Abstract
Background
Although prior observational studies indicate an association between cardiovascular diseases (CVDs) and frozen shoulder (FS), the potential causal relationship between them remains uncertain. This study aims to explore the genetic causal relationship between CVDs and FS using Mendelian randomization (MR).
Methods
Genetic variations closely associated with FS were obtained from the FinnGen Consortium. Summary data for CVD, including atrial fibrillation (AF), coronary artery disease (CAD), heart failure (HF), myocardial infarction (MI), stroke, and ischemic stroke (IS), were sourced from several large-scale genome-wide association studies (GWAS). MR analysis was performed using inverse variance weighting (IVW), MR Egger, and weighted median methods. IVW, as the primary MR analysis method, complemented by other sensitivity analyses, was utilized to validate the robustness of the results. Further reverse MR analysis was conducted to explore the presence of reverse causal relationships.
Results
In the forward MR analysis, genetically determined risk of stroke and IS was positively associated with FS (OR [95% CI] = 1.58 (1.23–2.03), P < 0.01; OR [95% CI] = 1.46 (1.16–1.85), P < 0.01, respectively). There was no strong evidence of an effect of genetically predicted other CVDs on FS risk. Sensitivity analyses confirmed the robustness of the results. In the reverse MR analysis, no causal relationships were observed between FS and various CVDs.
Conclusion
The study suggests that stroke increases the risk of developing FS. However, further basic and clinical research is needed to substantiate our findings.
Similar content being viewed by others
Introduction
Frozen shoulder (FS) is a fibroproliferative disorder characterized by pain and progressive restriction of shoulder motion [1]. Despite its self-limiting nature, with spontaneous recovery typically occurring within 1–2 years, research suggested that numerous FS-related symptoms could persist in 20–50% of patients, encompassing stiffness and pain [2, 3]. The treatment of FS emphasizes physical therapy, pharmacological relief, and potential surgery. Meanwhile, preventive measures are crucial for alleviating patient symptoms and reducing the socio-medical burden [4]. Certain preventive measures can regulate the occurrence and progression of FS; thus, identifying novel FS risk factors and interventions is imperative.
Cardiovascular diseases (CVDs), primarily encompassing coronary artery disease (CAD), heart failure (HF), myocardial infarction (MI), and stroke, have emerged as the leading causes of morbidity and mortality in the global population [5]. World Health Organization statistics indicated an annual death toll of about 17.8 million due to CVD, contributing to roughly 30% of the total global fatalities [6]. Recent studies have demonstrated a close association between various CVDs and FS. A clinical retrospective study involving 262 FS patients reported that the overall risk of FS was more than three times higher in CAD patients [7]. FS patients have a 1.22 times higher risk of experiencing a stroke compared to the healthy control group [8]. Similarly, a large cohort study indicated that FS patients face an elevated risk of MI compared to the general population [9]. Overall, the existing findings in the observational studies section demonstrate the correlation between CVDs and FS. Despite the association, the mentioned studies have not adequately distinguished the causal relationship between CVDs and FS, nor have they addressed the evidence gap that may result from unmeasured confounding or reverse causation. Therefore, caution is still needed in interpreting these relationships.
Observational studies face limitations in establishing causality due to the inability to control interventions, confounding, and bias. In recent years, in order to overcome the limitations of observational studies, Mendelian randomization (MR) has emerged as a novel approach to explore causal association between etiology and diseases [10, 11]. Based on the genome-wide association study (GWAS), MR uses natural genetic variation to simulate randomized controlled trials (RCTs) and uses genetic variation as instrumental variables (IVs) to assess the causal relationship between exposures and outcomes. In MR studies, single-nucleotide polymorphisms (SNPs) are utilized as IVs to estimate the causal associations between exposures and target outcomes [12]. These SNPs adhere to the random distribution principle of genetic variation, effectively mitigating the potential impacts of confounding factors and reverse causality, given that genetic variations manifest prior to the onset of the disease [13]. Meanwhile, the advancing GWASs have furnished robust and dependable IV support for MR study.
Although Lv et al. have reported the causal relationship between CVD and FS using MR methods, their study was limited to the association between stroke and FS and did not analyze the reverse causal relationship between them [14]. Therefore, the current study employs newly released GWAS data to conduct a two-sample bidirectional MR study, aiming to investigate the presence of a direct causal association between multiple CVDs and FS, providing a theoretical basis for clinical practice. To our knowledge, this is the first two-sample MR study endeavoring to unveil the causality between multiple CVDs and FS. By circumventing certain limitations of observational studies in the process of causal inference, this study holds the potential to offer a clearer perspective for us to delve deeper into understanding this association.
Methods
Data source
In order to mitigate potential confounding bias arising from racial stratification, our study exclusively focuses on participants of European descent to ensure the reliability and consistency of outcomes.
Aggregated statistical data for FS were obtained from FinnGen, encompassing a total of 170,583 Europeans (2942 cases and 167,641 controls). FinnGen constitutes a large-scale public–private partnership aimed at gathering and analyzing genomic and health data from 500,000 Finnish biobank participants. SNPs were analyzed using a mixed-model logistic regression, adjusting for gender, age, 10 principal components, genetic relatedness, and genotyping batch. Further comprehensive information regarding FinnGen can be accessed on its official website [https://www.finngen.fi/en].
We extracted genetic association data related to CVDs from five large-scale meta-analyses. Data on atrial fibrillation (AF) were derived from a GWAS of susceptibility genes published in 2018, the study included 60,620 patients with atrial fibrillation, 970,216 healthy controls and revealed 142 independent risk variants at 111 loci and prioritized 151 functional candidate genes likely to be involved in AF [15]. This database compares data from six cohort studies (the Nord-Trøndelag Health Study, the Michigan Genomics Initiative, deCODE, DiscovEHR, AFGen Consortium, and the UK Biobank). The GWAS of CAD data from the CARDIoGRAMplusC4D consortium and UK Biobank includes 122,733 cases and 424,528 controls [16]. Data on HF compilation included 47,309 cases and 930,014 controls from 26 cohorts of European ancestry, showing 12 independent variants at 11 genomic loci were associated with HF. The definition of HF was based on the Heart Failure Molecular Epidemiology (HERMES) Consortium targeting therapeutic endpoints [17]. The MI GWAS dataset originated from another GWAS analysis, including 14,825 cases and 44,000 controls of European ancestry [18]. The summary statistical data for stroke were derived from the MEGASTROKE consortium, which comprised 446,696 participants of European ancestry (40,585 stroke cases and 406,111 controls). Among these stroke cases, 34,217 individuals were classified as suffering from ischemic stroke (IS) [19]. Within this study, the definition of strokes is in accordance with guidelines from the World Health Organization (WHO). Detailed information regarding data sources and definitions is presented in Table 1.
The involved published GWAS meta-analyses and the FinnGen study have obtained approvals from relevant ethical review bodies and have received informed consent from participants. Our study solely analyzes publicly available summary-level statistical data; thus, new ethical review committee approval is unnecessary.
MR assumptions and genetic instrument selection
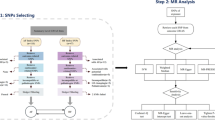
Genetic variations serve as tools for establishing causal association in MR analysis. To attain unbiased estimates of the association between CVD and FS-related features, the following conditions must be met: (1) The selected instrumental variable (IV) is exclusively linked to CVD; (2) The eligible exposure IV remains independent of any confounding factors; (3) The IV solely affects FS through CVD (Fig. 1). With a genome-wide significance threshold set at P < 5.0 × 10–8, we selected potential IVs from each SNP associated with CVD. Based on genome-wide significance, to ensure independence among the utilized IVs, we set a linkage disequilibrium (LD) threshold of R2 < 0.001 and employed a window size of 1000 kb.
In cases where exposure-associated SNPs are absent in the outcome dataset, we employed alternative SNPs significantly correlated with the SNPs of interest (R2 > 0.8). If suitable replacement SNPs were not available, the SNP was discarded. Simultaneously, we set a minimum allele frequency (MAF) of 0.3 to ensure SNP commonality. To eliminate bias, we harmonized effect alleles between the exposure and outcome datasets and excluded all SNPs with palindromic characteristics. Weak IVs typically lack strong correlation with the exposure factor, leading to diminished effectiveness in explaining the genetic variation in the exposure. The presence of weak IVs results in an augmentation of bias between estimated and actual values. To quantify IV strength, we utilize the F-statistic, where an F-statistic > 10 is considered to indicate sufficient instrument strength [20]. The formula for calculating the F-statistic is as follows: F = ((N − 2) × R2 / (1 − R2)), where R2 signifies the extent to which the SNP explains the exposure, and N denotes the sample size [20]. The calculation formula for R2 is as follows: R2 = 2 × MAF × (1—MAF) × β2, where MAF refers to the minor allele frequency and β represents the effect size of the exposure [21]. In the absence of MAF, we utilize the formula R2 = β2/(β2 + SE2 × N)to compute R2 [22].
Statistical analysis
In the MR analysis, we employed the inverse variance weighted (IVW), weighted median (WM), and MR Egger methods. Compared to the conventional fixed-effect IVW method, the random-effects IVW method demonstrates enhanced robustness in the presence of instrument selection heterogeneity, resulting in more conservative and realistic parameter estimates [23] (Additional file 1: Table S1). Given this, we consider the results of the IVW method as the basis for primary outcome assessment. To enhance result reliability, we stipulated that the IVW results should be statistically significant at minimum, and the outcomes of WM and MR-Egger methods must align directionally with the IVW results. To control for the type I error rate, the Benjamini–Hochberg method was used to adjust for multiple testing. The false discovery rate (FDR) threshold was set at 0.05 for significance.
Following the MR analysis, we utilized Cochran's Q test to evaluate heterogeneity in the effect of CVD-related SNPs on FS risk [24]. When the test results indicate heterogeneity (P < 0.05), we introduce the MR-PRESSO method to remove IVs with heterogeneity and then perform a re-analysis of the IVs not identified as heterogeneous [25]. We also employed the MR-Egger intercept method to assess evidence of horizontal pleiotropy among the selected SNPs (presence of horizontal pleiotropy was speculated at P < 0.05) [26]. Moreover, we conducted leave-one-out analysis to verify if any individual SNP significantly influences the outcomes. To assess the reliability and heterogeneity of the estimates, we employed forest plots and funnel plots. Scatter plots were generated to visually present the estimated effect sizes.
In the reverse causal analysis, we replicated the aforementioned methodology, employing an SNP set associated with FS to explore the causal impact of FS on CVDs. It should be noted that given the restricted count of SNPs closely associated with FS (P < 5 × 10–8), we employed a more relaxed threshold (P < 5 × 10–6) for SNP identification [27, 28].
“Two SampleMR” (version 0.5.6) of R software (version 4.2.1) was used for all analyses. P values less than 0.05 were considered statistically significant. We adhered to the guidance of the STROBE-MR guidelines in reporting the MR study [29].
Results
Characteristics of the genetic instruments
Adhering to established stringent quality control criteria, we selected for a set of SNPs associated with CVDs and FS to serve as IVs. Specifically, we selected 98 SNPs associated with AF (rs4642101 replaced by rs11717013), 61 SNPs associated with CAD, 9 SNPs associated with HF, 67 SNPs associated with MI (rs10404176, rs112374545, rs12897285, rs180803, rs433903, rs9865841 replaced by rs10423961, rs113722226, rs1958320, rs77036345, rs355788, rs6779146, respectively), 16 SNPs associated with stroke, 17 SNPs associated with IS (rs9909858 replaced by rs60460011), and 12 SNPs associated with FS. The F-statistics for all these genetic variations are above the threshold of 10 (range: 30–45951). Because higher F-statistics indicate stronger instruments, the IVs we selected have a robust power to predict CVD and FS, rendering them suitable for application as IVs in MR analysis. Additional file 1: Table S2 and S3 and Additional file 1: Fig. S1 furnish comprehensive details about these SNPs.
Causal effects of CVDs on FS
The IVW, MR Egger, and WM were employed to assess the causal association between CVDs and FS. The IVW was employed as the principal method. When conducting the analysis using the IVW, we noted a significant elevation in the risk of FS due to genetic propensity toward stroke and IS (OR = 1.58, P < 0.05; OR = 1.46, P < 0.05, respectively). Nevertheless, in the causal association analysis between other CVDs (including AF, CAD, HF, and MI) and FS, we did not observe significant results (all P > 0.05 & FDR > 0.05). The MR Egger and WM also demonstrated trends resembling IVW, providing additional support for the robustness of the causal relationship inference between FS and CVDs (Fig. 2).
Forest plots of MR study using genetically predicted CVDs with frozen shoulder. IVW, inverse variance weighted; AF, atrial fibrillation; CAD, coronary artery disease; HF, heart failure; MI, myocardial infarction; IS, ischemic stroke. High risk: P < 0.05 and FDR < 0.05; potential risk: P < 0.05 and FDR > 0.05; unclear: P > 0.05; protective: P < 0.05 and FDR < 0.05
Table 2 provides a compilation of detailed results concerning heterogeneity and horizontal pleiotropy. Regarding the MR Egger regression, our analysis findings indicated none of the examined SNPs exhibited signs of horizontal pleiotropy. Simultaneously, while conducting the Cochran's Q test, the P-values for the Q statistics significantly surpassed the threshold of 0.05. This indicated the absence of noteworthy heterogeneity among IVs we employed. To further confirm the robustness of our results, we conducted leave-one-out analysis, and the relevant results are presented in Additional file 1: Fig. S3. Additionally, we also provided scatter plots (Fig. 3), funnel plots (Additional file 1: Fig. S4), and forest plots (Additional file 1: Fig. S2) to visually present the MR analysis. Building upon our sensitivity analysis results, we possess substantial confidence in the reliability and stability of the conclusions derived from the IVW method.
Causal effects of FS on CVDs
In the reverse MR analysis, we effectively screened and identified 12 SNPs associated with FS. It is worth noting that the SNP rs28599891 was excluded from the analysis due to its palindromic characteristics. Unfortunately, genetic data for SNP rs35464280 could not be obtained in the stroke, IS, and HF aspects. Consequently, in the final MR studies involving AF, CAD, HF, MI, stroke, IS, and FS, analyses were conducted with 11, 11, 10, 11, 10, and 10 SNPs, respectively (Additional file 1: Fig. S1). Nevertheless, our study did not uncover any causality between CVDs and FS (AF, OR [95% CI] = 0.99 (0.96 to 1.02), P = 0.33; CAD, OR [95% CI] = 1.00(0.97 to 1.04), P = 0.85; HF, OR [95% CI] = 0.99(0.95 to 1.02), P = 0.44; MI, OR [95% CI] = 1.00(0.95 to 1.05), P = 0.96; Stroke, OR [95% CI] = 0.97(0.90 to 1.03), P = 0.32; IS, OR [95% CI] = 0.96(0.90 to 1.03), P = 0.24), as indicated in Fig. 4. Encouragingly, these sensitivity analyses did not reveal any prominent anomalies, further reinforcing the reliability of our research conclusions (Additional file 1: Table S4). Additional detailed information, including characteristics of each SNP, can be found in Additional file 1: Table S3. To visually present our research findings more intuitively, Additional file 1: Figs. S5, S6, S7 and S8 depict the visual results of the relationship between FS and the 6 types of CVD.
Forest plots of MR study using genetically predicted frozen shoulder with CVDs. IVW, inverse variance weighted; AF, atrial fibrillation; CAD, coronary artery disease; HF, heart failure; MI, myocardial infarction; IS, ischemic stroke. High risk: P < 0.05 and FDR < 0.05; potential risk: P < 0.05 and FDR > 0.05; unclear: P > 0.05; protective: P < 0.05 and FDR < 0.05
Discussion
We utilized a bidirectional two-sample MR study for the first time to investigate the causal relationships between CVDs and FS. The results indicated that some types of CVDs were related to the risk of FS. Specifically, genetic susceptibility to stroke and IS was significantly associated with FS risk, and this association remains significant even after FDR correction. Nevertheless, we lacked substantial evidence to establish a link between AF, CAD, HF, MI, and FS. However, we did not find sufficient evidence to support connections between AF, CAD, HF, MI, with FS. These findings exhibited robustness and consistency across various sensitivity analyses, further enhancing the reliability of our study results.
Previous studies have shown that stroke increases the risk of FS. This observation aligns with the conclusion of our study. Relevant studies indicated that the most common complication one year after stroke was shoulder pain, accounting for approximately 40% [30]. Most shoulder pain occurs within 2 months after onset, with 50% of them attributed to FS [31]. Over time, the risk of developing FS in participants 2 to 6 months after stroke occurrence increases four times [32]. Furthermore, MR studies have also verified a causality between IS and FS [14]. Firstly, stroke might trigger an inflammatory response in the shoulder joint, stimulate fibrosis of the muscles around the shoulder area, and subsequently elevate the risk of developing FS. Stroke results in cerebral ischemia or hemorrhage, causing the release of chemical compounds called inflammatory mediators when brain cells are damaged or die. These compounds include cytokines and inflammatory proteins, which then activate immune cells and inflammatory cells, initiating an inflammatory response [33, 34]. A broad consensus exists that the progression of FS is closely linked to inflammation and fibrosis. Studies have indicated a significant elevation of inflammatory cytokines such as interleukin (IL)-1α, IL-1β, tumor necrosis factor (TNF)-α, and cyclooxygenase-2 in the joint capsules of FS patients [35]. Extensive presence of inflammatory cells like T cells, B cells, macrophages, and mast cells has been widely detected in supraspinatus muscle interval samples from FS patients [36]. Secondly, central nervous system lesions following a stroke often lead to limb paralysis, progressively increasing the risk of joint effusion and muscle adhesions, ultimately contributing to the occurrence of FS [37, 38]. Finally, Some medications used in stroke treatment, especially those affecting blood coagulation and circulation, may have adverse effects on joints and muscles, thereby increasing the risk of developing FS. These medications may lead to blood vessel narrowing, affecting local blood supply and causing damage to joint tissues [39]. Additionally, drugs may modulate the immune response in joints through the immune system, leading to inflammation and damage. Side effects of medications may include discomfort or pain in muscles and joints, while a decrease in mobility may restrict normal shoulder joint movement, creating unfavorable conditions for the development of FS [40]. This complex interplay may make patients more prone to developing shoulder periarthritis.
It is noteworthy that there is currently no consensus on whether FS increases the risk of stroke. One study indicated that the risk of stroke in FS patients was 1.46-times (95% CI 1.32–1.62; P < 0.001) that of the control group, and even after adjusting for confounding factors, the risk remained elevated at 1.22-times(95% CI 1.06–1.40; P = 0.002) [8]. Subsequently, another follow-up study based on a large propensity score-matched population found no association between FS and an increased risk of stroke (OR [95% CI] = 0.93 (0.83–1.04), P = 0.178) [41]. This is consistent with our study findings. We mainly attribute this result to the substantial imbalance in demographic characteristics and distribution of vascular risk factors between the FS and non-FS groups in the study mentioned above [8]. Compared to the non-FS group, the FS group had a significantly older age, and the prevalence of diabetes and hyperlipidemia was 1.5 times higher. Despite adjustments in the multivariate regression analysis, the marked imbalances in these potential confounding variables could present challenges in effectively addressing these factors.
Interestingly, this study found no clear causal relationship between MI, CAD, and FS, which differs from previous study results and presents an intriguing trend. One study revealed that the risk of FS in CAD patients was 3.128-times (95% CI, 1.428–7.019; P = 0.005) that of non-FS patients [7]. On the one hand, shoulder pain in CAD patients may result in muscle contraction due to heart disease, thereby restricting shoulder joint movement. On the other hand, insufficient coronary artery blood supply and impaired cardiac function in CAD patients affect local vascular circulation, resulting in tissue ischemia and hypoxia, which can also cause shoulder pain [42]. Another follow-up study on the risk of FS patients indicated that over a 3-year follow-up period, the incidence risk of MI increased by 1.49-times (95% CI, 1.25–1.77; P < 0.001) [9]. They indicated that FS patients may exhibit elevated levels of C-reactive protein (CRP) and alterations in related immune complexes [43]. However, CRP plays a significant role in CVDs and is closely associated with their prognosis [44]. We speculate that the reasons for the inconsistency in study results may include the following two aspects: First, CAD, MI, and FS share some pathogenic factors, such as age, hyperlipidemia, and diabetes. CAD and MI may indirectly affect FS through these common pathogenic factors. MR studies may adopt more stringent methods, potentially better controlling confounding factors, while cross-sectional studies may yield inconsistent results due to the incomplete elimination of confounding factors. Second, MR studies focus more on the causal relationship's time sequence, while cross-sectional studies mainly reflect the correlation at the observed time points, potentially leading to differences in study outcomes. Importantly, the existing literature concerning this area of research is limited. Therefore, a cautious evaluation of these study results remains necessary, and future investigations should be conducted to attain a more comprehensive understanding of the interrelationships between MI, CAD, and FS.
However, our study results currently do not support a causal relationship between AF, HF, and FS, and no related research reports have been found to date. We speculate that AF and HF are CVD involving disruptions in heart structure and function, while FS is an inflammatory disorder around the joints. Since they impact distinct physiological systems and mechanisms, establishing a direct causal relationship is difficult.
We found that stroke increases the risk of developing FS, similar to Lv et al.'s study. However, we focus on the bidirectional causal relationship between six CVDs and FS, going beyond the singular relationship between stroke and FS. The results confirm that stroke increases the risk of FS, but there is no evidence that FS affects stroke. This deepens the understanding of the impact of FS on different CVDs. Although no causal relationship was found between FS and the other four CVDs, observational studies on the relationship between CAD, MI, and FS provided stronger evidence. Overall, by broadening the scope of our study, we ultimately confirmed the causal relationship between stroke and FS, providing a comprehensive and in-depth perspective on understanding the relationship between CVDs and FS.
The roles of various CVDs in the occurrence and progression of FS are complex and potentially interactive. Although our MR analysis was able to account for their interactive effects and assess their causality from a genetic perspective, our study has inherent limitations. Firstly, the results of this study are products of statistical analysis, and some correlations between CVDs and FS have not been reported, lacking theoretical support. Further, additional fundamental and clinical research is necessary to substantiate our observations. Secondly, our data were obtained from public databases that do not permit subgroup analyses for specific factors (such as age, gender, etc.). Future investigations into the correlation between CVDs and FS should account for the overall impact of these factors. Lastly, we exclusively utilized genetic data from individuals of European ancestry, limiting the generalizability of our study findings.
In summary, stroke is a high risk factor for FS. Orthopedic physicians should prioritize shoulder care for stroke patients, promptly assessing potential restrictions in shoulder range of motion to mitigate FS risk. However, further research is needed to validate and extend these findings.
Availability for data and materials
All the Mendelian randomization study files are available from GWAS. (URL https://gwas.mrcieu.ac.uk).
Abbreviations
- CVD:
-
Cardiovascular disease
- FS:
-
Frozen shoulder
- MR:
-
Mendelian randomized
- RCTs:
-
Randomized controlled trials
- AF:
-
Atrial fibrillation
- CAD:
-
Coronary artery disease
- HF:
-
Heart failure
- MI:
-
Myocardial infarction
- IS:
-
Ischemic stroke
- GWAS:
-
Genome-wide association studies
- IVW:
-
Inverse variance weighting
- WM:
-
Weighted median
- IV:
-
Instrumental variable
- FDR:
-
False discovery rate
- MAF:
-
Minimum allele frequency
- SNP:
-
Single-nucleotide polymorphism
- OR:
-
Odds ratio
- CI:
-
Confidence interval
- β :
-
Regression coefficient
- SE:
-
Standard error
References
Dias R, Cutts S, Massoud S. Frozen shoulder. BMJ. 2005;331(7530):1453–6.
Kim DH, Kim YS, Kim BS, Sung DH, Song KS, Cho CH. Is frozen shoulder completely resolved at 2 years after the onset of disease? J Orthop Sci. 2020;25(2):224–8.
Hand C, Clipsham K, Rees JL, Carr AJ. Long-term outcome of frozen shoulder. J Shoulder Elbow Surg. 2008;17(2):231–6.
Date A, Rahman L. Frozen shoulder: overview of clinical presentation and review of the current evidence base for management strategies. Future Sci OA. 2020;6(10):FSO647.
Arnett DK, Blumenthal RS, Albert MA, Buroker AB, Goldberger ZD, Hahn EJ, Himmelfarb CD, Khera A, Lloyd-Jones D, McEvoy JW, et al. 2019 ACC/AHA guideline on the primary prevention of cardiovascular disease: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice guidelines. Circulation. 2019;140(11):e596–646.
Collaborators GBDCoD: Global, regional, and national age-sex-specific mortality for 282 causes of death in 195 countries and territories, 1980–2017: a systematic analysis for the Global Burden of Disease Study 2017. Lancet 2018, 392(10159):1736–1788.
Tie K, Wang H, Yang X, Ni Q, Chen L. Analysis of risk factors for advanced age in patients with frozen shoulder. Aging Clin Exp Res. 2023;35(3):615–20.
Kang JH, Sheu JJ, Lin HC. Increased risk of stroke after adhesive capsulitis: a population-based study. Stroke. 2010;41(5):1044–7.
Kang JH, Keller JJ, Lin HC. A population-based follow-up study on the risk of acute myocardial infarction following adhesive capsulitis. Int J Cardiol. 2012;157(2):289–91.
Davey Smith G, Hemani G. Mendelian randomization: genetic anchors for causal inference in epidemiological studies. Hum Mol Genet. 2014;23(R1):R89-98.
Sekula P, Del Greco MF, Pattaro C, Kottgen A. Mendelian randomization as an approach to assess causality using observational data. J Am Soc Nephrol. 2016;27(11):3253–65.
Burgess S, Small DS, Thompson SG. A review of instrumental variable estimators for Mendelian randomization. Stat Methods Med Res. 2017;26(5):2333–55.
Lawlor DA, Harbord RM, Sterne JA, Timpson N, Davey Smith G. Mendelian randomization: using genes as instruments for making causal inferences in epidemiology. Stat Med. 2008;27(8):1133–63.
Lv X, Hu Z, Liang F, Liu S, Gong H, Du J, Deng X, Qian JH, Nie Q, Luo J. Causal relationship between ischemic stroke and its subtypes and frozen shoulder: a two-sample Mendelian randomization analysis. Front Neurol. 2023;14:1178051.
Nielsen JB, Thorolfsdottir RB, Fritsche LG, Zhou W, Skov MW, Graham SE, Herron TJ, McCarthy S, Schmidt EM, Sveinbjornsson G, et al. Biobank-driven genomic discovery yields new insight into atrial fibrillation biology. Nat Genet. 2018;50(9):1234–9.
van der Harst P, Verweij N. Identification of 64 novel genetic loci provides an expanded view on the genetic architecture of coronary artery disease. Circ Res. 2018;122(3):433–43.
Shah S, Henry A, Roselli C, Lin H, Sveinbjornsson G, Fatemifar G, Hedman AK, Wilk JB, Morley MP, Chaffin MD, et al. Genome-wide association and Mendelian randomisation analysis provide insights into the pathogenesis of heart failure. Nat Commun. 2020;11(1):163.
Hartiala JA, Han Y, Jia Q, Hilser JR, Huang P, Gukasyan J, Schwartzman WS, Cai Z, Biswas S, Tregouet DA, et al. Genome-wide analysis identifies novel susceptibility loci for myocardial infarction. Eur Heart J. 2021;42(9):919–33.
Malik R, Chauhan G, Traylor M, Sargurupremraj M, Okada Y, Mishra A, Rutten-Jacobs L, Giese AK, van der Laan SW, Gretarsdottir S, et al. Multiancestry genome-wide association study of 520,000 subjects identifies 32 loci associated with stroke and stroke subtypes. Nat Genet. 2018;50(4):524–37.
Burgess S, Thompson SG, Collaboration CCG. Avoiding bias from weak instruments in Mendelian randomization studies. Int J Epidemiol. 2011;40(3):755–64.
Zhang Y, Xiong Y, Shen S, Yang J, Wang W, Wu T, Chen L, Yu Q, Zuo H, Wang X, et al. Causal association between tea consumption and kidney function: a Mendelian randomization study. Front Nutr. 2022;9: 801591.
Shim H, Chasman DI, Smith JD, Mora S, Ridker PM, Nickerson DA, Krauss RM, Stephens M. A multivariate genome-wide association analysis of 10 LDL subfractions, and their response to statin treatment, in 1868 Caucasians. PLoS ONE. 2015;10(4): e0120758.
Borenstein M, Hedges LV, Higgins JP, Rothstein HR. A basic introduction to fixed-effect and random-effects models for meta-analysis. Res Synth Methods. 2010;1(2):97–111.
Bowden J, Del Greco MF, Minelli C, Zhao Q, Lawlor DA, Sheehan NA, Thompson J, Davey Smith G. Improving the accuracy of two-sample summary-data Mendelian randomization: moving beyond the NOME assumption. Int J Epidemiol. 2019;48(3):728–42.
Verbanck M, Chen CY, Neale B, Do R. Detection of widespread horizontal pleiotropy in causal relationships inferred from Mendelian randomization between complex traits and diseases. Nat Genet. 2018;50(5):693–8.
Hartwig FP, Davey Smith G, Bowden J. Robust inference in summary data Mendelian randomization via the zero modal pleiotropy assumption. Int J Epidemiol. 2017;46(6):1985–98.
Dong Q, Chen D, Zhang Y, Xu Y, Yan L, Jiang J. Constipation and cardiovascular disease: a two-sample Mendelian randomization analysis. Front Cardiovasc Med. 2023;10:1080982.
Wang Q, Hu S, Qi L, Wang X, Jin G, Wu D, Wang Y, Ren L. Causal associations between sleep traits and brain structure: a bidirectional Mendelian randomization study. Behav Brain Funct. 2023;19(1):17.
Skrivankova VW, Richmond RC, Woolf BAR, Yarmolinsky J, Davies NM, Swanson SA, VanderWeele TJ, Higgins JPT, Timpson NJ, Dimou N, et al. Strengthening the reporting of observational studies in epidemiology using mendelian randomization: the STROBE-MR statement. JAMA. 2021;326(16):1614–21.
Pinedo S, de la Villa FM. Complications in the hemiplegic patient in the first year after the stroke. Rev Neurol. 2001;32(3):206–9.
Lo SF, Chen SY, Lin HC, Jim YF, Meng NH, Kao MJ. Arthrographic and clinical findings in patients with hemiplegic shoulder pain. Arch Phys Med Rehabil. 2003;84(12):1786–91.
Suethanapornkul S, Kuptniratsaikul PS, Kuptniratsaikul V, Uthensut P, Dajpratha P, Wongwisethkarn J. Post stroke shoulder subluxation and shoulder pain: a cohort multicenter study. J Med Assoc Thai. 2008;91(12):1885–92.
Xu S, Lu J, Shao A, Zhang JH, Zhang J. Glial cells: role of the immune response in ischemic stroke. Front Immunol. 2020;11:294.
Zeng J, Bao T, Yang K, Zhu X, Wang S, Xiang W, Ge A, Zeng L, Ge J. The mechanism of microglia-mediated immune inflammation in ischemic stroke and the role of natural botanical components in regulating microglia: a review. Front Immunol. 2022;13:1047550.
Lho YM, Ha E, Cho CH, Song KS, Min BW, Bae KC, Lee KJ, Hwang I, Park HB. Inflammatory cytokines are overexpressed in the subacromial bursa of frozen shoulder. J Shoulder Elbow Surg. 2013;22(5):666–72.
Hand GC, Athanasou NA, Matthews T, Carr AJ. The pathology of frozen shoulder. J Bone Joint Surg Br. 2007;89(7):928–32.
de Oliveira RA, de Andrade DC, Machado AG, Teixeira MJ. Central poststroke pain: somatosensory abnormalities and the presence of associated myofascial pain syndrome. BMC Neurol. 2012;12:89.
Wilson RD, Chae J. Hemiplegic shoulder pain. Phys Med Rehabil Clin N Am. 2015;26(4):641–55.
Winter Y, Uphaus T, Sandner K, Klimpe S, Stuckrad-Barre SV, Groppa S. Efficacy and safety of antiseizure medication in post-stroke epilepsy. Seizure. 2022;100:109–14.
Kilkenny MF, Olaiya MT, Dalli LL, Kim J, Andrew NE, Sanfilippo FM, Thrift AG, Nelson M, Pearce C, Sanders L, et al. Treatment with multiple therapeutic classes of medication is associated with survival after stroke. Neuroepidemiology. 2022;56(1):66–74.
Wu CH, Wang YH, Huang YP, Pan SL. Does adhesive capsulitis of the shoulder increase the risk of stroke? A population-based propensity score-matched follow-up study. PLoS ONE. 2012;7(11): e49343.
Elmissbah TE, Iderous ME, Al-Qahtani FM, Elaskary A, Dahlawi H. Assessment of antithrombin III and protein C in Saudi myocardial infarction patients. Clin Lab. 2021;67(10). https://doi.org/10.7754/Clin.Lab.2021.201206.
Bulgen DY, Binder A, Hazleman BL, Park JR. Immunological studies in frozen shoulder. J Rheumatol. 1982;9(6):893–8.
Verma S. C-reactive protein incites atherosclerosis. Can J Cardiol. 2004;20(Suppl B):291–311.
Acknowledgements
YQW will act as the guarantors for the paper.
Funding
This work was supported by the Special Research Project of Traditional Chinese Medicine in Sichuan Province (Academic Thought Compilation and Experience Study of Orthopedics by Renowned Traditional Chinese Medicine Practitioner Wang Zhaopeng, Grant No. 2021MS570).
Author information
Authors and Affiliations
Contributions
WSL and BP designed the study, wrote, reviewed, and edited the manuscript. SW, MZL, and YA analyzed data. JL and WYQ reviewed and edited the manuscript. All authors approved the final version of the manuscript to be published. WYQ is the guarantor of this work.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
No ethical review or approval is required for this study, and all data from Mendelian randomization are publicly accessible. In the initial investigation, all subjects obtained informed consent. The topics provided a written informed consent form to participate in this study.
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Additional file 1. Table S1.
Characteristics of SNPs associated with cardiovascular disease. Table S2. Characteristics of SNPs associated with frozen shoulder. Table S3. Heterogeneity and pleiotropy analysis in reverse MR analysis. Fig S1. The forest plots for causal effect of cardiovascular disease on frozen shoulder. Fig S2. Leave-one-out sensitivity analysis for causal effect of cardiovascular disease on frozen shoulder. Fig S3. The funnel chart for causal effect of cardiovascular disease on frozen shoulder. Fig. S4. The scatter plots for causal effect of frozen shoulder on cardiovascular disease. Fig. S5. The forest plots for causal effect of frozen shoulder on cardiovascular disease. Fig. S6. Leave-one-out sensitivity analysis for causal effect of frozen shoulder on cardiovascular disease. Fig. S7. The funnel chart for causal effect of frozen shoulder on cardiovascular disease.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Lu, W., Pu, B., Wang, S. et al. Bidirectional two-sample Mendelian randomization analysis identifies causal associations between cardiovascular diseases and frozen shoulder. J Orthop Surg Res 19, 116 (2024). https://doi.org/10.1186/s13018-024-04600-7
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s13018-024-04600-7