Abstract
Background
Acute thrombotic thrombocytopenic purpura (TTP) is an aggressive thrombotic microangiopathy that if not treated, can have a 90% mortality rate. Timely, extensive plasma exchange (PEX) has been indicated to reduce the mortality rate to < 10%, but its side effects are not well-known. We present here a case of a patient presented with Comb (+) TTP and developed catheter-associated deep vein thrombosis (DVT).
Case presentation
A 27-year-young man presented with persistent thrombocytopenia and Coombs positive anemia was firstly diagnosed with Evans syndrome. However, he was refractory to a methylprednisolone pulse therapy with a combination of platelet transfusion and eventually developed microangiopathy of central nerve system. Several pathological manifestations of the disease were prevented by PEX. The immediate start of PEX (1500 mL/d) induced a complete remission of acquired TTP and disappearance of neurological signs and symptoms. However, external iliac and femoro-popliteal venous thrombosis was diagnosed subsequently, inferior vena cava filter (IVC) filter was immediately implanted accompanied with anticoagulation therapy. Meanwhile, PEX session was sustained as well as oral anticoagulant (rivaroxaban). 14 days later, the patient got full recovery.
Conclusions
Catheter-related DVT under the setting of TTP should be cautious. It is necessary to start anticoagulation and antiplatelet therapy for thrombosis early, especially in such cases when PLT count > 50 × 109/L.
Similar content being viewed by others
Background
Thrombotic thrombocytopenic purpura (TTP) is a rare and potentially fatal hematologic disease. Fifty years ago, before the era of effective treatment, the diagnosis of TTP was based on the progressive appearance of the “pentad” of clinical manifestations: microangiopathic hemolytic anemia, thrombocytopenia, renal and neurological abnormalities, and fever [1]. Recent studies have demonstrated that deficiency in the von Willebrand factor (vWF) cleaving protease ADAMTS13 (a disintegrin and metalloproteinase with thrombospondin motifs 13) causes TTP. The deficiency of ADAMTS13 can be genetic or more common, acquired, resulting from autoimmune production of inhibitory anti-ADAMTS13 antibodies [2].
PEX (plasma exchange) has now become the cornerstone of the management of TTP. Timely, extensive PEX has been indicated to reduce the mortality rate to < 10%, resulting in > 90% short-term effectiveness. However, few attentions have been paid to the complication of PEX since PEX requires insertion of a central venous dialysis catheter, with its risk for hemorrhage, thrombosis and infection [3].
In this case, we describe a young male who was firstly defined by either simultaneous or sequential combination of immune thrombocytopenia and autoimmune hemolytic anemia with a positive direct anti globulin test (DAT) in the absence of known underlying etiology and diagnosed as TTP. He responded well to the timely PEX but suffered catheter-associated thrombosis.
Case presentation
Presenting clinical features
A 27-year-old previously healthy male patient (height 176 cm, and weight 79 kg) presented with fever, macrohematuria, and purpura in the lower legs developed 4 days before admission, respectively. He denied drug exposures and recent infectious illness. He had no abdominal pain or diarrhea. Vital signs were normal and physical examination was unremarkable except for petechiae. His mother died of anemia (details unclear). Laboratory findings revealed hemolytic anemia, thrombocytopenia (Table 1), and renal damage (Urinalysis disclosed a proteinuria score of 2+, a red blood cell count of 8.4 per high-power field, a white blood cell count of 4.4 per high-power field, and serum creatinine: normal). Peripheral smear showed numerous schistocytes (1.2%). Prothrombin time, partial thromboplastin time, and renal function test was all within normal limits (Table 2). The laboratory tests showed a direct antiglobulin test (+), indicating peripheral cytopenias, particularly autoimmune cytopenias (AIC) such as autoimmune thrombocytopenia, Anti-SSA, Jo-52 (+). A bone marrow biopsy was also performed, showing only erythroid hyperplasia without other abnormalities. A diagnosis of ES was made given the evidence of immune-mediated hemolysis with thrombocytopenia in the absence of a known etiology, we administered methylprednisolone pulse therapy with the dose of 500 mg/d for 3 consecutive days. At the following days, he had a drop in his Hgb was from 15.2 to 7.4 g/dL, with an elevated LDH level soaring to 4136 U/L.
Neurologic abnormalities
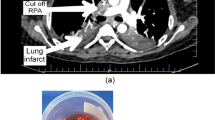
He remained asymptomatic but over 9 days, he experienced several episodes of headache, blurred vision and minor mental status changes, with fever high up to 38.5. Moreover, Peripheral smear showed an increased number of schistocytes (1.3%) (Fig. 1). PEX through a right femoral venous hemodialysis catheter was carried out daily immediately after the onset of neurologic abnormalities immediately even if ADAMTS-13 levels remained unknown given the high risk of morbidity and mortality of TTP within the first 24 h if plasma replacement therapy is not given [4]. However, because the shortage of serum, we collected 1000 mL serum, then added 500 mL volume of albumin. The PEX procedure resulted in a dramatic response with improvements. His neurologic abnormalities resolved immediately and did not recur.
On the 3th day post PEX therapy, the PLT rise to 156 × 109/L with the LDH level down to 478 U/L. Because complete response of PEX was defined by a full resolution of any neurological manifestations and platelet count recovery (> 150 × 109/L) for at least two days based on previous studies and in accordance with international guidelines [5]. Therefore, we continued PEX therapy. On the 4th day post PEX, continuous improvement was noted on the blood test, with platelets peaked to 195 × 109/L and LDH down to 331 U/L. However, the patient presented with a sudden onset right leg swelling and pain. There were no associated signs or symptoms such as dyspnea or fever. The color Doppler ultrasound demonstrated evidence of DVT in the right lower extremity which showed total thrombosis of the right external iliac and femoral veins and nearly total thrombosis of the right popliteal vein (Fig. 2).
We consulted vascular surgeons. Based on their recommendation, the patient was implanted an inferior vena cava filter and underwent catheter thrombolysis and perfusion catheter insertion with continued administration of thrombolytic agent (Fig. 3). At the meantime, PEX session was sustained for another 2 days. The symptoms of DVT relieved markedly, and we shifted to an oral anticoagulant (rivaroxaban). After another twice PEX, he continued to remain asymptomatic, his hematological parameters stabilized with a platelet count of 200 × 109/L at discharge and plasma D-dimer levels returned to normal. The patient is now under follow-up in the outpatient clinic and is undertaking rivaroxaban daily, while progressively tapering oral corticosteroids. In a yearly follow-up, there has been no anemia and the platelet count also remains normal to date.
Discussion
Hemolysis in idiopathic TTP is mechanical and nonimmune mediated, thus Coombs testing is usually negative. Nowadays, autoimmune diseases caused acquired TTP. have been explored broadly, which caused vascular endothelial cell damage, release of a large number of vWF, lack of vWF-cp or inhibition of vWF-cp activity, leading to microaggregation of platelets and vWF-fibrinogen, vessel occlusion, and rapid reduction of platelets, and finally resulting in occurrence of TTP [6]. Coombs testing could be positive in that case, and usually ended with fatality in adult literature [7]. A variety of autoimmune disorders may develop several years after the recovery of TTP and such observations highlight the necessity of clinical surveillance [6], however, the guidelines are still missing.
The diagnosis of TTP requires clinical judgment in addition to measurement of ADAMTS13 activity [8]. Since rapid ADAMTS13 activity assessment is not available in routinely, leading to diagnostic wanderings with potentially severe consequences on prognosis by delaying therapeutic plasma exchange (TPE) in cases of diagnosis uncertainty. Our current practice continues to treat patients with PEX if they have clinical features of TTP with no alternative diagnosis, even if the ADAMTS13 activity is not available. However, we must balance the risks and benefits for PEX procedure at the first place. Common risks are as follows: hemorrhage or pneumothorax complicating the insertion of central venous catheter, thrombosis or sepsis attributed to central venous catheter, anaphylactic reaction to plasma and cardiac tamponade related to catheter insertion [9]. As in this case, the patient responded well with treatment of timely PEX and corticosteroids, however, femoral catheterization associated DVT occurred. It is most probably caused by endothelial damage secondary to intravenous catheters. Then, loss of physiological thromboresistance, leukocyte adhesion to damaged endothelium, complement consumption, abnormal vWF release and fragmentation, and increased vascular shear stress may then sustain and amplify the microangiopathic process. We hypothesis that tries to explain the complications of PEX considers that PEX removed autoantibodies and corrected PLT deficiency, resulting in thrombosis. Low-molecular-weight heparin thromboprophylaxis plus antiplatelet when the platelet count > 50 × 109/L were suggested in clinical work [10]. Previous studies have shown that the frequency of mechanical complications is greater with femoral catheterization than with subclavian. And internal jugular catheterization [11]. However, no specific guidelines regarding catheterization pathway was made yet.
Although the PEX-based method is the recommended acquired TTP treatment worldwide [12], other options could be considered for treating recurring or refractory TTP cases, or when severe adverse effects related to PEX such as bleeding or thrombosis appear [13]. Since 2002, therapeutic interventions aiming to B-cell depletion and reduction of autoantibodies, with rituximab, appear very effective both as induction therapy for the initiation of remission, as well as maintenance therapy, some even advocate the use of rituximab as routine initial treatment together with PEX and corticosteroids [14]; however, the frequencies of severe neurologic abnormalities, exacerbations, and death have not changed, while the frequency of relapse has decreased [15]. Recently, treatment of acute episodes of TTP with increasing use of rituximab and the addition of new agents, such as caplacizumab [16] and recombinant ADAMTS13 showed to be more effective.
We anticipate that more effective treatment will improve the quality and duration of life for patients in remission from TTP. With more effective treatments, the need for PEX and the risks for complications from PEX may decrease.
Conclusion
Catheter-related DVT under the setting of TTP or TTP recovery stage may be presented as a more fulminant form. Still, long-term follow-up of TTP patients is crucial to identify the occurrence of other autoimmune diseases, to control relapses and to evaluate psychophysical sequelae. Further development of both patients’ registries worldwide and innovative drugs is still needed to improve TTP management.
Availability of data and materials
All data generated during our study is included in this article.
Abbreviations
- TTP:
-
Thrombotic thrombocytopenic purpura
- vWF:
-
Von Willebrand factor
- ADAMTS13:
-
A disintegrin and metalloproteinase with thrombospondin motifs 13
- PEX:
-
Plasma exchange
- DAT:
-
Direct antiglobulin test
- AIC:
-
Autoimmune cytopenias
- DVT:
-
Deep vein thrombosis
- IVC:
-
Inferior vena cava filter
- TPE:
-
Therapeutic plasma exchange
- PT:
-
Prothrombin time
- APTT:
-
Activated partial thromboplastin time
- PLT:
-
Platelets
- LDH:
-
Lactate dehydrogenase
References
George JN, Nester CM. Syndromes of thrombotic microangiopathy. N Engl J Med. 2014;371:654–66.
Chapman K, Seldon M, Richards R. Thrombotic microangiopathies, thrombotic thrombocytopenic purpura, and ADAMTS-13. Semin Thromb Hemost. 2012;38:47–54.
George JN, Sandler SA, Stankiewicz J. Management of thrombotic thrombocytopenic purpura without plasma exchange: the Jehovah’s Witness experience. Blood Adv. 2017;1:2161–5.
Scully M, Hunt BJ, Benjamin S, Liesner R, Rose P, Peyvandi F, Cheung B, Machin SJ. British Committee for Standards in H: guidelines on the diagnosis and management of thrombotic thrombocytopenic purpura and other thrombotic microangiopathies. Br J Haematol. 2012;158:323–35.
Froissart A, Buffet M, Veyradier A, Poullin P, Provot F, Malot S, Schwarzinger M, Galicier L, Vanhille P, Vernant JP, et al. Efficacy and safety of first-line rituximab in severe, acquired thrombotic thrombocytopenic purpura with a suboptimal response to plasma exchange. Experience of the French Thrombotic Microangiopathies Reference Center. Crit Care Med. 2012;40:104–11.
Dimopoulou D, Dimosiari A, Mandala E, Dimitroulas T, Garyfallos A. Autoimmune thrombotic thrombocytopenic purpura: two rare cases associated with juvenile idiopathic arthritis and multiple sclerosis. Front Med (Lausanne). 2017;4:89.
Zhang C, Chen XH, Zhang X. Quick development and sudden death: evans syndrome followed by thrombotic thrombocytopenic purpura. Am J Emerg Med. 2014;32(1156):e1153–4.
Page EE, Kremer Hovinga JA, Terrell DR, Vesely SK, George JN. Thrombotic thrombocytopenic purpura: diagnostic criteria, clinical features, and long-term outcomes from 1995 through 2015. Blood Adv. 2017;1:590–600.
Howard MA, Williams LA, Terrell DR, Duvall D, Vesely SK, George JN. Complications of plasma exchange in patients treated for clinically suspected thrombotic thrombocytopenic purpura-hemolytic uremic syndrome. Transfusion. 2006;46:154–6.
Oshima T, Ikutomi M, Shinohara H, Ishiwata J, Fukino K, Amaki T, Nakamura F. Acute myocardial infarction caused by thrombotic microangiopathy complicated with myelodysplastic syndrome. Int Heart J. 2016;57:634–6.
McGee DC, Gould MK. Preventing complications of central venous catheterization. N Engl J Med. 2003;348:1123–33.
Rottenstreich A, Kalish Y, Tvito A, Hauschner H, Arad A. Acquired thrombotic thrombocytopenic purpura in pregnancy: the role of placental and breast-milk mediated transfer of ADAMTS13-autoantibodies. Thromb Res. 2017;156:80–1.
Nakao H, Ishiguro A, Ikoma N, Nishi K, Su C, Nakadate H, Kubota M, Hayakawa M, Matsumoto M. Acquired idiopathic thrombotic thrombocytopenic purpura successfully treated with intravenous immunoglobulin and glucocorticoid: a case report. Medicine (Baltimore). 2017;96:e6547.
Jestin M, Benhamou Y, Schelpe A-S, Roose E, Galicier L,Girault S, et al. Rituximab prevents long-term relapses in TTP. Blood. 2018;132:2210.
Thumma S, Idrees S, Phuyal P, Manchala V, Mattana J. When the standard treatment fails: rituximab therapy for refractory TTP. Am J Ther. 2019;26:e552–3.
Peyvandi F, Scully M, Kremer Hovinga JA, Cataland S, Knobl P, Wu H, Artoni A, Westwood JP, Mansouri Taleghani M, Jilma B, et al. Caplacizumab for acquired thrombotic thrombocytopenic purpura. N Engl J Med. 2016;374:511–22.
Acknowledgements
None.
Funding
The work was supported by the National Natural Science Foundation of China (NSFC, 81570305 and 81600265).
Author information
Authors and Affiliations
Contributions
MZ and JY designed the methods, analyzed the data and results, MZ wrote the manuscript and prepared figures. All authors have read and agreed to the published version of the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Our study has received Ethics approval of Qianfoshan Hospital and patients’ informed consent.
Consent for publication
Our study has received all authors’ consent for publication.
Competing interests
Authors declare that they have no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Zhou, M., Yin, J. Complete recovery of deep venous thrombosis from Coombs (+) thrombotic thrombocytopenic purpura: case report. J Cardiothorac Surg 17, 43 (2022). https://doi.org/10.1186/s13019-022-01789-8
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s13019-022-01789-8