Abstract
Background
Continuing therapy for aggressive non-small-cell lung cancer (NSCLC) after first-line treatment (FLT) is challenging. The clinical efficacy of second-line chemotherapy (SLCT) for progressive NSCLC is limited. In this meta-analysis, we aim to evaluate the clinical efficacy of the combination of I-125 seeds brachytherapy (ISB) and SLCT in progressive NSCLC after FLT.
Methods
The PubMed, Embase, Cochrane Library, CNKI, Wanfang, and VIP databases were screened for relevant publications until September 2021. Meta-analyses are conducted by RevMan 5.3 and Stata 12.0.
Results
Our meta-analysis encompassed 6 studies (4 retrospective studies and 2 randomized controlled trials), which included 272 patients that underwent ISB with SLCT (combined group) and 257 patients that received SLCT alone (chemotherapy alone group). The complete response (24.7% vs. 7.0%, P < 0.00001), treatment response (65.7% vs. 38.1%, P = 0.0002), and disease control (95.2% vs. 80.4%, P < 0.00001) rates are markedly elevated for patients receiving combined therapy versus those receiving chemotherapy alone. Moreover, pooled progression-free survival (P = 0.0001) and overall survival (P < 0.00001) were remarkably extended for patients that received the combination therapy, while no obvious differences were detected in the pooled myelosuppression (39.0% vs. 30.6%, P = 0.05) and gastrointestinal response (38.5% vs. 35.9%, P = 0.52) rates between 2 groups. Significant heterogeneity was found in the endpoints of the treatment response and progression-free survival.
Conclusions
This meta-analysis demonstrated that ISB could enhance the clinical efficacy of SLCT in patients with progressive NSCLC after FLT without inducing major toxic side effects.
Similar content being viewed by others
Introduction
Lung cancer is the leading cause of global cancer death. The most (approximate 75–80%) prominent type of lung cancer is non-small-cell lung cancer (NSCLC) [1,2,3]. For the patients suffering from inoperable NSCLC, concurrent chemoradiotherapy (CCRT) is regarded as the standard first-line treatment (FLT) [4,5,6]. When performing the CCRT, traditional external radiotherapy is commonly used [4,5,6]. However, traditional external radiotherapy is typically associated with radiation- related complications, and the radiological dosing is limited by the distance between the tumor and the surrounding healthy tissue and vital organs [7].
Recently, I-125 seeds brachytherapy (ISB) has been widely used for the treatment of various malignant tumors [7,8,9,10]. Compared to the traditional external radiotherapy, ISB has many advantages, including the ease of delivery, direct interaction with the tumor surface, and the sustained application of low-dose radiation to the tumor site over a prolonged duration of time [10]. ISB is often used in combination with systematic chemotherapy or transcatheter arterial chemical infusion in patients with progressive NSCLC [7]. However, a majority of the previous studies focused on the combination of ISB and chemotherapy as the FLT for severe NSCLC patients [7]. However, the number of studies investigating the combination of ISB and chemotherapy treatment for progressive NSCLC after FLT is still limited [11,12,13,14,15,16]. Therefore, a meta-analysis should be performed to increase the statistical power of the small sample study.
In this meta-analysis, we aimed to evaluate the clinical effectiveness of combined ISB with second-line chemotherapy (SLCT) for treating progressive NSCLC after the FLT.
Methods
This meta-analysis abided by the Preferred Reporting Items for Systematic reviews and Meta-Analyses (PRISMA) statement [17], and it was registered at INPLASY.COM (No. INPLASY2021100120).
Study selection
Relevant studies were searched in the following databases: PubMed, Embase, Cochrane Library, CINK, Wanfang, and VIP (until September 2021) using the following search terminologies: ((((((Iodine-125) OR (I125)) OR (125I)) OR (brachytherapy)) AND ((lung cancer) OR (NSCLC))) AND (chemotherapy)) AND ((((recurrent) OR (failure)) OR (second line)) OR (progressive)).
The following articles were included in the current meta-analysis:
-
a.
Type of investigation: comparative studies;
-
b.
Disease: advanced NSCLC after first-line chemotherapy or CCRT;
-
c.
Type of interventions: ISB with SLCT versus SLCT alone;
-
d.
Languages: all.
The following articles were excluded from the current meta-analysis:
-
a.
studies without any control group;
-
b.
case reports;
-
c.
meta-analyses and reviews.
Data extraction
Two independent researchers retrieved the relative data and endpoints, and the disagreements were resolved by a third researcher. The baseline data from each publication included the first author’s name, publication year, countries, types of study design, cancer types, tumor stage, FLT protocol, SLCT protocol, sample size, age, and gender. The outcomes of each study included the following parameters: complete response (CR), treatment response (TR), disease control (DC), myelosuppression, progression-free survival (PFS), and overall survival (OS).
Quality assessment
Potential bias was evaluated by using the Cochrane risk of bias tool for randomized controlled trials (RCTs) [18]. The items of Cochrane risk of bias tool included the following six domains of bias: performance, attrition, detection, selection, reporting, and other sources of bias.
The overall quality of the non-RCTs were analyzed with the 9-point Newcastle–Ottawa scale (NOS) [19], which classified the studies exhibiting into low, intermediate, or high levels of risk, with the scores of ≥ 7, 4–6, and < 4, respectively. The items of NOS included the non-RCT articles based on nine aspects related to study selection (4 points), comparability (2 points), and exposure (3 points).
Definitions
Therapeutic efficacy was examined with the Response Evaluation Criteria in Solid Tumors [20]. TR = CR + partial response; DC = CR + partial response + stable disease. PFS was defined as the time from the initial testing to radiographic monitoring of disease progression. OS was calculated as the time from the treatment to death.
CR was described as the complete absence of all target lesions. Partial response was described as a minimum of 30% reduction in the sum of the largest size of the target lesions. Progressive disease referred to a minimum of 20% increase in the sum of the largest size of the target lesions, or formation of new lesions. Stable disease is regarded as not reaching the partial response or progressive disease standard [20].
Statistical analyses
RevMan v5.3 and Stata v12.0 were employed for this meta-analysis. Dichotomous variables were determined by pooled odds ratios (ORs) with 95% confidence intervals (CIs). Pooled PFS and OS were calculated by hazard ratios (HRs) with 95% CI. Heterogeneity was determined by the I2statistic and Q test. I2 > 50% was defined as high heterogeneity, and then the random effect model was utilized; otherwise, the fixed effects model was utilized. Heterogeneity sources were evaluated by sensitivity and subgroup analyses. Egger test was used to evaluate publication bias. P < 0.05 is the threshold for statistical significance in publication bias.
Results
Included studies
We identified 117 relevant studies by using the research strategy described above. After a full review, only 6 studies fulfilled the selection criteria and they were included into the current meta-analysis (Fig. 1). Among the 6 studies, 272 patients underwent ISB with SLCT (combined group), and 257 patients underwent SLCT alone (chemotherapy alone group, Table 1). All included studies were from China. Two studies were RCTs [11, 14], and 4 studies were retrospective studies [12, 13, 15, 16]. Furthermore, 4 studies used CCRT [11,12,13,14] and 2 studies used chemotherapy only as the FLTs [15, 16]. The raw data for CR, PR, SD, and PD are shown in Table 2.
Quality assessments
The included RCTs had an unclear risk of performance, detection, reporting, and other bias (Fig. 2). The NOS for all the retrospective studies are all 8 (Table1).
CR rates
CR rates could be extracted from all included studies. The pooled CR rate was significantly larger in the combined group than that in the chemotherapy alone group (24.7% vs. 7.0%, P < 0.00001, Fig. 3a). The heterogeneity was not significant (I2 = 13%). The publication bias did not reach statistical significance (Egger test, P = 0.503).
TR rates
TR rates could be extracted from all studies. The pooled TR rate was significantly larger in the combined group than that in the chemotherapy alone group (65.7% vs. 38.1%, P = 0.0002, Fig. 3b). The heterogeneity was significant (I2 = 67%). The publication bias did not reach statistical significance (Egger test, P = 0.709).
The sensitivity analysis indicated that the significant heterogeneity disappeared (I2 = 39%) upon excluding the study from Zhang et al. [15] study. Under this condition, the pooled TR rate was still significantly larger in the combined group than that in the chemotherapy alone group (P = 0.0005).
DC rates
DC rates could be extracted from all studies. The pooled DC rate was significantly higher in the combined group than that in the chemotherapy alone group (95.2% vs. 80.4%, P < 0.00001, Fig. 3c). The heterogeneity was not significant (I2 = 0%). The publication bias did not reach statistical significance (Egger test, P = 0.662).
PFS
The logHR and SE for PFS could be extracted from all studies. The pooled logHR and SE indicated that PFS was significantly longer in the combined group than that in the chemotherapy alone group (P = 0.0001, Fig. 3d). The heterogeneity was significant (I2 = 55%). The publication bias did not reach statistical significance (Egger test, P = 0.637).
The sensitivity analysis indicated that the significant heterogeneity disappeared (I2 = 0%) upon excluding the study from Zhang et al. [15] study. Under this condition, the PFS was still significantly longer in the combined as compared to the chemotherapy alone group (P < 0.00001).
OS
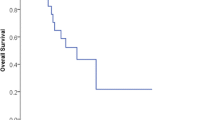
The logHR and SE for OS could be extracted from 5 studies [12,13,14,15]. The pooled logHR and SE indicated that OS was significantly longer in the combined group than that in the chemotherapy alone group (P < 0.00001, Fig. 3e). The heterogeneity was not significant (I2 = 15%). The publication bias did not reach statistical significance (Egger test, P = 0.682).
Myelosuppression
Myelosuppression rates could be extracted from 3 studies [12, 13, 15]. The pooled myelosuppression rates were comparable between 2 groups (39.0% vs. 30.6%, P = 0.05, Fig. 3f). The heterogeneity was not significant (I2 = 0%). The publication bias did not reach statistical significance (Egger test, P = 0.758).
Gastrointestinal response
Gastrointestinal response rates could be extracted from 3 studies [12, 13, 15]. The pooled myelosuppression rates were comparable between 2 groups (38.5% vs. 35.9%, P = 0.52, Fig. 3g). The heterogeneity was not significant (I2 = 0%). The publication bias did not reach statistical significance (Egger test, P = 0.896).
Subgroup analyses
Table 3 shows the subgroup analyses based on the study types (RCT or retrospective). According to the RCTs, combination therapy showed better efficacy in terms of the CR rate (P = 0.001) and PFS (P = 0.0005). Based on the retrospective studies, combination therapy showed better efficacy in the terms of the CR (P < 0.00001), TR (P = 0.001), DC (P < 0.0001) rates, PFS (P = 0.009), and OS (P < 0.0001). Nevertheless, the significant heterogeneity was found in the endpoints of TR rates (I2 = 78%) and PFS (I2 = 67%).
Table 4 shows the subgroup analyses based on the different FLTs (CCRT or chemotherapy alone). Based on studies with the first-line CCRT, the combination therapy showed better efficacy in terms of the CR (P < 0.0001), TR (P = 0.001), DC (P = 0.0008) rates, PFS (P < 0.00001), and OS (P < 0.00001). Based on the studies with chemotherapy alone as the FLT, the combination therapy showed a better efficacy in terms of CR (P = 0.0007), TR (P < 0.00001), DC (P = 0.002) rates, and OS (P = 0.0004). The significant heterogeneity was found in the endpoint of PFS (I2 = 87%).
Discussion
In recent years, the treatment for locally advanced, and unresectable NSCLC has been revolutionized by immunotherapy and conventional CCRT [21]. The first-line platinum-based CCRT or chemotherapy is the gold standard for treating resectable, and locally advanced NSCLC [21]. Durvalumab, a PD-L1 suppressor, gained recent FDA approval as a consolidation drug following CCRT; this medication represents a major progress in the treatment of unresectable stage III NSCLC [22]. With consolidation immunotherapy becoming common in managing unresectable stage III NSCLC, the OS has been significantly improved, with the 3-year OS rate reaching up to 57% [23]. Recently, a meta-analysis reported that Endostar could increase the clinical efficacy of CCRT [24].
Approximately 80% of NSCLC patients experienced recurrence within 1–2 years after first-line CCRT [25]. The SLCT is commonly used for treating progressive NSCLC after the FLT [26,27,28]. However, the TR rates only ranged from 7.7%-18.6%, with the median time to progression of 3.07–3.5 months and the median OS of 7.6–7.83 months [26]. A RCT showed that the 1-year OS rate was 30% after SLCT for progressive NSCLC [28]. These findings indicated that systemic SLCT was not sufficient for treating progressive NSCLC, suggesting that some additional local treatment for the tumor would also required to improve the survival outcome of these patients. Percutaneous CT-guided ISB decreases the local recurrence rate of NSCLC with a few complications [29]. Therefore, many researchers have also combined ISB and first-line chemotherapy for patients with severe NSCLC [7].
In this meta-analysis, we compared the clinical effectiveness of the combination of ISB with SLCT and SLCT alone for patients with progressive NSCLC after they received the FLT. Firstly, the combination therapy promoted a better tumor response rate, which was indicated by the CR, TR, and DC rates. The current results validated the findings from a prior meta-analysis that compared the combination of ISB and chemotherapy with chemotherapy alone as the FLT for severe NSCLC [7]. However, in some of the selected publications in the previously reported meta-analysis, the CR rates in the chemotherapy alone group were less than 5% [12, 16]. These data suggested that ISB could improve the therapeutic efficacy of systemic SLCT by providing the local treatment.
However, the TR (OR = 0.50, P = 0.13) and DC (OR = 0.38, P = 0.14) rates were comparable between the 2 groups based on the RCT subgroup analysis. This could be because of the limited number of RCTs that were included in this meta-analysis. Furthermore, the 2 included RCTs had only enrolled the patients with stage III NSCLC [11, 14], which might have potentially increased the tumor response rates following the chemotherapy alone regimen.
Our meta-analysis combination group showed a PFS. Furthermore, the combination group showed a longer PFS, and this was true for both the RCTs and retrospective studies that were included in this study. Although, in some cases, SLCT alone might provide similar DC rates when compared to the combination therapy, the combination therapy could effectively prolong the time to tumor progression. OS was also improved, as the PFS was improved by the combination therapy. The effectiveness of the SLCT alone was limited by the dosage-related complications and the individual patients’ tolerance while ISB facilitated the continuous irradiation of the tumors [7].
Myelosuppression and gastrointestinal response are the toxicities associated with chemotherapy. But, we found no obvious differences in myelosuppression and gastrointestinal response between the 2 groups in the present meta-analysis. These findings suggested that ISB did not increase the chemotherapy-related toxic side effects, potentially due to the site-specific nature of ISB therapy.
Apart from the subgroup analysis according to the differing study designs, we also have conducted another subgroup analysis based on the different FLTs (CCRT or chemotherapy alone). We found that both the tumor response rates and the OS were favorable in the combination therapy group, irrespective of the kind of FLT administered. Based on these findings, we concluded that different FLTs did not affect the effectiveness of the combination therapy. Although the PFS duration was similar (HR = 1.41, P = 0.20) between the 2 groups based on the subgroup analysis of first-line chemotherapy alone, a significant heterogeneity (I2 = 87%) indicated that this result required further validation.
This meta-analysis had some limitations. Firstly, a majority of the selected publications were retrospective studies. When we performed the subgroup analysis based on the retrospective studies, many endpoints (TR and PFS) were presented with significant heterogeneity. Further RCTs are therefore required to address this heterogeneity. Secondly, the first-line and SLCT protocols, which included the types of medicines, dosage of the medicines, and the number of cycles of the of treatment, were not same for all the included studies. These parameters may further contribute to the increase in the risk of bias. Thirdly, all the included studies were from China and could contribute to cause the patient selection bias. Therefore, further comprehensive studies involving patients across the globe are required in order to generalize our findings for the wider population.
Conclusion
In summary, the current meta-analysis demonstrated that ISB could enhance the clinical efficacy of SCLT in patients with progressive NSCLC following FLT without inducing toxic side effects.
Availability of data and materials
The data that support the findings of this study are available from the corresponding author upon reasonable request.
Abbreviations
- CCRT:
-
Concurrent chemoradiotherapy
- CR:
-
Complete response
- DC:
-
Disease control
- FLT:
-
First-line treatment
- ISB:
-
I-125 seeds brachytherapy
- NOS:
-
Newcastle–Ottawa scale
- NSCLC:
-
Non-small-cell lung cancer
- OS:
-
Overall survival
- PFS:
-
Progression-free survival
- RCT:
-
Randomized controlled trial
- SLCT:
-
Second-line chemotherapy
- TR:
-
Treatment response
References
Duma N, Santana-Davila R, Molina JR. Non-small cell lung cancer: epidemiology, screening, diagnosis, and treatment. Mayo Clin Proc. 2019;94:1623–40.
Patel SA, Weiss J. Advances in the treatment of non-small cell lung cancer: immunotherapy. Clin Chest Med. 2020;41:237–47.
Edelman MJ, Khanwani SL. Advanced non-small cell lung cancer. Curr Treat Options Oncol. 2001;2:51–62.
Faivre-Finn C, Snee M, Ashcroft L, Appel W, Barlesi F, Bhatnagar A, et al. Concurrent once-daily versus twice-daily chemoradiotherapy in patients with limited-stage small-cell lung cancer (CONVERT): an open-label, phase 3, randomised, superiority trial. Lancet Oncol. 2017;18:1116–25.
Eberhardt WE, Pöttgen C, Gauler TC, Friedel G, Veit S, Heinrich V, et al. Phase III study of surgery versus definitive concurrent chemoradiotherapy boost in patients with resectable stage IIIA(N2) and selected IIIB non-small-cell lung cancer after induction chemotherapy and concurrent chemoradiotherapy (ESPATUE). J Clin Oncol. 2015;33:4194–201.
Conibear J, Limited AUK. Rationale for concurrent chemoradiotherapy for patients with stage III non-small-cell lung cancer. Br J Cancer. 2020;123:10–7.
Wu H, Li L, Yang J, Yang ZM. Radioactive seeds insertion with chemotherapy for advanced non-small-cell lung cancer: a meta-analysis. Clin Respir J. 2021;15:187–95.
Sha KH, Liu TG, Yang F, Zhang LG, Jiao ZS, Xia FF. Irradiation stent insertion for inoperable malignant biliary obstruction: a meta-analysis of randomized controlled trials. Abdom Radiol (NY). 2021;46:2173–81.
Yang ZM, Geng HT, Wu H. Radioactive stent for malignant esophageal obstruction: a meta-analysis of randomized controlled trials. J Laparoendosc Adv Surg Tech A. 2021;31:783–9.
Yuan D, Gao Z, Zhao J, Zhang H, Wang J. 125I seed implantation for hepatocellular carcinoma with portal vein tumor thrombus: a systematic review and meta-analysis. Brachytherapy. 2019;18:521–9.
Luo HL, Yu XJ, Li J, Chen XF, He JD. 125I implantation combined with chemotherapy for treatment for local recurrent stage III non-small cell lung cancer. Chin J Nucl Med Mol Imaging. 2013;33:195–8.
Xiang Z, Li G, Liu Z, Huang J, Zhong Z, Sun L, et al. 125I brachytherapy in locally advanced nonsmall cell lung cancer after progression of concurrent radiochemotherapy. Medicine (Baltimore). 2015;94:e2249.
Xiang Z, Zhong Z, Mu L, Li G, Zhou C, Wang H, et al. The clinical value of computed tomography (CT)-guided 125I brachytherapy for locally advanced non-small cell lung cancer after progression of concurrent radiochemotherapy. Cancer Manag Res. 2021;13:5297–307.
Yu X, Li J, Zhong X, He J. Combination of Iodine-125 brachytherapy and chemotherapy for locally recurrent stage III non-small cell lung cancer after concurrent chemoradiotherapy. BMC Cancer. 2015;15:656.
Zhang T, Lu M, Peng S, Zhang W, Yang G, Liu Z, et al. CT-guided implantation of radioactive 125I seed in advanced non-small-cell lung cancer after failure of first-line chemotherapy. J Cancer Res Clin Oncol. 2014;140:1383–90.
Zheng X, Li HF, Li KW, Tan W. CT-guided I-125 seeds insertion for local advanced non-small-cell lung cancer. Yin Shi Bao Jian. 2020;7:85–7.
Moher D, Shamseer L, Clarke M, Ghersi D, Liberati A, Petticrew M, et al. Preferred reporting items for systematic review and meta-analysis protocols (PRISMA-P) 2015 statement. Syst Rev. 2015;4:1.
Higgins JP, Altman DG, Gøtzsche PC, Jüni P, Moher D, Oxman AD, et al. The Cochrane Collaboration's tool for assessing risk of bias in randomised trials. BMJ. 201;343:d5928.
Cook DA, Reed DA. Appraising the quality of medical education research methods: the Medical Education Research Study Quality Instrument and the Newcastle-Ottawa Scale-Education. Acad Med. 2015;90:1067–76.
Therasse P, Arbuck SG, Eisenhauer EA, Wanders J, Kaplan RS, Rubinstein L, et al. New guidelines to evaluate the response to treatment in solid tumors. European Organization for Research and Treatment of Cancer, National Cancer Institute of the United States, National Cancer Institute of Canada. J Natl Cancer Inst. 2000;92:205–16.
Puri S, Saltos A, Perez B, Le X, Gray JE. Locally advanced, unresectable non-small cell lung cancer. Curr Oncol Rep. 2020;22:31.
Antonia SJ, Villegas A, Daniel D, Vicente D, Murakami S, Hui R, et al. Durvalumab after chemoradiotherapy in stage III non-small-cell lung cancer. N Engl J Med. 2017;377:1919–29.
Gray JE, Villegas A, Daniel D, Vicente D, Murakami S, Hui R, et al. Three-year overall survival with durvalumab after chemoradiotherapy in stage III NSCLC-update from PACIFIC. J Thorac Oncol. 2020;15:288–93.
Yuan M, Zhai Y, Men Y, Wang J, Deng L, Wang W, et al. Endostar (rh-endostatin) improves efficacy of concurrent chemoradiotherapy for locally advanced non-small cell lung cancer: a systematic review and meta-analysis. Thorac Cancer. 2021 Oct 21. doi: https://doi.org/10.1111/1759-7714.14188. Epub ahead of print.
Jassem J. Combined chemotherapy and radiation in locally advanced non-small-cell lung cancer. Lancet Oncol. 2001;2:335–42.
Pallis AG, Syrigos K, Kotsakis A, Karachaliou N, Polyzos A, Chandrinos V, et al. Second-line paclitaxel/carboplatin versus vinorelbine/carboplatin in patients who have advanced non-small-cell lung cancer pretreated with non-platinum-based chemotherapy: a multicenter randomized phase II study. Clin Lung Cancer. 2011;12:100–5.
Michael A, Ainsley A, Joseph A, Jahan N. First and second line chemotherapeutic regimens for non-small cell lung carcinomas: the efficacy of platinum, non-platinum and combination therapy: a literature review. Cureus. 2020;12:e11619.
Sun Y, Wu YL, Zhou CC, Zhang L, Zhang L, Liu XY, et al. Second-line pemetrexed versus docetaxel in Chinese patients with locally advanced or metastatic non-small cell lung cancer: a randomized, open-label study. Lung Cancer. 2013;79:143–50.
Martínez-Monge R, Pagola M, Vivas I, López-Picazo JM. CT-guided permanent brachytherapy for patients with medically inoperable early-stage non-small cell lung cancer (NSCLC). Lung Cancer. 2008;61:209–13.
Acknowledgements
None.
Funding
This work is supported by the Gansu Natural Science Fund (No. 21JR7RA573).
Author information
Authors and Affiliations
Contributions
SYZ and YSX designed this work. JF and FQL searched the articles. ZKC, YSX, and SYZ performed the data extraction and statistical analyses. ZKC was a major contributor in writing the manuscript. All authors read and approved the final manuscript.
Corresponding authors
Ethics declarations
Ethics approval and consent to participate
This is a meta-analysis and ethics approval and consent to participate are not required.
Consent for publication
This is a meta-analysis and consent for publication is not required.
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Chen, ZK., Fan, J., Li, FQ. et al. I-125 seeds with chemotherapy for progressive non-small-cell lung cancer after first-line treatment: a meta-analysis. J Cardiothorac Surg 17, 75 (2022). https://doi.org/10.1186/s13019-022-01820-y
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s13019-022-01820-y