Abstract
Periarticular infiltration following total knee and hip arthroplasty has been demonstrated to be equivalent to peripheral nerve blocks for postoperative pain management. The ideal cocktail has not been established yet. We have conducted a literature search on PubMed and Embase. Our search criteria included randomized controlled trials (RCTs) and systematic reviews (SRs). We tried to only include the most recent studies to keep the information current. The included research focused at Dexmedetomidine, Liposomal Bupivacaine, Ropivacaine, Epinephrine, Ketorolac, Morphine, Ketamine and Glucocorticosteroids. Each medication’s mode of action, duration, ideal dosage, contraindications, side effects and effectiveness have been summarized in the review article. This article will help the clinician to make an informed evidence-based decision about which medications to include in their ideal cocktail.
Similar content being viewed by others
Introduction
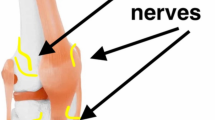
Periarticular infiltration is an effective adjunct to multimodal pain management following a joint replacement. Multiple drugs have been proposed to use in the periarticular cocktail. Unfortunately, there is no consensus on which medications to use. This review article summarizes the most recent evidence available for the most commonly used medicines in periarticular blocks. In addition, there are multiple sites proposed for injection. Ross et al. have summarized the best potential locations for periarticular injection based on nociceptor prevalence [1]. The highest concentrations of nociceptors in the knee were found in the medial and lateral retinacula, infrapatellar fat pad, pes anserine bursa, tibial, femoral, and patellar periosteum, and the bony insertions of the MCL, LCL and IT band [1]. In the hip articulation, there is limited research, but higher concentrations of nociceptors have been found in the labral base, ligamentum teres and the hip capsule [1]. No data exist regarding nociceptor concentration for tendon insertions around the hip joint.
We have conducted a literature search on PubMed and Embase. Our search criteria included randomized controlled trials (RCTs) and systematic reviews (SR). We tried only to include the most recent studies to keep the information current. We included only studies published in the English language. We aimed to have at least ten studies available for each drug. If insufficient RCTs and systematic reviews (SRs) were available, we broadened the search to include other specialties that also utilize local infiltration following surgery. Our search terms included: dexmedetomidine; Presedex, liposomal ketorolac, ketorolac, Toradol, tranexamic acid, morphine, glucocorticosteroids, triamcinolone acetate, methylprednisolone, betamethasone, ropivacaine, epinephrine, total knee arthroplasty, total knee replacement, periarticular infiltration, local anesthetic, peripheral block in combination with Boolean operators.
Dexmedetomidine (Presedex)
Using the search criteria, we identified 12 randomized controlled trials evaluating dexmedetomidine (Table 1) [2,3,4,5,6,7,8,9,10,11,12,13]. Dexmedetomidine (DM) is a highly selective alpha-2 adrenoceptor agonist with a half-life of 2 h [2]. It binds an alpha-2 receptor eight times more than clonidine [3]. Though multiple theories have been proposed, the exact mechanism of action is not completely understood. The first prevalent theory suggests that DM activates the alpha-2 adrenoreceptors on the peripheral smooth muscle cells, leading to vasoconstriction with subsequent delayed absorption of the local anesthetic [2]. Secondly, it was proposed that DM can block the activity-dependent cation current [2]. The activation of the Na–K pump causes hyperpolarization of the membrane in the peripheral fibers. DM could enhance hyperpolarization by blocking the Na–K currents, thus inhibiting the action potentials [7].
Because of the sedating effects, anesthesiologists routinely use intravenous (IV) DM to help sedate patients during a surgical procedure. If DM is used in the periarticular block, the sedating effects can improve sleep at night, a common problem after hip or knee replacements. Additional benefits include: reduced opioid usage, prolonged neuraxial analgesia, decreased postoperative delirium and reduced postoperative nausea [14]. In addition to the sedative effects described above, it also has anxiolytic, analgesic, anesthetic-sparing and sympatholytic effects [3].
Yang et al. [14] demonstrated a dose-dependent response using IV DM and postoperative hypotension and bradycardia. They suggested using the lowest possible dose (< 50ug) to minimize hemodynamic side effects. Even though there was a statistical difference in hemodynamic side effects between patients receiving DM compared to patients not receiving DM, they did not find a statistical difference comparing side effects between different doses of DM (< 50; 50–99; > 100ug). It is important to note that conclusions cannot be extrapolated from IV administration of DM to periarticular infiltration. Fritsch et al. [15] found that blood concentration was extremely low at 180 min after surgery. In this study, 150ug DM was added to the interscalene brachial plexus block [15].
The dosing of DM is not clearly defined, with different dose recommendations per route administration. Typically, IV dosing should be as minimal as possible (< 50ug), while intramuscular (2.5ug/kg) or periarticular dosing (1–2 ug/kg) can be more generous due to the slower absorption and low blood concentrations [2, 15, 16]. In the RCTs in Table 1, the dosages varied between 0.5 and 2 ug/kg. There was no correlation between dosage and duration of analgesia. DM showed a benefit in combination with longer-acting anesthetic drugs, such as ropivacaine and levobupivacaine, demonstrating longer-lasting pain relief [12].
There were minimal complications observed with the addition of DM. Only two studies from the twelve RCTs listed in Table 1 had observed side effects (bradycardia, hypotension and sedation) [6, 8]. Therefore, with careful screening, DM can be a valuable addition to the periarticular block. The most commonly used exclusion criteria in the studies in Table 1 include allergies to the medications [2, 4, 5, 7,8,9,10,11,12,13], diagnosis of severe cardiovascular disease [2, 5,6,7,8,9,10,11,12], diagnosis of hepatic or renal disease [2, 4], psychiatric disorders [4, 9,10,11, 13], pregnancy or lactation [5, 6, 10, 12], and history of neuromuscular disorders [2, 8,9,10]. Care should be exercised in these groups of patients. It is advisable to discuss patients with these conditions with your anesthesiologist and consider omitting or using smaller doses of DM (e.g., 0.5ug/kg).
Epinephrine
Epinephrine is commonly used in periarticular injections. It is a sympathomimetic catecholamine that affects alpha- and beta-adrenergic receptors. Its effect on Alpha-1 receptors produces increased vascular smooth muscle contraction. It is thought by this mechanism to create a synergistic effect when used with local anesthetics [17]. Epinephrine is believed to reduce the peripheral blood flow, prolonging the duration of local anesthetics and increasing the maximal dose that can be used without fear of systemic toxicity [17]. Its effect on the duration of local anesthetic is controversial, as it has not been well demonstrated. It is also thought to cause potentially harmful effects on wound healing through the local vasoconstriction at the level of the skin when blood flow is poor [17].
A literature review concerning the use of epinephrine in periarticular blocks does not reveal a clear consensus on its utility (Table 2) [18,19,20,21,22,23,24]. Some studies have reported statistically decreased visual analog pain scores when epinephrine is used in the periarticular block [19, 22], but this did not reach clinical significance. Contrarily, Kong et al. [18] showed no significant differences in pain scores postoperatively nor on opioid use after arthroplasty. There is some evidence that epinephrine can reduce postoperative bleeding when used in the periarticular block without increasing deep venous thrombosis (DVT) risk [21, 23]. However, it does not influence intraoperative blood loss, postoperative hemoglobin levels, or postoperative transfusion rates [21, 22].
Dosing of epinephrine as part of a periarticular cocktail does not have clear recommendations for administration. In the reviewed studies, epinephrine was typically diluted at concentrations of 1:1000 for total dosages ranging between 25 and 60 mcg. No studies compared different dosages of epinephrine.
No complications were recorded in any of the reviewed studies regarding the use of epinephrine in periarticular infiltrations. Specifically, no wound-healing problems related to using epinephrine in the periarticular cocktail were recorded.
Therefore, the benefits and recommended use of epinephrine as part of a periarticular block still need to be determined.
Glucocorticosteroids
Triamcinolone acetonide is a synthetic corticosteroid with a half-life of 18–36 h [25]. The steroids' acetate form is insoluble and postulated to remain in the tissues to give anti-inflammatory properties. This can be potentiated by the vasoconstricting effects of epinephrine [25, 26]. Methylprednisolone is a synthetic glucocorticosteroid with a half-life of 1.8–2.6 h [27, 28]. Betamethasone and dexamethasone have half-lives of 36–54 h [29,30,31]. All the corticosteroids examined in this review paper are primarily metabolized in the liver and excreted by the kidneys.
Glucocorticosteroids (GCSs) are thought to reduce the stress response, decrease edema and blood loss, prevent nausea and vomiting and achieve a better permanent range of motion by reducing the inflammatory response [32]. Pain is induced by inflammation. Inflammatory markers (IL-1Beta, IL-6, TGF-alpha) are released after surgery, leading to a decrease in the nociceptor threshold with subsequent occurrence of pain. GCS inhibits phospholipase A-2, which reduces the proinflammatory derivatives of arachidonic acid, decreasing inflammation [33, 34]. Interestingly, only one study [26] evaluated the inflammatory markers in patients receiving GCS and found a reduction in the CRP values until postoperative day four compared to the control group.
GCS also leads to decreased production of prostaglandins with their vasodilatory effects and a subsequent diminution in blood loss [33, 34]. Again, only two studies from the RCTs listed evaluated blood loss in patients receiving GCS as part of the PAI and found a non-statistical decrease in blood loss in the GCS group [26, 33].
There is, however, hesitancy in administering GCS routinely after joint replacements because of a fear of poor wound healing, postoperative infections or ligament/tendon ruptures. When evaluating the injection sites in the PAI, most studies injected GCS as part of the cocktail into the MCL [25,26,27,28, 30, 31, 35, 36] and LCL [26,27,28, 30, 31, 35, 36]. Four studies injected GCS into the patella tendon and fat pad [27, 28, 31, 35], while others did not inject GCS into the fat pad or patella tendon in fear of late rupture [25, 32, 36]. Two studies did not inject GCS in the subcutaneous skin to avoid steroid-induced skin atrophy [31, 33].
Most studies excluded patients with a history of renal insufficiency, uncontrolled diabetes mellitus, local infection, history of cardiac arrhythmias or prolonged QT intervals, immunosuppression (e.g., inflammatory conditions), history of congestive heart failure, psychiatric illnesses or history of gastrointestinal bleeding [25,26,27,28, 30, 31, 33, 35, 36].
No clear conclusions can be derived from the results of the randomized controlled trials in Table 3 [25,26,27,28,29,30,31,32,33, 35, 36]. Multiple studies demonstrated lower VAS scores in the immediate postoperative period. Most studies showed an effect in the 12–24-h postoperative period [26,27,28, 30, 32, 33, 35], while longer duration up to 48 h is supported only by several studies [27, 28, 33]. 72 h or more of lower VAS scores were only observed in a few studies [28, 33]. Even though some studies did not report any difference in VAS scores with the addition of corticosteroids [25, 31, 36], it does seem plausible to assume GCS can improve pain in the early postoperative period.
The increase in range of motion with the addition of GCS still needs to be clarified with an equal number of studies demonstrating benefits [28,29,30, 32, 33] compared to studies showing no apparent benefit [25,26,27, 31, 36].
Most studies demonstrated a decrease in length of stay in the cohort of patients receiving GCS in their PAI [29, 33, 36] compared to only one study, which did not show any benefit [31].
GCS added to the PAI is a safe option, with most studies not demonstrating an increase in adverse events [26, 28, 30,31,32,33, 35]. Two studies did show complications after GCS administration. The first study had one periprosthetic joint infection (PJI) with triamcinolone acetonide [25], and the second study demonstrated one PJI and two manipulations under anesthesia with methylprednisolone [30].
Ketorolac (Toradol)
We identified 12 articles exploring the effectiveness of ketorolac (Toradol; ketorolac tromethamine) as an element of periarticular injection (PAI) for total knee arthroplasty (TKA) (Table 4) [3, 37,38,39,40,41,42,43,44,45,46,47]. Ketorolac is a non-steroidal anti-inflammatory drug (NSAID) that has been an additive agent to various high-volume local infiltration analgesia cocktails, often including additional medications such as ropivacaine and epinephrine [48]. However, the efficacy of the individual elements of such regimens has limited evidence [37]. Ketorolac is a non-specific cyclooxygenase (COX) inhibitor, limiting the conversion of arachidonic acid to thromboxane, prostacyclin, and prostaglandins, thus decreasing sensitization of afferent nerves [49]. There is also evidence of an impact on the central hypothalamic prostaglandin response by inhibiting prostaglandin synthetase systems [50]. It was first approved for parenteral use in 1990 and thus has been available as an anti-inflammatory for over three decades. Ketorolac has a rapid onset of action following IM and IV administration, with peak analgesic effects at 75–150 min [49]. The half-life of ketorolac is 5–6 h [49]. Adverse effects of ketorolac are similar to those of other NSAIDs, including gastrointestinal bleeding, nausea/vomiting, peptic ulceration, renal failure and increased bleeding due to inhibited platelet function [49], which may limit its effectiveness in many patients receiving total knee arthroplasty.
Ketorolac's recommended IM and IV dose is 30 mg as a one-time dose or 30 mg every 6 h up to a maximum total of 120 mg in 24 h [51]. Still, some studies also describe 60 mg as a dosage option[39, 40] with a possible dose-dependent response [49, 52]. Many studies included visual analog scale (VAS) scores and opioid usage as measures of effectiveness for postoperative pain management. All but two studies [39, 45] that explored subjective pain scores found improved postoperative pain when ketorolac was added to the PAI. Decreased opioid usage was reported by Nikhar [3], Tammachote [47] and Andersen [37], but opioid usage was otherwise not significantly reduced [38,39,40]. Improvements in postoperative function, including range of motion [39, 40, 42, 44,45,46] and time to home readiness [37], were also investigated and were generally favorable, with some exceptions [39, 45]. Complications in the studies with ketorolac infiltration were rare, with one episode of hematoma [37] and one episode of DVT [43] highlighted across the studies. There were no documented statistical increases in complication rates for PAIs containing ketorolac. In the studies examined, the duration of action of ketorolac was reliably improved in the first 4–8 h [3, 37, 41,42,43,44, 46, 47], and pain relief was seen up to 96 h [43].
Overall, 9 out of the 12 studies did show a benefit of using ketorolac as part of multimodal pain management compared to a management plan not containing ketorolac. Exclusion criteria included previous surgery on the joint in question [37, 40, 42, 43, 46], previous infection [3, 41,42,43], bilateral knee joint involvement [44, 45], cardiac disease [3, 42, 43, 47], history of venous thromboembolism [42, 43], coagulopathies [3, 37, 40, 44], hepatic [3, 40, 46, 47] or kidney disease [3, 40, 42, 43, 46, 47], gastrointestinal ulcer [42, 43] or hemorrhage [42, 43], cerebrovascular disease [40, 42,43,44], neurocognitive disorders [41, 44, 47], rheumatoid arthritis [37, 40, 46], or allergies to the medications [3, 37, 40,41,42,43,44, 46, 47]. Patients under these exclusion criteria should be given extended clinical consideration before Toradol is included in periarticular infiltration during TKA.
Liposomal bupivacaine
Local anesthetics have proven beneficial in intraoperative and postoperative pain relief. Using local drugs, pain relief is mediated by impairing the voltage-gated sodium channels, which leads to decreased depolarization and therefore decreased conduction of pain signals [53]. However, their use is limited by their relatively short duration of action. Bupivacaine is a hepatically metabolized amide-type local anesthetic with an expected analgesic effect of up to 8–10 h [54].
Formulations of bupivacaine encapsulated in liposomes (liposomal bupivacaine) have been developed with the proposed benefit of sustained analgesia. These liposomes are composed of a lipid bilayer which encapsulates the anesthetic. This results in a delayed release of drugs based on lipid permeability and lipid bilayer breakdown prolonging the duration of action [55]. Initial studies have suggested an improvement of up to 72–96 h of effect [55]. The first formulations gained FDA approval in 2011. Subsequently, they began to be studied for the possibility of opioid-sparing effects [55].
Although some studies have shown benefits to liposomal bupivacaine over standard formulations[56], many have failed to show statistically significant improvements in opioid usage, time to discharge, or functional status in long-term follow-up [57,58,59,60,61,62] (Table 5). Several studies demonstrated no difference in pain experienced with rest [57,58,59,60,61,62,63]. Three studies did demonstrate decreased pain with physiotherapy [60, 64, 65], while only one study demonstrated the opposite [66]. Most studies showed no difference in the total narcotic usage [57,58,59,60,61, 64,65,66]. There were similar satisfaction rates in three studies [57, 62, 63], while only one study demonstrated better satisfaction with LB [56]. This has raised the question of the role of liposomal bupivacaine, especially given the greater-than-average cost of the medication60.
The most commonly used dosage in the RCTs examined was 20 mL (266 mg) of liposomal bupivacaine mixed with 40 mL of normal saline solution. Additional variations to the periarticular cocktail include adding epinephrine, ketorolac, or standard bupivacaine solutions.
The most commonly used exclusion criteria in the tabled studies include allergies to amide anesthetics [57, 59, 62, 63, 65, 66], chronic pain or opioid dependence [57, 59, 62, 64,65,66], abnormal hepatic, renal or cardiac function [56, 57, 65, 66] and elevated BMI [56, 62, 65, 66].
No specific complications were associated with liposomal bupivacaine described in the reviewed studies. Most reported complications involved arthroplasty complications such as infection, wound dehiscence or periprosthetic fracture following a fall [67]. One study involved a patient exceeding bupivacaine's toxicity threshold in a continuous femoral nerve block. However, this patient had no symptoms of systemic local toxicity [57]. The most reported serious complications of bupivacaine are related to cardiac and neurologic toxicity. These are more likely to occur when exceeding the recommended safe dose (2–2.5 mg/kg) but have been described in individuals even at lower doses [53]. The potentially life-threatening side effects highlight the importance of coordination between care team members to avoid reaching toxic doses.
Morphine
Morphine is an opioid medication that primarily acts through μ opioid receptors [68]. Activation of μ opioid receptors in the central nervous system has a long-standing history of use in pain control and sedation. Opioid receptors in the peripheral nervous system have also been described. However, the mechanism and effects are less well known.
The evidence described in arthroscopy literature shows the benefit of pain control with intra-articular injections. In animal studies, there is evidence for the benefit of local infiltration of morphine [69]. However, our literature review shows limited data for this in human studies (Table 6). Previous systematic reviews on periarticular morphine have identified a scarcity in the number of studies examining peri/intra-articular morphine [70]. An equivalent number of studies examined in Table 6 showed benefit [32, 47, 71,72,73] and no difference [74,75,76,77,78] in pain scores. The duration of pain improvement varied between 2 and 24 h [32, 47, 71,72,73]. Most studies showed a better range of motion with Morphine usage [47, 74,75,76,77,78]. This was similar to opioid consumption postoperatively. Six studies demonstrated less morphine consumption [32, 47, 71,72,73, 75], while some demonstrated no difference [74, 76,77,78]. This reduction in opioid consumption was observed in the first 48 h. Dosages used in studies reviewed showed ranges from 1 mg of morphine up to 10 mg. Most studies used 5 mg Morphine Sulfate [32, 47, 74, 78]. Three studies used a weight-based regime of 0.1 mg/kg [75,76,77]. Heine et al.’s [72] results suggested a likely dose-dependent effect in the case of intra-articular morphine.
Given the mixed data, its use should be considered in the context of possible adverse effects of systemic morphine, including nausea, vomiting, sedation, constipation, and pruritus being most described. Iwakiri et al.’s [77] study showed concerns about the possibility of nausea, vomiting and increased requirements for anti-emetics even in the case of local infiltration. However, there is again heterogeneity in the data as other articles reviewed showed no statistical difference in the development of nausea/vomiting postoperatively [32, 47, 71,72,73,74,75,76, 78].
The exclusion criteria most commonly utilized include but are not limited to morphine sensitivity [32, 47, 73,74,75,76,77,78]; alcohol or narcotic dependency [72,73,74,75,76,77]; previous surgical procedure involving the same knee [32, 75,76,77]; inability to do a spinal anesthetic [47, 72, 73, 78]; and a history of cardiac or thrombotic events [47, 75,76,77].
Ropivacaine
Ropivacaine is a local anesthetic agent initially described in reports of the first periarticular cocktails used in joint arthroplasty. Subsequent studies have used different drug cocktails and combinations of local anesthetics and compared their efficacy to ropivacaine [44, 45, 79, 80]. Ropivacaine is commonly used in epidurals and major nerve blocks. It is a long-acting local anesthetic that reversibly inhibits sodium ion influx in nerve fibers [81]. Some properties of ropivacaine that make it unique are that it is less lipophilic than other local anesthetics and, therefore, less likely to penetrate large, myelinated motor fibers, selectively acting on the nociceptive fibers [81]. It usually has an onset of action in 1–15 min with a duration of 2–6 h[82]. It is reported to have significantly less cardiotoxicity and neurotoxicity [81, 83, 84]. It is generally well tolerated with few side effects [85]. The most commonly reported adverse reactions are hypotension, nausea and vomiting [85].
When used in periarticular blocks in the context of arthroplasty, ropivacaine has demonstrated the capacity to lower patient-reported pain scores in a dose-dependent manner [82]. Newer literature has sought to compare its efficacy to bupivacaine and liposomal bupivacaine (Table 7). Based on our literature review, there has been no consistent statistically significant difference in the performance of these three drugs (see liposomal bupivacaine section) [45, 67, 79, 80]. One of the highlighted studies did show better pain management with ropivacaine compared to bupivacaine [44].
The usual dosage for ropivacaine used in a field block with 0.5% concentration is 5–200 mg (1–40 ml) [82]. In the tabled studies, the dosing of ropivacaine was weight-based, with Ropivacaine concentrations varying between 0.25 and 0.75% [86]. Van Haagen et al. [82] specifically looked at the effects of varying concentrations of ropivacaine in the periarticular block, comparing doses of 150–300 mg, and suggested that the higher dosage provided better pain relief.
Although ropivacaine use is commonly associated with nausea and vomiting, only one study commented on this side effect. Teratani et al. [87] reported that the group that received only ropivacaine in normal saline instead of a cocktail (including morphine, epinephrine, and betamethasone) said higher rates of nausea and vomiting postoperatively. However, this did not achieve statistical significance.
Ropivacaine is metabolized in the liver, and the metabolites are excreted through the renal system [82]. Therefore, dose adjustments should be considered for patients with hepatic or renal involvement. Caution should also be practiced in elderly debilitated patients and patients with cardiac disease [82].
Tranexamic acid (TXA)
Using the search criteria, there were five randomized control trials [88,89,90,91,92] evaluating the effect of TXA as an additive to periarticular infiltration (Table 8). Tranexamic acid (TXA) is a competitive inhibitor of plasminogen, a component of the fibrinolytic pathway necessary for hemostasis [91]. As such, TXA has shown promise in orthopedic surgery in reducing bleeding-related complications, such as hemarthrosis or postoperative bleeding. In most cases, TXA is administered intravenously, but TXA can also be administered intra-articularly [93,94,95], using drain clamping [92, 96,97,98,99], or via intraoperative TXA soak [91].
Peng et al. [91] hypothesized that TXA might be better functionally used as an element of PAI to infiltrate damaged tissues locally, prolonging the drug’s effects. They proposed that local infiltration may also reduce the risk of adverse effects [91], which include nausea, intraoperative hypotension, deep venous thrombosis, and blood transfusion [88,89,90, 92]. Peng et al. showed a significant decrease in HBL and blood loss in the PAI TXA group compared to the TXA administered intravenously [91]. Kim et al. showed decreased bleeding when PAI and IV administration are combined [88]. Pinsornak et al. [89] also showed decreases in blood loss and transfusion rates compared to intra-articular TXA.
However, the effect of TXA administered in a PAI may be limited to reduced blood loss. All the studies included showed decreased [89,90,91] or equivalent bleeding [88, 92] compared to other routes of TXA administration. Only one study by Zhang et al. [90] showed improved VAS scores and range of motion; it was only displayed in the short term. No studies demonstrated increased complications, including venous thromboembolism [88,89,90,91] or the need for transfusions [88,89,90,91], with the administration of TXA in a PAI.
Exclusion criteria for the studies listed include age < 18 [92] or > 80 [91], allergy to TXA [89, 91], secondary osteoarthritis [88, 89], bilateral TKA [88], cruciate-retaining prostheses [88], renal dysfunction [88, 91, 92], ischemic heart disease [88, 89], hepatic disease [88], malignancy [90], respiratory disease [88, 91], cerebrovascular disease [89], subarachnoid hemorrhage [89], acquired color-blindness [89], coagulopathy [88,89,90,91], or anticoagulation [89, 90], thrombocytopenia [88, 91], history of a pro-thrombotic condition or previous venous thromboembolism [88, 89, 91], pregnancy [91], breastfeeding [91], donated preoperative autologous blood [91, 92], postoperative allogenic blood transfusion [92], use of an unexpected prosthesis [92], severe synovectomy during the procedure [92] or low preoperative hemoglobin [89, 91]. Special consideration should be given to patients in these categories before providing TXA in a periarticular infiltration.
Conclusion
The ideal cocktail for pain control has not been established yet. Multiple drugs can be administered safely in this “cocktail” to help with pain control following a total knee and hip replacement. The medications should be individualized to avoid administering the medications to high-risk patients. Risk factors should be weighed against the benefits of the medications included in the periarticular injection. Even though the surgeon is administering the drug, there should be communication with the other team members (anesthesiologist, Internal medicine, ward physician, and pharmacists) to collaborate regarding the medications used and dosages. Certainly, other drugs might also prove beneficial in the future to optimize periarticular injections, and hopefully, future research might identify the optimal combination. The senior author uses periarticular injections around total knee and total hip replacements consistently with great success and have done so for the past 5 years. During this time, the “cocktail” has evolved and currently consists of 0.5% Ropivicaine 200 mg, Epinephrine 0.3 ml (1:1000), Ketorolac 30 mg, Dexmedetomidine 50ug, Methylprednisolone 40 mg and 1 g of Tranexamic acid. The senior author tries to adjust the “cocktail” if certain risk factors are present. That includes omitting Dexmedetomidine if patients have a history of bradycardia, hypotension etc., or Methylprednisolone if patients are a higher risk for infections among other things or adjusting the dosage of ropivacaine if a peripheral block was performed by the anesthesiologist. The main injection points utilized in total hip and knee replacements are at the highest concentrations of the nociceptors as described above.
Availability of data and materials
Not applicable – review article.
References
Ross JA, Greenwood AC, Sasser P III, Jiranek WA. Periarticular injections in knee and hip arthroplasty: where and what to inject. J Arthroplasty. 2017;32(9):S77–80.
Zhao E, Zhou K, Liu Z, Ding Z, Lu H, Chen J, et al. Dexmedetomidine prolongs the analgesic effects of periarticular infiltration analgesia following total knee arthroplasty: a prospective, double-blind, randomized controlled trial. J Arthroplasty. 2023.
Nikhar SA, Yadav M, Damera S, Mohan L, Ch VJ, Ramachandran G. A Comparative study of periarticular infiltration with dexmedetomidine versus ketorolac as an additive to ropivacaine after total knee arthroplasty: a prospective, randomized double-blind study. Anesth Essays Res. 2020;14(4):550.
Salem DAE, Nabi SMA, Alagamy SA, Kamel AAF. Comparative study between dexmedetomidine and fentanyl as an adjuvant to intraarticular bupivacaine for postoperative analgesia after knee arthroscopy. Pain Phys. 2021;24(7):E989.
Panigrahi R, Roy R, Mahapatra AK, Prasad A, Priyadarshi A, Palo N. Intra-articular adjuvant analgesics following knee arthroscopy: comparison between single and double dose dexmedetomidine and ropivacaine a multicenter prospective double-blind trial. Orthop Surg. 2015;7(3):250–5.
Mohamed S, Sayed D, El Sherif F, Abd E-R. Effect of local wound infiltration with ketamine versus dexmedetomidine on postoperative pain and stress after abdominal hysterectomy, a randomized trial. Eur J Pain. 2018;22(5):951–60.
Hao J, Wu Z, Luo Z, Dong B. Addition of dexmedetomidine to ropivacaine for local infiltration anaesthesia improves analgesic efficacy after tonsillectomy and adenoidectomy: a randomized controlled trial. Int J Pediatr Otorhinolaryngol. 2020;137:110168.
Azemati S, Pourali A, Aghazadeh S. Effects of adding dexmedetomidine to local infiltration of bupivacaine on postoperative pain in pediatric herniorrhaphy: a randomized clinical trial. Korean J Anesthesiol. 2020;73(3):212–8.
Elfadl GMA, AbdelRady MM, Osman HM, Gad MO, Abd el-Rady NM, Ali WN. Efficacy of Levobupivacaine Versus Levobupivacaine Plus dexmedetomidine infiltration for post-tonsillectomy analgesia: a randomized controlled trial. Pain Res Manag. 2022;2022.
Mitra S, Purohit S, Sharma M. Postoperative analgesia after wound infiltration with tramadol and dexmedetomidine as an adjuvant to ropivacaine for lumbar discectomies: a randomized-controlled clinical trial. J Neurosurg Anesthesiol. 2017;29(4):433–8.
Li J, Yang J-S, Dong B-H, Ye J-M. The effect of dexmedetomidine added to preemptive ropivacaine infiltration on postoperative pain after lumbar fusion surgery: a randomized controlled trial. LWW; 2019.
Yu J-M, Sun H, Wu C, Dong C-S, Lu Y, Zhang Y. The analgesic effect of ropivacaine combined with dexmedetomidine for incision infiltration after laparoscopic cholecystectomy. Surg Laparosc Endosc Percutaneous Tech. 2016;26(6):449–54.
Luan H, Zhu P, Zhang X, Tian L, Feng J, Wu Y, et al. Effect of dexmedetomidine as an adjuvant to ropivacaine for wound infiltration in patients undergoing open gastrectomy: a prospective randomized controlled trial. Medicine. 2017;96(38).
Yang SS, Gelinas C, Yim E, Li MM, Kardash K, Zhang M, et al. Association of intraoperative dexmedetomidine use with postoperative hypotension in unilateral hip and knee arthroplasties: a historical cohort study. Can J Anesth. 2022;69(12):1459–70.
Fritsch G, Danninger T, Allerberger K, Tsodikov A, Felder TK, Kapeller M, et al. Dexmedetomidine added to ropivacaine extends the duration of interscalene brachial plexus blocks for elective shoulder surgery when compared with ropivacaine alone: a single-center, prospective, triple-blind, randomized controlled trial. Reg Anesth Pain Med. 2014;39(1):37–47.
Karaaslan D, Peker TT, Alaca A, Ozmen S, Kirdemir P, Yorgancigil H, et al. Comparison of buccal and intramuscular dexmedetomidine premedication for arthroscopic knee surgery. J Clin Anesth. 2006;18(8):589–93.
Bylund DB. Epinephrine. xPharm: The Comprehensive Pharmacology Reference. In: Elsevier eBooks [Internet]. 2007; p.1–5. https://doi.org/10.1016/b978-008055232-3.61695-2.
Kong DY, Oh JH, Choi WR, Ko Y-I, Choi CH. The impact of epinephrine in the periarticular injection cocktail using ropivacaine for total knee arthroplasty: a prospective, randomized, double-blind comparison study. J Arthroplasty. 2020;35(9):2439–43.
Chareancholvanich K, Tantithawornwat S, Ruangsomboon P, Narkbunnam R, Chatmaitri S, Pornrattanamaneewong C. Efficacy of epinephrine in local infiltration analgesia on pain relief and opioid consumption following total knee arthroplasty: a randomized controlled trial. Acta Orthop. 2023;94:97.
Liu J, Zeng W, Wang F, Chen C, Gong X, Yang H, et al. Effects of low-dose epinephrine on perioperative hemostasis and inflammatory reaction in major surgical operations: a randomized clinical trial. J Thromb Haemost. 2018;16(1):74–82.
Teng Y, Ma J, Ma X, Wang Y, Lu B, Guo C. The efficacy and safety of epinephrine for postoperative bleeding in total joint arthroplasty: a PRISMA-compliant meta-analysis. Medicine. 2017;96(17).
Villatte G, Engels E, Erivan R, Mulliez A, Caumon N, Boisgard S, et al. Effect of local anaesthetic wound infiltration on acute pain and bleeding after primary total hip arthroplasty: the EDIPO randomised controlled study. Int Orthop. 2016;40:2255–60.
Gao F, Sun W, Guo W, Li Z, Wang W, Cheng L. Topical administration of tranexamic acid plus diluted-epinephrine in primary total knee arthroplasty: a randomized double-blinded controlled trial. J Arthroplasty. 2015;30(8):1354–8.
Mikjunovikj-Derebanova L, Kartalov A, Kuzmanovska B, Donev L, Lleshi A, Toleska M, et al. Epinephrine and dexamethasone as adjuvants in upper extremity peripheral nerve blocks in pediatric patients. Prilozi. 2021;42(3):79–88.
Chia SK, Wernecke GC, Harris IA, Bohm MT, Chen DB, MacDessi SJ. Peri-articular steroid injection in total knee arthroplasty: a prospective, double blinded, randomized controlled trial. J Arthroplasty. 2013;28(4):620–3.
Kwon SK, Yang IH, Bai SJ, Han CD. Periarticular injection with corticosteroid has an additional pain management effect in total knee arthroplasty. Yonsei Med J. 2014;55(2):493–8.
Reddy AG, Thayi C, Natarajan N, Sankineani SR, Daultani D, Khanna V, et al. Validating the role of steroid in analgesic cocktail preparation for local infiltration in total knee arthroplasty: a comparative study. Anesth Essays Res. 2018;12(4):903.
Kulkarni M, Mallesh M, Wakankar H, Prajapati R, Pandit H. Effect of methylprednisolone in periarticular infiltration for primary total knee arthroplasty on pain and rehabilitation. J Arthroplasty. 2019;34(8):1646–9.
El-Boghdadly K, Short AJ, Gandhi R, Chan V. Addition of dexamethasone to local infiltration analgesia in elective total knee arthroplasty: double-blind, randomized control trial. Reg Anesth Pain Med. 2021;46(2):130–6.
Wang Q, Tan G, Mohammed A, Zhang Y, Li D, Chen L, et al. Adding corticosteroids to periarticular infiltration analgesia improves the short-term analgesic effects after total knee arthroplasty: a prospective, double-blind, randomized controlled trial. Knee Surg Sports Traumatol Arthrosc. 2021;29:867–75.
Yue D-b, Wang B-l, Liu K-p, Guo W-s. Efficacy of multimodal cocktail periarticular injection with or without steroid in total knee arthroplasty. Chin Med J. 2013;126(20):3851–5.
Kim TW, Park SJ, Lim SH, Seong SC, Lee S, Lee MC. Which analgesic mixture is appropriate for periarticular injection after total knee arthroplasty? Prospective, randomized, double-blind study. Knee Surg Sports Traumatol Arthrosc. 2015;23(3):838–45. https://doi.org/10.1007/s00167-014-3366-x.
Seah V, Chin P, Chia S, Yang K, Lo N, Yeo S. Single-dose periarticular steroid infiltration for pain management in total knee arthroplasty: a prospective, double-blind, randomised controlled trial. Singap Med J. 2011;52(1):19.
Li Z, Li Z, Cheng K, Weng X. The efficacy and safety of glucocorticoid on periarticular infiltration analgesia in total knee arthroplasty: a systematic review and meta-analysis of randomized controlled trials. J Arthroplasty. 2021;36(9):3340–50.
Tsukada S, Wakui M, Hoshino A. The impact of including corticosteroid in a periarticular injection for pain control after total knee arthroplasty: a double-blind randomised controlled trial. Bone Joint J. 2016;98(2):194–200.
Christensen CP, Jacobs CA, Jennings HR. Effect of periarticular corticosteroid injections during total knee arthroplasty: a double-blind randomized trial. JBJS. 2009;91(11):2550–5.
Andersen KV, Nikolajsen L, Haraldsted V, Odgaard A, Søballe K. Local infiltration analgesia for total knee arthroplasty: should ketorolac be added? Br J Anaesth. 2013;111(2):242–8. https://doi.org/10.1093/bja/aet030.
Hannon CP, Fillingham YA, Spangehl MJ, Karas V, Kamath AF, Casambre FD, et al. The efficacy and safety of periarticular injection in total joint arthroplasty: a direct meta-analysis. J Arthroplasty. 2022;37(10):1928-38.e9. https://doi.org/10.1016/j.arth.2022.03.045.
Apinyankul R, Lilakhunakon K, Witayakom W, Vechvitvarakul M, Goodman SB. Efficacy of periarticular multimodal analgesic injection containing high-dose ketorolac versus triamcinolone in early postoperative total knee arthroplasty: a randomized controlled trial. Surg Technol Int. 2022;40:321–6. https://doi.org/10.52198/22.Sti.40.Os1591.
Hinzpeter J, Barahona M, Aliste J, Barrientos C, Zamorano A, Palet M, et al. Gonyautoxins 2/3 local periarticular injection for pain management after total knee arthroplasty: a double-blind, Randomized Study. J Knee Surg. 2023;36(4):389–96. https://doi.org/10.1055/s-0041-1735312.
Kopitkó C, Czermann R, Orosz M, Hangody G, Kiss D, Szabó Z, et al. A randomized comparative evaluation of local infiltration analgesia, extended nerve blocks, and conventional analgesia in pain management after total knee arthroplasty. Joint Dis Relat Surg. 2021;32(2):290–8. https://doi.org/10.52312/jdrs.2021.68.
Laoruengthana A, Rattanaprichavej P, Mahatthanatrakul A, Tantimethanon T, Lohitnavy M, Pongpirul K. Periarticular Injection of ketorolac augmenting intravenous administration of ketorolac for postoperative pain control: a randomized controlled trial in simultaneous bilateral total knee arthroplasty. J Knee Surg. 2022;35(8):868–73. https://doi.org/10.1055/s-0040-1721088.
Laoruengthana A, Rattanaprichavej P, Reosanguanwong K, Chinwatanawongwan B, Chompoonutprapa P, Pongpirul K. A randomized controlled trial comparing the efficacies of ketorolac and parecoxib for early pain management after total knee arthroplasty. Knee. 2020;27(6):1708–14. https://doi.org/10.1016/j.knee.2020.10.005.
Liu M, Zhang D, Shi B. Comparison of the post-total knee arthroplasty analgesic effect of intraoperative periarticular injection of different analgesics. J Coll Physicians Surg Pak. 2019;29(12):1169–72. https://doi.org/10.29271/jcpsp.2019.12.1169.
Danoff JR, Goel R, Henderson RA, Fraser J, Sharkey PF. Periarticular ropivacaine cocktail is equivalent to liposomal bupivacaine cocktail in bilateral total knee arthroplasty. J Arthroplasty. 2018;33(8):2455–9. https://doi.org/10.1016/j.arth.2018.02.083.
Motififard M, Omidian A, Badiei S. Pre-emptive injection of peri-articular-multimodal drug for post-operative pain management in total knee arthroplasty: a double-blind randomized clinical trial. Int Orthop. 2017;41(5):939–47. https://doi.org/10.1007/s00264-016-3357-2.
Tammachote N, Kanitnate S, Manuwong S, Panichkul P. Periarticular multimodal drug injection is better than single anesthetic drug in controlling pain after total knee arthroplasty. Eur J Orthop Surg Traumatol. 2018;28(4):667–75. https://doi.org/10.1007/s00590-017-2110-x.
Kerr DR, Kohan L. Local infiltration analgesia: a technique for the control of acute postoperative pain following knee and hip surgery: a case study of 325 patients. Acta Orthop. 2008;79(2):174–83. https://doi.org/10.1080/17453670710014950.
Vadivelu N, Gowda AM, Urman RD, Jolly S, Kodumudi V, Maria M, et al. Ketorolac tromethamine–routes and clinical implications. Pain Pract. 2015;15(2):175–93.
Rice AS, Lloyd J, Bullingham RE, O’Sullivan G. Ketorolac penetration into the cerebrospinal fluid of humans. J Clin Anesth. 1993;5(6):459–62. https://doi.org/10.1016/0952-8180(93)90061-i.
Mahmoodi AN, Kim PY. Ketorolac. 2019.
Mroszczak EJ, Jung D, Yee J, Bynum L, Sevelius H, Massey I. Ketorolac tromethamine pharmacokinetics and metabolism after intravenous, intramuscular, and oral administration in humans and animals. Pharmacother J Hum Pharmacol Drug Ther. 1990;10(6P2):33S-9S.
Aronson JK. Meyler’s side effects of drugs: the international encyclopedia of adverse drug reactions and interactions. Amsterdam: Elsevier; 2015.
Vyas KS, Rajendran S, Morrison SD, Shakir A, Mardini S, Lemaine V, et al. Systematic review of liposomal bupivacaine (Exparel) for postoperative analgesia. Plast Reconstr Surg. 2016;138(4):748e-e756.
Prabhakar A, Ward CT, Watson M, Sanford J, Fiza B, Moll V, et al. Liposomal bupivacaine and novel local anesthetic formulations. Best Pract Res Clin Anaesthesiol. 2019;33(4):425–32.
Dysart SH, Barrington JW, Del Gaizo DJ, Sodhi N, Mont MA. Local infiltration analgesia with liposomal bupivacaine improves early outcomes after total knee arthroplasty: 24-hour data from the PILLAR study. J Arthroplasty. 2019;34(5):882–6.
Marino J, Scuderi G, Dowling O, Farquhar R, Freycinet B, Overdyk F. Periarticular knee injection with liposomal bupivacaine and continuous femoral nerve block for postoperative pain management after total knee arthroplasty: a randomized controlled trial. J Arthroplasty. 2019;34(3):495–500.
Dizdarevic A, Aviles B, Kosharskyy B, Kim SJ, Nolasco L, Kumar R, et al. Feasibility and efficacy trial comparing liposomal bupivacaine and bupivacaine mixture with bupivacaine only in pre-operative four compartments periarticular infiltration block for patients undergoing total knee arthroplasty; an assessor-blinded single-center randomized trial. 2020.
Hyland SJ, Deliberato DG, Fada RA, Romanelli MJ, Collins CL, Wasielewski RC. Liposomal bupivacaine versus standard periarticular injection in total knee arthroplasty with regional anesthesia: a prospective randomized controlled trial. J Arthroplasty. 2019;34(3):488–94.
Smith EB, Kazarian GS, Maltenfort MG, Lonner JH, Sharkey PF, Good RP. Periarticular liposomal bupivacaine injection versus intra-articular bupivacaine infusion catheter for analgesia after total knee arthroplasty: a double-blinded, randomized controlled trial. JBJS. 2017;99(16):1337–44.
Perets I, Walsh JP, Mu BH, Yuen LC, Ashberg L, Battaglia MR, et al. Intraoperative infiltration of liposomal bupivacaine vs bupivacaine hydrochloride for pain management in primary total hip arthroplasty: a prospective randomized trial. J Arthroplasty. 2018;33(2):441–6.
Alijanipour P, Tan TL, Matthews CN, Viola JR, Purtill JJ, Rothman RH, et al. Periarticular injection of liposomal bupivacaine offers no benefit over standard bupivacaine in total knee arthroplasty: a prospective, randomized, controlled trial. J Arthroplasty. 2017;32(2):628–34.
Ali I, Gupta HO, Khazzam M, Thomas GL, Vattigunta S, Shi BY, et al. Do local liposomal bupivacaine and interscalene nerve block provide similar pain control after shoulder arthroplasty? A dual-center randomized controlled trial. J Shoulder Elbow Surg. 2021;30(7):S145–52.
Zlotnicki JP, Hamlin BR, Plakseychuk AY, Levison TJ, Rothenberger SD, Urish KL. Liposomal bupivacaine vs plain bupivacaine in periarticular injection for control of pain and early motion in total knee arthroplasty: a randomized, prospective study. J Arthroplasty. 2018;33(8):2460–4.
Johnson RL, Amundson AW, Abdel MP, Sviggum HP, Mabry TM, Mantilla CB, et al. Continuous posterior lumbar plexus nerve block versus periarticular injection with ropivacaine or liposomal bupivacaine for total hip arthroplasty: a three-arm randomized clinical trial. JBJS. 2017;99(21):1836–45.
Talmo CT, Kent SE, Fredette AN, Anderson MC, Hassan MK, Mattingly DA. Prospective randomized trial comparing femoral nerve block with intraoperative local anesthetic injection of liposomal bupivacaine in total knee arthroplasty. J Arthroplasty. 2018;33(11):3474–8.
Amundson AW, Johnson RL, Abdel MP, Mantilla CB, Panchamia JK, Taunton MJ, et al. A three-arm randomized clinical trial comparing continuous femoral plus single-injection sciatic peripheral nerve blocks versus periarticular injection with ropivacaine or liposomal bupivacaine for patients undergoing total knee arthroplasty. Anesthesiology. 2017;126(6):1139–50.
Listos J, Łupina M, Talarek S, Mazur A, Orzelska-Górka J, Kotlińska J. The mechanisms involved in morphine addiction: an overview. Int J Mol Sci. 2019;20(17):4302.
Buvanendran A, Kroin JS, Della Valle CJ, Moric M, Tuman KJ. Local drug infiltration analgesia during knee surgery to reduce postoperative pain in rats. Reg Anesth Pain Med. 2016;41(3):374–9.
Zhang Y, Mi F, Zhao H, Xie D, Shi X. Effect of morphine added to multimodal cocktail on infiltration analgesia in total knee arthroplasty: a meta-analysis of randomized controlled trials. Medicine. 2019;98(41).
Garcia JBSG, Barbosa Neto JO, Vasconcelos JW, Ferro LSG. Analgesic efficacy of the intra-articular administration of high doses of morphine in patients undergoing total knee arthroplasty. Rev Bras Anestesiol. 2010;60:1–12.
Heine M, Tillet E, Tsueda K, Loyd G, Schroeder J, Vogel R, et al. Intra-articular morphine after arthroscopic knee operation. Br J Anaesth. 1994;73(3):413–5.
Li Y, Wulamu W, Yushan N, Guo X, Gu W, Cao L, et al. Effects of adding morphine to periarticular infiltration analgesia combined with single dose epidural morphine in total knee arthroplasty: a randomized controlled study. Orthop Surg. 2023;15(4):1021–7.
Han C-D, Lee D-H, Yang IH. Intra-synovial ropivacaine and morphine for pain relief after total knee arthroplasty-a prospective, randomized, double blind study. Yonsei Med J. 2007;48(2):295–300.
Wang Q, Sun J, Hu Y, Zeng Y, Hu J, Yang J, et al. Effects of morphine on peri-articular infiltration analgesia in total knee arthroplasty: a prospective, double-blind, randomized controlled trial. Int Orthop. 2020;44:2587–95.
Iwakiri K, Minami Y, Ohta Y, Kobayashi A. Effect of periarticular morphine injection for total knee arthroplasty: a randomized, double-blind trial. J Arthroplasty. 2017;32(6):1839–44.
Iwakiri K, Ohta Y, Kobayashi A, Minoda Y, Nakamura H. Local efficacy of periarticular morphine injection in simultaneous bilateral total knee arthroplasty: a prospective, randomized, double-blind trial. J Arthroplasty. 2017;32(12):3637–42.
Mauerhan DR, Campbell M, Miller JS, Mokris JG, Gregory A, Kiebzak GM. Intra-articular morphine and/or bupivacaine in the management of pain after total knee arthroplasty. J Arthroplasty. 1997;12(5):546–52.
Leeuw MAD, Perez RG. Posterior lumbar plexus block in postoperative analgesia for total hip arthroplasty: a comparative study between 0.5% bupivacaine with epinephrine and 0.5% ropivacaine. Rev Bras Anestesiol. 2010;60:215.
Hungerford M, Neubauer P, Ciotola J, Littleton K, Boner A, Chang L. Liposomal bupivacaine vs ropivacaine for adductor canal blocks in total knee arthroplasty: a prospective randomized trial. J Arthroplasty. 2021;36(12):3915–21.
Ropivacaine PN. In: Watkins-Pitchford JM, Jahr JS, Sinatra RS, editors. The essence of analgesia and analgesics. Cambridge: Cambridge University Press; 2010. p. 276–8.
van Haagen MH, Verburg H, Hesseling B, Coors L, van Dasselaar NT, Langendijk PN, et al. Optimizing the dose of local infiltration analgesia and gabapentin for total knee arthroplasty, a randomized single blind trial in 128 patients. Knee. 2018;25(1):153–60.
Scott DB, Lee A, Fagan D, Bowler GM, Bloomfield P, Lundh R. Acute toxicity of ropivacaine compared with that of bupivacaine. Anesth Analg. 1989;69(5):563–9.
Sztark F, Malgat M, Dabadie P, Mazat J-P. Comparison of the effects of bupivacaine and ropivacaine on heart cell mitochondrial bioenergetics. J Am Soc Anesth. 1998;88(5):1340–9.
Sinatra RS, Jahr JS, Watkins-Pitchford JM. The essence of analgesia and analgesics. London: Cambridge University Press; 2010.
Xiao Q, Xu B, Wang H, Luo Z, Yuan M, Zhou Z, et al. Analgesic effect of single-shot ropivacaine at different layers of the surgical site in primary total hip arthroplasty: a randomised, controlled, observer-blinded study. J Orthop Surg Res. 2021;16(1):1–10.
Teratani T. Effect of cocktail therapy after arthroscopic rotator cuff repair: a randomized, double-blind trial. J Shoulder Elbow Surg. 2020;29(7):1310–5.
Kim KI, Bae JK, Kim JH, Gwak HG, Lee SH. Tranexamic acid in a periarticular multimodal cocktail injection for blood management in total knee arthroplasty: a prospective randomized study. BMC Musculoskelet Disord. 2021;22(1):675. https://doi.org/10.1186/s12891-021-04551-8.
Pinsornsak P, Phunphakchit J, Boontanapibul K. Efficacy and systemic absorption of peri-articular versus intra-articular administration of tranexamic acid in total knee arthroplasty: a prospective randomized controlled trial. Arthroplasty Today. 2021;11:1–5.
Zhang S, Wang C, Shi L, Xue Q. Multi-route applications of tranexamic acid to reduce blood loss after total knee arthroplasty: a randomized controlled trial. Medicine. 2019;98(30):e16570. https://doi.org/10.1097/md.0000000000016570.
Peng HM, Wang W, Lin J, Weng XS, Qian WW, Wang WD. Multimodal Peri-articular injection with tranexamic acid can reduce postoperative blood loss versus intravenous tranexamic acid in total knee arthroplasty: a randomized controlled trial. J Orthop Surg Res. 2021;16(1):546. https://doi.org/10.1186/s13018-021-02685-y.
Hishimura R, Onodera T, Ohkoshi Y, Okada K, Matsuoka M, Matsubara S, et al. The effect of local injection of tranexamic acid into peri-articular tissue versus drain clamping in total knee arthroplasty: a randomized controlled trial. BMC Musculoskelet Disord. 2022;23(1):111. https://doi.org/10.1186/s12891-022-05058-6.
Seo J-G, Moon Y-W, Park S-H, Kim S-M, Ko K-R. The comparative efficacies of intra-articular and IV tranexamic acid for reducing blood loss during total knee arthroplasty. Knee Surg Sports Traumatol Arthrosc. 2013;21:1869–74.
Mao Z, Yue B, Wang Y, Yan M, Dai K. A comparative, retrospective study of peri-articular and intra-articular injection of tranexamic acid for the management of postoperative blood loss after total knee arthroplasty. BMC Musculoskelet Disord. 2016;17:1–8.
Alshryda S, Mason J, Vaghela M, Sarda P, Nargol A, Maheswaran S, et al. Topical (intra-articular) tranexamic acid reduces blood loss and transfusion rates following total knee replacement: a randomized controlled trial (TRANX-K). JBJS. 2013;95(21):1961–8.
Tsumara N, Yoshiya S, Chin T, Shiba R, Kohso K, Doita M. A prospective comparison of clamping the drain or post-operative salvage of blood in reducing blood loss after total knee arthroplasty. J Bone Joint Surg Br Vol. 2006;88(1):49–53.
Liao L, Chen Y, Tang Q, Chen Y-Y, Wang W-C. Tranexamic acid plus drain-clamping can reduce blood loss in total knee arthroplasty: a systematic review and meta-analysis. Int J Surg. 2018;52:334–41.
Onodera T, Majima T, Sawaguchi N, Kasahara Y, Ishigaki T, Minami A. Risk of deep venous thrombosis in drain clamping with tranexamic acid and carbazochrome sodium sulfonate hydrate in total knee arthroplasty. J Arthroplasty. 2012;27(1):105–8.
Chareancholvanich K, Siriwattanasakul P, Narkbunnam R, Pornrattanamaneewong C. Temporary clamping of drain combined with tranexamic acid reduce blood loss after total knee arthroplasty: a prospective randomized controlled trial. BMC Musculoskelet Disord. 2012;13(1):1–6.
Funding
No funding was provided for the completion of this systematic review.
Author information
Authors and Affiliations
Contributions
JV + BS + AL + GK +MN all wrote the main manuscript text and all prepared the tables 1-8. All authors reviewed the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Ethics approval and consent to participate
Not applicable – review article.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
King, G.A., Le, A., Nickol, M. et al. Periarticular infiltration used in total joint replacements: an update and review article. J Orthop Surg Res 18, 859 (2023). https://doi.org/10.1186/s13018-023-04333-z
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s13018-023-04333-z