Abstract
An ongoing outbreak of severe respiratory illness and pneumonia caused by the severe acute respiratory coronavirus 2 (SARS-CoV-2) commenced in December 2019, and the disease was named as coronavirus disease 2019 (COVID-19). Soon after, scientists identified the characteristics of SARS-CoV-2, including its genome sequence and protein structure. The clinical manifestations of COVID-19 have now been established; and nucleic acid amplification is used for the direct determination of the virus, whereas immunoassays can determine the antibodies against SARS-CoV-2. Clinical trials of several antiviral drugs are ongoing. However, there is still no specific drugs to treat COVID-19. Traditional Chinese medicine (TCM) was used in the treatment of COVID-19 during the early stages of the outbreak in China. Some ancient TCM prescriptions, which were efficacious in the treatment of severe acute respiratory syndrome (SARS) in 2002–03 and the influenza pandemic (H1N1) of 2009, have been improved by experienced TCM practitioners for the treatment of COVID-19 based on their clinical symptoms. These developed new prescriptions include Lianhua Qingwen capsules/granules, Jinhua Qinggan granules and XueBiJing injection, among others. In this review, we have summarized the presenting features of SARS-CoV-2, the clinical characteristics of COVID-19, and the progress in the treatment of COVID-19 using TCMs.
Similar content being viewed by others
Introduction
Coronavirus disease 2019 (COVID-19), an acute respiratory infectious disease caused by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), was first reported in December 2019 [1]. As of June 16, 2021, over 176,156,662 cases of COVID-19 and more than 3,815,486 deaths have been confirmed worldwide, which includes over 116,665 cases and more than 5306 deaths in China alone [2]. COVID-19 was officially declared a pandemic by the World Health Organization (WHO) on March 11, 2020 [3]. At present, there are no specific drugs to treat COVID-19 [4]. Most treatment schemes proposed by Chinese clinicians stemmed from the extension and improvement of their experience in treating severe acute respiratory syndrome (SARS), middle east respiratory syndrome (MERS) and the influenza pandemic (H1N1) of 2009 earlier this century.
China has a long history of the use of traditional Chinese medicine (TCM) of over 2000 years [5]. This system of treatment has widely spread to Japan, South Korea and other countries [6]. TCMs, including Sang Ju Yin and Yu Ping Feng San among others, have been used in the prevention and treatment of SARS and H1N1 [7]. In a controlled clinical trial [8] performed in 11 hospitals in Hong Kong, China, none of the 1063 health care workers who used the herbal supplement consisting of Sang Ju Yin formula and Yu Ping Feng San formula [9] for two weeks contracted SARS compared to 0.4% of the 36,111 health care workers who did not receive the supplement. During the COVID-19 wave, Chinese clinicians summarized the previous outcomes of the use of TCM in the treatment of viral infections, thoroughly explored several ancient classics formulae, combined their use with clinical practice, and screened six effective prescriptions, namely, Lianhua Qingwen capsules/granules, Jinhua Qinggan granules, XueBiJing injection, Lung Cleansing and detoxifying decoction, Huashibaidu formula and Xuanfeibaidu granules [10]. As of March 23, 74,187 of the confirmed cases of COVID-19 in China, including 61,449 cases in Hubei Province, used TCM [10]. The early clinical effects showed that for mild and moderate patients, it was easy to recover after treatment using TCM; moreover, the progression of the infection from moderate to severe was significantly reduced in patients. In severe and critical patients, TCM therapy could stabilize the blood oxygen saturation, improve dyspnea, and exert a certain auxiliary effect [11]. Whether TCM can be used in the clinical treatment of viral infections depends on two main aspects, namely, the clinical symptoms of patients, and the efficacy of TCM and its previous effects [12]. In this review, we summarize the features of coronaviruses (CoVs), the presenting clinical characteristics of COVID-19, and its treatment using TCMs, which will be helpful to clinicians and medical practitioners.
Features of CoVs
CoVs are enveloped positive-sense single-stranded RNA viruses [13]. CoVs belong to the Coronavirinae subfamily, Coronaviridae family, and the order Nidovirales. They are divided into four genera, namely, α, β, γ, and δ, among which mainly α and β CoVs infect mammals [14]. CoVs from γ and δ genera mainly infect birds [15]. Prior to 2019, the following six species were known to infect humans: human coronavirus (HCoV)-NL63 and HCoV-229E (belonging to genus α); HCoV-HKU1, HCoV-OC43, SARS-CoV and MERS-CoV (belonging to genus β) [16]. Apart from SARS-CoV and MERS-CoV, the other four HCoVs mainly cause self-limiting diseases [17]. The SARS-CoV and MERS-CoV outbreaks in 2002–03 and 2012, respectively, caused severe acute respiratory illnesses [18]. Currently, SARS-CoV-2 is the seventh HCoV that has been identified, and belongs to the genus β [19].
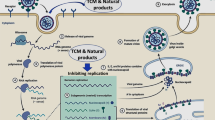
The size of the CoVs genome ranges from 26–32 Kb, and is currently the largest genome known among RNA viruses [20]. It contains 7–10 different open reading frames (ORFs) with methylation at the 5ʹ cap structure and 3ʹ polyadenylation [20]. CoVs contain four major structural proteins, namely, the spike (S), membrane (M), envelope (E), and nucleocapsid (N) protein, as well as a number of accessory ORF proteins [21]. The S protein is a large oligomeric transmembrane protein responsible for receptor binding and cell fusion [21]. The M protein participates in budding and envelope formation, and plays a key role in virus assembly [22]. SARS-CoV-2 shares 76.7%, 33.8% and 96.2% overall genome sequence identity with SARS-CoV, MERS-CoV and bat CoV RaTG13, respectively [18]. A structural study of the S protein suggests that the SARS-CoV-2 S protein retains sufficient affinity to the human angiotensin-converting enzyme 2 (ACE2) protein [23], and uses ACE2 protein as the primary receptor for cellular entry (Fig. 1), as that for SARS-CoV S protein [24].
Clinical characteristics of COVID-19
COVID-19 symptoms
Huang et al. [25] reported that the common symptoms were fever (98%), cough (76%), and myalgia or fatigue (44%) at the onset of the infection, based on the presentation of 41 COVID-19 patients in Wuhan by Jan 2, 2020. Sputum production (28%), headache (8%), hemoptysis (5%), and diarrhea (3%) were rare. All 41 patients had pneumonia and abnormal imaging patterns, which were observed in chest computerized tomography (CT) scans. The plasma concentrations of interleukin (IL) 2, IL7, IL10, granulocyte-colony stimulating factor (G-CSF), IFN-γ-inducible protein 10 (IP-10/CXCL10), monocyte chemoattractant protein-1 (MCP-1/CCL2), macrophage inflammatory protein-1α (MIP-1α/CCL3), and tumor necrosis factor-α (TNF-α) were higher in patients in the intensive care unit (ICU) than that of non-ICU patients. The report published by Guan et al. [26] was a retrospective study comprising 1099 patients with laboratory-confirmed COVID-19, which found that the most common symptoms were fever (43.8% on admission and 88.7% during hospitalization) and cough (67.8%); while vomiting (5.0%) and diarrhea (3.8%) were rare. About 23.7% of patients had at least one coexisting illness (e.g., hypertension or chronic obstructive pulmonary disease) and 86.2% (840/975) patients on admission were found to have abnormalities based on the results of their CT scan. Among them, 56.4% of chest CT scans reveals a ground-glass opacity pattern, while 51.8% had bilateral patchy shadowing. In addition, there were many asymptomatic patients who did not exhibit fever, fatigue or other clinical manifestations, other than positive nucleic acid or antibody tests. Such asymptomatic patients can also spread infections. Since their condition is stable and the viral load is relatively low, the transmission ability is relatively weak [27].
Diagnosis of COVID-19
As the symptoms of COVID-19 and other viral pneumonia may overlap, nucleic acid detection using real-time polymerase chain reaction (RT-PCR), is considered essential in the diagnosis [28]. However, false-negative RT-PCR test results [29] lead to inconsistencies between clinical symptoms and findings of lung imaging. Additionally, the process of RT-PCR is time consuming (usual 2–3 h). Compared to RT-PCR, CT imaging for chest is more popular and accurate, and has the added advantage of the ease of operation [30]. Moreover, it can be used to obtain near real-time images, helping medical practitioners promptly assess the lung condition of patients. However, CT imaging may not be a useful tool to differentiate between COVID-19 and other viral pneumonia; thus it cannot completely replace nucleic acid detection [27, 31]. It is well known that immunoglobulin (Ig) M is produced in the early stages of viral infections, which can indicate current or the most recent infection [32]. IgG is the main antibody response for long-term immunity and immunological memory, and indicates that either the disease is in its recovery period or there is a prior infection [32]. Therefore, the presence of the IgM and IgG antibodies could likely indicate a SARS-CoV-2 infection [33]. Several clinical test statistics show that the combined IgM-IgG antibody detection can effectively reduce false-negative results during nucleic acid detection [34]. However, there are some false-positive results while performing an IgM-IgG immunoassay because (1) some weak positive results near the Cut-off value are likely to be misinterpreted as false positive; (2) the presence of endogenous or exogenous interfering substances in patient samples could lead to false-positive results [35].
Treatment of COVID-19 with TCM
So far, no therapeutic agents have been proven to be effective for SARS-CoV-2-caused diseases [36]. TCM is based on the central premise that when pathogens invade the body to cause diseases, they reduce the immune function of the body [37]. In general, the reduced immune function is further weakened if only simple anti-pathogen treatment is used. TCM improves immune function by improving the body’s resistance to disease, physical conditions and by reducing the side effects of Western medicine [38]. Based on China’s experience in using TCM for more than 2000 years and its success in the treatment of patients during the 2002–03 SARS and 2009 H1N1 epidemics [39,40,41], some TCM prescriptions have been rapidly developed and used for the management of COVID-19 [42,43,44,45,46]. Moreover, some bioactive components, such as glycyrrhizic acid, a component of licorice [47], baicalin, a flavonoid [48], and ginsenosides [49], have been reported to inhibit the replication of SARS-CoV, indicating their potential in the treatment of COVID-19.
Lianhua Qingwen (LH) capsules/granules
Lianhua Qingwen (LH) is a Chinese patent medicine produced by the reduction of a combination of Yinqiao San and Maxing Shigan decoctions [50]. Maxing Shigan decoction was originally described in a classic Chinese medicine book, Shanghan Lun written by Zhang Zhongjing of the Eastern Han Dynasty about 1800 years ago [51]. Its ingredients include Ephedra sinica Stapf (6 g), Prunus amygdalus Batsch (9 g), Gypsum (24 g) and Glycyrrhiza glabra L. (6 g). Yinqiao San is a prescription from the TCM monograph,Wen bing Tiao bian, of the Qing Dynasty about 300 years ago [52]. Its main ingredients include Forsythia suspensa (Thunb.) Vahl (30 g), Lonicera japonica Thunb. (30 g), Platycodon grandiflorus (Jacq.) A.DC. (18 g), Mentha canadensis L. (18 g), Bamboo leaf (12 g), Glycyrrhiza glabra L. (15 g), Brassica juncea (L.) Czern. (12 g), Tempeh (15 g) and Arctium lappa L. (18 g). LH is a prescription formulated under the TCM guidance of collateral disease theory and the treatment method of “clearing away plague and detoxification”, and composed of 13 herbs [53], including Isatis tinctoria L. (Banlangen), Forsythiae fructus (Lianqiao), Lonicera japonica Thunb. (Jinyinhua), Dryopteris crassirhizoma Nakai (Mianmaguanzhong), Ephedra sinica Stapf (Mahuang), Prunus armeniaca L. (Kuxingren), Houttuynia cordata Thunb. (Yuxingcao), Pogostemon cablin (Blanco) Benth. (Guanghuoxiang), Rhodiola crenulata (Hook.f. & Thomson) H. Ohba (Hongjingtian), Rheum officinale Baill. (Dahuang), Glycyrrhiza inflata Batalin (Gancao), Gypsum Fibrosum (Shigao), and l-Menthol (Bohenao) (Table 1).
LH has been recommended for the treatment of influenza A viral infection [54] and influenza complicated with bronchial pneumonia in humans [55]. LH exerts a broad-spectrum antiproliferative effect against influenza viruses, including those that caused the H1N1 and H7N9 pandemics. LH exerts its effects by blocking the early cell entry of the virus in the lung, and suppresses the virus-induced NF-kB activation and release of the inflammatory cytokines, including IL-6, TNF-α, and MCP-1, thereby particularly improving the immune response to prevent viral infection [56] (Fig. 2). Besides, the role of LH in the treatment of 2002–03 SARS in China [57] preliminarily affirmed its efficacy in the management of coronavirus infection. In addition, LH is efficacious in the animal models of MERS.
The replication cycle of SARS-CoV-2 and the targets of various therapeutic drugs. SARS-CoV-2 recognizes the ACE2 receptor on the host cell membrane via the RBD of spike protein, and enters host cell. Once uncoating, the viral genomic RNA will be used to synthesize the genomic RNA (- sense). Next, the genomic RNA is used as a template to form genomic and subgenomic mRNA (+ sense). The viral RNA and nucleocapsid protein are synthesized in the cytoplasm, while the viral spike, envelop, membrane proteins are transcribed and translated in the ER. After that, the SARS-CoV-2 are assembled to form mature virion and encapsulated in the Golgi vesicle. Viruses are released outside the host cell membrane via vesicle-mediated exocytosis, and further activate host immune system. RBD receptor-binding domain, ACE2 angiotensin-converting enzyme 2, Fc immunoglobulin fragment, scFv single-chain variable region fragments against spike protein of SARS-CoV2, ER endoplasmic reticulum
Dong et al. [58] investigated the effects of LH on airway inflammation in 100 patients with acute exacerbation of chronic obstructive pulmonary disease. They reported that LH treatment decreased the expression of IL-8, TNF-α, IL-17, and IL-23 in the sputum, and of IL-8 and IL-17 in the blood, suggesting that the mechanism of action of LH was related to the decreased release of the inflammatory mediators. A retrospective, not double blind study comprising 42 patients with COVID-19 in three hospitals in Wuhan was performed to compare the effect of the combination of LH plus standard care (21 patients) and standard care alone (21 patients). The result showed that the disappearance rate of fever (85.7% vs 57.1%), cough (46.7% vs 5.6%), expectoration (64.3% vs 9.1%), and shortness of breath (77.8% vs 0) in the combined treatment group was better (P < 0.05) than that in the control group. The duration of fever in the combined treatment group was 1.5 days shorter than that in the control group (4.6 ± 3.2 vs 6.1 ± 3.1) [59]. Another multicentre retrospective, not double blind study compared the clinical data of 51 patients receiving LH combined with standard care and 51 receiving standard care alone in three hospitals in Wuhan during January 2020. The combined treatment showed a better (P < 0.05) therapeutic effect than the control in the disappearance rate of fever (83.7% vs 61.0%), fatigue (61.3% vs 34.3%), cough (62.2% vs 35.9%), expectoration (55.0% vs 15.8%), shortness of breath (61.5% vs 14.3%), chest distress (54.6% vs 15.8%), anorexia (34.8% vs 7.7%), and the rate of progressing to severe symptoms (7.8% vs 21.6%). There was no difference in lung improvement rate (54.9% vs 45.1%, P > 0.05) [60]. A prospective, multicentre, open-label randomized controlled trial evaluating LH capsules in 284 confirmed cases of COVID-19 was performed [61]. Patients were randomized to receive standard treatment alone (142 patients) or a combination of standard treatment and LH capsules (4 capsules, thrice daily, 142 patients) for 14 days. The combined treatment group exhibited a higher recovery rate than the control group (91.5% vs 82.4%, P = 0.022). The median time to symptom recovery, recovery from fever, fatigue, and cough was significantly shorter in the combined treatment group versus the control group (median: 7 vs 10 days, 2 vs 3 days, 3 vs 6 days, 7 vs 10 days, respectively, all P ≤ 0.001). The recovery rate of abnormal manifestations within the chest evaluated by chest CT (83.8% vs 64.1%, P ≤ 0.001) and clinical cure (78.9% vs 66.2%, P = 0.017) was markedly higher in the treatment group compared to the control group, respectively. There was no difference in the rate of conversion to severe cases and in the median viral assay conversion time between the two groups. These results indicated that LH was effective in improving fever, cough, and other symptoms in patients with a mild infection of COVID-19. A meta-analysis of 154 COVID-19 patients showed that the disappearance rate of the main clinical symptoms (fever, cough and fatigue) was higher in LH treated group, and the disappearance rate of other clinical secondary symptoms (runny nose, sputum, nasal congestion, muscle pain, difficulty breathing, chest tightness, nausea and vomiting, and loss of appetite) was also higher, while the duration of fever was significantly reduced by LH treatment [62]. In addition, results from a recent meta-analysis of 8 clinical trials with 924 COVID-19 patients indicated that patients treated by LH combined with conventional treatment (e.g. oxygen therapy, antiviral, antimicrobial) had a higher overall effective rate and CT recovery rate than those with conventional treatment [63].
The antiviral activity of LH in SARS-CoV-2 was studied using Vero E6 cells. LH significantly inhibited the replication of SARS-CoV-2 obtained from a clinical isolated and reduced mRNA levels of the pro-inflammatory cytokines, including TNF-α, IL-6, MCP-1/CCL2, and IP-10/CXCL10, suggesting that LH might inhibit the cytokine storm induced by SARS-CoV-2 [43]. According to a recent study published on ChemRxiv [64], 20 components in LH were identified using ultra-performance liquid chromatography coupled to quadrupole time of flight mass spectrometry. The active components of LH include rutin, forsythoside E, hyperoside, liquiritin apioside, emodin, chlorogenic acid, amygdalin, cryptochlorogenic acid, isoliquiritin apioside, neochlorogenic acid, chrysophanol-8-O-glucoside, rhein, isoliquiritin, emodin 8-O-β-d-glucoside, sweroside, formononetin, salidroside, liquiritigenin, loqanic acid, and secolologanin, among others. Among them, three compounds, namely hyperoside, rutin, and forsythoside, were found to be bound with the main protease of SARS-CoV-2 and had docking scores of at − 9.1, − 9.0 and − 8.7 kcal/mol, respectively, which were better than that of lopinavir (− 7.3 kcal/mol). Using the component-target-pathway network analysis, the authors indicated that LH alleviates the symptoms of COVID-19 by activating the antiviral and anti-inflammatory responses of cells. LH has been recommended by the Evidence-Based Medicine Chapter of China International Exchange and Promotive Association for Medical and Health Care (CPAM) to treat patients with mild or moderate COVID-19 in combination with conventional therapy [65], and some clinical evidences showed the conjunction therapy could improve COVID-19 patients, suggesting LH would be beneficial as a supplymentary strategy for treating COVID-19.
Jinhua Qinggan (JH) granules
JH was developed during the H1N1 epidemic in 2009 [66]. This prescription is composed of two prescriptions, namely Maxing Shigan decoction and Yinqiao San, and is similar to LH [67]. JH comprises 12 herbs, including Lonicera japonica Thunb. (Jinyinhua), Gypsum Fibrosum (Shigao), Ephedra sinica Stapf (Mahuang), Prunus armeniaca L. (Kuxingren), Scutellaria baicalensis Georgi (Huangqin), Forsythia suspensa (Thunb.) Vahl (Lianqiao), Fritillaria thunbergii Miq. (Zhebeimu), Anemarrhena asphodeloides Bunge (Zhimu), Arctium lappa L. (Niubangzi), Artemisia annua L. (Qinghao), Mentha canadensis L. (Bohe), and Glycyrrhiza inflata Batalin (Gancao) [68]. Molecular docking analysis showed that the key compounds of JH, namely formononetin, stigmasterol, β-sitosterol, and anhydroicaritin, had a certain degree of affinity to SARS-CoV-2 3CL hydrolase (Mpro) and ACE2 [69, 70]. Another study by Simayi et al. [71]. indicated that the active components, kaempferol, baicalein, and oroxylin A of JH granules had a high affinity to the M protein of SARS-CoV-2, and were related to the anti-inflammatory response and apoptotic signaling pathways by binding to ACE2 to exert a therapeutic effect in COVID-19.
JH is efficacious in treating mild and moderate COVID-19 patients, helps restore the lymphocyte and white blood cell count, and reduces the rate of mild patients worsening to the severe form of the infection [72]. A recently concluded clinical study performed in Wuhan showed that the disappearance rate of the symptoms of fever (80.3% vs 53.1%), cough (66.1% vs 42.9%), fatigue (77.6% vs 53.8%), and expectoration (85.3% vs 46.2%) in the treatment group was significantly higher than that in the control group. The study compared 82 patients with mild COVID-19 treated with JH combined with standard care to 41 mild COVID-19 patients treated with standard care alone [73]. Another retrospectively, not double blind clinical trial has also been performed to evaluate the efficacy and safety of JH in 80 patients with COVID-19 in Beijing. The results indicated that the average duration of viral nucleic acid detection (test negative) and the pneumonia recovery time were shorter in JH treatment group (7 ± 4) d and (8 ± 4) d than that control group (10 ± 4) d and (10 ± 5) d, P = 0.010 and P = 0.021, respectively. Further, the 7-day viral clearance rate was significantly higher in the JH group (56.82%) compared with the control group (27.78%, P = 0.009), and no adverse effects existed in the JH treatment [74]. Based on the experience of clinicians, patients with mild fever and severe headache should be treated with JH, while those with high fever and dry stools should be treated with LH [72]. Shi et al. [75] found that both LH and JH had multiple antiviral activities by targeting viral life cycle and regulating host immune responses and inflammation through literature mining. Moreover, JH is more potent in modulating viral life cycle, whereas LH exhibits better efficacy in regulating host anti-viral responses, suggesting LH and JH could be potentially an alternative therapy for emerging viral diseases. They also found that both LH and JH are potentially being able to prevent the progress of COVID-19 into severe or critical conditions. Oral administration of LH could be more beneficial for patients with insufficient immune functions or for patients with alleviated symptoms after treatment with JH.
Xuebijing (XBJ) injection
XBJ was developed during the SARS epidemic in 2002–03 [76]. It is a Chinese herbal-based therapeutic injection consisting of Paeonia lactiflora Pall. (Chishao), Conioselinum anthriscoides “Chuanxiong” (Chuanxiong), Salvia miltiorrhiza Bunge (Danshen), Carthamus tinctorius L. (Honghua), and Angelica sinensis (Oliv.) Diels (Danggui). Ultra-high-performance liquid chromatography coupled with quadrupole Orbitrap high-resolution mass spectrometry (UHPLC-Q-Orbitrap HRMS) indicates that the primary components of XBJ are hydroxysafflor yellow A, oxypaeoniforin, senkyunolide I, and benzoylpaeoniflorin [77]. XBJ has been approved for use in the treatment of severe infections (sepsis) in critically ill patients (China Food and Drug Administration; Beijing, China, Number Z20040033). A prospective, randomized, controlled study on XBJ injection for critically ill patients with severe community-acquired pneumonia was performed in 33 hospitals in China [78]. Results from the study indicate that XBJ injection significantly improves the primary endpoint of the pneumonia severity index as well as brings about a significant improvement in the secondary clinical outcomes of mortality, reduces the duration of mechanical ventilation, and shortens the duration of ICU stay [78]. Chen et al. [79] showed that XBJ facilitates the expansion of IL-10+ Tregs and normalizes the pro-inflammatory Th17 population in mice with sepsis. XBJ also reduces the levels of the cytokines, TNF-α and IL-6, which are known to participate in the inflammatory response. Liu et al. [80] report that XBJ significantly improves the survival rate of mice with sepsis by promoting M2 polarization of the macrophages, thereby contributing to a therapeutic effect in sepsis and providing the mechanism for further treatment of COVID-19.
The observation in 11 severe or critical COVID-19 patients received XBJ treatment showed that the expression of TNF-α, IP-10, macrophage inflammatory protein-1β (MIP-1β) and RANTES protein was inhibited during day 7–8 of treatment. In addition, XBJ has been shown to protect Vero E6 cells from SARS-CoV-2-induced cell death[81]. A randomized, double-blinded trial with 60 COVID-19 patients (3 dropped out) indicated that the secretion of IL-6, IL-8 and TNF-α was significantly suppressed in the routine medication plus XBJ therapy group [82]. After 14 days of treatment, the lymphocyte level was higher, and the C-reactive protein level was lower in the combination therapy group than the routine medication plus saline group [82]. However, there was no difference between the 28-day mortality of the two groups. XBJ combined with routine treatment significantly reduced IL-6 levels and body temperature in a retrospective case–control study with 42 COVID-19 patients [83]. XBJ was recommended by China's National Health Commission to treat severe and critical cases of COVID-19, especially during systematic inflammatory response syndrome (SIRS) and/or multi-organ failure [84, 85].
Other TCMs in the treatment of COVID-19
Lung Cleansing and detoxifying decoction is composed of four compound prescriptions, i.e. Maxing shigan decoction, Shegan Mahuang decoction, Xiaochaihu decoction, and Wuling San, comprising a total of 21 herbal components [86]. It is mainly effective in reducing the symptoms of fever, cough, and fatigue, as well as in improving the lung condition in severe patients. It is a prescription for disease treatment and is not recommended to be used as a prophylactic [87]. Xuanfeibaidu granules (XFBD) are composed of four prescriptions, namely, Maxing shigan decoction, Maxingyigan decoction, Qianjinweigan decoction, and Tingledazaoxiefeng decoction [88], and is currently in the process to obtain permission for new drug research. It is effective in improving symptoms of COVID-19, including fever, cough, suffocation, and fatigue, especially when combined another TCM, Reduning injection [89]. In addition, mechanically XFBD has an obvious effect in reducing the levels of C-reactive protein, increasing the lymphocyte count, balancing immunity, eliminating inflammation, regulating and recovering metabolism [90]. Huashibaidu formula is composed of 14 herbal components and is approved for clinical trials as a new drug [91]. Moreover, according to the 7th trial version of “Diagnosis and Treatment Protocol for Novel Coronavirus Pneumonia”, Huoxiang Zhengqi capsules/oral solution and Shufeng Jiedu capsules/granules were suggested to use in medical observation period of COVID-19; Xiyanping injection, Reduning injection, Tanreqing injection, and Xingnaojing injection were also suggested to use in severe cases; and Reduning injection, Tanreqing injection, Xingnaojing injection, Shenfu injection, and Shengmai injection was used in critical cases [11, 85]. In addition, there are nine TCMs that have been approved for national clinical trials (Table 2).
Therapy of COVID-19 with modern medicine and vaccine
The present clinical methods for COVID-19 treatment mainly include antiviral and antibacterial drugs, anti-inflammatory drugs, anti-SARS-CoV-2 antibody, oxygen therapy, and intestinal microecological agents and plasma from patients in rehabilitation [92, 93]. It is widely understood that patients in the early stages of COVID-19 may benefit from antiviral drugs to reduce viral replication, while patients in severe or advanced stages may benefit from anti-inflammatory treatment [94].
The guidelines for the diagnosis and treatment of novel coronavirus pneumonia (the seventh edition) issued by the National Health Commission and the State Administration of TCM of the People’s Republic of China recommends the use of certain antiviral drugs, but not the anti-COVID-19 indications, such as the HIV-protease inhibitors lopinavir and ritonavir, and the broad-spectrum antiviral drug, ribavirin [36, 95, 96]. However, a randomized, controlled, open-label trial on lopinavir and ritonavir showed that the combination was not beneficial in 99 COVID-19 patients who were in a critical condition compared to the 100 serious patients who were receiving standard care to accelerate clinical improvement, reduce mortality, and reduce the viral RNA detectability from the throat. The efficacy of the combination of lopinavir and ritonavir and other antiviral drugs in COVID-19 remains to be determined [97]. Remdesivir was originally tested in Ebola-infected patients [98]. It previously received emergency use authorization (EUA) from the Food and Drug Administration (FDA) in May 2020, for use in patients with severe COVID-19. In August, the FDA relaxed its guidelines to extend the use of the drug in less serious cases. In October, the FDA gave full approval to remdesivir for the treatment of COVID-19 [99]. However, preliminary results from the Solidarity Trial by the WHO showed that remdesivir had no effect on mortality and had negligible outcomes on the length of hospital stay [100]. Moreover, umifenovir and favipiravir, both for influenza prophylaxis, have also been used in COVID-19 treatment, and the latter exerted the better clinical effect for moderate COVID-19 patients in a prospective randomized study [101]. It is worth mentioning that chloroquine/hydroxychloroquine, used for malaria and rheumatoid arthritis, have also been applied to treat COVID-19 in clinic, although their efficacy in inhibiting pneumonia progression and replication of SARS-CoV-2 needs to be further considered [97, 102]. On September 2, 2020, the WHO issued an interim guideline on the use of dexamethasone and other corticosteroids for the treatment of COVID-19. Those drugs are used in the management of several conditions, owing to their anti-inflammatory and immunosuppressant effects. The WHO strongly recommends the oral or intravenous administrations of corticosteroids in patients with severe and critical COVID-19 and advises against its use in those with non-severe COVID-19, unless the patient condition[103] (Fig. 2). In addition, many novel therapeutics, including convalescent plasma treatment, mono-antibody bamlanivimab derived from plasma, antibody against inflammatory (Tocilizumab), interferon-α/-β and stem cell therapy, etc. are also used or ongoing in the clinical treatment of COVID-19 [104]. More importantly, many teams worldwide have employed multiple strategies to develop vaccines against COVID-19, which need to undergo stringent clinical trials, as in the case of drugs [105]. These vaccines include nucleic acid vaccine (DNA or RNA), adenovirus vaccine, inactivated virus vaccine, attenuated live virus vaccine and recombinant protein vaccine. Presently, more than 287 vaccines are developed around the world at different stages according to WHO reports, and 102 vaccines are already in clinical use, which will build an effective barrier against COVID-19 (Table 3).
Conclusion
Currently, COVID-19 pandemic is still rapidly spreading around the worldwide. The genome sequence, protein structure and the features of SARS-CoV-2 were identified since the finding of SARS-CoV-2. The diagnoses of COVID-19 have made a great progress through nucleic acid amplification for the virus genome and immunoassay for the antibodies against SARS-CoV-2. Although COVID-19 has been propagated around the world for more than a year, there are still few effective drugs to prevent it. Fortunately, the worldwide vaccination of SARS-CoV-2 has brought hope for the prevention and elimination of COVID-19.
TCM is a whole set of treatment methods and prescriptions based on individual syndrome differentiation. It pursues to achieve the balance of Yin and Yang in the human body. It resists external infections by adjusting the overall immunity. Compared to Western medicine, which mostly uses chemical drugs with single and specific targets, TCM primarily uses compound plant formulations and mixtures with numerous and miscellaneous targets [106]. Due to the successful experience in treating SARS of 2002–03 and the H1N1 influenza pandemic of 2009 using ancient TCM prescriptions, TCM was rapidly applied to treat COVID-19 and attained effective results since the early outbreak of COVID-19 in China. Moreover, new TCM prescriptions have been quickly developed according to the clinical symptoms of COVID-19 patients. Among them, TCM “three medicines three parties” play a crucial role in against COVID-19 in China, which including “Three medicines”-Lianhua Qingwen capsules/granules, Jinhua Qinggan granules and XueBiJing injection, and “Three parties”-LungCleansing and detoxifying decoction, Huashibaidu formula, Xuanfeibaidu granule. Since then, the molecular mechanism of TCM against COVID-19 has been reported constantly.
Limitations of TCM and prospects for future research
It exists on the limitations for TCM treatment of COVID-19. For example, it is very difficult to maintain the stability of the various formulations of TCM. The time and method of decocting can alter the composition of the final product. At present, only limited data on COVID-19 cases are available. Therefore, additional prospective cohort studies and randomized controlled trials are needed to evaluate the efficacy of TCM in preventing and treating COVID-19, as well as to explore and identify suitable methods to combine TCM and Western medicine [107, 108]. However, owing to the control of the COVID-19 epidemic in China, there has been a reduction in the number of patients, thereby restricting the number of studies evaluating the efficacy of TCM. To address this challenge, researchers are in the process of establishing animal models to further determine the efficacy of TCM in vivo. Apart from the lack of a large sample of randomized controlled clinical research data, the following urgent problems should be addressed to promote evidence-based Chinese medicine: the efficacy and safety of TCM lack scientific evidence; A rugged evaluation index system and evaluation methodology for disease prevention and treatment using TCM are currently lacking.
Availability of data and materials
Not applicable.
Abbreviations
- SARS-CoV-2:
-
Severe acute respiratory coronavirus 2
- COVID-19:
-
Coronavirus disease 2019
- TCM:
-
Traditional Chinese medicine
- WHO:
-
World Health Organization
- MERS:
-
Middle east respiratory syndrome
- ORF:
-
Open reading frames
- ACE2:
-
Angiotensin-converting enzyme 2
- CT:
-
Computerized tomography
- IL:
-
Interleukin
- GCSF:
-
Granulocyte-colony stimulating factor
- IP-10/CXCL10:
-
IFN-γ-inducible protein 10
- MCP-1/CCL2:
-
Monocyte chemoattractant protein-1
- MIP-1α/CCL3:
-
Macrophage inflammatory protein-1α
- MIP-1β:
-
Macrophage inflammatory protein-1β
- TNF-α:
-
Tumor necrosis factor-α
- ICU:
-
Intensive care unit
- RT-PCR:
-
Real-time polymerase chain reaction
- Ig:
-
Immunoglobulin
- EUA:
-
Emergency use authorization
- FDA:
-
Food and Drug Administration
- LH:
-
Lianhua Qingwen
- JH:
-
Jinhua Qinggan
- Mpro:
-
3CL hydrolase
- XBJ:
-
Xuebijing
- XFBD:
-
Xuanfeibaidu granules
References
Lai CC, Shih TP, Ko WC, Tang HJ, Hsueh PR. Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) and coronavirus disease-2019 (COVID-19): the epidemic and the challenges. Int J Antimicrob Agents. 2020;55(3):105924.
WHO Coronavirus Disease (COVID-19) Dashboard. https://covid19.who.int/. Accessed 16 June 2021.
Zu ZY, Jiang MD, Xu PP, Chen W, Ni QQ, Lu GM, Zhang LJ. Coronavirus Disease 2019 (COVID-19) a perspective from China. Radiology. 2020;296(2):E15–25.
Huang Y, Yang C, Xu XF, Xu W, Liu SW. Structural and functional properties of SARS-CoV-2 spike protein: potential antivirus drug development for COVID-19. Acta Pharmacol Sin. 2020;41(9):1141–9.
Xiao LJ, Tao R. Traditional Chinese Medicine (TCM) therapy. Adv Exp Med Biol. 2017;1010:261–80.
Xia J, Inagaki Y, Song P, Sawakami T, Kokudo N, Hasegawa K, Sakamoto Y, Tang W. Advance in studies on traditional Chinese medicines to treat infection with the hepatitis B virus and hepatitis C virus. Biosci Trends. 2016;10(5):327–36.
Poon PM, Wong CK, Fung KP, Fong CY, Wong EL, Lau JT, Leung PC, Tsui SK, Wan DC, Waye MM, et al. Immunomodulatory effects of a traditional Chinese medicine with potential antiviral activity: a self-control study. Am J Chin Med. 2006;34(1):13–21.
Lau JT, Leung PC, Wong EL, Fong C, Cheng KF, Zhang SC, Lam CW, Wong V, Choy KM, Ko WM. The use of an herbal formula by hospital care workers during the severe acute respiratory syndrome epidemic in Hong Kong to prevent severe acute respiratory syndrome transmission, relieve influenza-related symptoms, and improve quality of life: a prospective cohort study. J Altern Complement Med. 2005;11(1):49–55.
He B, Dun B, Wang J. Shiyong Chufang Gangmu. Shan Xi: Science and Technique Publishing House; 1990.
State Council Information Office. http://ghs.satcm.gov.cn/gongzuodongtai/2020-03-27/14281.html. Accessed 27 Mar 2020.
Yang Y, Islam MS, Wang J, Li Y, Chen X. Traditional Chinese medicine in the treatment of patients infected with 2019-new coronavirus (SARS-CoV-2): a review and perspective. Int J Biol Sci. 2020;16(10):1708–17.
Zhang YS, Cong WH, Zhang JJ, Guo FF, Li HM. Research progress of intervention of Chinese herbal medicine and its active components on human coronavirus. Zhongguo Zhong Yao Za Zhi. 2020;45(6):1263–71.
Millet JK, Whittaker GR. Host cell proteases: critical determinants of coronavirus tropism and pathogenesis. Virus Res. 2015;202:120–34.
Almazan F, Sola I, Zuniga S, Marquez-Jurado S, Morales L, Becares M, Enjuanes L. Coronavirus reverse genetic systems: infectious clones and replicons. Virus Res. 2014;189:262–70.
Paim FC, Bowman AS, Miller L, Feehan BJ, Marthaler D, Saif LJ, Vlasova AN. Epidemiology of deltacoronaviruses (delta-CoV) and gammacoronaviruses (gamma-CoV) in wild birds in the United States. Viruses. 2019;11(10):897.
Corman VM, Lienau J, Witzenrath M. Coronaviruses as the cause of respiratory infections. Internist (Berl). 2019;60(11):1136–45.
Essaidi-Laziosi M, Brito F, Benaoudia S, Royston L, Cagno V, Fernandes-Rocha M, Piuz I, Zdobnov E, Huang S, Constant S, et al. Propagation of respiratory viruses in human airway epithelia reveals persistent virus-specific signatures. J Allergy Clin Immunol. 2018;141(6):2074–84.
Rabaan AA, Al-Ahmed SH, Haque S, Sah R, Tiwari R, Malik YS, Dhama K, Yatoo MI, Bonilla-Aldana DK, Rodriguez-Morales AJ. SARS-CoV-2, SARS-CoV, and MERS-COV: a comparative overview. Infez Med. 2020;28(2):174–84.
Zhou Y, Hou Y, Shen J, Huang Y, Martin W, Cheng F. Network-based drug repurposing for novel coronavirus 2019-nCoV/SARS-CoV-2. Cell Discov. 2020;6:14.
Weiss SR. Forty years with coronaviruses. J Exp Med. 2020;217(5):e20200537.
Chen Y, Liu Q, Guo D. Emerging coronaviruses: Genome structure, replication, and pathogenesis. J Med Virol. 2020;92(4):418–23.
Chen SC, Lo SY, Ma HC, Li HC. Expression and membrane integration of SARS-CoV E protein and its interaction with M protein. Virus Genes. 2009;38(3):365–71.
Lan J, Ge J, Yu J, Shan S, Zhou H, Fan S, Zhang Q, Shi X, Wang Q, Zhang L, et al. Structure of the SARS-CoV-2 spike receptor-binding domain bound to the ACE2 receptor. Nature. 2020;581(7807):215–20.
Wrapp D, Wang N, Corbett KS, Goldsmith JA, Hsieh CL, Abiona O, Graham BS, McLellan JS. Cryo-EM structure of the 2019-nCoV spike in the prefusion conformation. Science. 2020;367(6483):1260–3.
Huang C, Wang Y, Li X, Ren L, Zhao J, Hu Y, Zhang L, Fan G, Xu J, Gu X, et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. 2020;395(10223):497–506.
Guan WJ, Ni ZY, Hu Y, Liang WH, Ou CQ, He JX, Liu L, Shan H, Lei CL, Hui DSC, et al. Clinical characteristics of coronavirus disease 2019 in China. N Engl J Med. 2020;382(18):1708–20.
Yang W, Cao Q, Qin L, Wang X, Cheng Z, Pan A, Dai J, Sun Q, Zhao F, Qu J, et al. Clinical characteristics and imaging manifestations of the 2019 novel coronavirus disease (COVID-19): a multi-center study in Wenzhou city, Zhejiang, China. J Infect. 2020;80(4):388–93.
Silva Junior JVJ, Merchioratto I, de Oliveira PSB, Rocha Lopes TR, Brites PC, de Oliveira EM, Weiblen R, Flores EF. End-point RT-PCR: a potential alternative for diagnosing coronavirus disease 2019 (COVID-19). J Virol Methods. 2019;2020:114007.
Xiao AT, Tong YX, Zhang S. False negative of RT-PCR and prolonged nucleic acid conversion in COVID-19: Rather than recurrence. J Med Virol. 2020;92:1755–6.
Ai T, Yang Z, Hou H, Zhan C, Chen C, Lv W, Tao Q, Sun Z, Xia L. Correlation of chest CT and RT-PCR testing for coronavirus disease 2019 (COVID-19) in China: a report of 1014 cases. Radiology. 2020;296(2):E32–40.
Bao C, Liu X, Zhang H, Li Y, Liu J. Coronavirus disease 2019 (COVID-19) CT findings: a systematic review and meta-analysis. J Am Coll Radiol. 2020;17(6):701–9.
Pan Y, Li X, Yang G, Fan J, Tang Y, Zhao J, Long X, Guo S, Zhao Z, Liu Y, et al. Serological immunochromatographic approach in diagnosis with SARS-CoV-2 infected COVID-19 patients. J Infect. 2020;81(1):e28–32.
Zhong L, Chuan J, Gong B, Shuai P, Zhou Y, Zhang Y, Jiang Z, Zhang D, Liu X, Ma S, et al. Detection of serum IgM and IgG for COVID-19 diagnosis. Sci China Life Sci. 2020;63(5):777–80.
Li Z, Yi Y, Luo X, Xiong N, Liu Y, Li S, Sun R, Wang Y, Hu B, Chen W, et al. Development and clinical application of a rapid IgM-IgG combined antibody test for SARS-CoV-2 infection diagnosis. J Med Virol. 2020;92:1518–2152.
Cassaniti I, Novazzi F, Giardina F, Salinaro F, Sachs M, Perlini S, Bruno R, Mojoli F, Baldanti F, Members of the San Matteo Pavia C-TF. : Performance of VivaDiag COVID-19 IgM/IgG Rapid Test is inadequate for diagnosis of COVID-19 in acute patients referring to emergency room department. J Med Virol. 2020;92:1724–7.
Jean SS, Lee PI, Hsueh PR. Treatment options for COVID-19: the reality and challenges. J Microbiol Immunol Infect. 2020;53(3):436–43.
Pan J, Yang C, Jiang Z, Huang J. Trametes robiniophila Murr: a traditional Chinese medicine with potent anti-tumor effects. Cancer Manag Res. 2019;11:1541–9.
Chan K, Zhang H, Lin ZX. An overview on adverse drug reactions to traditional Chinese medicines. Br J Clin Pharmacol. 2015;80(4):834–43.
Luo H, Tang QL, Shang YX, Liang SB, Yang M, Robinson N, Liu JP. Can Chinese medicine be used for prevention of corona virus disease 2019 (COVID-19)? A review of historical classics, research evidence and current prevention programs. Chin J Integr Med. 2020;26(4):243–50.
Liu J, Manheimer E, Shi Y, Gluud C. Chinese herbal medicine for severe acute respiratory syndrome: a systematic review and meta-analysis. J Altern Complement Med. 2004;10(6):1041–51.
Li JH, Wang RQ, Guo WJ, Li JS. Efficacy and safety of traditional Chinese medicine for the treatment of influenza A (H1N1): a meta-analysis. J Chin Med Assoc. 2016;79(5):281–91.
Huang YF, Bai C, He F, Xie Y, Zhou H. Review on the potential action mechanisms of Chinese medicines in treating Coronavirus Disease 2019 (COVID-19). Pharmacol Res. 2020;158:104939.
Runfeng L, Yunlong H, Jicheng H, Weiqi P, Qinhai M, Yongxia S, Chufang L, Jin Z, Zhenhua J, Haiming J, et al. Lianhuaqingwen exerts anti-viral and anti-inflammatory activity against novel coronavirus (SARS-CoV-2). Pharmacol Res. 2020;156:104761.
Zhang D, Zhang B, Lv JT, Sa RN, Zhang XM, Lin ZJ. The clinical benefits of Chinese patent medicines against COVID-19 based on current evidence. Pharmacol Res. 2020;157:104882.
Huang K, Zhang P, Zhang Z, Youn JY, Wang C, Zhang H, Cai H. Traditional Chinese Medicine (TCM) in the treatment of COVID-19 and other viral infections: efficacies and mechanisms. Pharmacol Ther. 2021;225:107843.
Zhao Z, Li Y, Zhou L, Zhou X, Xie B, Zhang W, Sun J. Prevention and treatment of COVID-19 using traditional Chinese medicine: a review. Phytomedicine. 2021;85:153308.
Cinatl J, Morgenstern B, Bauer G, Chandra P, Rabenau H, Doerr HW. Glycyrrhizin, an active component of liquorice roots, and replication of SARS-associated coronavirus. Lancet. 2003;361(9374):2045–6.
Chen F, Chan KH, Jiang Y, Kao RY, Lu HT, Fan KW, Cheng VC, Tsui WH, Hung IF, Lee TS, et al. In vitro susceptibility of 10 clinical isolates of SARS coronavirus to selected antiviral compounds. J Clin Virol. 2004;31(1):69–75.
Ma X, Bi S, Wang Y, Chi X, Hu S. Combined adjuvant effect of ginseng stem-leaf saponins and selenium on immune responses to a live bivalent vaccine of Newcastle disease virus and infectious bronchitis virus in chickens. Poult Sci. 2019;98(9):3548–56.
Liu CY, Li Q, Cai SQ. Advances in pharmacology and clinical research of lianhuaqingwen capsules. Pharmacol Clin Chin Mater Med. 2010;26(6):84–5.
Jiang T, Fu H. Progress of experimental studies on prescriptions designed by Zhang Zhongjing. J Trad Chin Med Chung i tsa chih ying wen pan. 1996;16(1):55–64.
Chen Y, Xu L. Analysis of modified Yinqiao Powder in Wen Bing Tiao Bian based on defensive Qi and nutrient blood. J Gansu College Trad Chin Med. 2014;31(5):13–4.
Wang CH, Zhong Y, Zhang Y, Liu JP, Wang YF, Jia WN, Wang GC, Li Z, Zhu Y, Gao XM. A network analysis of the Chinese medicine Lianhua-Qingwen formula to identify its main effective components. Mol BioSyst. 2016;12(2):606–13.
Mo HYM, Ke CW, Zheng JP, Zhong NS. Anti viral effects of Lianhua Qingwen capsule against influenza a virus in vitro. Trad Chin Drug Res Clin Pharmacol. 2007;18(1):5–9.
Li J. Efficacy of Lianhua Qingwen capsule on influenza combined with bronchial pneumonia. Guang Ming Zhong Yi. 2011;26(12):2450–1.
Ding Y, Zeng L, Li R, Chen Q, Zhou B, Chen Q, Cheng PL, Yutao W, Zheng J, Yang Z, et al. The Chinese prescription lianhuaqingwen capsule exerts anti-influenza activity through the inhibition of viral propagation and impacts immune function. BMC Complement Altern Med. 2017;17(1):130.
Jia W, Wang C, Wang Y, Pan G, Jiang M, Li Z, Zhu Y. Qualitative and quantitative analysis of the major constituents in Chinese medical preparation Lianhua-Qingwen capsule by UPLC-DAD-QTOF-MS. Sci World J. 2015;2015:731765.
Dong L, Xia JW, Gong Y, Chen Z, Yang HH, Zhang J, He J, Chen XD. Effect of lianhuaqingwen capsules on airway inflammation in patients with acute exacerbation of chronic obstructive pulmonary disease. Evid Based Complement Altern Med eCAM. 2014;2014:637969.
Yao K, Liu M, Li X, Huang J, Cai H. Retrospective Clinical Analysis on Treatment of Coronavirus Disease 2019 with Traditional Chinese Medicine Lianhua Qingwen. Chinese J Exp Trad Med Form. 2020;26(11):8–12.
Cheng DZ, Wang WJ, Li Y, Wu XD, Zhou B, Song Q. Analysis of curative effect of 51 patients with novel coronavirus pneumonia treated with Chinese medicine Lianhua Qingwen:a multicentre retrospective study. Tianjin J Trad Chinese Med. 2020;37(5):509–16.
Hu K, Guan WJ, Bi Y, Zhang W, Li L, Zhang B, Liu Q, Song Y, Li X, Duan Z, et al. Efficacy and safety of Lianhuaqingwen capsules, a repurposed Chinese herb, in patients with coronavirus disease 2019: a multicenter, prospective, randomized controlled trial. Phytomedicine. 2019;2020:153242.
Zeng M, Li L, Wu Z. Traditional Chinese medicine Lianhua Qingwen treating corona virus disease 2019(COVID-19): meta-analysis of randomized controlled trials. PLoS ONE. 2020;15(9):e0238828.
Liu M, Gao Y, Yuan Y, Yang K, Shi S, Tian J, Zhang J. Efficacy and safety of herbal medicine (Lianhuaqingwen) for treating COVID-19: a systematic review and meta-analysis. Integr Med Res. 2021;10(1):10644.
Ye CH, Gao M, Lin WQ, Yu KQ, Chen G. Theoretical Study of the anti-NCP Molecular Mechanism of Traditional Chinese Medicine Lianhua-Qingwen Formula (LQF). ChemRxiv. 2020. https://doi.org/10.26434/chemrxiv.12016236
Jin YH, Zhan QY, Peng ZY, Ren XQ, Yin XT, Cai L, Yuan YF, Yue JR, Zhang XC, Yang QW, et al. Chemoprophylaxis, diagnosis, treatments, and discharge management of COVID-19: an evidence-based clinical practice guideline (updated version). Mil Med Res. 2020;7(1):41.
Tao Z, Yang Y, Shi W, Xue M, Yang W, Song Z, Yao C, Yin J, Shi D, Zhang Y, et al. Complementary and alternative medicine is expected to make greater contribution in controlling the prevalence of influenza. Biosci Trends. 2013;7(5):253–6.
Zeng L, Ding Y, Wang Y, Yang Z, Zhang F. Comparative Study of Pharmacological Properties of Maxing Shigan Tang and Yinqiao San Against Influenza Virus. Tradit Chin Drug Res Clini Pharmacol. 2016;27(3):381–5.
Wei M, Xie L, Xi L. Interpretation of “Jinhua Qinggan prescription.” Home Med. 2010;3:20–3.
Gao Y, Yan L, Huang Y, Liu F, Zhao Y, Cao L, Wang T, Sun Q, Ming Z, Zhang L, et al. Structure of the RNA-dependent RNA polymerase from COVID-19 virus. Science. 2020;368(6492):779–82.
Gong PY, Guo YJ, Li X, Wang N, Gu J. Exploring active compounds of Jinhua Qinggan Granules for prevention of COVID-19 based on network pharmacology and molecular docking. Chin Tradit Herbal Drugs. 2020;51(7):1685–93.
Simayi J, Noormaimaiti M, Wumaier A, Yusufu M, Noor M, Mahemuti N, Zhou WT. Study on the Active Components in the Adjuvant Treatment of Novel Coronavirus Pneumonia (COVID-19) with Jinhua Qinggan Granules Based on Network Pharmacology and Molecular Docking. J Chin Med Mater. 2020;43(5):1271–9.
Ren Y, Yao MC, Huo XQ, Gu Y, Zhu WX, Qiao YJ, Zhang YL. Study on treatment of “cytokine storm” by anti-2019-nCoV prescriptions based on arachidonic acid metabolic pathway. China J Chin Mater Med. 2020;45(6):1225–31.
Duan C, Xia WG, Zhen CJ, Sun G, Li ZL, Li Q, Li P, Zhang HL, Yang FW, ZB L et al.: Clinical Observation on Jinhua Qinggan Granule Combined with Conventional Western Medicine Therapy in Treating Mild Cases of Coronavirus Disease 2019. J Tradit Chin Med. 2020;61(17):1473–7.
Liu Z, Li X, Gou C, Li L, Luo X, Zhang C, Zhang Y, Zhang J, Jin A, Li H, et al. Effect of Jinhua Qinggan granules on novel coronavirus pneumonia in patients. J Tradit Chin Med. 2020;40(3):467–72.
Shi M, Peng B, Li A, Li Z, Song P, Li J, Xu R, Li N. Broad Anti-viral capacities of Lian-Hua-Qing-Wen capsule and Jin-Hua-Qing-Gan granule and rational use against COVID-19 based on literature mining. Front Pharmacol. 2021;12:640782.
Editorial Board Of Chinese Critical Care M. Xuebijing injection versus placebo for critically ill patients with severe community-acquired pneumonia: a randomized controlled trial: research results and clinical value. Zhonghua Wei Zhong Bing Ji Jiu Yi Xue. 2019;31(10):1199–203.
Zuo L, Sun Z, Wang Z, Ding D, Xu T, Liu L, Gao L, Du S, Kang J, Zhang X. Tissue distribution profiles of multiple major bioactive components in rats after intravenous administration of Xuebijing injection by UHPLC-Q-Orbitrap HRMS. Biomed Chromatogr. 2019;33(2):e4400.
Song Y, Yao C, Yao Y, Han H, Zhao X, Yu K, Liu L, Xu Y, Liu Z, Zhou Q, et al. XueBiJing injection versus placebo for critically ill patients with severe community-acquired pneumonia: a randomized controlled trial. Crit Care Med. 2019;47(9):e735–43.
Chen X, Feng Y, Shen X, Pan G, Fan G, Gao X, Han J, Zhu Y. Anti-sepsis protection of Xuebijing injection is mediated by differential regulation of pro- and anti-inflammatory Th17 and T regulatory cells in a murine model of polymicrobial sepsis. J Ethnopharmacol. 2018;211:358–65.
Liu YC, Yao FH, Chai YF, Dong N, Sheng ZY, Yao YM. Xuebijing injection promotes M2 polarization of macrophages and improves survival rate in septic mice. Evid Based Complement Altern Med eCAM. 2015;2015:352642.
Ma Q, Qiu M, Zhou H, Chen J, Yang X, Deng Z, Chen L, Zhou J, Liao Y, Chen Q, et al. The study on the treatment of Xuebijing injection (XBJ) in adults with severe or critical Corona Virus Disease 2019 and the inhibitory effect of XBJ against SARS-CoV-2. Pharmacol Res. 2020;160:105073.
Luo Z, Chen W, Xiang M, Wang H, Xiao W, Xu C, Li Y, Min J, Tu Q. The preventive effect of Xuebijing injection against cytokine storm for severe patients with COVID-19: a prospective randomized controlled trial. Eur J Integr Med. 2021;42:101305.
Guo H, Zheng J, Huang G, Xiang Y, Lang C, Li B, Huang D, Sun Q, Luo Y, Zhang Y, et al. Xuebijing injection in the treatment of COVID-19: a retrospective case-control study. Ann Palliat Med. 2020;9(5):3235–48.
Song Y, Zhang M, Yin L, Wang K, Zhou Y, Zhou M, Lu Y. COVID-19 treatment: close to a cure? A rapid review of pharmacotherapies for the novel coronavirus (SARS-CoV-2). Int J Antimicrob Agents. 2020;56(2):106080.
National Health Commission (NHC) of the People's Republic of China: Diagnosis and treatment protocol for COVID-19 pneumonia caused by novel coronavirus infection (7th ed.), NHC (2020). http://en.nhc.gov.cn/2020-03/29/c_78469.htm. Accessed 29 Mar 2020.
Cao P, Wu S, Wu T, Deng Y, Zhang Q, Wang K, Zhang Y. The important role of polysaccharides from a traditional Chinese medicineLung cleansing and detoxifying decoction against the COVID-19 pandemic. Carbohyd Polym. 2020;240:116346.
Liu C, Wang Y, Zhang H, Tian C, Huang H, Zhang T. Attach importance to research and development of Chinese materia medica based on prevention and control needs of SARS-CoV-2 infection. Chin Tradit Herbal Drugs. 2020;51(6):1361–74.
Xiong WZ, Wang G, Du J, Ai W. Efficacy of herbal medicine (Xuanfei Baidu decoction) combined with conventional drug in treating COVID-19: a pilot randomized clinical trial. Integr Med Res. 2020;9(3):100489.
Wang Y, Han L, Zhang W, Sun J. The curative effect of reduning injection combined with Xuanfeibaidu formula on COVID-19: a protocol for systematic review and meta-analysis. Medicine. 2020;99(46):e22830.
Wang Y, Li X, Zhang JH, Xue R, Qian JY, Zhang XH, Zhang H, Liu QQ, Fan XH, Cheng YY, et al. Mechanism of Xuanfei Baidu Tang in treatment of COVID-19 based on network pharmacology. Zhongguo Zhong yao za zhi Zhongguo zhongyao zazhi China J Chin Mater Med. 2020;45(10):2249–56.
Yang X. Material basis research of Huashibaidu granule formula on anti-COVID-19 Modern Chinese Medicine. Mod Chin Med. 2020;5:672–89.
Nicola M, O’Neill N, Sohrabi C, Khan M, Agha M, Agha R. Evidence based management guideline for the COVID-19 pandemic—review article. Int J Surg. 2020;77:206–16.
Baum A, Fulton BO, Wloga E, Copin R, Pascal KE, Russo V, Giordano S, Lanza K, Negron N, Ni M, et al. Antibody cocktail to SARS-CoV-2 spike protein prevents rapid mutational escape seen with individual antibodies. Science. 2020;369(6506):1014–8.
Asselah T, Durantel D, Pasmant E, Lau G, Schinazi RF. COVID-19: discovery, diagnostics and drug development. J Hepatol. 2021;74(1):168–84.
Jin YH, Cai L, Cheng ZS, Cheng H, Deng T, Fan YP, Fang C, Huang D, Huang LQ, Huang Q, et al. A rapid advice guideline for the diagnosis and treatment of 2019 novel coronavirus (2019-nCoV) infected pneumonia (standard version). Mil Med Res. 2020;7(1):4.
Zeng YM, Xu XL, He XQ, Tang SQ, Li Y, Huang YQ, Harypursat V, Chen YK. Comparative effectiveness and safety of ribavirin plus interferon-alpha, lopinavir/ritonavir plus interferon-alpha, and ribavirin plus lopinavir/ritonavir plus interferon-alpha in patients with mild to moderate novel coronavirus disease 2019: study protocol. Chin Med J (Engl). 2020;133(9):1132–4.
Cao B, Wang Y, Wen D, Liu W, Wang J, Fan G, Ruan L, Song B, Cai Y, Wei M, et al. A trial of lopinavir-ritonavir in adults hospitalized with severe Covid-19. N Engl J Med. 2020;382(19):1787–99.
Gordon CJ, Tchesnokov EP, Feng JY, Porter DP, Gotte M. The antiviral compound remdesivir potently inhibits RNA-dependent RNA polymerase from Middle East respiratory syndrome coronavirus. J Biol Chem. 2020;295(15):4773–9.
Lim S, DeBruin DA, Leider JP, Sederstrom N, Lynfield R, Baker JV, Kline S, Kesler S, Rizza S, Wu J, et al. Developing an ethics framework for allocating remdesivir in the COVID-19 pandemic. Mayo Clin Proc. 2020;95(9):1946–54.
Singh AK, Singh A, Singh R, Misra A. Remdesivir in COVID-19: a critical review of pharmacology, pre-clinical and clinical studies. Diabetes Metab Syndr. 2020;14(4):641–8.
Sanders JM, Monogue ML, Jodlowski TZ, Cutrell JB. Pharmacologic treatments for Coronavirus Disease 2019 (COVID-19): a review. JAMA. 2020;323(18):1824–36.
Meo SA, Klonoff DC, Akram J. Efficacy of chloroquine and hydroxychloroquine in the treatment of COVID-19. Eur Rev Med Pharmacol Sci. 2020;24(8):4539–47.
Jensen MP, George M, Gilroy D, Sofat R. Beyond dexamethasone, emerging immuno-thrombotic therapies for COVID-19. Br J Clin Pharmacol. 2020;87:845–57.
Salian VS, Wright JA, Vedell PT, Nair S, Li C, Kandimalla M, Tang X, Carmona Porquera EM, Kalari KR, Kandimalla KK. COVID-19 transmission, current treatment, and future therapeutic strategies. Mol Pharm. 2021;18(3):754–71.
Ahn DG, Shin HJ, Kim MH, Lee S, Kim HS, Myoung J, Kim BT, Kim SJ. Current status of epidemiology, diagnosis, therapeutics, and vaccines for novel coronavirus disease 2019 (COVID-19). J Microbiol Biotechnol. 2020;30(3):313–24.
Li H, Yang L, Liu FF, Ma XN, He PL, Tang W, Tong XK, Zuo JP. Overview of therapeutic drug research for COVID-19 in China. Acta Pharmacol Sin. 2020;41(9):1133–40.
Ren JL, Zhang AH, Wang XJ. Traditional Chinese medicine for COVID-19 treatment. Pharmacol Res. 2020;155:14743.
Ni L, Chen L, Huang X, Han C, Xu J, Zhang H, Luan X, Zhao Y, Xu J, Yuan W, et al. Combating COVID-19 with integrated traditional Chinese and Western medicine in China. Acta Pharm Sin B. 2020;10(7):1149–62.
Acknowledgements
We would like to thank Prof. Xiao-yong Fan for suggestions to design the paper and for writing assistance.
Funding
This work was supported by the National Natural Science Fund (31671348, 31301064, 81803069) for Liang Chu, Fang Huang; Zhejiang Provincial Natural Science Foundation of China (LY18C070002) and the Grant for 521 talent project of ZSTU for Yigang Wang and Chen Xiao-ping Foundation for the Development of Science and Technology of Hubei Province (CXPJJH12000001-2020317) for Liang Chu.
Author information
Authors and Affiliations
Contributions
LC and YW designed the paper; LC, YW, FH, MZ, and BH wrote the paper; YW and LC collected the data and checked the paper. All authors read and approved the final manuscript.
Corresponding authors
Ethics declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
We declare that the Publisher has the author’s permission to publish the relevant contribution.
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Chu, L., Huang, F., Zhang, M. et al. Current status of traditional Chinese medicine for the treatment of COVID-19 in China. Chin Med 16, 63 (2021). https://doi.org/10.1186/s13020-021-00461-y
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s13020-021-00461-y