Abstract
Background
Bone fracture is a common orthopedic disease that needs over 3 months to recover. Promoting the osteogenic differentiation of bone mesenchymal stem cells (BMSCs) is beneficial for fracture healing. Therefore, this research aimed to study the roles of long non-coding RNA (lncRNA) KCNQ10T1 in osteogenic differentiation of BMSCs.
Methods
BMSCs were treated with osteogenic medium and assessed by CCK-8 and flow cytometry assays. Alkaline phosphatase (ALP) staining, alizarin red staining (ARS), as well as concentration of osteoblast markers were measured to evaluate osteogenic differentiation of BMSCs. Western blot was employed to detect proteins; while, qRT-PCR was for mRNA levels. Additionally, targeted relationships between KCNQ10T1 and miR-19a-3p, as well as miR-19a-3p and SMAD5 were verified by dual luciferase reporter gene assay along with RNA pull-down method.
Results
Upregulation of KCNQ10T1 promoted the ALP staining and ARS intensity, increased the cell viability and decreased the apoptosis rate of BMSCs. Besides, KCNQ10T1 overexpression increased the ALP, OPG, OCN and OPN protein levels. KCNQ10T1 sponges miR-19a-3p, which targets Smad5. Upregulated miR-19a-3p reversed the overexpressed KCNQ10T1-induced effects, and depletion of SMAD5 reversed the miR-19a-3p inhibitor-induced effects on osteogenic medium-treated BMSCs.
Conclusions
Upregulation of KCNQ10T1 promoted osteogenic differentiation of BMSCs through miR-19a-3p/SMAD5 axis in bone fracture.
Similar content being viewed by others
Introduction
Bone fracture is a common orthopedic disease that needs over 3 months to recover. Delayed fracture union and nonunion are common and worrisome complications in fracture treatment, placing a significant burden on individuals and society [1, 2]. Despite the increasingly comprehensive exploration of fracture healing mechanisms, these influencing factors remain a major clinical challenge for fracture treatment [3]. Mesenchymal stem cells (MSCs), which are pluripotent stromal cells, have attracted much attention as powerful tools for tissue regeneration [4]. MSCs have the ability to self-renew and differentiate into multiple lineages, such as osteoblasts (bone), chondrocytes (cartilage), muscle cells (muscle), and fat cells (adipocytes) [5]. In addition, bone MSCs (BMSCs) are widely available from bone marrow, adipose tissue, cord blood, and any other tissue [6]. In particular, BMSCs tend to promote osteoblasts and stimulate bone formation. There is much convincing evidence that MSCs can repair bone and related defects in animal models [7, 8]. Systemic and local administration of allogeneic BMSCs promotes fracture healing in rats [9]. However, the ability of BMSCs to differentiate into functional osteoblasts remains limited in terms of bone regeneration in vivo. Therefore, stimulating osteogenic differentiation of BMSCs may be considered as a potential therapeutic approach to promote bone regeneration.
Long non-coding RNAs (lncRNAs) are endogenous cellular ribonucleic acid RNAs with a length of 200 nt ~ 100 kb [10]. Recently, several lncRNAs have been found to play an important role in the pathophysiological processes of various orthopedic diseases, such as LncRNA ROR [11], LncRNA THUMPD3-AS1 [12], lncRNA-CRNDE [13], etc. LncRNA-KCNQ1OT1 (KCNQ1OT1) is located on human chromosome 11p and is a chromatin regulatory RNA [14]. KCNQ1OT1 is a well-studied lncRNA, which has a profound impact on the regulation of colon cancer [15], non-small cell lung cancer [16], ischemic stroke [17] and osteogenic differentiation [18]. In the field of orthopedics, KCNQ1OT1 has been shown to accelerate osteoblast differentiation through up-regulating the Wnt/β-catenin signaling pathway [18]. In addition, KCNQ1OT1 silencing inhibited osteogenic differentiation and downregulated expression of osteogenic differentiation related proteins [19, 20]. However, the relationship between KCNQ1OT1 and growth or osteogenic differentiation of BMSCs is still not well defined.
MicroRNAs (miRNAs) are short non-coding RNAs, which are common in the expression of post transcriptional regulatory genes and mainly bind to the 3 '- untranslated region of targeted messenger RNA to regulate cell biological processes, including BMSCs differentiation [21,22,23,24,25]. miR-19a-3p is confirmed to be broadly conserved among vertebrates [26] and participate in the pathogenesis of preeclampsia and atherosclerosis [27, 28]. Recent studies have demonstrated the involvement of miR-19a-3p in the progression of various cancers including glioma, lung cancer, breast cancer, osteosarcoma, gastric cancer and hepatocellular carcinoma [29,30,31,32]. However, the precise mechanism by which miR-19a-3p in bone fracture treatment remains unknown. Here, through the Starbase and TargetScan on line database, we found that KCNQ1OT1 targeted miR-19-3p and Smad5 was a target gene of miR-19-3p. Smad5 is a receptor regulated SMAD protein that is a key transcription factor for osteogenic differentiation. Under physiological conditions, Smad5 is mainly located in the cytoplasm. When Smad5 is phosphorylated, it is directed to the nucleus, thereby regulating the expression of osteogenic genes and inducing osteogenic differentiation [33]. Inhibiting nuclear translocation of Smad5 can inhibit osteogenic differentiation of BMSCs [34].
Therefore, our study tended to investigate the molecular mechanisms of KCNQ1OT1 in bone fracture in vitro. We hypothesized that KCNQ1OT1 sponges miR-19-3p to regulate Smad5 expression to regulate osteogenic differentiation of BMSCs. Our research provided a novel understanding of bone fracture treatment.
Materials and methods
Cell culture
The mouse Bone Mesenchymal Stem Cells (BMSCs) were purchased from Beijing Baiou Bowei Biotechnology Co., Ltd (Beijing, China). The cells were cultured in complete α-MEM, and 10% fetal bovine serum and 1% penicillin streptomycin were added to the culture medium. The cultivation environment is set to 95% air and 5% CO2, and the temperature is set to 37 °C.
Cell transfection
Overexpressed KCNQ10T1 vector (pcDNA3.1-KCNQ1OT1), overexpressed miR-19a-3p (miR-19a-3p mimic), suppressed miR-19a-3p (miR-19a-3p inhibitor), downregulated Smad5 (si-Smad5), as well as their negative control plasmids (pcDNA3.1, NC mimic, NC inhibitor, si-NC) in this study were all bought from GenePharma (Shanghai, China). BMSCs were cultured at a concentration of 2 × 105 cells per well in 6-well plates. After the cells had grown to about 60% confluence, corresponding vectors were transfected into BMSCs using Lipofectamine 3000 reagent (Life Technologies,CA, USA), with all procedures following the manufacturer's protocol.
Osteogenic differentiation
BMSCs were inoculated into a 12-well plate at 5 × 105 cells/well, and cultured in medium containing 5 mmol/L β-glycerophosphate sodium, 50μg/mL vitamin C, 100 mmol/L dexamethasone and 10% FBS. The supernatant was discarded after 7 days, then BMSCs were fixed with 4% paraformaldehyde for 15 min, and stained with alkaline phosphatase (ALP) and Alizarin Red according to the instructions of kits (Beyotime, Shanghai, China) for observation and semi-quantitative analysis of mineralized nodules.
Cell counting kit (CCK)-8 assay
BMSCs were planted into 96-well plates, followed by incubating for 0, 24, 48, and 72 h. 10 μl CCK-8 (KeyGEN, Jiangsu, China) was added into each well and incubated for 4 h. The absorption was measured at 450 nm using a microplate reader (BioTek, VT, USA).
Flow cytometry
BMSCs were digested by trypsin and washed by PBS, and the cell suspension concentration was adjusted to 1 × 104 cells/mL. The cells were incubated and stained by Annexin V-FITC and propidium iodide in dark for 15 min. The apoptosis rate of each group was detected by flow cytometry (BD FACSCalibur, NJ, USA).
RT-qPCR
BMSCs of each group were inoculated into 6-well plates. RNA was isolated with TRIzol (PrimeScript™ RT Kit, Takara, Japan) and the mRNA was reversed into complementary DNA (cDNA) for RT-qPCR. The RT-qPCR was carried out using TaKaRa Ex Taq® kit (Takara, Japan). GAPDH was used as a housekeeping gene for KCNQ10T1 as well as Smad5 while U6 was applied as an internal reference of miR-19a-3p, and the relative expression levels of target genes were calculated using 2−ΔΔCt (ΔCt = Ct target gene − Ct reference gene, Ct value represents the number of cycles when the fluorescence signal in each reaction tube reaches the set threshold).
Western blot
Osteogenic differentiation-related proteins including ALP, osteocalcin (OCN), osteoprotegerin (OPG) and osteopontin (OPN) were measured in BMSCs. Total protein extracted from BMSCs was determined with the BCA kit (Sigma-Aldrich, MO, USA), and then subjected to 10% SDS-PAGE gel electrophoresis, and transferred onto the PVDF membrane (Millipore, MA, USA). The membranes were blocked with 5% milk for 2 h and then incubated with primary antibodies including anti-ALP(1/1000, Abcam, MA, USA), anti-OCN (1/1500, Abcam), anti-OPG (1/800, Abcam), anti-OPN (1/1000, Abcam) and GADPH (1/3000, Abcam) for 12 h at 4 °C. Subsequently, immune complexes were incubated with horseradish peroxidase-labeled Immunoglobulin G (IgG; 1/2000, Abcam) for 1 h and visualized using a chemiluminescence kit (Beyotime, Shanghai, China). Finally, the brands were photographed by IS gel image analysis system and analyzed using the Image J.
Verification of binding relationship between mRNA and miRNA
Dual luciferase reporter assay as well as RNA pull-down methods were carried out to confirm the prediction of bioinformatics concerning the interactions between miR-19a-3p and KCNQ1OT1 of Smad5. The wild-type (WT) KCNQ10OT1, mutant-type (MUT) KCNQ1OT1, WT Smad5 3′-UTR, MUT Smad5 3′-UTR were synthesized and cloned into pmirGLO luciferase vectors (Promega, Beijing, China) to determine whether miR-19a-3p directly targets KCNQ1OT1 and the Smad5 3′-UTR. The miR-19a-3p mimic negative control plasmids (nc mimic), miR-19a-3p mimic (mimic) along with Renilla luciferase plasmid (Promega, Beijing, China) were co-transfected into BMSCs. Firefly and Renilla luciferase activities were determined by a Dual-Luciferase Reporter Assay kit (BioVision Tech, Guangdong, China). As for RNA pull-down method, 500 μg streptavidin magnetic beads were combined with 200 pmol biotin-labeled miR-19a-3p mimic, and added into RNA extracted from BMSCs. Eluting buffer was added to collect the pulled RNA complex after 30 min incubation at room temperature, and the KCNQ1OT1 and Smad5 levels were quantitatively analyzed by RT-qPCR.
Statistical analysis
For bioinformatic analysis, the target miRNA of KCNQ1OT1 was predicated by Starbase online database (http://starbase.sysu.edu.cn/), and the target gene of miR-19a-3p was predicated by TargetScan online database (http://www.targetscan.org/). The results were statistically analyzed using GraphPad Prism 9.0 (MacKiev Software). Normal distribution (Shapiro–Wilk) and homogeneity of variance test were carried out for multiple groups of data, and those who met the criteria were represented in the form of "\(\overline{x} \pm {\text{sd}}\)". One-way ANOVA and paired T test of two independent samples were used for comparison between groups and populations. Each experiment was conducted independently three times (n = 3). p < 0.05 on both sides was considered statistically significant.
Results
KCNQ1OT1 overexpression promoted the osteogenic differentiation of BMSCs
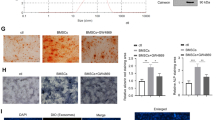
Firstly, we explored the role of KCNQ1OT1 in the osteogenic differentiation of BMSCs. After pcDNA3.1-KCNQ1OT1 (over-KCNQ1OT1) transfection, KCNQ1OT1 was obviously upregulated (Fig. 1A). KCNQ1OT1 overexpression significantly increased the cell viability of osteogenic medium-treated BMSCs (Fig. 1B). In addition, positive mineralized nodules stained by ALP (Fig. 1C) as well as Alizarin Red (Fig. 1D) in osteogenic medium-treated BMSCs transfected with over-KCNQ10T1 were markedly more than that in negative control group. Besides, KCNQ1OT1 overexpression significantly decreased the apoptosis rate of the osteogenic medium-treated BMSCs (Fig. 1E). Moreover, overexpression of KCNQ1OT1 induced the significant upregulation of mRNA (Fig. 1F) and protein (Fig. 1G) expressions of ALP, OPG, OCN and OPN in osteogenic medium-treated BMSCs.
KCNQ1OT1 overexpression promoted the osteogenic differentiation of BMSCs. A Overexpression efficiency of pcDNA3.1-KCNQ1OT1 was detected by RT-qPCR assay. Then the BMSCs were cultured in osteogenic medium and transfected with pcDNA3.1-KCNQ1OT, B the cell viability was detected by CCK-8 assay. The positive mineralized nodules were stained by ALP (C) as well as Alizarin Red (D). E The apoptosis rate was measured by flow cytometry. The mRNA (F) and protein (G) expressions of ALP, OPG, OCN and OPN were detected by RT-qPCR and western blot assays. **P < 0.01
KCNQ1OT1 targets miR-19a-3p
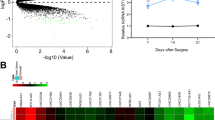
The results of bioinformatic analysis showed that KCNQ1OT1 could binds to miR-19a-3p (Fig. 2A). MiR-19a-3p mimic rather than nc mimic dramatically decreased luciferase activity after co-transfected with WT-KCNQ1OT1; whereas, miR-19a-3p co-transfected with MUT-KCNQ1OT1 did not affect luciferase activity (Fig. 2B). Results of RNA pull-down method revealed that KCNQ1OT1 was obviously captured by biotin-miR-19a-3p, compared with biotin-nc (Fig. 2C). In addition, after KCNQ1OT1 overexpression, the miR-19a-3p levels were significantly decreased in the BMSCs (Fig. 2D).
KCNQ1OT1 targets miR-19a-3p. A The binding site between KCNQ1OT1 and miR-19a-3p was predicted by Starbase online database. The binding interaction between KCNQ1OT1 and miR-19-3p was tested by decreased luciferase reporter (B) and RNA pull-down (C) assays. D The miR-19a-3p levels were detected by RT-qPCR after KCNQ1OT1 overexpression. **P < 0.01
Elevated KCNQ10T1 accelerates osteogenic differentiation of BMSCs via sponging miR-19a-3p
After successfully transfection, miR-19a-3p in BMSCs was notably increased in the mimic group (Fig. 3A). Increased cell viability (Fig. 3B), positive mineralized nodules intensity stained by ALP (Fig. 3C) and Alizarin Red (Fig. 3D), and decreased apoptosis were induced by overexpressed KCNQ1OT1 (Fig. 3E), which were obviously reversed by overexpressed miR-19a-3p (Fig. 3B–E). Likewise, the increase in mRNA (Fig. 3F) and protein (Fig. 3G) levels of ALP, OPG, OCN and OPN induced by overexpressed KCNQ1OT1 was significantly decreased after miR-19a-3p overexpression.
Elevated KCNQ10T1 accelerates osteogenic differentiation of BMSCs via sponging miR-19a-3p. A Overexpression efficiency of miR-19a-3p mimic was detected by RT-qPCR assay. Then the BMSCs were cultured in osteogenic medium and transfected with pcDNA3.1-KCNQ1OT and miR-19a-3p mimic, B the cell viability was detected by CCK-8 assay. The positive mineralized nodules were stained by ALP (C) as well as Alizarin Red (D). E The apoptosis rate was measured by flow cytometry. The mRNA (F) and protein (G) expressions of ALP, OPG, OCN and OPN were detected by RT-qPCR and western blot assays. **P < 0.01
miR-19a-3p directly targets Smad5
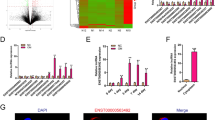
The results of bioinformatics indicated that Smad5 has the binding sites in miR-19a-3p (Fig. 4A). MiR-19a-3p mimic rather than nc mimic dramatically decreased luciferase activity after co-transfected with WT-Smad5; whereas, miR-19a-3p co-transfected with MUT-Smad5 did not affect luciferase activity (Fig. 4B). Results of RNA pull-down method revealed that Smad5 was obviously captured by biotin-miR-19a-3p, compared with biotin-nc (Fig. 4C). The expression of Smad5 in the osteogenic medium-treated BMSCs was dramatically increased after KCNQ1OT1 overexpression, which was decreased after miR-19a-3p overexpression (Fig. 4D).
miR-19a-3p directly targets Smad5. A The binding site between Smad5 and miR-19a-3p was predicted by TargetScan online database. The binding interaction between Smad5 and miR-19-3p was tested by decreased luciferase reporter (B) and RNA pull-down (C) assays. D The Smad5 levels were detected by RT-qPCR after KCNQ1OT1 and miR-19a-3p overexpression. **P < 0.01
Silenced Smad5 inhibited the role of downregulated miR-19-3p in BMSCs
The knockout efficiency of miR-19a-3p inhibitor and si-Smad5 was test by PCR assay. MiR-19a-3p inhibitor significantly decreased the miR-19a-3p levels (Fig. 5A) and si-Smad5 significantly decreased the Smad5 levels (Fig. 5B). Increased cell viability (Fig. 5C), positive mineralized nodules intensity stained by ALP (Fig. 5D) and Alizarin Red (Fig. 5E), and decreased apoptosis were induced by miR-19a-3p inhibitor (Fig. 5F), which were obviously reversed by Smad5 knockdown (Fig. 5C–F). In addition, the increase in mRNA (Fig. 5G) and protein (Fig. 5H) levels of Smad5, ALP, OPG, OCN and OPN induced by miR-19a-3p inhibitor was significantly decreased after Smad5 knockdown.
Silenced Smad5 inhibited the role of downregulated miR-19-3p in BMSCs. Knockout efficiency of miR-19a-3p inhibitor (A) and si-Smad5 (B) was detected by RT-qPCR assay. Then the BMSCs were cultured in osteogenic medium and transfected with miR-19a-3p inhibitor and si-Smad5, C the cell viability was detected by CCK-8 assay. The positive mineralized nodules were stained by ALP (D) as well as Alizarin Red (E). F The apoptosis rate was measured by flow cytometry. The mRNA (G) and protein (H) expressions of Smad5, ALP, OPG, OCN and OPN were detected by RT-qPCR and western blot assays. **P < 0.01
Discussion
In current study, we clarified the role of KCNQ1OT1 in bone fracture and the molecular mechanism in vitro. Heightening of KCNQ1OT1 helped to protect BMSCs from dysfunction by promoting osteogenic differentiation. Furthermore, KCNQ1OT1 played its role via the miR-19a-3p/Smad5 axis.
Accumulating evidences have showed that lncRNAs are regarded as the regulator of bone fracture occurrence and development [35]. The abnormally expressed lncRNAs played a different role in osteogenic differentiation of BMSCs. For example, Zhang et al. [36] demonstrated that lncRNA-NEAT1 upregulated the expression of osteogenic differentiation proteins to improve mitochondrial function. It indicated that lncRNA-NEAT1 might be a potential therapeutic target for skeletal aging. Yin et al. [37] suggested that lncRNA-Malat1 knockdown suppressed the osteogenic differentiation of BMSCs, which was reversed by decreasing the expression of miR-129-5p. In the pathogenesis of osteoporosis, high levels of lncRNA SNHG1 increased the expression of DNMT1 via interacting with PTBP1. LncRNA SNHG1 contributed to osteoporosis through leading to osteoprotegerin hypermethylation and downregulated osteoprotegerin expression [38]. Thus, it can be seen, focusing on the role of differentially expressed lncRNAs in osteogenic differentiation of BMSCs may be the key to treating orthopedic diseases such as fractures and osteoporosis. KCNQ1OT1, a widely studied lncRNA, has been shown to exhibit different expression levels in different diseases. For instance, in the osteosarcoma [39], ovarian cancer [40], lung squamous cell carcinoma [41], etc. High levels of KCNQ1OT1 promoted the malignant behaviors, such as excessive proliferation. In other diseases, such as atherosclerosis, high levels of KCNQ1OT1 prevented cholesterol efflux and induced lipid accumulation in THP-1 macrophages. In contrast, KCNQ1OT1 silencing protected against atherosclerosis in apoE-/-mice and inhibited the lipid accumulation in THP-1 macrophages [42]. However, in the process of cellular senescence, high levels of KCNQ1OT1 inhibited senescence-associated heterochromatin foci, transposon activation and retrotransposition as well as cellular senescence, suggesting KCNQ1OT1 inhibited the cellular senescence. Here, we found that KCNQ1OT1 overexpression promoted the growth and osteogenic differentiation of BMSCs. Our results were similar to a previous study, which also demonstrated KCNQ1OT1 promoted osteogenic differentiation of BMSCs through inhibiting miR-205-5p [43].
More and more evidence suggests that lncRNAs act as competitive endogenous RNAs (ceRNAs) to sponge miRNAs [44]. As reported by previous studies, KCNQ1OT1 has been demonstrated to sponge miR-34c-5p in osteosarcoma [39], miR-125b-5p in ovarian cancer [40], miR-26a-5p in ischemia reperfusion [45]. Here, through the Starbase online database, we found that KCNQ1OT1 targeted to miR-19a-3p. miR-19a-3p has been demonstrated to participated in various diseases such as myocardial ischemia/reperfusion injury [46], sepsis-induced lung injury [47], in multiple myeloma [48], etc. Most studies have found that miR-19a-3p acts as a sponge for lncRNAs, thereby participating in the progression of diseases. For example, Xiang et al. [49] found that miR-19a-3p promoted the migration and epithelial–mesenchymal transition of breast cancer cells through sponge adsorbing by LINC00094. In osteoporosis, Chen et al. [50] demonstrated that lncRNA Xist was a sponge of miR-19a-3p to inhibit BMSCs osteogenic differentiation. Similarly, this study found that miR-19a-3p overexpression inhibited the BMSCs osteogenic differentiation and reversed the role of KCNQ1OT1 in the BMSCs. However, there are contradictions with previous research, Chen et al. [50] exhibited the promoting effect of miR-19a-3p on osteogenic differentiation of BMSCs, while we confirmed the inhibitory effect of miR-19a-3p on osteogenic differentiation of BMSCs. We speculated that this may be due to they performed the study using BMSCs in aging cell models, while we are explored the osteogenic differentiation of normal BMSCs. In addition, different lncRNAs and target genes may lead to different expressions and functions of miR-19a-3p. Therefore, further research is still needed to explore the specific mechanism of miR-19a-3p in BMSCs.
Finally, we confirmed that Smad5 was a target gene of miR-19a-3p. Smad5 is a receptor regulated Smad protein that is a key transcription factor for osteogenic differentiation [34]. Under physiological conditions, Smad5 is mainly located in the cytoplasm. When Smad5 is phosphorylated, it is directed to the nucleus, thereby regulating the expression of osteogenic genes and inducing osteogenic differentiation [51]. According to reports, inhibiting nuclear translocation of p-Smad5 can inhibit osteogenic differentiation of BMSCs [52]. Here, we found that Smad5 knockdown reversed the effects of miR-19a-3p on the growth and osteogenic differentiation of BMSCs. The findings suggested that miR-19a-3p targets Smad5 to promote bone fracture development. Taken together, Upregulation of KCNQ10T1 attenuated bone fracture progression by the miR-19-3p/Smad5 axis.
In conclusion, our research suggested KCNQ10T1 overexpression promoted the osteogenic differentiation of BMSCs. MiR-19a-3p overexpression reversed the role of KCNQ10T1 accelerates osteogenic differentiation of BMSCs via sponging miR-19a-3p. In addition, silenced Smad5 inhibited the role of downregulated miR-19-3p in BMSCs. KCNQ10T1 acted as a ceRNA to regulate osteogenic differentiation of BMSCs via miR-19-3p/Smad5 axis. Overexpression of KCNQ10T1 may be an alternative for the treatment of bone fracture. However, there was still a limitation in this study. Due to limitations in hospital research conditions, we did not conduct animal experiments to verify the role of KCNQ10T1 in bone growth and development in vivo. In the future, we will aim to establish a fracture mouse model and inject KCNQ10T1 overexpression lentivirus to further investigate the role of KCNQ10T1 in vivo.
Availability of data and materials
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
References
Camal RI, Cicero AM, Issa J, Feldman S. Bone fracture healing: perspectives according to molecular basis. J Bone Miner Metab. 2021;39(3):311–31.
Liu Y, Jia Z, Akhter MP, Gao X, Wang X, Wang X, et al. Bone-targeting liposome formulation of Salvianic acid A accelerates the healing of delayed fracture Union in Mice. Nanomed Nanotechnol Biol Med. 2018;14(7):2271–82.
Nicholson JA, Makaram N, Simpson A, Keating JF. Fracture nonunion in long bones: a literature review of risk factors and surgical management. Injury. 2021;52:S3-11.
Jo H, Brito S, Kwak BM, Park S, Lee M, Bin B. Applications of mesenchymal stem cells in skin regeneration and rejuvenation. Int J Mol Sci. 2021;22(5):2410.
Almalki SG, Agrawal DK. Key transcription factors in the differentiation of mesenchymal stem cells. Differentiation. 2016;92(1):41–51.
Pajarinen J, Lin T, Gibon E, Kohno Y, Maruyama M, Nathan K, et al. Mesenchymal stem cell-macrophage crosstalk and bone healing. Biomaterials. 2019;196:80–9.
Wang Y, Yao J, Cai L, Liu T, Wang X, Zhang Y, et al. Bone-targeted extracellular vesicles from mesenchymal stem cells for osteoporosis therapy. Int J Nanomed. 2020;15:7967–77.
Du GQ, Gong ZH, Liang B, Li P, Yang SY, Jia L, et al. Concentration changes of peripheral blood mesenchymal stem cells of Sprague Dawley rats during distraction osteogenesis. Orthop Surg. 2021;13(2):623–31.
Zhang D, Wu Y, Li Z, Chen H, Huang S, Jian C, et al. MiR-144-5p, an exosomal miRNA from bone marrow-derived macrophage in type 2 diabetes, impairs bone fracture healing via targeting Smad1. J Nanobiotechnol. 2021;19(1):226.
Ransohoff JD, Wei Y, Khavari PA. The functions and unique features of long intergenic non-coding RNA. Nat Rev Mol Cell Biol. 2018;19(3):143–57.
Fu Y, Hu X, Gao Y, Li K, Fu Q, Liu Q, et al. LncRNA ROR/miR-145-5p axis modulates the osteoblasts proliferation and apoptosis in osteoporosis. Bioengineered. 2021;12(1):7714–23.
Wang Y, Li T, Yang Q, Feng B, Xiang Y, Lv Z, et al. LncRNA THUMPD3-AS1 enhances the proliferation and inflammatory response of chondrocytes in osteoarthritis. Int Immunopharmacol. 2021;100:108138.
Shi C, Zheng W, Wang J. lncRNA-CRNDE regulates BMSC chondrogenic differentiation and promotes cartilage repair in osteoarthritis through SIRT1/SOX9. Mol Cell Biochem. 2021;476(4):1881–90.
Xia F, Wang Y, Xue M, Zhu L, Jia D, Shi Y, et al. LncRNA KCNQ1OT1: molecular mechanisms and pathogenic roles in human diseases. Genes Dis. 2022;9(6):1556–65.
Li Y, Li C, Li D, Yang L, Jin J, Zhang B. lncRNA KCNQ1OT1 enhances the chemoresistance of oxaliplatin in colon cancer by targeting the miR-34a/ATG4B pathway. Oncotargets Ther. 2019;12:2649–60.
Zheng L, Zhang F, Wang L, Hu H, Lian Y. LncRNA KCNQ1OT1 is overexpressed in non-small cell lung cancer and its expression level is related to clinicopathology. Eur Rev Med Pharmacol. 2019;23(16):6944.
Yu S, Yu M, He X, Wen L, Bu Z, Feng J. KCNQ1OT1 promotes autophagy by regulating miR-200a/FOXO3/ATG7 pathway in cerebral ischemic stroke. Aging Cell. 2019;18(3):e12940.
Gao X, Ge J, Li W, Zhou W, Xu L. LncRNA KCNQ1OT1 promotes osteogenic differentiation to relieve osteolysis via Wnt/β-catenin activation. Cell Biosci. 2018;8(1):19.
Wang C, Liao Z, Xiao H, Liu H, Hu Y, Liao Q, et al. LncRNA KCNQ1OT1 promoted BMP2 expression to regulate osteogenic differentiation by sponging miRNA-214. Exp Mol Pathol. 2019;107:77–84.
Yu Y, Chen Y, Zhang X, Lu X, Hong J, Guo X, et al. Knockdown of lncRNA KCNQ1OT1 suppresses the adipogenic and osteogenic differentiation of tendon stem cell via downregulating miR-138 target genes PPARγ and RUNX2. Cell cycle (Georgetown, Tex). 2018;17(19–20):2374–85.
Giordano L, Porta GD, Peretti GM, Maffulli N. Therapeutic potential of microRNA in tendon injuries. Brit Med Bull. 2020;133(1):79–94.
Oliviero APGG. MicroRNA in osteoarthritis: physiopathology, diagnosis and therapeutic challenge. Brit Med Bull. 2019;130:137–47.
Gargano G, Oliviero A, Oliva F, Maffulli N. Small interfering RNAs in tendon homeostasis. Brit Med Bull. 2021;138(1):58–67.
Gargano G, Oliva F, Oliviero A, Maffulli N. Small interfering RNAs in the management of human rheumatoid arthritis. Brit Med Bull. 2022;142(1):34–43.
Gargano G, Asparago G, Spiezia F, Oliva F, Maffulli N. Small interfering RNAs in the management of human osteoporosis. Br Med Bull. 2023:ldad023. https://doi.org/10.1093/bmb/ldad023.
Guo Q, Chen Y, Guo L, Jiang T, Lin Z. miR-23a/b regulates the balance between osteoblast and adipocyte differentiation in bone marrow mesenchymal stem cells. Bone Res. 2016;4(2):110–8.
Chen H, Li X, Liu S, Gu L, Zhou X. MircroRNA-19a promotes vascular inflammation and foam cell formation by targeting HBP-1 in atherogenesis. Sci Rep-UK. 2017;7(1):12010–89.
Wang N, Li R, Xue M. Potential regulatory network in the PSG10P/miR-19a-3p/IL1RAP pathway is possibly involved in preeclampsia pathogenesis. J Cell Mol Med. 2019;23(2):852–64.
Qiao F, Gong P, Song Y, Shen X, Su X, Li Y, et al. Downregulated PITX1 modulated by MiR-19a-3p promotes cell malignancy and predicts a poor prognosis of gastric cancer by affecting transcriptionally activated PDCD5. Cell Physiol Biochem. 2018;46(6):2215–31.
Zhang B, Liu Y, Zhang J. Silencing of miR-19a-3p enhances osteosarcoma cells chemosensitivity by elevating the expression of tumor suppressor PTEN. Oncol Lett. 2019;17(1):414–21.
Wang Z, Shi Z, Zhang L, Zhang H, Zhang Y. Profilin 1, negatively regulated by microRNA-19a-3p, serves as a tumor suppressor in human hepatocellular carcinoma. Pathol Res Pract. 2019;215(3):499–505.
Gu Y, Liu S, Zhang X, Chen G, Liang H, Yu M, et al. Oncogenic miR-19a and miR-19b co-regulate tumor suppressor MTUS1 to promote cell proliferation and migration in lung cancer. Protein Cell. 2017;8(6):455–66.
Ye Y, Ke Y, Liu L, Xiao T, Yu J, Christian M, et al. CircRNA FAT1 Regulates osteoblastic differentiation of periodontal ligament stem cells via miR-4781-3p/SMAD5 pathway. Stem Cells Int. 2021;2021:5177416–88.
Zhang X, Li H, Chen F, Chen Y, Chai Y, Liao J, et al. Icariin regulates miR-23a-3p-mediated osteogenic differentiation of BMSCs via BMP-2/Smad5/Runx2 and WNT/β-catenin pathways in osteonecrosis of the femoral head. Saudi Pharm J. 2021;29(12):1405–15.
Wu Y, Lu X, Shen B, Zeng Y. The therapeutic potential and role of miRNA, lncRNA, and circRNA in osteoarthritis. Curr Gene Ther. 2019;19(4):255–63.
Zhang H, Xu R, Li B, Xin Z, Ling Z, Zhu W, et al. LncRNA NEAT1 controls the lineage fates of BMSCs during skeletal aging by impairing mitochondrial function and pluripotency maintenance. Cell Death Differ. 2022;29(2):351–65.
Yin J, Zheng Z, Zeng X, Zhao Y, Ai Z, Yu M, et al. lncRNA MALAT1 mediates osteogenic differentiation of bone mesenchymal stem cells by sponging miR-129-5p. PeerJ. 2022;10:e13355.
Yu X, Song MS, Rong PZ, Chen XJ, Shi L, Wang CH, et al. LncRNA SNHG1 modulates adipogenic differentiation of BMSCs by promoting DNMT1 mediated Opg hypermethylation via interacting with PTBP1. J Cell Mol Med. 2022;26(1):60–74.
Shen Y, Xu J, Pan X, Zhang Y, Weng Y, Zhou D, et al. LncRNA KCNQ1OT1 sponges miR-34c-5p to promote osteosarcoma growth via ALDOA enhanced aerobic glycolysis. Cell Death Dis. 2020;11(4):278.
Chen P, Sun L, Shen H, Qu B. LncRNA KCNQ1OT1 accelerates ovarian cancer progression via miR-125b-5p/CD147 axis. Pathol Res Pract. 2022;239:154135.
Liu J, Jiang M, Guan J, Wang Y, Yu W, Hu Y, et al. LncRNA KCNQ1OT1 enhances the radioresistance of lung squamous cell carcinoma by targeting the miR-491-5p/TPX2-RNF2 axis. J Thorac Dis. 2022;14(10):4081–95.
Yu XH, Deng WY, Chen JJ, Xu XD, Liu XX, Chen L, et al. LncRNA kcnq1ot1 promotes lipid accumulation and accelerates atherosclerosis via functioning as a ceRNA through the miR-452-3p/HDAC3/ABCA1 axis. Cell Death Dis. 2020;11(12):1043.
Yang JJ, Peng WX, Zhang MB. LncRNA KCNQ1OT1 promotes osteogenic differentiation via miR-205-5p/RICTOR axis. Exp Cell Res. 2022;415(1):113119.
Wang L, Cho KB, Li Y, Tao G, Xie Z, Guo B. Long noncoding RNA (lncRNA)-mediated competing endogenous RNA networks provide novel potential biomarkers and therapeutic targets for colorectal cancer. Int J Mol Sci. 2019;20(22):5758.
Xu X, Huang C, Oka S. LncRNA KCNQ1OT1 promotes Atg12-mediated autophagy via inhibiting miR-26a-5p in ischemia reperfusion. Int J Cardiol. 2021;339:132–3.
Liu M, Yang P, Fu D, Gao T, Deng X, Shao M, et al. Allicin protects against myocardial I/R by accelerating angiogenesis via the miR-19a-3p/PI3K/AKT axis. Aging (Albany, NY). 2021;13(19):22843–55.
Ren H, Mu W, Xu Q. miR-19a-3p inhibition alleviates sepsis-induced lung injury via enhancing USP13 expression. Acta Biochim Pol. 2021;68(2):201–6. https://doi.org/10.18388/abp.2020_5505.
Li Y, Gao S, Xue W, Ma Y, Meng Y, Zhang D. miR-19a-3p functions as an oncogene by regulating FBXO32 expression in multiple myeloma. Balk Med J. 2021;38(1):43–9.
Xiang Y, Liu H, Hu H, Li LW, Zong QB, Wu TW, et al. LINC00094/miR-19a-3p/CYP19A1 axis affects the sensitivity of ER positive breast cancer cells to Letrozole through EMT pathway. Aging (Albany NY). 2022;14(11):4755–68.
Chen S, Li Y, Zhi S, Ding Z, Huang Y, Wang W, et al. lncRNA Xist regulates osteoblast differentiation by sponging miR-19a-3p in aging-induced osteoporosis. Aging Dis. 2020;11(5):1058–68.
Liu M, Sun F, Feng Y, Sun X, Li J, Fan Q, et al. MicroRNA-132-3p represses Smad5 in MC3T3-E1 osteoblastic cells under cyclic tensile stress. Mol Cell Biochem. 2019;458(1–2):143–57.
Lee JS, Kim ME, Seon JK, Kang JY, Yoon TR, Park Y, et al. Bone-forming peptide-3 induces osteogenic differentiation of bone marrow stromal cells via regulation of the ERK1/2 and Smad1/5/8 pathways. Stem Cell Res. 2018;26:28–35.
Acknowledgements
Not applicable.
Funding
The authors declare that no funds, grants, or other support were received during the preparation of this manuscript.
Author information
Authors and Affiliations
Contributions
All authors contributed to the study conception and design. Material preparation, data collection and analysis were performed by LN, GL, HW and TX. The first draft of the manuscript was written by HL and all authors commented on previous versions of the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Lin, H., Nie, L., Lu, G. et al. Long non-coding RNA KCNQ10T1/miR-19a-3p/SMAD5 axis promotes osteogenic differentiation of mouse bone mesenchymal stem cells. J Orthop Surg Res 18, 929 (2023). https://doi.org/10.1186/s13018-023-04425-w
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s13018-023-04425-w