Abstract
Objective
Dexmedetomidine (DEX) has been shown to have anti-apoptotic effects in diabetes mellitus, but its role in mitigating diabetic cardiomyopathy (DCM) through ferroptosis regulation is unclear.
Methods
An in vitro DCM model was established using H9C2 cells induced with high glucose (HG) and treated with DEX at varying doses and a nuclear factor erythroid 2-realated factor 2 (Nrf2) specific inhibitor ML385. Cell viability was evaluated using the MTT method after treatment with DEX or mannitol (MAN), and the dosage of DEX used in subsequent experimentation was determined. The effects of HG-induced high osmotic pressure were assessed using MAN as a control. Cell apoptosis was evaluated using flow cytometry. Protein levels of Bcl2, Bax, nuclear Nrf2, and glutathione peroxidase 4 (GPX4) were measured using Western blot. Superoxide dismutase (SOD) activity, malondialdehyde (MDA) levels, Fe2+ concentration and reactive oxygen species (ROS) levels were measured using corresponding kits and dichlorodihydrofluorescein diacetate, respectively.
Results
Treatment with DEX or MAN had no effect on H9C2 cell viability. HG induction reduced H9C2 cell viability, increased cell apoptosis, upregulated levels of Bax, Fe2+, MDA, and ROS, and downregulated Bcl2 protein levels, SOD activity, and protein levels of nuclear Nrf2 and GPX4. DEX inhibited HG-induced H9C2 cell apoptosis, promoted Nrf2 nuclear translocation, and activated the Nrf2/GPX4 pathway. Inhibition of Nrf2 partially reversed the protective effects of DEX against HG-evoked H9C2 cell injury.
Conclusion
Our findings demonstrate that DEX attenuates HG-induced cardiomyocyte injury by inhibiting ferroptosis through the Nrf2/GPX4 pathway, providing potential therapeutic targets for DCM treatment.
Similar content being viewed by others
Introduction
The surging prevalence of diabetes mellitus and heart failure represents a significant public health burden worldwide, and both conditions are independent risk factors for one another [1]. Diabetic cardiomyopathy (DCM) is a pathophysiological abnormality of cardiac structure and function in diabetic patients without hypertension, coronary artery disease, and other types of heart diseases [2]. The pathogenesis of DCM involves a complex interplay of systemic metabolic disorders, subcellular component abnormalities, oxidative stress, inflammation, dysfunctional immune modulation, and inappropriate activation of the renin-angiotensin-aldosterone system [3]. Ferroptosis, a novel form of iron-dependent cell death, has emerged as a potential contributor to the development of DCM, given its critical involvement in the pathological processes of iron accumulation and lipid peroxidation [4, 5]. However, the precise molecular mechanisms underlying the role of ferroptosis in DCM remain poorly understood and warrant further investigation.
Dexmedetomidine (DEX), a highly selective α2 adrenergic agonist primarily used for sedation [6], possesses an array of diverse pharmacological properties, including cardiac protection, anti-inflammatory, sedative, anesthesia, sleep-enhancing, bowel recovery, and sore throat-relieving effects [7]. In rats with diabetes, DEX has been shown to alleviate cardiac dysfunction and improve autophagic dysfunction, indicating its potential anti-autophagic effects in DCM patients [8]. Furthermore, DEX has been demonstrated to exert cardioprotective effects by inhibiting ferroptosis in cardiac ischemia/reperfusion injury [9]. Despite these promising findings, it remains unclear whether DEX can attenuate DCM by suppressing ferroptosis in cardiomyocytes, and this question remains an active area of investigation.
Nuclear factor erythroid 2-related factor 2 (Nrf2) serves as a regulator of cellular antioxidant responses, lipid peroxidation, and ferroptosis [10]. Nrf2 demonstrates its protective function by translocating into the nucleus to counteract organ dysfunction [11]. Additionally, glutathione peroxidase 4 (GPX4) is a crucial regulator of ferroptosis and can be transcriptionally regulated by Nrf2 [12]. DEX has been shown to exert myocardial protection by activating the Nrf2/heme oxygenase-1/solute carrier family 7 member 11/GPX4 axis [13]. In contrast, protein arginine methyltransferase 4 inhibits the Nrf2/GPX4 pathway, thereby accelerating ferroptosis in doxorubicin-induced cardiomyopathy [14]. Furthermore, curcumin has been found to mitigate glucose-induced ferroptosis of cardiomyocytes by facilitating Nrf2 nuclear translocation and reducing excessive GPX4 loss [15]. However, the precise mechanism by which DEX attenuates high glucose (HG)-induced cardiomyocyte ferroptosis by activating the Nrf2/GPX4 pathway requires further investigation. Therefore, the primary objective of this study is to explore whether DEX can enhance the nuclear translocation of Nrf2 and upregulate GPX4 to repress ferroptosis and alleviate HG-induced cardiomyocyte injury.
Materials and methods
Cell culture and treatment
Rat cardiomyocytes H9C2 cells (The Cell Bank of Type Culture Collection of The Chinese Academy of Sciences, Shanghai, China) were cultured overnight in Dulbecco’s modified Eagle’s medium (DMEM, Hyclone, Logan, UT, USA) containing 1% penicillin-streptomycin (Hyclone) and 10% fetal bovine serum (FBS, Hyclone) at 37oC with in humidified 95% air and 5% CO2.
H9C2 cells were divided into the following six groups: control (Con) group (treated with 5.5 mmol/L glucose), mannitol (MAN) group (treated with 5.5 mmol/L MAN to exclude the effect of osmotic pressure [16]), DEX group (treated with 5.5 mmol/L glucose + 0.1, 1, 5, 10, and 20 µM DEX for 72 h), HG group (treated with 30 mmol/L glucose), HG + DEX group (treated with 30 mmol/L glucose + 0.1, 1, 5, 10, and 20 µM DEX for 72 h [17]), HG + DEX + ML385 group (treated with 30 mmol/L glucose + 10 µM DEX + 20 µM ML385 for 72 h [17]). ML385 is a specific Nrf2 inhibitor. MAN (SM8120), DEX (YZ-1,179,333), and ML385 (IM1020) were purchased from Solarbio (Beijing, China). DMEMs with normal glucose and HG were obtained from Hyclone. Cells were harvested after 72 h of treatment.
Cell viability detection
Cell viability of H9C2 cells was measured using the 3-(4, 5-dimethylthiazol-2-yl)-2, 5-diphenyl tetrazolium bromide (MTT) assay. H9C2 cells were seeded in 96-well plates (1 × 104 cells/well) and cultured for 24 h in normal glucose DMEM supplemented with 10% FBS. Next, 0.5 mg/mL MTT solution was added and incubated for 4 h of treatment at 37oC, followed by the addition of 100 µL dimethyl sulfoxide solution to each well. A microplate reader (Thermo Fisher Scientific, Rockford, IL, USA) was used to measure absorbance at 490 nm. Cell viability was defined as the optical density ratio of the sample to the control.
Cell apoptosis detection
H9C2 cells were collected and stained with Annexin V-FITC and propidium iodide (Beyotime, Shanghai, China) for 30 min at room temperature, followed by two rinses with phosphate buffer saline. The CellQuest software (BD Biosciences, Franklin Lakes, NJ, USA) was used to analyze the fluorescence data.
Measurement of SOD activity and MDA level
The activity of total superoxide dismutase (SOD) and the level of malondialdehyde (MDA) in H9C2 cells were determined using the Total SOD Activity Assay kit (S0109, Beyotime) and MDA Assay kit (S0131, Beyotime), respectively, in strict compliance with the manufacturer’s specifications.
Measurement of ferrous iron (Fe2+) concentration
To determine the levels of Fe2+ in H9C2 cells, the iron assay kit (MAK025, Sigma-Aldrich, St. Louis, MO, USA) was utilized following the manufacturer’s instructions.
Measurement of reactive oxygen species (ROS) level
To determine the levels of ROS in H9C2 cells, dichlorodihydrofluorescein diacetate (DCFH-DA) staining (10 µmol/L at 37 °C) was performed for 20 min according to the instructions of the ROS assay kit (S0033M, Beyotime). Data quantification was carried out using Image J (NIH, Bethesda, MD, USA).
Western blot
Protein extraction was performed by lysing H9C2 cells using a lysis buffer (Beyotime) containing protease inhibitor cocktails. Protein extraction and concentration determination were performed using the nuclear protein extraction and BCA kits (Beyotime), respectively. The cell lysates were mixed with 5 × Gel Sample Loading Buffer (New Cell & Molecular Biotech, Suzhou, China) and heated at 100 °C for 8 min to denature the proteins. Next, the proteins was then separated by 10% SDS-PAGE and transferred to a PVDF membrane (Merck Millipore, Billerica, MA, USA). After blocking with 5% bovine serum albumin in TBS-Tween, the membrane was incubated overnight at 4 °C with primary antibodies, washed with TBS-Tween, and probed with horseradish peroxidase-conjugated goat anti-rabbit antibody (1/2000, ab6721, Abcam, Cambridge, MA, USA). The protein bands were visualized using ECL detection reagents (Thermo Fisher Scientific), and the signal intensities were quantified using Image J (NIH). The primary antibodies used were anti-Bcl2 (1/1000, ab196495, Abcam), anti-Bax (1/1000, ab32503, Abcam), anti-Nrf2 (1/500, ab62352, Abcam), anti-GPX4 (1/1000, ab125066, Abcam), anti-β-actin (cytoplasmic protein control; 1/1000, ab8227, Abcam) and anti-Lamin A/C (nuclear protein control; 1/10,000, ab133256, Abcam).
Statistical analysis
Data analysis and graph plotting were performed using GraphPad Prism 8.01 (GraphPad Software, San Diego, CA, USA). Measurement data were presented as mean ± standard deviation. The independent sample t test and one-way analysis of variance with Tukey’s test were used for group comparisons. Two-sided tests were used to obtain p values, and p < 0.05 was considered statistically significant.
Results
DEX suppresses HG-induced cardiomyocyte apoptosis
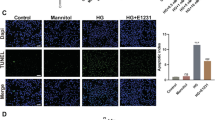
H9C2 cells were exposed to either normal glucose or HG and treated with different concentrations of DEX (0.1, 1, 5, 10, and 20 µM). The effect of high osmotic pressure on cells was excluded using MAN treatment as a control. DEX or MAN alone did not affect H9C2 cell viability under normal glucose conditions (Fig. 1A, all p > 0.05). However, HG-induced cells showed reduced viability compared to the control group (Fig. 1B, p < 0.001). Treatment with medium and low dose levels of DEX (0.1–10 µM) significantly increased cell viability in a dose-dependent manner, (Fig. 1B, all p < 0.05), while 20 µM DEX showed only a slight reduction in cell viability compared to 10 µM DEX, and the difference was not significant (Fig. 1B, p > 0.05). Thus, dosage of 10 µM DEX was used in subsequent experiments. Flow cytometry and Western blot analysis showed that HG-induced cells had a higher cell apoptotic rate and Bax protein levels, and lower Bcl2 protein levels relative to the control group (Fig. 1-C, all p < 0.001). Treatment with DEX reduced the apoptotic rate and increased Bcl2 protein levels while decreasing Bax protein levels in HG-induced cells (Fig. 1C-D, all p < 0.01). These findings suggest that DEX can suppress HG-induced H9C2 cell apoptosis.
DEX suppresses HG-induced H9C2 cell apoptosis. (A/B) Cell viability was detected using the MTT method; (C) Cell apoptotic rate was estimated by flow cytometry; (D) The protein levels of Bcl2 and Bax were determined by Western blot. Three replicates were guaranteed in cell experiments. Mean ± standard deviation was introduced to represent data. The comparison among multiple groups was made using one-way analysis of variance with Tukey’s test. ns p > 0.05, * p < 0.05, ** p < 0.01, *** p < 0.001
DEX ameliorates HG-induced oxidative stress and ferroptosis of cardiomyocytes
Subsequently, the effects of DEX on HG-induced oxidative stress and ferroptosis of cardiomyocytes were investigated. H9C2 cells were exposed to HG and treated with different concentrations of DEX. Results showed that HG induction led to a significant reduction in SOD activity and a significant increase in MDA level and Fe2+ concentration compared to the control group (Fig. 2A-B, all p < 0.001). However, treatment with DEX resulted in a dose-dependent increase in SOD activity and a reduction in MDA level and Fe2+ concentration in HG-induced H9C2 cells (Fig. 2A-B, all p < 0.05). Furthermore, the use of DCFH-DA demonstrated that ROS levels increased after HG induction, but decreased after DEX treatment (Fig. 2C, p < 0.001). These findings indicate that DEX ameliorates oxidative stress and ferroptosis of HG-induced H9C2 cells.
DEX ameliorates HG-induced oxidative stress and ferroptosis of H9C2 cells. (A) Total SOD activity and MDA levels; (B) Fe2+ concentration; (C) ROS levels assessed by DCFH-DA. Three replicates were guaranteed in cell experiments. Mean ± standard deviation was introduced to represent data. The comparison among multiple groups was made using one-way analysis of variance with Tukey’s test. * p < 0.05, ** p < 0.01, *** p < 0.001
DEX potentiates Nrf2 nuclear translocation and activates the Nrf2/GPX4 pathway
The effects of DEX on Nrf2 nuclear translocation and the Nrf2/GPX4 pathway were investigated. Western blot analysis revealed that HG induction led to a downregulation of nuclear Nrf2 expression and GPX4 expression in H9C2 cells (Fig. 3, all p < 0.001). In contrast, the HG + DEX group exhibited higher expression levels of both nuclear Nrf2 and GPX4 compared to the HG group (Fig. 3, all p < 0.01). These findings suggest that DEX promotes the nuclear translocation of Nrf2 and activates the Nrf2/GPX4 pathway.
DEX potentiates Nrf2 nuclear translocation and activates the Nrf2/GPX4 pathway. Nuclear Nrf2 expression and GPX4 expression were determined by Western blot. Three replicates were guaranteed in cell experiments. Mean ± standard deviation was introduced to represent data. The comparison among multiple groups was made using one-way analysis of variance with Tukey’s test. ** p < 0.01, *** p < 0.001
Nrf2 inhibition reduces the protective function of DEX on HG-induced cardiomyocyte injury
To investigate the role of Nrf2 in the protective effect of DEX against HG-induced cardiomyocyte injury, H9C2 cells were treated with both DEX and ML385, a specific Nrf2 inhibitor. The HG + DEX + ML385 group showed lower nuclear Nrf2 levels and GPX4 levels compared to the HG + DEX group (Fig. 4A, all p < 0.01), indicating successful downregulation of Nrf2 and GPX4. Furthermore, the HG + DEX + ML385 group exhibited decreased cell viability, increased cell apoptotic rate, downregulated Bcl2 protein and SOD activity, and upregulated Bax protein, Fe2+ concentration and levels of MDA and ROS compared to the HG + DEX group (Fig. 4A-F, all p < 0.05). These findings suggest that Nrf2 inhibition partially reverses the protective action of DEX against HG-induced H9C2 cell injury.
Nrf2 inhibition partially invalidates protective function of DEX on HG-induced cardiomyocyte injury. (A) The protein levels of nuclear Nrf2, GPX4, Bcl2 and Bax were determined by Western blot; (B) Cell viability was detected using the MTT method; (C) Cell apoptotic rate was estimated by flow cytometry; (D) Total SOD activity and MDA levels; (E) Fe2+ concentration; (F) DCFH-DA was used to assess ROS levels. Three replicates were guaranteed in cell experiments. Mean ± standard deviation was introduced to represent data. The comparison between 2 groups was made using the independent sample t test. * p < 0.05, ** p < 0.01
Discussion
Despite the identification of numerous targets for the prevention and treatment of DCM, few therapeutic strategies have demonstrated efficacy [18]. Targeting ferroptosis may offer a promising approach to prevent cardiomyopathy [19], as ferroptosis has been shown to contribute to the pathogenesis of DCM and interfering with its pathways may represent a promising strategy to reduce reducing cardiovascular injury [20, 21]. DEX has shown potential as a therapeutic agent for targeting septic heart injury and myocardial ischemia/reperfusion (I/R) injury by modulating ferroptosis [22, 23]. The present study aimed to investigate the therapeutic effects of DEX on HG-induced cardiomyocyte ferroptosis and injury in vitro.
Increased cardiac apoptosis is a major risk factor for for the development of DCM [24]. Consistent with the first set of results presented in this study, HG induction led to a decline in cell viability in cardiomyocytes, while medium and low doses of DEX resulted in enhanced cardiomyocyte viability. The regulation of apoptosis is complex and involves opposing activities of Bcl2 proteins, with apoptosis occurring when Bax and Bak outnumber Bcl2 activities [25]. In this study, DEX treatment resulted in a significant decline in Bax protein expression and an increase in Bcl2 protein expression in HG-induced cardiomyocytes. This is in agreement with previous studies that have documented a reduction in cardiomyocyte apoptosis following DEX treatment in the context of myocardial infarction, as evidenced by an increase in the Bcl-2/Bax ratio [26], as well as in I/R-induced cardiomyocyte injury [27]. These findings suggest that DEX may have a protective effect against HG-induced cardiomyocyte apoptosis.
Mounting evidence suggests that ferroptosis, an iron and ROS-dependent form of cell death, is closely associated with the occurrence and progression of DCM [28,29,30,31]. Ferroptosis, mediated by oxidative stress, is critical to the pathogenesis of several cardiovascular diseases [32]. Additionally, oxidative stress is one of the most typical pathogenic characteristics of DCM [33]. Oxidative stress is provoked when ROS levels increase and are not compensated by endogenous antioxidant systems such as SOD [34]. Excessive instability of Fe2+ increases the risks of oxidative stress-evoked injury [35]. MDA is one of the most commonly evaluated markers of oxidative stress [36]. Our results demonstrate that after DEX treatment, HG-induced cardiomyocytes exhibited decreased MDA and ROS levels and Fe2+ concentration, as well as increased SOD activity. Previous studies have shown that DEX can reduce oxidative stress and H9C2 cell necroptosis and apoptosis [37, 38]. Furthermore, DEX has been shown to attenuate cardiomyocyte ferroptosis in myocardial I/R injury [13, 23]. These findings suggest that DEX may exert anti-ferroptotic effects on cardiomyocytes in DCM.
Recent studies indicate that the post-translational modification of GPX4 could be a potential target for treating ferroptosis-associated conditions [12]. Nrf2, as a transcription factor, plays a critical role in regulating ferroptosis [39]. Herein, we uncovered that Nrf2 nuclear levels and GPX4 levels were diminished in HG-induced cardiomyocytes. However, DEX treatment has been shown to increase Nrf2 nuclear translocation in H9C2 cells exposed to cobalt chloride [40]. In addition, DEX has been shown to elevate the levels of GPX4 and nuclear Nrf2 in HG-induced cardiomyocytes. Moreover, a prior study indicated that DEX could significantly augment the levels of Nrf2 and GPX4 in cardiomyocytes exposed to hypoxia/reoxygenation [13]. These findings suggest that DEX may activate the Nrf2/GPX4 pathway by facilitating Nrf2 nuclear translocation. Nrf2 has been demonstrated to protect cardiac cells and the heart from high glucose-induced injury in vitro [41]. Interestingly, Nrf2 can modulate the levels of oxidant signaling protein levels, which play a crucial role in programmed cellular functions [42]. Inhibition of Nrf2 using the specific inhibitor ML385 resulted in reduced cardiomyocyte viability, increased apoptosis, downregulated Bcl2 and SOD, and upregulated Bax, Fe2+, MDA, and ROS. The activation of the Nrf2/ferroportin1 pathway has been shown to mitigate myocardial I/R injury in diabetic rats by modulating ferroptosis and iron homeostasis [43]. Moreover, Nrf2 deficiency exacerbates cardiac injury induced by Angiotensin II [44]. Transcriptional activation of Nrf2 has been found to be protective against ferroptosis, while Nrf2 inhibition averts resistance to GPX4 inhibitor-induced ferroptosis in head and neck cancer [45]. These findings suggest that DEX up-regulates GPX4 and represses ferroptosis by increasing Nrf2 nuclear translocation, thus alleviating HG-induced cardiomyocyte injury. However, Nrf2 inhibition could partly negate the protective effect of DEX on HG-induced cardiomyocytes.
Overall, the current study suggests that DEX has the potential to alleviate HG-induced cardiomyocyte injury by upregulating GPX4 and increasing the nuclear translocation of Nrf2, thus inhibiting ferroptosis. However, there are still limitations to this study, such as the lack of immunofluorescence validation for Nrf2 expression through immunofluorescence and the absence of animal experiments. Further research is needed to determine the optimal dosage of DEX and explore other regulatory mechanisms involved in DCM. Nonetheless, the findings of this study provide insights into the potential therapeutic effects of DEX in the treatment of DCM.
Data Availability
The data that support the findings of this study are available from the corresponding author upon reasonable request.
References
Siao WZ, Chen YH, Tsai CF, Lee CM, Jong GP. Diabetes Mellitus and Heart failure. J Pers Med. 2022;12(10).
Zhao X, Liu S, Wang X, Chen Y, Pang P, Yang Q, et al. Diabetic cardiomyopathy: clinical phenotype and practice. Front Endocrinol (Lausanne). 2022;13:1032268.
Jia G, Whaley-Connell A, Sowers JR. Diabetic cardiomyopathy: a hyperglycaemia- and insulin-resistance-induced heart disease. Diabetologia. 2018;61(1):21–8.
Chen Y, Hua Y, Li X, Arslan IM, Zhang W, Meng G. Distinct types of cell death and the implication in Diabetic Cardiomyopathy. Front Pharmacol. 2020;11:42.
Li J, Cao F, Yin HL, Huang ZJ, Lin ZT, Mao N, et al. Ferroptosis: past, present and future. Cell Death Dis. 2020;11(2):88.
Yuki K. The immunomodulatory mechanism of dexmedetomidine. Int Immunopharmacol. 2021;97:107709.
Liu X, Li Y, Kang L, Wang Q. Recent advances in the clinical value and potential of Dexmedetomidine. J Inflamm Res. 2021;14:7507–27.
Oh JE, Jun JH, Hwang HJ, Shin EJ, Oh YJ, Choi YS. Dexmedetomidine restores autophagy and cardiac dysfunction in rats with streptozotocin-induced diabetes mellitus. Acta Diabetol. 2019;56(1):105–14.
Yu P, Zhang J, Ding Y, Chen D, Sun H, Yuan F, et al. Dexmedetomidine post-conditioning alleviates myocardial ischemia-reperfusion injury in rats by ferroptosis inhibition via SLC7A11/GPX4 axis activation. Hum Cell. 2022;35(3):836–48.
Dodson M, Castro-Portuguez R, Zhang DD. NRF2 plays a critical role in mitigating lipid peroxidation and ferroptosis. Redox Biol. 2019;23:101107.
Sadrkhanloo M, Entezari M, Orouei S, Zabolian A, Mirzaie A, Maghsoudloo A, et al. Targeting Nrf2 in ischemia-reperfusion alleviation: from signaling networks to therapeutic targeting. Life Sci. 2022;300:120561.
Cui C, Yang F, Li Q. Post-translational modification of GPX4 is a Promising Target for treating ferroptosis-related Diseases. Front Mol Biosci. 2022;9:901565.
Wang Z, Yao M, Jiang L, Wang L, Yang Y, Wang Q, et al. Dexmedetomidine attenuates myocardial ischemia/reperfusion-induced ferroptosis via AMPK/GSK-3beta/Nrf2 axis. Biomed Pharmacother. 2022;154:113572.
Wang Y, Yan S, Liu X, Deng F, Wang P, Yang L, et al. PRMT4 promotes ferroptosis to aggravate doxorubicin-induced cardiomyopathy via inhibition of the Nrf2/GPX4 pathway. Cell Death Differ. 2022;29(10):1982–95.
Wei Z, Shaohuan Q, Pinfang K, Chao S. Curcumin attenuates Ferroptosis-Induced Myocardial Injury in Diabetic Cardiomyopathy through the Nrf2 pathway. Cardiovasc Ther. 2022;2022:3159717.
Ren BC, Zhang YF, Liu SS, Cheng XJ, Yang X, Cui XG, et al. Curcumin alleviates oxidative stress and inhibits apoptosis in diabetic cardiomyopathy via Sirt1-Foxo1 and PI3K-Akt signalling pathways. J Cell Mol Med. 2020;24(21):12355–67.
Wei Z, Jing Z, Pinfang K, Chao S, Shaohuan Q. Quercetin inhibits pyroptosis in Diabetic Cardiomyopathy through the Nrf2 pathway. J Diabetes Res. 2022;2022:9723632.
Tan Y, Zhang Z, Zheng C, Wintergerst KA, Keller BB, Cai L. Mechanisms of diabetic cardiomyopathy and potential therapeutic strategies: preclinical and clinical evidence. Nat Rev Cardiol. 2020;17(9):585–607.
Fang X, Wang H, Han D, Xie E, Yang X, Wei J, et al. Ferroptosis as a target for protection against cardiomyopathy. Proc Natl Acad Sci U S A. 2019;116(7):2672–80.
Sha W, Hu F, Xi Y, Chu Y, Bu S. Mechanism of ferroptosis and its role in type 2 diabetes Mellitus. J Diabetes Res. 2021;2021:9999612.
Lu LQ, Tian J, Luo XJ, Peng J. Targeting the pathways of regulated necrosis: a potential strategy for alleviation of cardio-cerebrovascular injury. Cell Mol Life Sci. 2021;78(1):63–78.
Wang C, Yuan W, Hu A, Lin J, Xia Z, Yang CF, et al. Dexmedetomidine alleviated sepsis–induced myocardial ferroptosis and septic heart injury. Mol Med Rep. 2020;22(1):175–84.
Ma X, Xu J, Gao N, Tian J, Song T. Dexmedetomidine attenuates myocardial ischemia-reperfusion injury via inhibiting ferroptosis by the cAMP/PKA/CREB pathway. Mol Cell Probes. 2023;68:101899.
Jia G, Hill MA, Sowers JR. Diabetic Cardiomyopathy: an update of Mechanisms contributing to this clinical entity. Circ Res. 2018;122(4):624–38.
Edlich F. BCL-2 proteins and apoptosis: recent insights and unknowns. Biochem Biophys Res Commun. 2018;500(1):26–34.
Sun T, Gong Q, Wu Y, Shen Z, Zhang Y, Ge S, et al. Dexmedetomidine alleviates cardiomyocyte apoptosis and cardiac dysfunction may be associated with inhibition of RhoA/ROCK pathway in mice with myocardial infarction. Naunyn Schmiedebergs Arch Pharmacol. 2021;394(7):1569–77.
Yang FY, Zhang L, Zheng Y, Dong H. Dexmedetomidine attenuates ischemia and reperfusion-induced cardiomyocyte injury through p53 and forkhead box O3a (FOXO3a)/p53-upregulated modulator of apoptosis (PUMA) signaling signaling. Bioengineered. 2022;13(1):1377–87.
Xie Y, Hou W, Song X, Yu Y, Huang J, Sun X, et al. Ferroptosis: process and function. Cell Death Differ. 2016;23(3):369–79.
Wang X, Chen X, Zhou W, Men H, Bao T, Sun Y, et al. Ferroptosis is essential for diabetic cardiomyopathy and is prevented by sulforaphane via AMPK/NRF2 pathways. Acta Pharm Sin B. 2022;12(2):708–22.
Wei J, Zhao Y, Liang H, Du W, Wang L. Preliminary evidence for the presence of multiple forms of cell death in diabetes cardiomyopathy. Acta Pharm Sin B. 2022;12(1):1–17.
Ge ZD, Lian Q, Mao X, Xia Z. Current Status and Challenges of NRF2 as a potential therapeutic target for Diabetic Cardiomyopathy. Int Heart J. 2019;60(3):512–20.
Yu Y, Yan Y, Niu F, Wang Y, Chen X, Su G, et al. Ferroptosis: a cell death connecting oxidative stress, inflammation and cardiovascular diseases. Cell Death Discov. 2021;7(1):193.
Peng ML, Fu Y, Wu CW, Zhang Y, Ren H, Zhou SS. Signaling pathways related to oxidative stress in Diabetic Cardiomyopathy. Front Endocrinol (Lausanne). 2022;13:907757.
Pena E, El Alam S, Siques P, Brito J. Oxidative stress and Diseases Associated with High-Altitude exposure. Antioxid (Basel). 2022;11(2).
Hassannia B, Van Coillie S, Vanden Berghe T, Ferroptosis. Biological rust of lipid membranes. Antioxid Redox Signal. 2021;35(6):487–509.
Czerska M, Mikolajewska K, Zielinski M, Gromadzinska J, Wasowicz W. Today’s oxidative stress markers. Med Pr. 2015;66(3):393–405.
Yin W, Wang C, Peng Y, Yuan W, Zhang Z, Liu H, et al. Dexmedetomidine alleviates H(2)O(2)-induced oxidative stress and cell necroptosis through activating of alpha2-adrenoceptor in H9C2 cells. Mol Biol Rep. 2020;47(5):3629–39.
Cai S, Liu Y, Cheng Y, Yuan J, Fang J. Dexmedetomidine protects cardiomyocytes against hypoxia/reoxygenation injury via multiple mechanisms. J Clin Lab Anal. 2022;36(7):e24119.
Wang Y, Zhao Y, Ye T, Yang L, Shen Y, Li H. Ferroptosis Signaling and regulators in atherosclerosis. Front Cell Dev Biol. 2021;9:809457.
Wu W, Du Z, Wu L. Dexmedetomidine attenuates hypoxia-induced cardiomyocyte injury by promoting telomere/telomerase activity: possible involvement of ERK1/2-Nrf2 signaling pathway. Cell Biol Int. 2022;46(7):1036–46.
Chen J, Zhang Z, Cai L. Diabetic cardiomyopathy and its prevention by nrf2: current status. Diabetes Metab J. 2014;38(5):337–45.
Ma Q. Role of nrf2 in oxidative stress and toxicity. Annu Rev Pharmacol Toxicol. 2013;53:401–26.
Tian H, Xiong Y, Zhang Y, Leng Y, Tao J, Li L, et al. Activation of NRF2/FPN1 pathway attenuates myocardial ischemia-reperfusion injury in diabetic rats by regulating iron homeostasis and ferroptosis. Cell Stress Chaperones. 2021;27(2):149–64.
Chen D, Li Z, Bao P, Chen M, Zhang M, Yan F, et al. Nrf2 deficiency aggravates angiotensin II-induced cardiac injury by increasing hypertrophy and enhancing IL-6/STAT3-dependent inflammation. Biochim Biophys Acta Mol Basis Dis. 2019;1865(6):1253–64.
Shin D, Kim EH, Lee J, Roh JL. Nrf2 inhibition reverses resistance to GPX4 inhibitor-induced ferroptosis in head and neck cancer. Free Radic Biol Med. 2018;129:454–62.
Acknowledgements
Not applicable.
Funding
This work was supported by Natural Science Foundation Project of Xinjiang Uygur Autonomous Region. No. (2019D01C293)
Author information
Authors and Affiliations
Contributions
FL is the guarantor of integrity of the entire study and contributed to the statistical analysis; FL, ZFH contributed to the study concepts, study design; YDH contributed to the definition of intellectual content, literature research; HTZ contributed to the data acquisition; YDH, HTZ contributed to the data analysis; All authors contributed to the preparation, editing and review manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Ethics approval and consent to participate
This article does not contain any studies with human participants or animals performed by any of the authors.
Consent for publication
Not applicable.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Li, F., Hu, Z., Huang, Y. et al. Dexmedetomidine ameliorates diabetic cardiomyopathy by inhibiting ferroptosis through the Nrf2/GPX4 pathway. J Cardiothorac Surg 18, 223 (2023). https://doi.org/10.1186/s13019-023-02300-7
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s13019-023-02300-7