, all these candidates have complied with Liu’s and our principles (Table 4).
Liu’s principles might also be used to explain why “glycyrrhetinic acid” has been selected as the Pharmacopoeia Q-marker previously. The Q-marker was applied to characterize Gancao via a tedious and unreliable TLC operation [3]. However, in the study, the so-called “glycyrrhetinic acid” was clearly identified as 18β-glycyrrhetinic acid (45) rather than 18α-glycyrrhetinic acid. The complete separation of two glycyrrhetinic acids mainly relied on the difference in R.T. values, as seen in Additional file 44: S44. This difference however originated from molecular polarity. As seen in Table 4, two glycyrrhetinic acids displayed different dipole moments: 4.6173 Debye for 18α- and 3.1347 Debye for 18β-. In other words, different dipole moments have brought about different molecular polarities. Different molecular polarities have facilitated two glycyrrhetinic acids to be separated through a C18 adsorption column [61]. Obviously, the TLC analysis was incapable for separation of two glycyrrhetinic acids. In summary, it was necessary and feasible to revised the old Pharmacopoeia Q-marker “glycyrrhetinic acid” as 18β-glycyrrhetinic acid (45). As mentioned above, the revision can avoid the safety-incident similar to Thalidomide Disaster (1960s).
The revised Q-marker (45), together with four new candidates (7, 28, 40, and 44) and the old Q-marker (hesperidin 23) have re-constructed a new Q-markers system for Pharmacopoeia. In the system, 6 Q-markers showed different molecular polarities from each other; their dipole moments varied from -5.8110 to 5.8740 Debye (Table 4). This ensures that the six can be completely separated by a C18 adsorption column (Fig. 2).
From the perspective of analytic approach, the present UHPLC-Q-orbitrap MS/MS apparatus would be not an ideal choose, because it was too expensive and could not be afforded by most of pharmaceutical factories or drug control institute. For this reason, a lower revision LC–MS, i.e., UHPLC-ESI-Q-TOF–MS/MS, was used for anti-counterfeiting validation experiments, for its relative cheapness and popularity. The study thus used the UHPLC-ESI-Q-TOF–MS/MS to analyze 6 counterfeit Wushicha Granules, i.e., CWG 1 ~ CWG 6.
This first anti-counterfeiting validation experiment focused on CWG 1, one counterfeit Wushicha Granule without Chenpi and Zhishi. As seen in Fig. 8, CWG 1 showed no hesperidin (23) peak. In contrast, Wushicha Granule displayed a strong peak (2.0 × 106) through the extraction of hesperidin formula (C28H33O15), which was equipped in LC–MS software. Owing hesperidin was the old Q-marker and could be only from either Chenpi or Zhishi (Table 3). The great contrast has clearly suggested the old Q-marker hesperidin was absent in CWG 1, implying that both Chenpi and Zhishi were absent in CWG 1. This has successfully recognized the counterfeit regarding both Chenpi and Zhishi. The successful instance further indicated that, our experiment based on LC–MS equipped extraction technology was feasible for anti-counterfeiting validation.
The results of anti-counterfeiting validation experiment of CWG 1–CWG 6. CWG 1, extraction of C28H33O15 (m/z 610, hesperidin); CWG 2, extraction of C42H68O13 (m/z 780, saikosaponin A); CWG 3, extraction of C8H10N4O2 (m/z 194, caffeine); CWG 4, extraction of C12H16O2 (m/z 193, S-senkyunolide A); CWG 5, extraction of C18H18O2 (m/z 266, magnolol); CWG 6, extraction of C30H46O4 (m/z 470.69, 18β-glycyrrhetinic acid). The analytic technology was UHPLC-ESI-Q-TOF–MS/MS
Using the LC–MS equipped extraction technology, CWG 2 was also analyzed in anti-counterfeiting validation experiment. As seen in Fig. 8, CWG 2 gave no saikosaponin A (28) peak; while Wushicha Granule gave a strong peak (7 × 105). This difference suggested that, saikosaponin A was absent in CWG 2; and thus its corresponding CHM Chaihu was absent in CWG 2. Thus, the counterfeit regarding Chaihu has been successfully recognized by the UHPLC-ESI-Q-TOF–MS/MS analysis of Q-marker saikosaponin A (28).
Similarly, the counterfeits involved in Hongcha, Chuanxiong, and Houpo have also been successfully recognized by detection of their corresponding Q-markers, i.e., caffeine (7), S-senkyunolide A (40), magnolol (44), and 18β-glycyrrhetinic acid (45), respectively. Finally, the counterfeit concerning Gancao could be easily recognized by detection of 18β-glycyrrhetinic acid (45), a revised Q-marker. In summary, the validation experiments have successfully recognized 6 counterfeit Wushicha Granules (i.e., CWG 1 ~ CWG 6, Fig. 8), by means of analysis the corresponding Q-markers.
These Q-markers included one old Q-marker (23), one revised Q-marker (45), and 4 new Q-markers (7, 28, 40, and 44). All these have constructed a new Q-markers system and corresponded 7 CHMs (Chenpi, Zhishi, Houpo, Chaihu, Gancao, Hongcha, and Chuanxiong). Accordingly, the Q-markers system can effectively recognize their counterfeits in Wushicha Granule. The Q-markers system, along with experimental description in 2.3 Section and 2.8 Section, have proposed an available procedure for analysis new Q-markers system (Fig. 9). Through analysis of new Q-markers system, it can be judged whether there are counterfeits regarding Houpo, Chaihu, Gancao, Hongcha, Chuanxiong, Chenpi, and Zhishi. Apparently, all these will provide Pharmacopoeia with a reliable, feasible, and effective quality-control method concerning Wushicha Granule.
Finally, it should be noted that, (1) although the so-called “database-aided UHPLC-Q-orbitrap MS/MS putative identification strategy” has laid a solid foundation for Q-markers system update, however, the total amount of identified compounds is lower, compared with other “tentative identification strategy”. This will be improved through expanding the database in future.
(2) As indicated by our UV–vis scanning experiment (Fig. 10), there was no co-wavelength for simultaneous analysis of all 6 Q-markers (7, 23, 28, 40, 44, and 45). All these have constructed). Therefore, the conventional HPLC–UV was not recommended as analytic approach for new Q-markers system.
(3) Platycodin D (38) is specific saponin for Jiegeng. As seen in Table 3, it has also been detected out in the study. However, its testability was not so good, for its low peak response (Fig. 2). Thus, it could be recommended as one optional Q-marker, when the analytic apparatus has high accuracy.
(4) As stated above, Wushicha Granule is a prescription consisting 19 CHMs. One CHM however is well known to enrich a good number of compounds; On other hand, one compound may distribute in different CHMs. This has made the Grandule to become an extremely complicated system, from the perspective chemistry. Thus, it is impossible to characterize all these CHMs. Nonetheless, our new Q-markers system has great improved the efficiency and reliability of quality-assessment method regarding Wushicha Granule in Pharmacopoeia. In the current Pharmacopoeia (2020), there was only one ole Q-marker (23) for reliable HPLC analysis. Aa a result, the total characterizing rate (TCR) was 10.5% (2 ÷ 19); while the specifically characterizing rate (SCR) was 0.0%, according to our recent definition [17]. For new Q-markers system, the TCR and SCR values were calculated as 36.8% (7 ÷ 19) and 26.3% (5 ÷ 19), respectively.
(5) The present study regarding Pharmacopoeia is not identical with the Pharmacopoeia itself. As stated by our tem [15, 19], the studies Pharmacopoeia have neither administrative compulsion nor legal authority. However, these studies can help Pharmacopoeia Commission to find a new and applicable Q-marker. This has highlighted the mission of our studies [15, 17, 19].
Conclusions
By means of a novel database-aided UHPLC-Q-orbitrap MS/MS strategy, 48 non-isomeric compounds have been putatively identified; and two pairs of isomers have been successfully discriminated from Wushicha Granule; while A total of 52 compounds have been found from the Granule. Of these, 18β-glycyrrhetinic acid is recommended to replace the old Q-marker “glycyrrhetinic acid”, to prevent safety-incident. Meanwhile, four compounds (saikosaponin A, caffeine, S-senkyunolide A, and magnolol) are recommended as new Q-markers. Even through low version LC–MS technology, analysis of these Q-markers can effectively recognize six counterfeit Wushicha Granules. Thereby, they can prevent the counterfeiting in Wushicha Granule, and will improve the efficiency and reliability of Pharmacopoeia.
Availability of data and materials
All the data used to support the findings of this study are available from the corresponding author upon reasonable request.
References
Zhou G, Luo Y, Zhang G. A study on the quality criteria for wushicha capsules. Zhongguo Zhong Yao Za Zhi. 1999;24:217–20.
Yu W, Jiang Z, Zhang Z, Jiang L, Liu C, Lu C, Liang Z, Wang G, Yan J. The Wu-Shi-Cha formula protects against ulcerative colitis by orchestrating immunity and microbiota homeostasis. J Ethnopharmacol. 2023;304: 116075.
Chinese-Pharmacopoeia-Commission, Chinese Pharmacopoeia (Part 1). Vol. 1, Beijing: Chinese Medical Science and Technology Press; 2020.
China, N.M.P.A.o. Data Search from National Medical Products Administration of China (Website). National Medical Products Administration of China. 2017, 2022
China-Quality-News-Network. China Quality News Wushicha. 2021. https://www.cqn.com.cn/ms/content/2021-04/25/content_8686378.htm. Accessed 2022
China-Quality-News-Network. China Quality News Wushicha. 2021. https://www.cqn.com.cn/ms/content/2021-06/08/content_8700836.htm. Accessed 2022
Shao Y, Songh LI. Study on the quality standard for Wushi Cha. China For Med Tr. 2009;3:24–7.
Cui Y, Liu T, Zhang Y, Wang R, Liu X, Zhang Q, Yu P, Zhao Y, Yu Z. Simultaneous determination of five bioactive components of Gancao in rat plasma by UHPLC-MS/MS and its application to comparative pharmacokinetic study of incompatible herb pair Gansui-Gancao and Gansuibanxia Decoction. J Pharm Biomed Anal. 2018;159:318–25.
Wang Z, Xia Q, Liu X, Liu W, Huang W, Mei X, Luo J, Shan M, Lin R, Zou D, Ma Z. Phytochemistry, pharmacology, quality control and future research of Forsythia suspensa (Thunb.) Vahl: a review. J Ethnopharmacol. 2018;210:318–39.
Classen-Houben D, Schuster D, Da Cunha T, Odermatt A, Wolber G, Jordis U, Kueenburg B. Selective inhibition of 11β-hydroxysteroid dehydrogenase 1 by 18α-glycyrrhetinic acid but not 18β-glycyrrhetinic acid. J Steroid Biochem Mol Biol. 2009;113:248–52.
Li XC. Solvent effects and improvements in the deoxyribose degradation assay for hydroxyl radical-scavenging. Food Chem. 2013;141:2083–8.
Xie Y, Li X, Xu J, Jiang Q, Xie H, He J, Chen D. Two phenolic antioxidants in Suoyang enhance viability of •OH-damaged mesenchymal stem cells: comparison and mechanistic chemistry. Chem Cent J. 2017. https://doi.org/10.1186/s13065-017-0313-1.
Jiang Q, Li XC, Tian YG, Lin QQ, Xie H, Lu WB, Chi YG, Chen DF. Lyophilized aqueous extracts of Mori Fructus and Mori Ramulus protect Mesenchymal stem cells from *OH-treated damage: bioassay and antioxidant mechanism. BMC Complement Altern Med. 2017;16:423.
Cai R, Li X, Li C, Zhu J, Zeng J, Li J, Tang B, Li Z, Liu S, Yan Y. Standards-based UPLC-Q-exactive orbitrap MS systematically identifies 36 bioactive compounds in ampelopsis grossedentata (Vine Tea). Separations. 2022;9:329.
Liu S, Li X, Cai R, Chen B, Zeng J, Li C, Zhou X, Li Y. UHPLC-quadrupole-exactive-orbitrap-MS/MS-based putative identification for Eucommiae Folium (Duzhongye) and its quality-marker candidate for pharmacopeia. J Sep Sci. 2023;46:00.
Chen B, Li X, Liu J, Qin W, Liang M, Liu Q, Chen D. Antioxidant and cytoprotective effects of Pyrola decorata H. Andres and its five phenolic components. BMC Complement Altern Med. 2019;19:275.
Chen S, Li X, Zeng J, Cai R, Li C, Chen CB. Library-based UHPLC-Q-exactive-orbitrap-MS putative identification of isomeric and non-isomeric bioactives from zibushengfa tablet and pharmacopoeia quality-marker chemistry. J Liq Chromatogr Relat Technol. 2023. https://doi.org/10.1080/10826076.2023.2223640.
Li XC, Zeng J, Cai R, Li C. New UHPLC-Q-orbitrap MS/MS-based library-comparison method simultaneously distinguishes 22 phytophenol isomers from desmodium styracifolium. Microchem J. 2023;190: 108938.
Zeng J, Li X, Cai R, Li C, Chen S. Jinhua Qinggan Granule UHPLC-Q-extractive-Orbitrap-MS assay: putative identification of 45 potential anti-Covid-19 constituents, confidential addition, and pharmacopoeia quality-markers recommendation. J Food Drug Anal. 2023. https://doi.org/10.38212/2224-6614.3466.
Grimme S, Ehrlich S, Goerigk L. Effect of the damping function in dispersion corrected density functional theory. J Comput Chem. 2011;32:1456–65.
Pritchard BP, Altarawy D, Didier B, Gibson TD, Windus TL. New basis set exchange: an open, up-to-date resource for the molecular sciences community. J Chem Inf Model. 2019;59:4814–20.
Zheng YZ, Chen DF, Deng G, Guo R, Fu ZM. The antioxidative activity of piceatannol and its different derivatives: antioxidative mechanism analysis. Phytochemistry. 2018;156:184–92.
Hua Y, Li XC, Zhang W, Chen B, Liu Y, Zhao X, Xie H, Chen D. Antioxidant product analysis of Folium Hibisci Mutabilis. J Saudi Chem. 2021;25: 101272.
Li X, Xie H, Jiang Q, Wei G, Lin L, Li C, Ou X, Yang L, Xie Y, Fu Z, Liu Y, Chen D. The mechanism of (+) taxifolin’s protective antioxidant effect for •OH-treated bone marrow-derived mesenchymal stem cells. Cell Mol Biol Lett. 2017;22:231.
Li XC, Ouyang X, Chen B, Liu S, Zeng J. Linkage and stereochemistry characters of phenolic antioxidant product formation. J Agric Food Chem. 2023;71:5382–90.
Xie H, Li XC, Ren ZX, Qiu WM, Chen JL, Jiang Q, Chen B, Chen DF. Antioxidant and cytoprotective effects of tibetan tea and its phenolic components. Molecules. 2018;23:179.
Li XC. 2-Phenyl-4,4,5,5-tetramethylimidazoline-1-oxyl 3-oxide (PTIO•) radical scavenging: a new and simple antioxidant assay in vitro. J Agric Food Chem. 2017;65:6288–97.
Xue Q, Wang Y, Fei C, Ren C, Li W, Li W, Yin F, Li L. Profiling and analysis of multiple constituents in Crataegi Fructus before and after processing by ultrahigh-performance liquid chromatography quadrupole time-of-flight mass spectrometry. Rapid Commun Mass Spectrom. 2021;35: e9033.
Li S, Lo CY, Pan MH, Lai CS, Ho CT. Black tea: chemical analysis and stability. Food Funct. 2013;4:10–8.
Dong Z, Lu X, Tong X, Dong Y, Tang L, Liu M. Forsythiae Fructus: a review on its phytochemistry, quality control pharmacology and pharmacokinetics. Molecules. 2017;22:1466.
Singh BN, Rawat AK, Bhagat RM, Singh BR. Black tea: phytochemicals, cancer chemoprevention, and clinical studies. Crit Rev Food Sci Nutr. 2017;57:1394–410.
Wang L, Zhang J, Hong Y, Feng Y, Chen M, Wang Y. Phytochemical and pharmacological review of da chuanxiong formula: a famous herb pair composed of chuanxiong rhizoma and gastrodiae rhizoma for headache. Evid Based Complement Alternat Med. 2013;2013: 425369.
Jiang H, Yang L, Hou A, Zhang J, Wang S, Man W, Zheng S, Yu H, Wang X, Yang B, Wang Q, Kuang H. Botany, traditional uses, phytochemistry, analytical methods, processing, pharmacology and pharmacokinetics of Bupleuri Radix: a systematic review. Biomed Pharmacother. 2020;131: 110679.
Chen XP, Li W, Xiao XF, Zhang LL, Liu CX. Phytochemical and pharmacological studies on Radix Angelica sinensis. Chin J Nat Med. 2013;11:577–87.
Zhou X, Zeng L, Chen Y, Wang X, Liao Y, Xiao Y, Fu X, Yang Z. Metabolism of gallic acid and its distributions in tea (Camellia sinensis) plants at the tissue and subcellular levels. Int J Mol Sci. 2020;21:5684.
Li F, Liu B, Li T, Wu Q, Xu Z, Gu Y, Li W, Wang P, Ma T, Lei H. Review of constituents and biological activities of triterpene saponins from Glycyrrhizae Radix et Rhizoma and its solubilization characteristics. Molecules. 2020;25:3904.
Wu L, Tan LX, Gong FF, Xia Y, Chu RG, Yang HS. Promoting effect of the Maillard reaction products produced during the stir-frying process of Hordei Fructus Germinatus on the intestinal absorption of active ingredients in Hordei Fructus Germinatus. Food Sci Biotechnol. 2021;30:631–42.
Shan L, Yang N, Zhao Y, Sheng X, Yang S, Li Y. A rapid classification and identification method applied to the analysis of glycosides in Bupleuri radix and liquorice by ultra high performance liquid chromatography coupled with quadrupole time-of-flight mass spectrometry. J Sep Sci. 2018;41:3791–805.
Wu Y, Jiang X, Zhang S, Dai X, Liu Y, Tan H, Gao L, Xia T. Quantification of flavonol glycosides in Camellia sinensis by MRM mode of UPLC-QQQ-MS/MS. J Chromatogr B. 2016;1017–1018:10–7.
Qiu J, Hu SY, Zhang CH, Shi GQ, Wang SE, Xiang T. The effect of Chaihu-Shugan-San and its components on the expression of ERK5 in the hippocampus of depressed rats. J Ethnopharmacol. 2014;152:320–6.
Wang C, Chen L, Xu C, Shi J, Chen S, Tan M, Chen J, Zou L, Chen C, Liu Z, Liu X. A comprehensive review for phytochemical, pharmacological, and biosynthesis Studies on Glycyrrhiza spp. Am J Chin Med. 2020;48:17–45.
Luo H, Wu H, Yu X, Zhang X, Lu Y, Fan J, Tang L, Wang Z. A review of the phytochemistry and pharmacological activities of Magnoliae officinalis cortex. J Ethnopharmacol. 2019;236:412–42.
Leng G, Wang H, Ming D. Identification and content determination of (+)-pinoresinol in Forsythiasuspensa. J Shanxi Med Univ. 2003;3:227–8.
Fei C, Dai H, Wu X, Li L, Lu T, Li W, Cai B, Yin W, Yin F. Quality evaluation of raw and processed Crataegi Fructus by color measurement and fingerprint analysis. J Sep Sci. 2018;41:582–9.
Rayyan S, Fossen T, Solheim Nateland H, Andersen ØM. Isolation and identification of flavonoids, including flavone rotamers, from the herbal drug Crataegi folium cum Flore (Hawthorn). Phytochem Anal. 2005;16:334–41.
Ma K, Tian P, Zhang D, Li J, Gao Q. Simultaneous determination of nine components in Massa Medicata Fermentata by HPLC with wavelength switching (in Chinese). Chin J Pharm Anal. 2019;39:526–30.
Wu J, Huang G, Li Y, Li X. Flavonoids from Aurantii Fructus Immaturus and Aurantii Fructus: promising phytomedicines for the treatment of liver diseases. Chin Med. 2020;15:89.
Liu EH, Zhao P, Duan L, Zheng GD, Guo L, Yang H, Li P. Simultaneous determination of six bioactive flavonoids in Citri Reticulatae Pericarpium by rapid resolution liquid chromatography coupled with triple quadrupole electrospray tandem mass spectrometry. Food Chem. 2013;141:3977–83.
Zhao BT, Lee KR, Lee JH, Min BS, Son JK, Woo MH. Quality evaluation of Perillae Folium by HPLC/PDA. Arch Pharm Res. 2015;38:1521–9.
Ho SC, Kuo CT. Hesperidin, nobiletin, and tangeretin are collectively responsible for the anti-neuroinflammatory capacity of tangerine peel (Citri reticulatae pericarpium). Food Chem Toxicol. 2014;71:176–82.
Yu XA, Li J, Azietaku JT, Liu W, He J, Chang YX. A Single standard to determine multi-components method coupled with chemometric methods for the quantification, evaluation and classification of Notopterygii Rhizoma et Radix from different regions. Molecules. 2019;24:3574.
Zeng P, Yi Y, Su HF, Ye CY, Sun YW, Zhou XW, Lu Y, Shi A, Tian Q. Key phytochemicals and biological functions of chuanxiong rhizoma against ischemic stroke: a network pharmacology and experimental assessment. Front Pharmacol. 2021;12: 758049.
Yuan B, Yang R, Ma Y, Zhou S, Zhang X, Liu Y. A systematic review of the active saikosaponins and extracts isolated from Radix Bupleuri and their applications. Pharm Biol. 2017;55:620–35.
Yin F, Li L, Chen Y, Lu T, Li W, Cai B, Yin W. Quality control of processed Crataegi Fructus and its medicinal parts by ultra high performance liquid chromatography with electrospray ionization tandem mass spectrometry. J Sep Sci. 2015;38:2630–9.
Cai L, Lun J, Liu Y, Guo X. Separation and quantitation of notopterol enantiomers in notopterygii rhizoma et radix using solid-phase extraction coupled with liquid chromatography-tandem mass spectrometry. J Pharm Biomed Anal. 2020;186: 113255.
Zhang LL, Huang MY, Yang Y, Huang MQ, Shi JJ, Zou L, Lu JJ. Bioactive platycodins from Platycodonis Radix: phytochemistry, pharmacological activities, toxicology and pharmacokinetics. Food Chem. 2020;327: 127029.
Gong AG, Li N, Lau KM, Lee PS, Yan L, Xu ML, Lam CT, Kong AY, Lin HQ, Dong TT, Tsim KW. Calycosin orchestrates the functions of Danggui Buxue Tang, a Chinese herbal decoction composing of Astragali Radix and Angelica Sinensis Radix: an evaluation by using calycosin-knock out herbal extract. J Ethnopharmacol. 2015;168:150–7.
Lin HQ, Gong AG, Wang HY, Duan R, Dong TT, Zhao KJ, Tsim KW. Danggui Buxue Tang (Astragali Radix and Angelicae Sinensis Radix) for menopausal symptoms: a review. J Ethnopharmacol. 2017;199:205–10.
Hu Y, Wang C. Extraction by microwave and determination by HPLC of 3,5,6, 7, 8, 3’,4’-heptamethphoxy flavone from citrus reticulata. J Yantai Univ. 2005;18:45–9 (in Chinese).
Liu S, Jin X, Shang Y, Wang L, Du K, Chen S, Li J, He J, Fang S, Chang Y. A comprehensive review of the botany, ethnopharmacology, phytochemistry, pharmacology, toxicity and quality control of Perillae Fructus. J Ethnopharmacol. 2023;304: 116022.
Tsai TH, Chen CF. High-performance liquid chromatographic determination of 18 α-glycyrrhetinic acid and 18 β-glycyrrhetinic acid in rat plasma: application to pharmacokinetic study. J Chromatogr. 1991;567:405–14.
Sun J, Li H, Sun J, Liu H, Chen J, Wang C. Chemical composition and antimigraine activity of essential oil of Angelicae dahuricae radix. J Med Food. 2017;20:797–803.
Azietaku JT, Ma H, Yu XA, Li J, Oppong MB, Cao J, An M, Chang YX. A review of the ethnopharmacology, phytochemistry and pharmacology of Notopterygium incisum. J Ethnopharmacol. 2017;202:241–55.
Kusari S, Sezgin S, Nigutova K, Cellarova E, Spiteller M. Spatial chemo-profiling of hypericin and related phytochemicals in Hypericum species using MALDI-HRMS imaging. Anal Bioanal Chem. 2015;407:4779–91.
Hou T, Netala VR, Zhang H, Xing Y, Li H, Zhang Z. Perilla frutescens: a rich source of pharmacological active compounds. Molecules. 2022;27:3578.
Haijie ZF. Determination of five components in Shenling Baizhu granules by QAMS. J Shenyang Pharma Univ. 2021;38:1144–51.
Qin LCZ. Study on HPLC characteristic fingerprint of Shenlingbaizhu powder and simultaneous determination of its five indicative components. China Pharmaceuticals. 2018;27:12–6.
Shi-jie WY. Determination of licorice flavonoids and ginseng saponins in Shenling Baizhu powder and antioxidant tests. Contem Chem Indu. 2020;49:1301–4.
Yilun LJ. Simultaneous determination of six constituents in Shenling Baizhu Pulvis and establishment of fingerprints by UPLC. China Pharm. 2019;22:214–8.
Xu X, Wang W, Chen Y, Zhang Q, Li B, Zhong Y, Tu Y, Zhang W, Xu G, Jiang L. Simultaneous determination of ten bioactive components from Shenling Baizhu San in Rat plasma by UHPLC-MS/MS: application to a comparative pharmacokinetic study in normal and two models of ulcerative colitis rats. Evid Based Complement Alternat Med. 2021;2021:3518241.
Wang P, Zhong L, Yang H, Zhang J, Hou X, Wu C, Zhang R, Cheng Y. Comprehensive comparative analysis of lipid profile in dried and fresh walnut kernels by UHPLC-Q-exactive Orbitrap/MS. Food Chem. 2022;386: 132706.
Wang N, Yang B, Zhang J, Zheng Y, Wang S, Zhang X, Situ H, Lin Y, Wang Z. Metabolite profiling of traditional Chinese medicine XIAOPI formula: an integrated strategy based on UPLC-Q-Orbitrap MS combined with network pharmacology analysis. Biomed Pharmacother. 2020;121: 109569.
Duan H, Wang GC, Khan GJ, Su XH, Guo SL, Niu YM, Cao WG, Wang WT, Zhai KF. Identification and characterization of potential antioxidant components in Isodon amethystoides (Benth). Hara tea leaves by UPLC-LTQ-Orbitrap-MS. Food Chem Toxicol. 2021;148:111961.
Liu CX, Liu L, Guo DA. Quality marker of TCMs: concept and applications. Phytomedicine. 2018;44:85–6.
Zhang T, Bai G, Chen C, Xu J, Han Y, Gong S, Zhang H, Liu C. Research approaches of quality marker (Q-marker) of Chinese materia medica formula based on five principles. Zhong Yao Cai. 2018;49:1–13.
Ventura J, Gutierrez-Sanchez G, Rodriguez-Herrera R, Aguilar CN. Fungal cultures of tar bush and creosote bush for production of two phenolic antioxidants (pyrocatechol and gallic acid). Folia Microbiol (Praha). 2009;54:199–203.
Murugesan A, Holmstedt S, Brown KC, Koivuporras A, Macedo AS, Nguyen N, Fonte P, Rijo P, Yli-Harja O, Candeias NR, Kandhavelu M. Design and synthesis of novel quinic acid derivatives: in vitro cytotoxicity and anticancer effect on glioblastoma. Future Med Chem. 2020;12:1891–910.
Deshpande S, Matei MF, Jaiswal R, Bassil BS, Kortz U, Kuhnert N. Synthesis, structure, and tandem mass spectrometric characterization of the diastereomers of quinic acid. J Agric Food Chem. 2016;64:7298–306.
Karagoz P, Mandair R, Manayil JC, Lad J, Chong K, Kyriakou G, Lee AF, Wilson K, Bill RM. Purification and immobilization of engineered glucose dehydrogenase: a new approach to producing gluconic acid from breadwaste. Biotechnol Biofuels. 2020;13:100.
Ma Y, Li B, Zhang X, Wang C, Chen W. Production of gluconic acid and its derivatives by microbial fermentation: process improvement based on integrated routes. Front Bioeng Biotechnol. 2022;10: 864787.
Upadhyay P, Lali A. Protocatechuic acid production from lignin-associated phenolics. Prep Biochem Biotechnol. 2021;51:979–84.
Li J, Ye BC. Metabolic engineering of Pseudomonas putida KT2440 for high-yield production of protocatechuic acid. Bioresour Technol. 2021;319: 124239.
Huang Y, Wu Y, Yin H, Du L, Chen C. Senkyunolide I: a review of its phytochemistry, pharmacology, pharmacokinetics, and drug-likeness. Molecules. 2023;28:3636.
Steinbrook RA, Garfield F, Batista SH, Urman RD. Caffeine for the prevention of postoperative nausea and vomiting. J Anaesthesiol Clin Pharmacol. 2013;29:526–9.
Sui C, Han WJ, Zhu CR, Wei JH. Recent progress in Saikosaponin biosynthesis in bupleurum. Curr Pharm Biotechnol. 2021;22:329–40.
Acknowledgements
Not applicable.
Funding
None.
Author information
Authors and Affiliations
Contributions
XL contributed to the project design and paper writing. SC, JZ, RC, and YL contributed to analysis experiments. CC contributed to literature review; BC contributed to computational chemistry. CL contributed to data analyses. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Additional file 1.
Identification of D-Gluconic acid (Cas 526-95-4, C6H12O7, M.W.196).
Additional file 2.
Identification of Quinic acid (Cas 77-95-2, C7H12O6, M.W. 192.17).
Additional file 3.
Identification of Gallic acid (Cas 149-91-7, C7H6O5, M.W. 170.12).
Additional file 4.
Identification of protocatechuate (Cas 99-50-3, C7H6O4, M.W. 154.12).
Additional file 5.
Identification of 5-Caffeoylquinic acid (Cas 906-33-2, C16H18O9, M.W. 354.311)
Additional file 6.
Identification of methyl gallate (Cas 99-24-1, C8H8O5, M.W. 184.147).
Additional file 7.
Identification of Caffeine (Cas 58-08-2,C8H10N4O2, M.W.194.191).
Additional file 8.
Identification of puerarin (Cas 3681-99-0, C21H20O9, M.W. 416.38).
Additional file 9.
Identification of Vicenin-2 (Cas 23666-13-9, C27H30O15, M.W. 594.52).
Additional file 10.
Identification of schaftoside (Cas 51938-32-0, C26H28O14, M.W. 564.5)
Additio.nal file 11.
Identification of Myricetin 3-O-galactoside (Cas 15648-86-9, C21H20O13, M.W. 480.37).
Additional file 12.
Identification of Liquiritin (Cas 551-15-5, C21H22O9, M.W. 418.4).
Additional file 13.
Identification of Vitexin (Cas 3681-93-4, C21H20O10, M.W. 432).
Additional file 14.
Identification of Acteoside (Cas 61276-17-3, C29H36O15, M.W. 624.59).
Additional file 15.
Identification of Scoparone (Cas 120-08-1, C11H10O4 M.W.206.19).
Additional file 16.
Identification of (-)-Pinoresinol (Cas 81446-29-9, C20H22O6, M.W.358.39).
Additional file 17.
Identification of isovitexin (Cas 38953-85-4, C21H20O10, M.W. 432.3775).
Additional file 18.
Identification of isoquercitrin (Cas 21637-25-2, C21H20O12, M.W. 464.38).
Additional file 19.
Identification of rutin (Cas 153-18-4, C27H30O16, M.W. 610.52).
Additional file 20.
Identification of Naringin (Cas 10236-47-2, C27H32O14, M.W.580.53).
Additional file 21.
Identification of Rosmarinic acid (Cas 20283-92-5, C18H16O8, M.W. 360.31).
Additional file 22.
Identification of Hesperidin (Cas 520-26-3, C28H34O15, M.W.610.565).
Additional file 23.
Identification of Isochlorogenic acid A (Cas 2450-53-5, C25H24O12, M.W.516.45).
Additional file 24.
Identification of Myricetin (Cas 529-44-2, C15H10O8, M.W. 318.24).
Additional file 25.
Identification of Astragalin (Cas 480-10-4, C21H20O11, M.W. 448.38).
Additional file 26.
Identification of Isorhamnetin-3-o-β-d-glucoside (Cas 5041-82-7, C22H22O12, M.W. 478.4).
Additional file 27.
Identification of Saikosaponin A (Cas 20736-09-8, C42H68O13, M.W. 780.982).
Additional file 28.
Identification of Daidzein (Cas 486-66-8, C15H10O4, M.W. 254.24).
Additional file 29.
Identification of Quercetin (Cas 117-39-5, C15H10O7, M.W. 302.23).
Additional file 30.
Identification of 7,4'-dihydroxyflavone (Cas 2196-14-7, C15H10O4, M.W. 254).
Additional file 31.
Identification of S-Naringenin (Cas 480-41-1, C15H12O5, M.W.272.25).
Additional file 32.
Identification of naringenin chalcone (Cas 73692-50-9, C15H12O5, M.W. 272.25).
Additional file 33.
Identification of Luteolin (Cas 491-70-3, C15H10O6, M.W.286.24).
Additional file 34.
Identification of Hesperetin (Cas 520-33-2, C16H14O6, M.W. 302.28).
Additional file 35.
Identification of Randaiol (Cas 87562-14-9, C15H14O3, M.W. 242.27).
Additional file 36.
Identification of Isoliquiritigenin (Cas 961-29-5, C15H12O4, M.W. 256.257).
Additional file 37.
Identification of Platycodin D (Cas 58479-68-8, C57H92O28, M.W. 1225.3).
Additional file 38.
Identification of Formononetin (Cas 485-72-3, C16H12O4, M.W. 268.26).
Additional file 39.
Identification of Senkyunolide A (Cas 63038-10-8, C12H16O2, M.W. 192.25).
Additional file 40.
Identification of 3,5,6,7,8,3,4,-7-Methoxy-2-phenyl-4H-chromen-4-one (Cas 1178-24-1, C22H24O9, M.W. 432.421).
Additional file 41.
Identification of Licoricesaponin H2 (Cas 118441-85-3, C42H62O16, M.W. 822.93).
Additional file 42.
Identification of 5-Hydroxyflavone (Cas 491-78-1, C15H10O3, M.W. 238.24).
Additional file 43.
Identification of Magnolol (Cas 528-43-8, C18H18O2, M.W. 266.32).
Additional file 44.
Identification of 18β-glycyrrhetinic acid and exclusion of 18α- glycyrrhetinic acid.
Additional file 45.
Identification of linoleic acid (Cas 60-33-3, C18H32O2, M.W. 280.4).
Additional file 46.
Identification of palmitic Acid (Cas 57-10-3, C16H32O2, M.W. 256.4).
Additional file 47.
Identification of Oleic Acid (Cas 112-80-1, C18H34O2, M.W. 282.468).
Additional file 48.
Identification of palmitic acid ethyl ester (Cas 628-97-7, C18H36O2, M.W. 284.484).
Additional file 49.
Identification of Ethyl Stearate (Cas 111-61-5, C20H40O2, M.W. 312.53).
Additional file 50.
Identification of Hypericin (Cas 548-04-9, C30H16O8, M.W. 504.45).
Additional file 51.
Identification of (+)-4-Cholesten-3-one (Cas 601-57-0, C27H44O, M.W. 384.65).
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Li, X., Chen, S., Zeng, J. et al. Database-aided UHPLC-Q-orbitrap MS/MS strategy putatively identifies 52 compounds from Wushicha Granule to propose anti-counterfeiting quality-markers for pharmacopoeia. Chin Med 18, 116 (2023). https://doi.org/10.1186/s13020-023-00829-2
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s13020-023-00829-2