Abstract
Background
Eosinophils (EOS) is one of the most important cells involved in the pathogenesis of chronic airway inflammation in asthma, and its apoptosis is part of the mechanisms of asthma. Therefore, this study aimed to observe the effect of Chinese medicine Wentong decoction (WTD) in EOS apoptosis in asthmatic rats. This work also explored the mechanism of WTD regulation in EOS apoptosis and provided a new target for clinical treatment of asthma.
Methods
Asthmatic rats induced by ovalbumin were treated with WTD. Lung function of rats in each group was detected, and lung tissue pathology, EOS counts in blood and bronchoalveolar lavage fluid were observed. The degree of the EOS apoptosis in rats was detected. The expression content of interleukin (IL)-5, IL-10, chemokine (C–C motif) ligand 5 (CCL5), granulocyte–macrophage colony-stimulating factor (GM-CSF), transforming growth factor beta 1 (TGF-β1), interferon (IFN)-γ, and other cytokines in rat serum and the genes of Eotaxin mRNA, Fas mRNA, FasL mRNA, Fas/FasL and Bcl-2 mRNA in the lung tissues were determined.
Results
WTD can reduced airway resistance in rat models and improved airway compliance. The pathological changes of lung tissue in WTD group were significantly alleviated, at the same time, WTD could reduce the EOS count in the blood and BALF smears of the asthmatic model rats. Compared with the model group, the apoptosis degree of EOS significantly increased in rats in the WTD group. The expression of IL-5, CCL5, and GM-CSF in the serum and the expression of Eotaxin mRNA, Bcl-2 mRNA in the lung tissues in rats in the WTD group rats decreased. Moreover, the expression of IL-10, TGF-β1, and IFN-γ in the serum and the expression of Fas mRNA, FasL mRNA in the lung tissues in rats in the WTD group rats increased compared with that in rats in the model group.
Conclusions
Wentong decoction may accelerate EOS apoptosis, reduce asthma inflammation, and alleviate the disease through regulating and controlling the factors related to the anti-apoptosis and pro-apoptosis.
Similar content being viewed by others
Background
Bronchial asthma (BA) is a chronic inflammatory disease of the airway associated with multiple cells (such as eosinophils, mast cells, T-lymphocytes, neutrophils, and airway epithelial cells) and the cellular elements [1]. This inflammation causes the susceptible person to exhibit high-airway reactivity to each kind of motivating factor causing airway constriction, wherein eosinophil (EOS) infiltration of airway leads to airway inflammation and airway pathology change is an important sign of clinical changes of asthma disease [2, 3]. Asthma is currently incurable, but it can be controlled by appropriate medications, self-management education, and avoidance of exposure to environmental allergens and irritants [4]. Rapid development of global industrialization, environmental pollution, and the change of climate and ecological environments cause rapid increase in global respiratory diseases. Asthma prevalence rate is significantly different in different countries and regions, and the asthma prevalence rate in children is 3.3–29% [5, 6]. The prevalence rate of adult asthma is 1.2–25.5% [7].
Many inflammatory cell infiltrations exist in the bronchial lung tissues of asthmatic patients, and EOS with abnormally long life is the main inflammatory component of allergic reaction in asthmatic patients [8]. BA animal model [9] showed that EOS infiltration is specifically correlated to the increase of airway reactivity and continues to accumulate and activate in the lungs. EOS infiltration also plays an important role in the formation of airway inflammation and the asthma onset [10]. EOS apoptosis plays a key role in the elimination of BA airway inflammation [11], which is associated with the increase of apoptosis, the improvement of BA symptom, and the decrease of inflammation.
Apoptosis, also known as programmed cell death (PCD), is an energy-consuming process, wherein cells obtain a certain signal or undergo some stimulations and an active apoptotic-related gene interacting to produce cells dies out. Simon et al. [12] suggested that EOS may be regulated by both apoptotic and anti-apoptotic signals, and the imbalance of EOS’ anti-apoptosis and pro-apoptosis mechanism may be the root cause of the delay of EOS apoptosis. Studies have shown that the expression of cytokines, growth factors, and cell-surface molecules and their ligands is directly related to EOS apoptosis. In addition, various factors can achieve the effect of resisting apoptosis and promoting apoptosis by different mechanisms, thereby constituting a complex regulatory network, which plays an important role in the occurrence, development, and outcome of asthma. Interleukin (IL)-5, granulocyte–macrophage colony-stimulating factor (GM-CSF), Eotaxin, Bcl-2 and chemokine (C–C motif) ligand 5 (CCL5) are the main factors that maintain the EOS survival and inhibit their apoptosis [13,14,15]. IL-10, transforming growth factor beta 1 (TGF-β1), interferon (IFN)-γ, and Fas antigens, Fas ligand are involved in the regulation of the EOS apoptosis. These cytokines can also promote EOS apoptosis [16,17,18].
This experiment reveals the biological effects of WTD on EOS apoptosis in asthmatic rat models and determines the molecular mechanism of WTD in EOS apoptosis by detecting IL-5, IL-10, GM-CSF, CCL5, TGF-β, IFN-γ, Eotaxin mRNA, Bcl-2 mRNA, Fas mRNA, FasL mRNA and other related factors.
Methods
The minimum standards of reporting checklist (Additional file 1) contains details of the experimental design, and statistics, and resources used in this study.
Herbal medicine and preparation of Wentong decoction
Twelve herbs, namely, Astragalus membranaceus, cassia twig, Rhizoma zingiberis, bighead atractylodes rhizome, Fructus Corni, Rhizoma anemarrhenae, Aster tataricus Linn, Aceranthus sagittatus S. et Z., Inula britannica chinensis, Magnolia officinalis, Schisandra chinensis, liquorice, for Wentong decoction were purchased from Tong Ren Tang (Tong Ren Pharmaceutical Co., Ltd., Beijing, China). Testing shows that the herbal medicines reached the Pharmacopoeia standard (version 2010). The extraction of active constituents from Wentong decoction was performed using water boiling method, and all herbs were made into medicinal extract according to the standard of the School of Chinese Materia Medica, Beijing University of Chinese Medicine.
Animal handling
Healthy male 4-week-old SD rats were purchased from Beijing Huafukang Bio-Tech Co., Ltd., Beijing, China (License No.: SCXK (Beijing) 2009-0007) and provided adaptive feeding, ambient temperature of 24 ± 2 °C, air humidity of 45–65%, separate cages for natural and artificial light, free diet, and drinking water. The experiment was started 1 week later. All experimental procedures were carried out in accordance with internationally recognized guidelines for the use and care of American Laboratory Animals (NIH Publication No. 85–23, revised in 1985).
Rats were randomly divided into four groups (10 per group) as follows: the normal control group (N), asthmatic rat model group (M), dexamethasone-positive control group (D), and Wentong decoction treatment group (W).
Establishment of asthmatic rat model
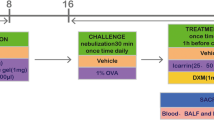
All rats except the normal control group were provided 0.2 mL 10% ovalbumin (OVA)/Al(OH)3-mixed liquid (OVA, a16951, Alfa Aesar, Ward Hill, MS, USA; Al(OH)3, a4682, Sigma Aldrich, St Louis, MO, USA) at the 1st and 8th days with five-point subcutaneous injection (bipedal, double groin, and peritoneal). At the same time, 0.0023‰ pertussis toxoid (p7208, Sigma-Aldrich) intraperitoneal injection was provided. After the initial sensitization, daily application of 1% OVA saline-atomization inhalation simulated for 1 h was performed using an ultrasonic atomizer (402ai; Yuyue Medical Equipment Co., Ltd., Jiangsu, China), and allergic asthmatic rat model was replicated for 9th–15th days. In the 16th–29th days, the dexamethasone control group and Wentong decoction treatment group were provided 0.5 mg/kg of dexamethasone (Sigma-Aldrich) and 1.34 g/kg of Wentong decoction, respectively, with daily lavage once each day. The normal control group and model group rats were provided equal saline (0.3 mL) for lavage. After 24 h of lavage, the lung tissue, BALF, abdominal aorta blood, and caudal venous blood were collected in rats.
Lung function test
After intraperitoneal injection of 4% pentobarbital sodium anesthesia in rats (2 mL/kg), rats were positioned in supine position in the enclosure, and 2 mm plastic tube was inserted into the trachea. The plastic tube was connected to a AniRes2005 small animal lung function analyzer (Beijing Bellambo High-tech Co., Ltd., Beijing, China). After 5 min, the inspiratory resistance, expiratory resistance, and dynamic compliance of the airway were observed.
Lung histopathology and detection of the EOS apoptosis
For lung histopathology and detection, the right lung middle lobe was used, and 4% of paraformaldehyde solution was added fixed for 24 h and paraffin-embedded at 4 µm thick continuous slices for routine hematoxylin and eosin (H&E) staining. In situ end labeling was performed using terminal deoxynucleotidyl transferase dUTP nick end labeling (TUNEL) alkaline phosphatase to detect the apoptotic cells, following the specific operation in strict accordance with the TUNEL reagent box manual (11684817910, Roche, United States). By combining the same H&E staining slices, one observer randomly selected five high-power fields in each slice to determine the index of EOS apoptosis and calculate its average as the representative value of the slice. The observation and analysis of the slices were performed under a light microscope (Olympus Corp., Tokyo, Japan).
EOS count in blood and BALF smears and flow cytometry technique for the detection of EOS apoptosis in blood
We took 20 µL of the rat tail vein blood, and 0.38 mL of the EOS count liquid was added at static pressure for 30 min. The EOS was counted on the cell count plate, and the procedure was repeated thrice to obtain the mean value.
The BALF was centrifuged (3500 r/min, 15 min), the centrifuged pellets were shaken with 8 mL PBS, and the supernatant was discarded by centrifugation and repeated three times. Take 0.1 mL liquid for smear, H&E staining, 200 cells were counted under a 400-fold light microscope, eosinophils were counted, repeated 3 times, and the average value was taken.
Up to 3 mL abdominal aorta blood was aseptically extracted from rats, and sodium citrate (130 mmol/L, ph 7.4) and 5% fetal bovine serum/Hanks liquid dilution were added for anti-coagulation. In a 15-mL centrifuge tube, we added 1 mL of Percoll liquid with a density of 1.100 and 1.085 g/mL successively. Finally, the diluted blood was added slowly to the cell suspension and horizontally centrifuged at 960×g for 15 min (20 °C). After centrifuging, we recycled the eosinophilic cell layer of Percoll liquid interfaces with different densities. D-Hanks liquid was used for centrifugal washing at 1500 rpm for 10 min twice. Then, using D-Hanks liquid of 1 mL heavy suspension, we calculated the absolute numbers of EOS under a microscope. We adjusted the cell count to approximately 2 × 106/mL and took 1 mL for flow detection.
Enzyme-linked immunosorbent assay (ELISA)
We took 2 mL of the rat’s blood at room temperature, centrifuged at 3000 r/min for 15 min at static pressure for 2 h, and then stored at − 80 °C after collecting the supernatant liquid. We performed the procedure in strict accordance with the ELISA Kit manual. We used the double antibody sandwich-ELISA assay test, to determine the IL-5, IL-10, GM-CSF, CCL5, TGF-β, and IFN-γ (Abcam, Cambridge, UK) in the serum.
RNA extraction and real-time polymerase chain reaction (PCR)
We evaluated the effect of Wentong decoction on the expression of Eotaxin mRNA and Fas mRNA in the lung tissues of asthmatic rats by using real-time fluorescent quantitative PCR. We determined the primer sequences using glyceraldehyde 3-phosphate dehydrogenase (GAPDH) and performed primer design (Table 1). We used Trizol total RNA extraction kit (DP405-02, Tiangen Biochemical Technology, Beijing Co., Ltd., Beijing, China) to extract total RNA from the 25 mg lung tissue according to the manufacturer’s instructions for inverse transcription of RNA samples to obtain the corresponding cDNA. After pretreatment of the upper machine mixture, we operated the real-time PCR device at 95 °C, 30 s, and 40 PCR loops (collected the fluorescence at 95 °C for 5 s and 60 °C for 40 s). The target and internal control genes of each sample underwent real-time PCR, and the data were analyzed using 2−△△CT method.
Data analysis
All data were analyzed using SPSS software (version 17.0, SPSS, Inc., Chicago, IL, USA). The result of each group is shown in mean ± standard deviation. P < 0.05 indicates statistically significant difference. Single factor variance analysis and Newman–Keuls test were used for group analysis. If the data do not conform to normal distribution, the nonparametric Kruskal–Wallis test was used for comparison.
Results
Lung function
Lung function examination plays an important role in evaluating the asthma severity, prognosis, and curative effect of drugs. To evaluate the effect of Wentong decoction in improving lung function in asthmatic rats, we tested the inspiratory resistance, expiratory resistance, and dynamic compliance of the airway in all groups of rats (Fig. 1). The results showed that compared with the normal group, the inspiratory resistance and expiratory resistance of rats in the model group increased significantly. Airway compliance significantly reduced, and the difference was statistically significant (p < 0.05). After drug intervention, dexamethasone (DXM) and WTD significantly reduced inspiratory resistance and expiratory resistance in rat models and improved airway compliance (p < 0.05). This finding suggested that WTD exhibited positive effect on the improvement of lung function of rats with OVA-sensitized asthma.
Changes of lung function in rats in each group. a Changes in inspiratory resistance of the airway in rats. Inspiratory resistance of asthmatic rat model was significantly higher than that of the normal control group, and inspiratory resistance decreased after DXM and WTD treatments. b Changes in airway expiratory resistance in rats. The expiratory resistance of the asthmatic rat model was significantly higher than that in the normal control group, and the expiratory resistance decreased after DXM and WTD treatments. c Changes in the dynamic compliance of the airway in rats. Airway compliance in asthmatic rat model was significantly lower than that in the normal control group, and the expiratory resistance increased after DXM and WTD treatments (*p < 0.05, compared with the normal group; #p < 0.05, compared with the model group; n = 8)
Pathology
The pathological changes of lung tissue in rats were evaluated using H&E staining in the paraffin section. In the model group, we can observe the irregular dark red hyperemia in the lung tissue, bronchial and vascular smooth muscle hyperplasia, airway and blood vessels with a large number of red-stained eosinophilic granulocyte and inflammatory cell infiltration taking purple-blue cluster-like lymphocytes as principal cells, lumen stenosis, tube wall thickening, arrangement disorder of bronchial mucosa epithelial cell, flakiness falling off, obvious goblet cell hyperplasia, and other typical pathological manifestations of BA. After the intervention of DXM and WTD, the pathological changes of the two groups significantly reduced, and the decrease in pathological changes in the DXM group was significant (Fig. 2). The results showed that WTD and DXM showed similar antiasthma effects in pathological changes of allergic asthma rats.
EOS count in blood and BALF
The inflammatory level of asthma was evaluated by calculating the EOS count of rat-tail-vein blood (Fig. 3a). Observation of BALF smears after HE staining (Fig. 4), counting the number of EOS per 200 cells (Fig. 3b). WTD and DXM can significantly reduce the level of EOS in the blood and BALF of OVA-sensitized-asthmatic rats. The results suggested that WTD may be able to reduce the inflammatory state of asthmatic rats to achieve the effect of antiasthma.
Analysis results of EOS count showing that WTD can reduce the inflammatory level of asthma. a EOS count in blood (1 × 109/L; *p < 0.05, compared with the normal control group; #p < 0.05, compared with the model group; n = 8); b EOS count in BALF smears (*p < 0.05, compared with the normal control group; #p < 0.05, compared with the model group; ▲p < 0.05, compared with the DXM group; n = 8)
Observation of EOS in BALF under the microscope. Eosinophils are round and 13–15 μm in diameter. The cytoplasm is filled with coarse, neat, even, and tightly arranged brick red or bright red eosinophilic particles. The nucleus is a characteristic lobular nucleus, usually 2–3 leaves, spectacle-shaped, dark purple. Eosinophils are easily broken and particles can be dispersed around the cells. Under the microscope, the expression of eosinophils from high to low was as follows: b asthma model group, d WTD group, c DXM group and a normal control group
Detection of EOS apoptosis
To evaluate the EOS apoptosis in asthmatic rats and the effect of WTD on the degree of EOS apoptosis, we adopted flow cytometry and TUNEL method, respectively, to determine the EOS apoptosis in the arterial blood and lung tissues of rats (Figs. 5, 6). The results showed that the degree of EOS apoptosis in arterial blood and lung tissues of rats was obviously consistent. Compared with the normal control group, the percentage of EOS apoptosis in asthmatic rat model decreased significantly, and the difference was statistically significant (p < 0.05). Compared with the model group, the WTD group could significantly increase the percentage of serum EOS in rats, and the difference was statistically significant (p < 0.05).
EOS apoptosis in lung tissue of rats: (a1, 2) Normal control group: The structure of bronchopulmonary tissue is normal, the lumen is smooth and the alveolar septum is intact. The eosinophil infiltration can be seen occasionally, and only a small amount of eosinophil apoptosis was found. (b1, 2) Model group: The bronchial lumen is stenotic, the epithelial cells proliferate and fall off, the tracheal smooth muscle and the alveolar septum thickens, and the structure is disordered. A large number of inflammatory cells, mainly eosinophils, infiltrating and apoptosis of eosinophils can be seen. (c1, 2) DXM group: Compared with the model group, the inflammatory cell infiltration of bronchopulmonary tissue in rats was alleviated, the pathological damage was significantly reduced, the apoptosis of EOS and the apoptotic index increased. (d1, 2) WTD group. Showed similar effects to DXM group in reducing pathological injury and increasing EOS apoptosis index in rats
Expression of cytokines in the serum
Several kinds of cytokines will exhibit a certain effect on EOS apoptosis. To further clarify whether the WTD can adjust the apoptosis state of EOS to achieve the purpose of alleviating asthma, we use ELISA method to detect IL-5, IL-10, CSF, CCL5, TGF-β, and IFN-γ in rat serum (Fig. 7). The results show that WTD and DXM can significantly reduce the expression of IL-5, GM-CSF, and CCL5 and significantly enhance the expression of IL-10, TGF-β, and IFN-γ in asthmatic rats.
Changes of cytokines in rat serum: a, c, and d Expression of IL-5, CCL5, and GM-CSF. The expression of IL-5, CCL5, and GM-CSF in asthmatic rat model was significantly higher than that in the normal control group, and the expression of IL-5, CCL5 and GM-CSF after DXM and WTD treatments significantly reduced. b, e, and f Expression of IL-10, IFN-γ, and TGF-β. The expression of IL-10, IFN-γ, and TGF-β in asthmatic rat model was significantly lower than that in the normal control group, and the expression of IL-10 and IFN-γ, and TGF-β significantly increased after DXM and WTD treatments. (*p < 0.05, compared with the normal group; #p < 0.05, compared with the model group; n = 8)
Expression of Eotaxin, Bcl-2, Fas and FasL mRNA in the lung tissues
Real-time PCR assay was used to detect the gene expression of Eotaxin, Bcl-2, Fas and FasL in lung tissues of rats. Quantitative results show that the expression of Eotaxin and Bcl-2 mRNA in lung tissue of the asthmatic model group significantly increased, whereas the expression of Fas and FasL mRNA decreased (compared with normal control group). Both DXM and WTD can significantly reduce the expression of Eotaxin and Bcl-2 mRNA in lung tissues of model rats and significantly increase Fas and FasL mRNA content in the lung tissues of rats (Fig. 8).
Gene expression of Eotaxin, Bcl-2, Fas and FasL in lung tissues. a, b Compared with the normal control group, the expression of Fas and FasL mRNA in lung tissues of the asthmatic model group significantly lower, the Fas and FasL mRNA increased after DXM and WTD treatment; c the expression of Fas and FasL was consistent, and the ratio of Fas/FasL showed no significant difference among groups. d, e Both the expression of Eotaxin and Bcl-2 mRNA in lung tissues of the asthmatic model group was significantly higher than that of the normal group. The Eotaxin and Bcl-2 mRNA decreased after DXM and WTD treatment (*p < 0.05, compared with the normal group; #p < 0.05, compared with the model group; n = 4)
Discussion
In this experiment, we successfully established asthmatic rat model by OVA sensitization, weakened pulmonary-function state in the model group, deteriorated histopathological changes, and aggregated and infiltrated the EOS. These undertakings can prove the success of our asthmatic rat model. After the intervention of WTD and DXM, the condition of rats improved obviously, indicating that WTD can achieve the asthma-like effect of DXM. In addition, we also found that WTD showed some anti-asthmatic effects in a dose-dependent manner. The WTD middle-dose group had more pronounced anti-asthma effects than the low-dose group, but the high-dose group and middle-dose groups had similar efficacy, Medium dose group is human equivalent group.
EOS infiltration is one of the important inflammatory mechanisms of asthma, and the activation and long-term survival of EOS are concentrated in the bronchial bronchi of allergic asthma [19], which induces a series of symptoms. However, the delay of EOS apoptosis maintained and aggravated the inflammatory state, which increased the difficulty of treatment. After bone marrow maturation, the EOS circulates briefly (~ 3 days) causing the PCD (apoptosis) in the peripheral blood [8]. Meanwhile, the apoptosis and necrosis are different, apoptosis indicating positive biological significance necessary for maintaining organism stability. The results of this experiment show that WTD can promote the EOS apoptosis in the lung tissue and blood and alleviate the inflammatory state of asthma.
IL-5 is a glycoprotein composed of 115 amino acids and mainly produced by T-helper cells and plays a biological effect through the binding of target cell surface of the specific receptor. IL-5 exhibits the role of promoting the proliferation, activation, and release of inflammatory mediators of EOS. IL-5 is also the most important eosinophil promoter. Suzuki [20] studies have confirmed that IL-5 inhibits EOS apoptosis. The high-affinity receptors on the EOS membrane combine with IL-5 to transmit the anti-apoptotic information into the cells, inhibit the apoptosis, and maintain the cell survival [13, 21].
CCL5 displays chemotaxis to EOS, which can show activation of EOS in vitro and increase EOS to express intercellular adhesion molecules. The study found that the CCL5 level in bronchoalveolar lavage fluid is significantly high in asthmatic patients [22], and the polymorphism of CCL5 gene is a risk factor for asthma by using meta-analysis of Huang et al. [23].
Eotaxin is one of the members of the CCL family. In normal respiratory tract, Eotaxin is mainly produced by epithelial cells. However, the exudation macrophages and EOS after antigen sensitization to some extent are the main sources of Eotaxin. This condition increases the Eotaxin formation [24]. Dexamethasone inhibits the production of Eotaxin mRNA in EOS, and IL-5 can induce Eotaxin expression in EOS [25]. Eotaxin increased synthesis after antigen stimulation, promoting the selective recruitment of EOS in local tissues. Once a large amount of EOS accumulated at the antigen-stimulating site, IL-5, together with other factors, causes the EOS to prolong survival and release a range of protein particles, leading to asthma attacks. Bcl-2 gene is a proto-oncogene that inhibits apoptosis. It not only plays an important role in the molecular regulation of apoptosis, but also has a close relationship with the occurrence and development of asthma. The high expression of Bcl-2 gene inhibits the apoptosis of eosinophils, causing a large number of eosinophils to infiltrate into the peripheral blood, lung tissue, and BALF. Eosinophils release inflammatory mediators and lead to asthma attacks.
GM-CSF is a cytokine closely related to asthma pathogenesis [26]. GM-CSF is mainly produced from T cells and macrophages. In asthma pathogenesis, GM-CSF can induce the growth of T cell precursors, cause EOS chemotaxis and activation, and promote EOS collection in the airway, causing airway-epithelial injury, inflammatory cell infiltration, and high-airway reactivity [27, 28].
In this study, we found that the content of serum IL-5, CCL5, GM-CSF, Eotaxin mRNA and Bcl-2 mRNA in asthmatic rat model was significantly higher than that in normal control group and negatively correlated with EOS apoptosis. WTD and DXM can effectively reduce the expression of the three cytokines in the serum of model rats. The effect of DXM on reducing CCL5 content was better than that of TCM Wentong decoction. However, no significant difference was observed between the two groups in IL-5, GM-CSF, and Eotaxin mRNA (p > 0.05).
IL-10 is an immunosuppressive agent with a multidirectional biological activity secreted by Th2 cells. IL-10 exhibits a direct inhibition effect on airway inflammatory cell activation, releases inflammatory factor in asthmatic patients [29, 30], can inhibit the IgE production, promotes IgG4 synthesis, and plays an important role in pathophysiology of allergic asthma [31]. Study found that IL-10 can inhibit the ability of Th2 cells to produce IL-5, and its inhibitory effect is concentration-dependent [32].
TGF-β induces EOS apoptosis and inhibits the IL-5, GM-CSF, and other cytokine-induced EOS to prolong the life cycle [33]. In addition to inhibiting cytokine synthesis and EOS survival, TGF-β also prevented EOS from releasing peroxidase and decreased the expression of EOS cell lines (Eol-1) \({\text{CD}}_{ 2 3}^{ + }\), indicating that TGF-β shows an inhibiting effect on growing EOS, differentiation, function, and all aspects of survival [34]. IFN-γ mainly originates from Th1 cells. In vivo experiments showed that IFN-γ can inhibit the infiltration of eosinophilic cells caused by allergens in the lungs [35], and inhibit the synthesis of Th2 cytokine IL-5, and its mechanism may be that IFN-γ inhibits the EOS collection by blocking the IL-5 synthesis by inhibiting GATA3 expression [36].
Fas gene is encoded as one of the members of the TNF receptor family, and its activation can induce apoptosis. Study found that interferon combined with tumor necrosis factor promoted the Fas expression on the surface of EOS and enhanced the FasL-mediated EOS apoptosis in vitro [37]. The expression of Fas receptor and FasL is relatively high in the cells of the immune system, which mediates apoptosis/proliferation plays an important role in asthma T cells with dysregulation of apoptosis/proliferation, Th1/Th2 imbalance, airway inflammation, airway hyperresponsiveness (AHR) and airway remodeling.
In this study, we found that the content of IL-10, TGF-β1, IFN-γ in the serum, Fas and FasL mRNA in the lung tissues of asthmatic rat model was significantly lower than that of the normal control group, which was positively correlated with the EOS apoptosis. Both WTD and DXM can effectively improve the expression of IL-10, TGF-β1, IFN-γ in the serum, and Fas mRNA in the lung tissues of model rats. This condition inhibits the EOS infiltration and promotes the EOS apoptosis to reduce the inflammatory response of asthma and achieve the goal of treating the disease.
In the complex internal environment, the local EOS apoptosis is not determined by a single factor but is controlled by the complex network system consisting of many cytokines, cell surface molecules, ligands, and chemokines. Maintaining homeostasis between pro-apoptotic factors and anti-apoptotic factors is the key to determine the EOS apoptosis.
Conclusions
Wentong decoction may promote the EOS apoptosis and reduce airway inflammation by increasing the expression of pro-apoptotic factors of IL-10, TGF-β1, IFN-γ, Fas mRNA and FasL mRNA and decreasing the anti-apoptotic factors of IL-5, GM-CSF, CCL5, Eotaxin mRNA and Bcl-2 mRNA. Thus, asthma prevention is achieved.
Abbreviations
- TCM:
-
traditional Chinese medicine
- EOS:
-
eosinophil
- WTD:
-
Wentong decoction
- IL:
-
interleukin
- CCL5:
-
chemokine (C–C motif) ligand 5
- TGF-β1:
-
transforming growth factor beta 1
- GM-CSF:
-
granulocyte–macrophage colony-stimulating factor
- IFN-γ:
-
interferon-γ
- BA:
-
bronchial asthma
- PCD:
-
programmed cell death
- DXM:
-
dexamethasone
References
Ray A, Kolls JK. Neutrophilic inflammation in asthma and association with disease severity. Trends Immunol. 2017. https://doi.org/10.1016/j.it.2017.07.003.
Kay AB. The role of eosinophils in the pathogenesis of asthma. Trends Mol Med. 2005;11(4):148.
Kulkarani NS, Hollins F, Sutcliffe A, et al. Eosinophil protein in airway macrophages: a novel biomarker of eosinophilic inflammation in patients with asthma. J Allergy Clin Immun. 2010;126(1):61.
Kliegman RM, Behrman RE, Jenson HB, Stanton BF. Nelson textbook of pediatrics. 18th ed. Philadelphia: Saunders Elsevier; 2007.
Akinbami LJ, Moorman JE, Garbe PL, et al. Status of childhood asthma in the Untied States. 1980–2007. Pediatrics. 2009;123(Suppl 3):S131–45.
Bai J, Zhao J, Shen KL, Xiang L, Chen AH, Huang S, Huang Y, Shu C, Wang JS, Rong-Wei YE. Prevalence of childhood asthma in Beijing, Chongqing, and Guangzhou. Chin J Allergy Clin Immunol. 2010;4:010.
Al Ghobain MO, Al-Hajjai MS, Al Moamary MS. Asthma prevalence among 16–18 year old adolescents in Saudi Arabia using the ISAAC questionnaire. BMC Public Health. 2012;12:239.
Oh J, Malter JS. Pin1-FADD interactions regulate Fas-mediated apoptosis in activated eosinophils. J Immunol. 2013;190(10):4937–45.
Blain JF, Sirois P. Involvement of LTD(4) in allergic pulmonary inflammation in mice: modulation by cysLT(1)antagonist MK-571. Prostaglandins Leukot Essent Fatty Acids. 2000;62(6):361–8.
Zietkowski Z, Tomasiak-Lozowska MM, Skiepko R, et al. Eotaxin-1 in exhaled breath condensate of stable and unstable asthma patients. Respir Res. 2010;11:110.
Kankaanranta H, Lindsay MA, Giembycz MA, Zhang X, Moilanen E, Barnes PJ. Delayed eosinophil apoptosis in asthma. J Allergy Clin Immunol. 2000;106(1 Pt 1):77–83.
Simon HU, Blaser K. Inhibition of programmed eosinophil death: a key pathogenic event for eosinophilia? Immunol Today. 1995;16(2):53–5.
Tai PC, Sun L, Spry CJ. Effects of IL-5, granulocyte/macrophage colony-stimulating factor (GM-CSF) and IL-3 on the survival of human blood eosinophils in vitro. Clin Exp Immunol. 1991;85(2):312–6.
Ganzalo JA, Jia GQ, Aguirre V, Friend D, Coyle AJ, Jenkins NA, Lin GS, Katz H, Lichtman A, Copeland N, et al. Mouse Eotaxin expression parallels eosinophil accumulation during lung allergic inflammation but it is not restricted to a Th2-type response. Immunity. 1996;4(1):1–14.
Lintomen L, Franchi G, Nowill A, Condino-Neto A, de Nucci G, Zanesco A, Antunes E. Human eosinophil adhesion and degranulation stimulated with Eotaxin and RANTES in vitro: lack of interaction with nitric oxide. BMC Pulm Med. 2008;8:13.
Beauvais F, Michel L, Dubertret L. The nitric oxide donors, azide and hydroxylamine, inhibit the programmed cell death of cytokine-deprived human eosinophils. FEBS Lett. 1995;361(2–3):229–32.
Aggarwal S, Gupta S. Increased apoptosis of T cell subsets in aging humans: altered expression of Fas (CD95), Fas ligand, Bcl-2, and Bax. J Immunol. 1998;160(4):1627–37.
Liu C, Wang Z, Feng Y, Lei S. A kinetic study on the relationship between of IL-5, IL-10 and eosinophil apoptosis in asthmatic airway inflammation. J West China Univ Med Sci. 2001;32(1):55–8.
Maret M, Ruffie C, Letuve S, Phelep A, Thibaudeau O, Marchal J, Pretolani M, Druilhe A. A role for bid in eosinophil apoptosis and in allergic airway reaction. J Immunol. 2009;182(9):5740–7.
Suzuki S, Okubo M, Kaise S, Ohara M, Kasukawa R. Gold sodium thiomalate selectivity inhibits interleukin-5-mediated eosinophil survival. J Allergy Clin Immunol. 1995;96(2):251–6.
Yousefi S, Hoessli DC, Blaser K, Mills GB, Simon HU. Requirement of Lyn and Syk tyrosine kinases for the prevention of apoptosis by cytokines in human eosinophils. J Exp Med. 1996;183(4):1407–14.
Alam R, York J, Boyars M, Stafford S, Grant JA, Lee J, Forsythe P, Sim T, Ida N. Increased MCP-1, RANTES, and MIP-1α in bronchoalveolar lavage fluid of allergic asthmatic patients. Am J Respir Crit Care Med. 1996;153(4 Pt 1):1398–404.
Huang H, Nie W, Zang Y, Chen J, Xiu Q. Association between CC motif chemokine ligand 5 (CCL5) polymorphisms and asthma risk: an updated meta-analysis. J Investig Allergol Clin Immunol. 2015;25(1):26–33.
Kampen GT, Stafford S, Adachi T, Jinquan T, Quan S, Grant JA, Skov PS, Poulsen LK, Alam R. Eotaxin induces degranulation and chemotaxis of eosinophils through the activation of ERK2 and p38 mitogen-activated protein kinases. Blood. 2000;95(6):1911–7.
Han SJ, Kim JH, Noh YJ, Chang HS, Kim CS, Kim KS, Ki SY, Park CS, Chung IY. Interleukin (IL)-5 downregulates tumor necrosis factor (TNF)-induced Eotaxin messenger RNA (mRNA) expression in eosinophils. Induction of Eotaxin mRNA by TNF and IL-5 in eosinophils. Am J Respir Cell Mol Biol. 1999;21(3):303–10.
Esnault S, Malter JS. Granulocyte macrophage-colony-stimulating factor mRNA is stabilized in airway eosinophils and peripheral blood eosinophils activated by TNF-alpha plus fibronectin. J Immunol. 2001;166(7):4658–63.
Weller PF, Lim K, Wan HC, Dvorak AM, Wong DT, Cruikshank WW, Kornfeld H, Center DM. Role of the eosinophil in allergic reactions. Eur Respir J Suppl. 1996;22:109s–15s.
Corrigan CJ, Hamid Q, North J, Barkans J, Moqbel R, Durham S, Gemou-Engesaeth V, Kay AB. Peripheral blood CD4 but not CD8 t-lymphocytes in patients with exacerbation of asthma transcribe and translate messenger RNA encoding cytokines which prolong eosinophil survival in the context of a Th2-type pattern: effect of glucocorticoid therapy. Am J Respir Cell Mol Biol. 1995;12(5):567–78.
Moniuszko M, Grubczak K, Kowal K, Eljaszewicz A, Rusak M, Jeznach M, Jablonska E, Dabrowska M, Bodzenta-Lukaszyk A. Development of asthmatic response upon bronchial allergen challenge is associated with dynamic changes of interleukin-10-producing and interleukin-10-responding CD4 + T cells. Inflammation. 2014;37(6):1945–56.
Palomares O, Martin-Fontecha M, Lauener R, Traidl-Hoffmann C, Cavkaytar O, Akdis M, Akdis CA. Regulatory T cells and immune regulation of allergic diseases: roles of IL-10 and TGF-β. Genes Immun. 2014;15(8):511–20.
Takeuchi M, Sato Y, Ohno K, Tanaka S, Takata K, Gion Y, Orita Y, Ito T, Tachibana T, Yoshino T. T helper 2 and regulatory T-cell cytokine production by mast cells: a key factor in the pathogenesis of IgG4-related disease. Mod Pathol. 2014;27(8):1126–36.
Codolo G, Mazzi P, Amedei A, Del Prete G, Berton G, D’Elios MM, de Bernard M. The neutrophil-activating protein of Helicobacter pylori down-modulates Th2 inflammation in ovalbumin-induced allergic asthma. Cell Microbiol. 2008;10(11):2355–63.
Komai M, Tanaka H, Nagao K, Ishizaki M, Kajiwara D, Miura T, Ohashi H, Haba T, Kawakami K, Sawa E, et al. A novel CC-chemokine receptor 3 antagonist, Ki19003, inhibits airway eosinophilia and subepithelial/peribronchial fibrosis induced by repeated antigen challenge in mice. J Pharmacol Sci. 2010;112(2):203–13.
Alam R, Forsythe P, Stafford S, Fukuda Y. Transforming growth factor beta abrogates the effects of hematopoietins on eosinophils and induces their apoptosis. J Exp Med. 1994;179(3):1041–5.
Iwamoto I, Nakajima H, Endo H, Yoshida S. Interferon gamma regulates antigen-induced eosinophil recruitment into the mouse airways by inhibiting the infiltration of CD4+ T cells. J Exp Med. 1993;177(2):573–6.
Li Q, Zhang YD, Sun CW, Chen YL, Du YH, Zhao GJ, Zhang DL. Treatment of allergic rhinitis rats by intranasal interferon gamma. Chin J Otorhinolaryngol Head Neck Surg. 2008;43(2):134–8.
Luttmann W, Dauer E, Schmidt S, Marx O, Hossfeld M, Matthys H, Virchow JC Jr. Effects of interferon-gamma and tumour necrosis factor-alpha on CD95/Fas ligand-mediated apoptosis in human blood eosinophils. Scand J Immunol. 2000;51(1):54–9.
Authors’ contributions
YY and YLL conceived and designed the research; CLL and TY performed the experiments; YHK and QS analyzed the data; HPB and YY wrote the first draft of the manuscript and other authors participated in revision. All authors read and approved the final manuscript.
Acknowledgements
Not applicable.
Competing interests
The authors declare that they have no competing interest.
Availability of data and materials
The datasets used in this study are available from the corresponding author upon reasonable request.
Consent for publication
The manuscript is approved by all authors for publication.
Ethics approval and consent to participate
Not applicable.
Funding
This study was supported by the National Natural Science Foundation of China (No. 81173245); NSFC Youth Foundation (No. 81302943); Class A Promotion Project of Young Scientific Talents in 2015 (No. 2015-QNYC-A-07); Beijing Ten Disease of Ten Drugs (No. Z151100003815025).
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Author information
Authors and Affiliations
Corresponding author
Additional file
Additional file 1.
The minimum standards of reporting checklist.
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated.
About this article
Cite this article
Yan, Y., Bao, HP., Li, CL. et al. Wentong decoction cures allergic bronchial asthma by regulating the apoptosis imbalance of EOS. Chin Med 13, 21 (2018). https://doi.org/10.1186/s13020-018-0180-2
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s13020-018-0180-2