Abstract
Background
In patients with unprotected left main coronary artery disease (ULMCAD), this study compared the long-term prognosis of drug-eluting stent insertion guided by intravascular ultrasonography (IVUS) vs. angiography.
Patients and methods
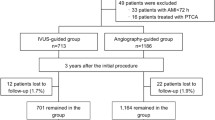
This retrospective consort investigation was performed in December 2021. This analysis included 199 patients who underwent IVUS-guided (IVUS group, n = 81) or angiography-guided (angiography group, n = 118) drug-eluting stent implantation at the Affiliated Hospital of Inner Mongolia Medical University between September 2013 and September 2018. Major adverse cardiac events (MACE) were defined as cardiovascular death, sudden cardiac death, myocardial infarction.
Results
The IVUS group had considerably lower proportions of MACE within 1 year postoperatively (P = 0.002) and cardiac mortality within 3 years postoperatively (P = 0.018) compared to the angiography group. However, after adjusting for confounding variables, the hazard ratio for 3-year cardiac mortality was similar between the two groups (P = 0.28). In the IVUS group, there was considerably greater minimum lumen diameter (MLD) (P = 0.046), and reduced frequencies of target vessel restenosis (P < 0.050) and myocardial infarction (MI) (P = 0.024) compared to the angiography group. Cox regression analysis for 3-year cardiac mortality found that MSD was independently associated with low cardiac mortality (HR = 0.1, 95% CI: 0.01–14.92, P = 0.030).
Conclusion
IVUS-guided drug-eluting stent implantation may lead to better long-term prognosis in patients with ULMCAD, and MSD may be a predictor for lower cardiac mortality.
Similar content being viewed by others
Introduction
Globally, coronary artery disease is a leading cause of illness and mortality. Percutaneous coronary intervention (PCI) is the primary therapeutic intervention for this condition [1]. Left main coronary artery disease (LMCAD) is a severe form of ischemic heart disease that poses a considerable risk of mortality and poorer clinical outcomes compared to other patients with ischemic heart disease. PCI was previously contraindicated for LMCAD, but with advances in drug-eluting stents (DES) and imaging techniques, it has become a more commonly used treatment option [2, 3].
Coronary angiography provides only a two-dimensional view of the vessel lumen and does not offer information about plaque burden or stent apposition. Angiography-guided stent implantation (SI) relies on visual estimation of the vessel size and lesion length, which may result in stent undersizing or oversizing, leading to stent malapposition and incomplete lesion coverage, which is inadequate for assessing complex lesions such as LMCAD [4]. Intravascular ultrasound (IVUS) and optical coherence tomography (OCT) are advanced intracoronary imaging techniques used to provide detailed 3D morphological information of coronary plaques, vessels, and stents. Studies have demonstrated that SI guided by IVUS can result in improved outcomes compared to angiography-guided implantation [5]. IVUS enables high-resolution, cross-sectional images of the coronary artery, allowing for precise assessment of the vessel lumen, plaque burden, and stent placement [6, 7]. By identifying stent malapposition, which is associated with an increased risk of stent thrombosis and restenosis, IVUS-guided SI can lead to better stent apposition and expansion, reducing the risk of adverse events. In addition, the decreased incidences of severe adverse cardiovascular events like death, myocardial infarction, and target lesion revascularization have been associated with IVUS-guided stenting [8,9,10]. These results make it possible to advise ideal SI with more thorough information [11, 12]. The effects of IVUS-guided stenting on coronary imaging findings before and after SI have been studied in a number of randomized controlled trials and observational studies [13], which have shown that IVUS-guided stenting is especially helpful for patients with complex coronary artery disease, such as severe calcifications, long lesions, unprotected left main disease, bifurcations, chronic total occlusion, and other complex conditions [8].
Despite the growing evidence supporting the use of IVUS to optimize PCI, its use in intracoronary imaging to guide stent placement during re-interventional clinical practice is still low. The lack of supporting clinical evidence and high costs have limited the popularity of this technology [14]. Therefore, we aim to investigate the clinical outcomes of PCI guided by angiography alone versus adjunctive IVUS imaging, as well as the clinical benefits of IVUS-guided SI in left main coronary artery (LMCA) stenosis.
Methods
Study design and participants
This study was a retrospective study that was performed on December 2021. This study reviewed the clinical data and follow up results of the patients treated at the Department of Cardiology, the Affiliated Hospital of Inner Mongolia Medical University, during the period from September 2013 to September 2018, in order to assess the effectiveness of PCI in patients with ULMCAD. ULMCAD was defined as stenosis of the LMCA causing at least a 50% reduction in blood vessel diameter [17]. Patients between 60 and 80 years of age with coronary LMCAD who received DES implantation and standard antiplatelet therapy after PCI were included. Patients who had a history of acute myocardial infarction (MI) within 24 h, cardiogenic shock, high risk for bleeding, liver insufficiency, malignant tumor, or had coronary chronic total occlusion (CTO) of left anterior descending (LAD) or left circumflex (LCX) artery and underwent rotational atherectomy within 3 months were excluded. The ethical committee of the Affiliated Hospital of Inner Mongolia Medical University authorized this study, and written informed permission were provided by all participants. All interventional treatments were performed according to the current standards.
Treatment methods
Four experienced surgeons conducted coronary angiography or IVUS procedures to assist in determining the optimal stenting method for patients, based on the criteria such as vessel diameter, stent expansion status, stent edge adherence, and presence of dissection at the edge. If optimal stenting was suggested with the assistance of IVUS and the patients agreed and underwent stenting with IVUS, then the patients were allocated to the IVUS group. If optimal stenting was suggested with the assistance of coronary angiography and the patients agreed and underwent stenting with coronary angiography, then the patients were allocated to the angiography group. Patients who had suboptimal stenting as determined by angiography or IVUS without further dilation were randomly included in the IVUS and angiography groups based on a random table method. The choice of medication and use of tools such as glycoprotein IIb/IIIa inhibitors, low molecular weight heparin, DES type, preconditioning, and intra-aortic balloon pump were at the discretion of the surgeon. Prior to surgery, patients received a 300 mg loading dose of clopidogrel. Post-dilation was recommended for both groups using a non-compliant balloon with a balloon/stent ratio of 1.0:1.0 as determined by angiography or IVUS. For patients with suboptimal stent expansion or stent malposition, post-expansion was performed as needed. IVUS was performed only if the patient was not at risk of circulatory failure. The effectiveness of stent placement was evaluated immediately after surgery using postoperative IVUS imaging with a Volcano’s s5 IVUS instrument. The ideal results as determined by IVUS were defined as thrombolysis in myocardial infarction (TIMI) blood flow grade 3, a minimum stent lumen cross-sectional area of > 6.9 mm2, fully expanded and adherent stent, no blood flow between the stent and vascular intima, and no vascular dissection as noted in references [15, 16]. Angiographic success was defined as TIMI grade 3 with < 10% residual stenosis. Following the procedure, patients were prescribed lifelong aspirin 100 mg/day and clopidogrel 75 mg/day for at least 12 months.
Data collection and definition
The study collected various baseline clinical characteristics of the patients, including demographic information such as age, gender, height, and weight, as well as comorbidities like hypertension, hyperlipidemia, diabetes, stroke, atrial fibrillation, smoking, chronic renal insufficiency, serum creatinine, history of myocardial infarction, acute myocardial infarction, non-ST-segment elevation myocardial infarction, ST segment elevation myocardial infarction, cardiogenic shock, left ventricular ejection fraction. Lesion features such as left anterior descending (LAD), left circumflex (LCX), right coronary artery (RCA), and LMCAD were also recorded, along with the location of ostial lesion, bifurcation lesion, coronary body, and LMCAD plaque characteristics like calcified lesions and medina classification. The study also collected information on the average number of stents, average implanted stent diameter, and average length of implanted stent, as well as post-expansion cases, synergy between PCI with TAXUS DES and cardiac surgery (SYNTAX) score, Numeric Rating Scale (NERS) score, thrombolysis in myocardial infarction (TIMI) blood flow grade, preoperative minimum lumen area (MLA), preoperative minimum lumen diameter (MLD), plaque burden, and minimum stent inner diameter (MSD). Furthermore, the study collected data on surgical hospitalization, outpatient follow-up, and 3-year follow-up records.
Outcomes
The primary outcomes of the study were the occurrence of main adverse cardiac events (MACE) at 1- and 3-year intervals. MACE were defined as cardiovascular death, sudden cardiac death, myocardial infarction. Cardiac mortality was considered the cause of all deaths, except in cases where clinical or autopsy findings indicated a non-cardiac origin. The secondary outcomes of the study were the risk of stent thrombosis (RST), and it was then divided into three categories: early (0–30 days after SI), late (31–360 days), and extremely late (> 360 days) [17]. This information was categorized as certain, likely, or possible. The study also evaluated target vessel lesion restenosis (TVR), myocardial infarction (MI), and CABG as secondary outcomes. During the follow up period, CT scan of coronary arteries were performed to check the patients’ status, and angiography was performed when the patients had obvious symptoms and unstable status.
Statistical analysis
The statistical analysis was performed using Stata version 12.0. Continuous variables were assessed for normality using the Kolmogorov-Smirnov test and reported as mean ± standard deviation or median. Normally distributed variables were compared using the Student’s t-test, while the Mann-Whitney U test was used for skewed variables. Categorical variables were presented as frequencies or percentages and compared using chi-square statistics or Fisher’s exact test. Kaplan-Meier method was used to generate survival curves, and the log-rank test was used to assess differences between them. To identify independent predictors of the primary endpoint, a multivariate Cox proportional hazards regression model was used with relevant variables from univariate analysis (with P-values ≤ 0.1) and previous research reports used as covariates. The Grønnesby-Borgan-May test was used to evaluate the goodness of fit of the Cox multivariate model. Hazard ratios, 95% confidence intervals, and P-values were reported as findings. A two-tailed P-value less than 0.05 was considered statistically significant.
Results
Comparison of baseline clinical characteristics between angiography and IVUS groups
Table 1 showed baseline data comparison between the angiography and IVUS groups. The angiography group had 118 patients with mean age of 68.1 ± 8.8 years, while the IVUS group had 81 patients with mean age of 65.6 ± 8.1 years. Age in the angiography group was older than the IVUS group (P = 0.042). The prevalence of hypertension and hyperlipidemia in the angiography group was greater than in the IVUS group (P < 0.05). The IVUS group had a considerably greater mean implanted stent diameter (3.7 ± 0.6 mm) than the angiography group (3.4 ± 0.2 mm)( P < 0.001). The proportion post-expansion cases in IVUS group was considerably higher than that in the angiography group (82.7% vs. 61.0%; P < 0.001). The mean minimum stent diameter (MSD) in the IVUS group was considerably larger than in the angiography group (3.8 ± 0.3 mm vs. 3.3 ± 0.3 mm; P < 0.001). The other variables were comparable between the two groups (P > 0.05).
Follow-up outcomes comparison between angiography and IVUS groups
Table 2shows follow-up outcomes of the patients. The incidence rate of MACE occurred in one year in the IVUS group was considerably lower than in the angiography group (6.2% vs. 22.9%; P = 0.002). The incidence rate of cardiac death in one year was considerably lower in the IVUS group than the angiography group (1.2% vs. 7.6%; P = 0.043). Similarly, the lower incidence of cardiac mortality was observed in the IVUS group compared to the angiography group after two and three years (P = 0.049 and P = 0.018, respectively). In one year, the IVUS group had a lower incidence of MI than the angiography group (6.2% vs. 16.9%; P = 0.024).
Target lesion restenosis occurred more frequently in the angiography group, compared to the IVUS group, in one, two and three years, respectively (P = 0.041, P = 0.041, P = 0.037, respectively). Within one year, neither group had a CABG (P = 1.000). The probability of stent thrombosis within one year had similar trend between the angiography and IVUS groups (IVUS: 8 cases, 6.7%; angiography: 1 case, 1.2%; P = 0.064). One year following surgery, the MLD in the IVUS group (3.5 ± 0.5) was considerably greater than in the angiography group (2.9 ± 0.8; P = 0.046).
The Cox regression analysis for 3-year cardiac mortality
The results of a Cox proportional hazard regression model study showed that the hazard ratios for 3-year cardiac mortality (HR = 1.89, 95%CI: 0.6-6.0, P = 0.280) were comparable between the angiography group and IVUS groups after accounting for average implanted stent diameter, post-expansion cases, MSD, and MLD at 1 year following surgery. However, it was discovered that MSD was independently correlated with 3-year cardiac mortality (HR = 0.1, 95% CI: 0.01–14.92, P = 0.030) (Table 3).
Discussion
The age, hypertension, hyperlipidemia, and hyperglycemia were considerably different between the angiography and IVUS groups. There were no considerable differences in gender, height, weight, atrial fibrillation, smoking, renal insufficiency, history of MI, left ventricular ejection fraction, or plaque burden. The MSD was considerably larger in the IVUS group than in the angiography group. Additionally, the study found that MSD may be an independent protective factor against cardiac mortality. From the follow up results, the mortality rate of IVUS group was lower than the angiography group. According to the study’s findings, IVUS-guided SI may offer patients with ULMCAD longer-lasting results than angiography-guided SI in a 3-year period.
With the advent of DES, LMCAD is no longer a contraindication for PCI [18, 19]. A landmark 5-year study of 1905 patients found no considerable difference in combined outcomes of mortality, stroke, or myocardial infarction between PCI and CABG in patients with LMCAD of low or moderate anatomical complexity [18].
Our results showed that angiography was associated with considerably better outcomes in terms of MACE, cardiac mortality, MI, and target lesion restenosis, compared to IVUS. The only variable that did not show a statistically considerable divergence between the angiography and IVUS groups was the risk of stent thrombosis. Additionally, IVUS was associated with a larger minimum lumen diameter after surgery. Overall, the results suggest that IVUS may be a preferable diagnostic tool for coronary artery disease compared to angiography. Because it enables a more precise evaluation of the amount and severity of coronary artery disease, IVUS is an important tool for guiding SI [20]. Compared to other invasive intravascular imaging modalities, High spatial resolution, real-time visualisation of the artery wall, and accurate assessment of stent growth and apposition are only a few benefits of IVUS [21, 22]. IVUS can also reveal whether plaques, calcification, or thrombus are present, which helps doctors choose the right stent size and model for a particular lesion [23]. Therefore, IVUS imaging can be considered an effective tool for guiding SI and improving the outcomes of PCI in patients with ULMCAD.
IVUS-guided treatment may yield better outcomes and lower mortality rate when compared to angiography-only guided treatment according to previous reports [24, 25]. The study did not find a difference in suspected stent thrombosis between the angiography group and IVUS groups. No diversity in postoperative TIMI blood flow was observed between the angiography group and IVUS groups. This suggests that stent thrombosis and TIMI blood flow alone may not be sufficient to evaluate SI, and the condition of the diseased blood vessel itself must be taken into consideration. If necessary, post-expansion or auxiliary stent placement can be performed to ensure that the stent is completely adherent and that no intima falls into the stent, in order to decrease the occurrence of stent thrombosis and the incidence of MACE.
However, it is important to note that the conclusions drawn from this study should be further verified with more case studies. The study highlights the potential of IVUS examination immediately after SI to evaluate its effectiveness. The anatomical grading technique used by IVUS combines circumference (180 degrees) and depth (intima, medium, and adventitia) to identify more anatomical features than angiography. This makes IVUS a valuable tool in predicting patency and complications following SI. Deep anatomical changes may trigger restenosis, while intimal tears may fall into the lumen, leading to acute or subacute thrombotic events, or requiring assisted stenting. The usefulness of IVUS in peripheral arterial studies has already been well established [26].
At the one-year follow-up, our results suggested that there was no considerable difference between the use of angiography and IVUS in terms of hazard ratio. The preoperative MLA and MLD did not considerably affect the choice of diagnostic tool. However, the mean stenosis diameter was considerably higher in the angiography group compared to the IVUS group, which may have influenced the selection of diagnostic tool. The MLD at 1 year post-surgery did not considerably differ between the angiography and IVUS groups. Therefore, the study indicated that the diagnostic tool chosen may not considerably impact post-operative outcomes in patients undergoing coronary artery bypass grafting surgery. But there was a statistically considerable reduction in the occurrence of MACE and cardiac mortality in the IVUS group, as compared to the angiography group.
Therefore, it can be concluded that IVUS guidance is superior to CAG guidance during PCI, regardless of the presence or absence of diabetes, acute coronary syndrome, hypertension, and location of LMCAD. This conclusion is consistent with recent meta-analyses and previous studies [25, 27]. Furthermore, the occurrence of target lesion restenosis and target vessel restenosis was considerably lower in the IVUS group compared to the angiography group. In-stent restenosis was also less frequent in the IVUS group compared to the angiography group, and this difference was statistically considerable. The minimum post-stent lumen area was found to be an independent predictor of the primary endpoint, as confirmed by COX analysis. These findings are consistent with the results of several previous studies [28, 29] and a meta-analysis [30].
The use of IVUS before SI also helps in assessing lipid plaque distribution and collateral access in the distal LMCA. IVUS can also aid in judging the true angle of the distal LMCA bifurcation and thus help determine the optimal PCI strategy. Additionally, IVUS guidance enables the selection of appropriate stent diameters and lengths, optimizing SI procedures for better clinical outcomes [31]. The use of IVUS guidance during SI has been shown to be a superior method for the treatment of left main coronary artery disease (LMCAD), according to a recent study. However, the study also had some limitations that should be considered.
Limitations
The study was carried out at a single facility with patients from a small geographic area and backgrounds, which may have limited the sample size and generalizability of the findings. Additionally, the study was retrospective and had a follow-up time of only three years, which may not have been long enough to detect any differences in outcomes that could occur with a longer latency period.
Conclusion
In patients with ULMCAD, IVUS-guided SI may result in better 3-year results than angiography-guided SI. Moreover, MSD could be an independent protective factor against cardiac mortality. However, these findings require further validation with multi-center studies and larger sample sizes.
Data availability
No datasets were generated or analysed during the current study.
References
Malakar AK, Choudhury D, Halder B, Paul P, Uddin A, Chakraborty S. A review on coronary artery disease, its risk factors, and therapeutics. J Cellu Physiol. 2019;234(10):16812–23.
Fajadet J, Chieffo A. Current management of left main coronary artery disease. Eur Heart J. 2012;33(1):36–50.
Dellard B, Taglierin. Successful manual thrombus as piration in anterior ST-segmentelevation myocardial infarction due to cardioembolicobstruction of the left main coronary artery. J Cardiol Cases. 2019;21(2):46–9.
Darmoch F, Alraies MC, Al-Khadra Y, Moussa Pacha H, Pinto DS, Osborn EA. Intravascular ultrasound imaging-guided versus coronary angiography-guided percutaneous coronary intervention: a systematic review and meta-analysis. J Am Heart Assoc. 2020;9(5):e013678.
Kumar A, Shariff M, Adalja D, Doshi R. Intravascular ultrasound versus angiogram guided drug eluting stent implantation. A systematic review and updated meta-analysis with trial sequential analysis. Int J Cardiol Heart Vasc. 2019;25:100419.
Malaiapan Y, Leung M, White AJ. The role of intravascular ultrasound in percutaneous coronary intervention of complex coronary lesions. Cardiovasc Diagn Ther. 2020;10(5):1371–88.
Hachinohe D, Mitomo S, Candilio L, Latib A. A practical approach to assessing stent results with IVUS or OCT. Methodist Debakey Cardiovasc J. 2018;14(1):32–41.
Lee SY, Choi KH, Song YB, et al. Use of intravascular ultrasound and long-term cardiac death or myocardial infarction in patients receiving current generation drug-eluting stents. Sci Rep. 2022;12(1):8237.
Choi KH, Song YB, Lee JM, et al. Impact of intravascular ultrasound-guided percutaneous coronary intervention on long-term clinical outcomes in patients undergoing complex procedures. JACC Cardiovasc Interv. 2019;12(7):607–20.
Wang Y, Mintz GS, Gu Z, et al. Meta-analysis and systematic review of intravascular ultrasound versus angiography-guided drug eluting stent implantation in left main coronary disease in 4592 patients. BMC Cardiovasc Disord. 2018;18(1):115.
Ali ZA, Galougahi KK, Maehara A, et al. Intracoronary optical coherence tomography 2018: current status and future directions. JACC Cardiovasc Interv. 2017;10:2473–87.
Schiele F, Meneveau N, Vuillemenot A, et al. Impact of intravascular ultrasound guidance in stent deployment on 6-month restenosis rate: a multicenter, randomized study comparing two strategies—with and without intravascular ultrasound guidance. J Am Coll Cardiol. 1998;32:320–8.
Oemrawsingh PV, Mintz GS, Schalij MJ, Zwinderman AH, Jukema JW, Wall EE. Intravascular ultrasound guidance improves angiographic and clinical outcome of stent implantation for long coronary artery stenoses: final results of a randomized comparison with angiographic guidance (TULIP study). Circulation. 2003;107:62–7.
Koskinas KC, Nakamura M, Räber L, et al. Current use of intracoronary imaging in interventional practice—results of a European Association of Percutaneous Cardiovascular Interventions (EAPCI) and Japanese Association of Cardiovascular Interventions and Therapeutics (CVIT) clinical practice survey. Circ J. 2018;82:1360–8.
Chaitman BR, Fisher LD, Bourassa MG, et al. Effect of coronary bypass surgery on survival patterns in subsets of patients with left main coronary artery disease. Report of the collaborative study in coronary artery surgery (CASS). Am J Cardiol. 1981;48:765–77.
Hong MK, Mintz GS, Lee CW, Park DW, Choi BR, Park KH, et al. Intravascular ultrasound predictors of angiographic restenosis after sirolimus-eluting stent implantation. Eur Heart J. 2006;27:1305–10.
Kang H-GS, Ahn J-M et al. Intravascular ultrasound assessment of optimal stent area to prevent in-stent restenosis after zotarolimus-, everolimus-, and sirolimus-eluting stent implantation. Catheter Cardiovasc Interv. 2014;1;83(6):873–78.
Secemsky EA, Matteau A, Robert W, Yeh et al. 3,. Comparison of Short- and Long-Term Cardiac Mortality in Early Versus Late Stent Thrombosis (from Pooled PROTECT Trials). Am J Cardiol. 2015;15;115(12):1678–1684.
Sano K, Mintz GS, Carlier SG, Ribamar Costa J Jr, Qian J, Missel E. Assessing intermediate left main coronary lesions using intravascular ultrasound. Am Heart J. 2007;154:983–8.
Ding D, Yang J, Westra J, et al. Accuracy of 3-dimensional and 2-dimensional quantitative coronary angiography for predicting physiological significance of coronary stenosis: a FAVOR II substudy. Cardiovasc Diagn Ther. 2019;9(5):481–91.
Porto I, Dato I, Todaro D. Comparison of two- and three-dimensional quantitative coronary angiography to intravascular ultrasound in the assessment of intermediate left main stenosis. Am J Cardiol. 2012;1(11):1600–7.
Garcìa-Garcìa HM, Gogas BD, Serruys PW, Bruining N. IVUS-based imaging modalities for tissue characterization: similarities and differences. Int J Cardiovasc Imaging. 2011;27(2):215–24.
Johnson TW, Rber L, Mario C. Clinical use of intracoronary imaging. Part 2: acute coronary syndromes, ambiguous coronary angiography findings, and guiding interventional decision-making: an expert consensus document of the European Association of Percutaneous Cardiovascular Interventions. Eur Heart J. 2019;40(31):2566–84.
Shammas NW, Radaideh Q, Shammas WJ, Daher GE, Rachwan RJ, Radaideh Y. The role of precise imaging with intravascular ultrasound in coronary and peripheral interventions. Vasc Health Risk Manag. 2019;15:283–90.
Case BC, Yerasi C, Forrestal BJ. Intravascular ultrasound guidance in the evaluation and treatment of left main coronary artery disease. Int J Cardiol. 2021;325:168–75.
Fuentes L, Gómez-Lara J, Salvatella N, et al. IVUS findings in late and very late stent thrombosis. A comparison between Bare-metal and drug-eluting stents. Rev Esp Cardiol (Engl Ed). 2018;71(5):335–43.
Koganti S, Kotecha T, Rakhit RD. Choice of intracoronary imaging: when to use intravascular ultrasound or optical coherence tomography. Interventional Cardiol Rev. 2016;11(1):11.
Shlofmitz E, Torguson R, Zhang C, et al. Impact of intravascular ultrasound on outcomes following PErcutaneous coronary interventioN for in-stent restenosis (iOPEN-ISR study). Inter J Cardiol. 2021;340:17–21.
Shlofmitz E, Torguson R, Mintz GS, et al. The IMPact on revascularization outcomes of intraVascular ultrasound-guided treatment of complex lesions and economic impact (IMPROVE) trial: study design and rationale. Am Heart J. 2020;228:65–71.
Bavishi C, Sardar P, Chatterjee S, et al. Intravascular ultrasound–guided vs angiography-guided drug-eluting stent implantation in complex coronary lesions: meta-analysis of randomized trials. Am Heart J. 2017;185:26–34.
Maehara A, Matsumura M, Ali ZA, Mintz GS, Stone GW. IVUS-guided versus OCT-guided coronary stent implantation: a critical appraisal. JACC: Cardiovasc Imaging. 2017;10(12):1487–503.
Acknowledgements
Not applicable.
Funding
Not applicable.
Author information
Authors and Affiliations
Contributions
QY contributed to conception and design of the research; QY, XM and YW contributed to the acquisition of data. QY and XM did the analysis and interpretation of the data and the statistical analysis. YW validated the data. All authors contributed to the writing of the manuscript and the critical revision of the manuscript. All authors have read and approved the final version of the manuscript for publication.
Corresponding author
Ethics declarations
Ethical approval
The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013) and was approved by the Institutional Review Board of the Affiliated Hospital of Inner Mongolia Medical University.
Consent to participate
The participants signed written informed consents before the study.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Yang, Q., Ma, X. & Wang, Y. Long-term outcomes of IVUS-guided and angiography-guided drug-eluting stent implantation for left main coronary artery disease: a retrospective consort study. J Cardiothorac Surg 19, 407 (2024). https://doi.org/10.1186/s13019-024-02800-0
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s13019-024-02800-0