Abstract
Background
Neurofibromatosis type 1 is a genetic disease that affects multiple organs and systems, leading to various clinical manifestations. In Neurofibromatosis type 1, rare intrathoracic meningoceles often occur alongside bone dysplasia. These meningoceles contain cerebrospinal fluid and can be mistakenly diagnosed as ‘pleural effusion’.
Case presentation
In this case report, we mistakenly identified ‘cerebrospinal fluid’ as ‘pleural effusion’ and proceeded with drainage. This error posed significant risks to the patient and holds valuable implications for the future diagnosis and treatment of similar patients.
Conclusions
In patients with Neurofibromatosis type 1 complicated by spinal deformity, there is a high incidence of intrathoracic meningoceles. Treatment strategies may differ based on the specific features of the lesions, and collaboration among multiple disciplines can significantly improve patient outcomes.
Similar content being viewed by others
Explore related subjects
Find the latest articles, discoveries, and news in related topics.Background
Intrathoracic meningocele is a rare condition characterized by the bulging of the meninges through the intervertebral foramen or a defect in the vertebral body. It is most associated with neurofibromatosis type 1 (NF-1), accounting for 60 to 85% of cases [1]. NF-1 is an uncommon chromosomal inherited disease with an incidence of approximately 1/2500-1/3000 in newborns [2]. These patients often develop scoliosis and meningocele in adulthood as a result of sclerotin dysplasia [3]. Initially, patients with intrathoracic meningocele may not exhibit any signs or symptoms. However, as the disease progresses, some patients may experience chest tightness or breathlessness due to compression of the lungs. Unfortunately, due to the rarity of the condition and a lack of expertise, the meningocele may be misdiagnosed as pleural effusion. As a result, patients may undergo unnecessary drainage of a mass pleural effusion, putting them at risk of complications and even death. In this article, we present a case of a patient with NF-1 combined with huge intrathoracic meningocele with NF-1 who was misdiagnosed as pleural effusion.
Case presentation
A 43-year-old female patient with a history of NF-1 presented to the local hospital on 16 February 2023, complaining of chest congestion and dyspnea that had been ongoing for a month. The patient had a history of skin conditions, such as brown macule, since the age of 4. At the age of 14, she underwent surgical treatment for a subcutaneous mass in her right leg, which was diagnosed as NF-1 through pathology. Although the patient had no prior family history of NF-1, her son also suffered from the condition. Over the past three years, she had experienced intermittent coughing but had not sought medical attention due to mobility problems and feelings of inferiority. One month ago, she started experiencing chest tightness and dyspnea at rest. She sought care at a local hospital, where a massive pleural effusion was discovered in the right hemithorax. To determine the cause, the local hospital performed closed chest drainage on the patient. However, only a small amount of transudate was drained, which was not consistent with the patient’s condition of massive pleural effusion. Subsequently, the patient was referred to our hospital. Upon admission, physical examination revealed decreased breath sounds in the right lung. The patient presented numerous skin nodules with pedicles and café-au-lait spots scattered across the trunk. Additionally, a large soft mass was noted in the lower lumbar region, and several masses were found on the right lower extremity, leading to difficulty in walking. No neurological signs or symptoms were observed during the examination.
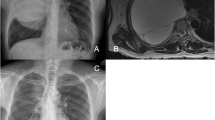
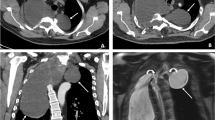
Chest computed tomography (CT) scan revealed a large low-density image in the right thoracic cavity, and a defect was observed in part of the vertebral plate in the thoracic spine (Fig. 1). The chest tube brought from local hospital was ineffective in draining the fluid, leading us to conduct a thoracoscopy to investigate the cause of the pleural effusion. The intraoperative exploration revealed that the drainage tube was in the thoracic cavity with only a small amount of fluid present. The pleurae appeared normal with no nodules, and the pleural biopsy results were normal as well (Fig. 2). Surprisingly, postoperative plain chest X-rays showed a significant pleural effusion still present in the right thorax, contradicting the findings of the thoracoscopy.
This raises the question of what caused the sudden accumulation of such a large amount of fluid in the pleural cavity and why it was not detected during the thoracoscopy. To address this issue, we replaced the original chest drainage tube with a new one guided by ultrasound. Following the insertion of the new tube, approximately 1000 ml of clear liquid was drained, leading to a significant relief in the patient’s chest tightness symptoms. However, she developed a severe headache the following day and found relief by lying flat on her back. Subsequent analysis of the hydrothorax revealed a white blood cell count of 11 × 106/L, lactate dehydrogenase level of 36U/L, total protein level of 3.7 g/L, and glucose level of 3.21 mmol/L. Cytology examination confirmed the absence of malignant tumor cells in the pleural effusion. Furthermore, thorough examination and data analysis ruled out heart failure, hepatic and renal dysfunction, hypothyroidism, and hypoproteinemia.
The pleural fluid was clear and transparent, with the patient developing a postural headache following the pleural effusion drainage. Upon analysis, we discovered that the fluid was cerebrospinal fluid (CSF) in actual. While CSF hydrothorax is commonly associated with traumatic injury or spinal surgery, this patient did not have a history of such events in the spinal column or chest. We reviewed the chest CT and identified a low-density image connected to the canalis vertebralis (Fig. 1). Subsequently, a thoracic magnetic resonance imaging (MRI) was performed (Fig. 3). The MRI revealed: 1) discontinuity between the T4-T8 vertebrae in both sagittal and horizontal axial views, 2) protrusion of the thoracic vertebrae towards the right, and 3) communication between the right pleural effusion and the spinal canal. Most importantly, the MRI revealed that the ‘pleural fluid’ had similar intensity to CSF, indicating the presence of an intrathoracic meningocele. During thoracoscopy, it was observed that the drainage tube, initially placed at a local hospital, had successfully entered the thoracic cavity instead of the bulging meninges, resulting in only a minimal amount of fluid being drained. Regrettably, our ‘successful’ fluid drainage procedure led to the patient experiencing postural headaches.
MRI revealed: 1) discontinuity between the T4-T8 vertebrae in both sagittal and horizontal axial views, 2) protrusion of the thoracic vertebrae towards the right, 3) communication between the right pleural effusion and the spinal canal. Most importantly, the MRI revealed that the ‘pleural fluid’ had similar intensity to CSF, indicating the presence of an intrathoracic meningocele
Upon recognizing the misdiagnosis, we promptly removed the drainage tube, disinfected the puncture site, and applied a sterile dressing. We invited neurosurgery, spine surgery, and thoracic surgery in a multidisciplinary discussion to consider surgical intervention. After comprehensive evaluation, it was determined that surgical procedures require collaboration across multiple disciplines, posing significant challenges and risks. Therefore, conservative treatment was advised for the time being, with a suggestion for patient to seek further care at a specialized hospital. After receiving conservative treatment, including anti-infection measures, the patient’s puncture site healed naturally, leading to a complication-free discharge without issues like pneumocephalus or meningitis. We conducted a follow-up call 3 months after the patient was discharged from the hospital. The patient sought surgical treatment at a more specialized hospital, where they were informed of the high risk and cost associated with the procedure. Ultimately, the patient opted for continued observation and follow-up, and is currently alive.
Discussion
NF-1 is a rare genetic disease that affects multiple systems, including the skin, bones, joints, respiratory system, and nervous system [4]. Intrathoracic meningocele, although relatively uncommon, is often associated with NF-1. It has been observed that 60–85% of thoracic meningoceles occur in individuals with NF-1 due to dural hypoplasia and intervertebral foramen enlargement, which are the primary contributing factors to the development of intrathoracic meningocele. Intrathoracic meningocele can be asymptomatic, but when the bulging area compresses the lungs, it can cause symptoms such as chest tightness and dyspnea. Asymptomatic patients can be treated conservatively, while patients with obvious symptoms may require surgical intervention [5]. Surgery is the primary method for fully addressing intrathoracic meningocele in individuals with NF-1, necessitating collaboration across multiple disciplines. The specific surgical approach is determined by factors such as the size of the meningocele, extent of spinal deformity, and overall medical conditions [6].
To enhance our understanding of recent surgical methods, we conducted a comprehensive literature review. Using the PubMed database, we searched for articles with the keywords ‘(Meningocele) AND (Neurofibromatosis)’ from 2000 to 2024, resulting in a total of 90 relevant articles. The literature review based on the titles and abstracts, those not relevant to the topic of intra-abdominal meningocele, non-surgical treatment, or lacking specific surgical details were excluded. Ultimately, 21 articles on surgical treatment for intrathoracic meningocele related to NF-1 were obtained. Among the articles, 20 were case reports and 1 was a case series. Literature review comprised 21 patients diagnosed with NF-1 who received surgical intervention for intrathoracic meningocele. The data gathered encompassed details such as patient age, gender, symptoms, presence or absence of spinal deformity, size of the meningocele and location, as well as any history of previous diseases. Additionally, information on spinal or meningocele surgery, surgical techniques employed, postoperative complications, and prognosis during follow-up was documented (Table 1).
The average age of the patients who underwent surgery was 49.9 years old, with 7 (33%) males and 14 (67%) females. The most common clinical symptom reported was dyspnea, with an incidence rate of 17/21 [5,6,7, 9, 10, 12,13,14,15,16,17,18, 20,21,22,23,24]. Followed by chest and back pain in 5/21, which was related to radiculopathy [5, 12, 15, 22, 25]. Spinal cord-related symptoms were observed in 4/21 patients, presenting as limb paresthesia, limb weakness, and ataxia [8, 11, 13, 18]. Additionally, hemorrhagic shock and syncope were each reported in one patient [16, 19].
Among the 21 patients, intrathoracic meningocele was observed on the left side in 9 cases and on the right side in 11 cases. Additionally, one patient presented with bilateral intrathoracic meningocele [20], while 5/21 patients had multiple intrathoracic meningoceles [9, 14, 20, 22, 25]. In 17/21 patients, the size of the intrathoracic meningeal cyst was greater than 10 cm, while in 4/21 patients, the size ranged between 5 and 10 cm [8, 17, 24, 25].
One patient had previously undergone cystoperitoneal (CP) shunt twice, but the meningocele recurred 6 years later. Following an unsuccessful attempt to transect the T2-T3 ribs and resect the meninges, the patient underwent thoracoscopic meningectomy repair and pleurodesis. Symptoms were completely resolved 5 months post-operation [6]. Another patient with a history of lumboperitoneal (LP) shunt developed symptoms of enlarged syringomyelia and lower limb weakness after surgery. This patient subsequently underwent ventriculoperitoneal (VP) shunt placement. While the syringomyelia reduced in size post-surgery, the lower limb weakness symptoms persist [11]. Five patients underwent CP shunt procedures for giant meningoceles measuring over 10 cm. Of these patients, 3 experienced a reduction in the size of their meningoceles within 1-year post-surgery [14, 20, 21], while 2 patients had recurring meningoceles that required additional surgical interventions, including thoracic surgery and thoracoscopic meningeal plication surgery [12, 15].
Among the 13 patients who underwent meningectomy repair for the bulge, 8 had thoracotomy [5, 9, 10, 16,17,18, 22, 25], 4 had meningectomy and repair following posterior laminectomy [8, 13, 23, 24], and 1 had meningectomy and repair through thoracoscopic surgery [7]. Out of the 8 patients with thoracotomy, only 1 experienced postoperative paraplegia complications, which resolved spontaneously after a few months [25], with the remaining patients showing a good prognosis. Of the 4 patients who had posterior laminectomy, 2 experienced postoperative walking difficulties and chest tightness, requiring further surgery [23, 24].
The primary objective of treatment is to alleviate lung compression and alleviate symptoms by resecting and repairing the meningocele. If clinical symptoms persist despite cerebrospinal fluid diversion, further surgical intervention may be necessary. Repairing bulging meninges can be achieved through either an internal or external approach. The internal approach may protect the nerve root, but performing a secure repair could be challenging due to the limited operating space. On the other hand, the external approach enables the resection or plication of the bulging meninges while completely ligating them. Whether it is thoracotomy, thoracoscopy, rib transection, or laminectomy, these methods all serve to expose the surgical field [6].
It is noted that many patients present with various spinal deformities, including scoliosis, kyphoscoliosis, costal deformities, and vertebral deformities such as spondylolisthesis, scalloping of vertebral borders, thinning of pedicles, enlargement of intervertebral foramen, and vertebral canal [26]. In our imaging analysis, 95% of the patients exhibited different levels of spinal deformity, with one patient having a history of spinal deformity correction surgery. The significance of spinal surgery in the treatment process should not be underestimated, especially for patients undergoing laminectomy or rib transection, as it may lead to spinal instability and require simultaneous spinal fusion. Hence, there is a strong emphasis on the importance of multidisciplinary collaboration.
Upon initial assessment of the patient in our study, we noted unexplained pleural effusion. Pleural effusion is a commonly observed condition in clinical practice, often associated with various underlying diseases. Despite advancements in diagnostic techniques, approximately 20% of pleural effusions still have an uncertain origin [26]. Thoracoscopy, a routine procedure, can be performed to identify the cause of unknown pleural effusions. Prior to performing thoracoscopy, it is important to thoroughly review chest CT images to identify any contraindications. For NF-1 patients with intrathoracic meningocele, the blind use of thoracoscopy can pose significant risks, including the development of meningitis, pneumocephalus, and even death [27].
CSF leakage into the chest cavity is a rare condition that usually occurs after trauma or surgery [28]. It is uncommon for patients to experience CSF leakage without a history of operation or trauma. β-2-Transferrin is a glycoprotein produced by neuraminidase in the brain and is predominantly found in CSF. It is present in only small amounts in cochlear perilymph, aqueous humor of the eye, and vitreous body. Laboratory techniques such as immunofixation, immunoblotting, gel electrophoresis, and isoelectric focusing are commonly used to determine β-2-transferrin content, all of which have high accuracy and specificity (over 90%) for CSF diagnosis. Moreover, studies have demonstrated that β-2-transferrin can be reliably detected in CSF samples stored at room temperature or refrigerated for 14 days [29]. Hence, β-2-transferrin serves as a reliable marker for determining CSF presence. In our case, although we strongly suspected the effusion to be CSF, we did not conduct a β-2-transferrin test on the extracted fluid. Instead, we selected MRI scanning due to its ability to clearly depict the relationship between the spinal canal, spinal cord, and nerve roots at the lesion site. Additionally, MRI can effectively differentiate fluids and distinguish between intrathoracic meningoceles, neurofibromas, and neuroblastomas. If the imaging reveals a correlation between pleural effusion and the spinal canal, along with spinal deformity and enlargement of the intervertebral foramen, intrathoracic meningocele should be taken into consideration [5].
Conclusions
In patients with NF-1 complicated by spinal deformity, there is a high incidence of intrathoracic meningoceles. However, inexperienced doctors may resort to blind fluid drainage or thoracoscopy when managing these patients, resulting in complications such as postural headache, meningitis, pneumocephalus, and potentially fatal outcomes. Thorough history taking is essential when treating patients with these conditions. Treatment strategies may differ based on the specific features of the lesions, and collaboration among multiple disciplines can significantly improve patient outcomes.
Data availability
No datasets were generated or analysed during the current study.
Abbreviations
- NF-1:
-
Neurofibromatosis Type 1
- CSF:
-
Cerebrospinal Fluid
- CT:
-
Computed Tomography
- MRI:
-
Magnetic Resonance Imagining
- CP:
-
Cystoperitoneal
- VP:
-
Ventriculoperitoneal
- LP:
-
Lumboperitoneal
References
Hirbe AC, Gutmann DH. Neurofibromatosis type 1: a multidisciplinary approach to care. Lancet Neurol. 2014;13:834–43.
Ferner RE. Neurofibromatosis 1 and neurofibromatosis 2: a twenty first century perspective. Lancet Neurol. 2007;6:340–51.
Modica R, Altieri B, D’Aniello F, Benevento E, Cannavale G, Minotta R, et al. Vitamin D and bone metabolism in adult patients with neurofibromatosis type 1. Metabolites. 2023;13:255.
Reynolds RM, Browning GGP, Nawroz I, Campbell IW. Von Recklinghausen’s neurofibromatosis: neurofibromatosis type 1. Lancet. 2003;361:1552–4.
Zuo T, Ni Z, Zhou M, Huang C, Tang Z, Yang Z. Large intrathoracic meningocele in a patient with neurofibromatosis type 1. Asian Cardiovasc Thorac Ann. 2014;22:219–21.
Sum CHF, Li L-F, Taw BBT, Lui W-M, Sit K-Y, Chow VLY, et al. Surgical repair of a large intrathoracic meningocele associated with neurofibromatosis type 1 after failed cystoperitoneal shunts: illustrative case. J Neurosurgery: Case Lessons. 2021;2:CASE21404.
Nakazawa R, Masui K, Goto T. Anesthesia management for thoracoscopic resection of a huge intrathoracic meningocele: a case report. JA Clin Rep. 2024;10:14.
Kang Y-R, Hong S-M, Choi J-H, Lee S-J, Kim J-M, Wook Kang K, et al. Sensory neuronopathy in a patient with neurofibromatosis type 1. Medicine. 2022;101:e31718.
Lehner K, Lubelski D, Witham T, Luciano M, Tuffaha S, Yang R, et al. Multidisciplinary approach for repair of a large ventral thoracic meningocele in neurofibromatosis-1; a systematic review of the literature and case report. Clin Neurol Neurosurg. 2021;210:106996.
Wang Y, He B, Yang J, Xu J, Liu J, Huang S. Individualized management of giant anterior meningoceles-case series. Medicine. 2020;99:e19631.
Close LN, Park B, Woodroffe RW, Hitchon PW. Thoracic meningocele and cervical Syringomyelia treated with Ventriculoperitoneal Shunt. World Neurosurg. 2019;129:322–6.
Das P, Goyal T, Hunt MA. Intrathoracic meningocele associated with neurofibromatosis type 1 and a novel technique for surgical repair: case report. J Neurosurgery: Spine. 2017;27:291–4.
Malla HP, Park BJ, Koh JS, Jo DJ. Giant intrathoracic meningocele and breast Cancer in a neurofibromatosis type I patient. J Korean Neurosurg Soc. 2016;59:650.
Cho DG, Chang YJ, Cho KD, Hong JT. Collaborative treatment of huge intrathoracic meningoceles associated with neurofibromatosis type 1: a case report. J Cardiothorac Surg. 2015;10:161.
Chen H-C, Chang P-H, Jhang S-W, Wang B-Y. Thoracoscopic plication for a huge thoracic meningocele in a patient with neurofibromatosis. J Cardiothorac Surg. 2014;9:85.
Yusuf A, Pillai A, Menon S, Panikar D. Massive spontaneous Hemothorax, Giant Intrathoracic Meningocele, and Kyphoscoliosis in neurofibromatosis type 1. J Surg Tech Case Rep. 2014;6:33.
Kim Y-J, Cho H-M, Yoon C-S, Lee C-K, Lee T-Y, Seok J-P. Surgical Treatment of Thoracic Menigocele Associated with neurofibromatosis and Kyphoscoliosis. Korean J Thorac Cardiovasc Surg. 2011;44:383–6.
Walcott BP, Kahle KT, Wain JC, Borges LF. Giant thoracic meningocele associated with neurofibromatosis 1. Neurology. 2011;76:1943–1943.
Kaneda H, Saito T, Konobu T, Saito Y. Chest wall bleeding with giant intrathoracic meningocele in neurofibromatosis type 1. Interact Cardiovasc Thorac Surg. 2011;12:328–30.
Tanaka K, Shimizu K, Kakegawa S, Oshima K, Takeyoshi I. Cystoperitoneal Shunt for a giant Intrathoracic Meningocele under Local Anesthesia. Ann Thorac Surg. 2011;91:317–9.
Vanhauwaert DJ, Deruytter MJ. Cystoperitoneal shunt as alternative treatment for a giant thoracic meningocele in a patient with neurofibromatosis. Surg Neurol. 2008;69:535–7.
de Andrade GC, Braga OP, Hisatugo MK-I. GIANT INTRATHORACIC MENINGOCELES ASSOCIATED WITH CUTANEOUS NEUROFIBROMATOSIS TYPE I. Arq Neuropsiquiatr.
Ebara S, Yuzawa Y, Kinoshita T, Takahashi J, Nakamura I, Hirabayashi H, et al. A neurofibromatosis type 1 patient with severe kyphoscoliosis and intrathoracic meningocele. J Clin Neurosci. 2003;10:268–72.
Mizuno J, Nakagawa H, Yamada T, Watabe T. Intrathoracic Giant Meningocele developing Hydrothorax: a Case Report. J Spin Disord Tech. 2002;15:529–32.
Nakai S, Ibe S, Otani K. Paraparesis after excision of intrathoracic meningoceles in a patient with neurofibromatosis. J Orthop Sci. 2002;7:143–6.
Heyde C-E, Völker A, von der Höh NH, Glasmacher S, Koller H. Wirbelsäulendeformitäten Bei Neurofibromatose Typ 1. Orthopäde. 2021;50:650–6.
Li J, Bao Q-L, Wang Y, Hu Y-M, Chen P. Diagnostic value of the FHIT and p16 mRNA loss and the K-ras gene mutation in pleural fluids for malignant pleural effusion. Cancer Biomarkers. 2013;13:49–58.
Trujillo-Reyes JC, Rami-Porta R, Navarro AK, Belda-Sanchis J. Tension pneumocephalus: a rare complication after resection of a neurogenic tumour by thoracoscopic surgery. Eur J Cardiothorac Surg. 2014;46:145.
Alves Júnior SF, Irion KL, de Melo ASA, Meirelles G, de Rodrigues SP, Souza RS Jr. et al. AS,. Neurofibromatosis type 1: evaluation by chest computed tomography. Radiol Bras. 2021;54:375–80.
Acknowledgements
Not applicable.
Funding
There is no relevant funding in this study.
Author information
Authors and Affiliations
Contributions
NYC and JRB wrote and revised the manuscript. WJL, LFM and QH conceived and directed this study. All authors read and approved the manuscript and agree to be accountable for all aspects of the research in ensuring that the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
This study was approved by the Human Research Ethics Committee of Northern Jiangsu Province Hospital.
Consent for publication
Written informed consent for publication of data was obtained from the patient. A copy of the consent form is available for review by the Editor of thejournal.
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Chen, N., Li, W., Min, L. et al. Neurofibromatosis type 1 with huge intrathoracic meningoceles misdiagnosed as pleural effusion: A case report and literature review. J Cardiothorac Surg 19, 303 (2024). https://doi.org/10.1186/s13019-024-02819-3
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s13019-024-02819-3